Abstract
The psychological mechanisms by which depressed mood can lead to impaired sleep and poorer overall health remain unclear. The goal of this study was to investigate the extent to which a tendency to ruminate accounts for the associations between depressed mood and both sleep quality and self-reported health in 165 healthy young adults. Self-reported assessments of anxiety, depressed mood, rumination, sleep quality, and general health were collected at two different time points approximately two months apart. Structural equation modeling revealed that rumination measured at the earlier time point mediated the relationships between depressed mood and both sleep quality and health, all measured at the later time point, in a model that was a good fit to the data overall, χ2 (50, N = 165) = 103.08, p < .001; RMSEA = .08 (.06 – .10), TLI = .91, CFI = .94. Results were similar whether or not anxiety was controlled. Results indicate that rumination may be a psychological mechanism by which negative mood leads to impaired sleep and poorer perceived health.
Keywords: rumination, anxiety, depressed mood, sleep quality, perceived health
Individuals with mood disorders as well as those with higher than average levels of depressed mood and anxiety often have poorer sleep quality (Thomsen et al., 2003; Tsypes et al., 2013) and poorer overall perceived health (Simon, 2003) than those without such issues. Although both poor sleep and health can contribute causally to negative mood, it is increasingly clear that depressed mood can contribute to less adaptive health behaviors and worse health, via a variety of routes (Kiecolt-Glaser & Glaser, 2002; Salovey et al., 2000). However, the psychological mechanisms underlying these associations are unclear. One likely candidate is rumination, or the tendency to engage in perseverative non-constructive thought and negative self-reflection on past or present problems and feelings (Nolen-Hoeksema & Morrow, 1991). Depression has been shown to involve heightened self-focused attention which can put one at risk for maladaptive rumination (Takano & Tanno, 2009). In turn, those who report more rumination in times of stress and bereavement have been shown to have higher depressive scores (Nolen-Hoeksema, 2000). A less-established but growing body of literature links ruminative tendencies with both sleep quality (Guastella & Moulds, 2007; Thomsen et al., 2003; Zawadzki et al., 2013) and overall physical health (Sansone & Sansone, 2012; Thomsen et al., 2004). Rumination has been significantly associated with sleep quality even after controlling for negative and anxious mood (e.g., Thomsen et al., 2003) and has been posited as a mediator of the effects of stressful experiences on various health outcomes (Brosschot et al., 2010; Gerin et al., 2012). The goal of the present research was to examine ruminative tendencies as a mediator of the relationships between depressed mood and both sleep quality and self-reported health.
Both self-reported general health and poor sleep quality are important health outcomes in the general population. Poor sleep quality and sleep deprivation are increasingly common (Buboltz et al., 2001; Gaultney, 2010); based on the Sleep in America Survey, 16% of Americans, including young adults 18 years and older, sleep fewer than 6 hours per night during weekdays, and 65% of the sample surveyed reported some sleep-related problem multiple times per week (National Sleep Foundation, 2008). Young adults and college students are at a growing risk for impaired sleep; dissatisfaction among college students about their levels of sleep increased from 24% in 1978 to 53% in 1988 to 71% in 2000 (Hicks et al., 2001). Sleep disturbances are also often comorbid with a number of mental and physical health issues such depression (Benca et al., 1992; Mosko et al., 1989; Van Moffaert, 1994), anxiety (Benca et al., 1992; Rosa et al., 1983), alcohol use (Graham & Streitel, 2010; Roehrs & Roth, 2001), obesity (Patel & Hu, 2008; Punjabi & Polotsky, 2005; Spiegel et al., 2005), hypertension (Gottlieb et al., 2006); and chronic pain (Graham & Streitel, 2010; Smith & Haythornthwaite, 2004). Studies of experimentally-induced sleep deprivation in young healthy adults (as reviewed in Knutson et al., 2007; Mullington et al., 2009) have shown changes in metabolic profiles that may place one at risk for conditions like diabetes mellitus (Spiegel et al., 2005), insulin resistance (Ayas et al., 2003), oxidative stress (Möller-Levet et al., 2013), and hypertension (Gottlieb et al., 2006).
Depression has been strongly linked with both sleep and health. Patients with diagnosed clinical depression have reported poorer quality of life, in terms of both psychological and physical health as compared to those individuals without clinical depression (Kuehner & Buerger, 2005). In terms of sleep, changes in sleeping patterns are part of the diagnostic criteria for diagnosing depression, and multiple studies have shown through polysomnographic techniques that depressed individuals have increased sleep latency and sleep disturbances (Walker & van Der Helm, 2009).
It is likely that cognitive tendencies akin to rumination drive connections between depressed mood and sleep quality and health. Rumination exacerbates negative thinking styles and negative mood; impairs coping, judgment, and problem-solving; and leads to more negative perseverative thought about the past, present, and future (Nolen-Hoeksema et al., 2008). According to the perseverative cognition hypothesis (Brosschot et al., 2006), rumination can lead to a sustained psychophysiological arousal that can cause short-term and long-term disturbances in sleep, either directly through immune system and neuroendocrine alterations or indirectly through the adoption of maladaptive health behaviors, such as substance abuse or lack of physical exercise. In keeping with this, impaired sleep has been linked with poor coping styles in response to stress (Sadeh et al., 2004). Moreover, in one study of college students, rumination mediated the relationships between loneliness and depressed mood and between loneliness and sleep quality (Zawadzki et al., 2013). Several other studies suggest that rumination is an independent predictor of sleep quality, even after controlling for other aspects of negative mood, such as anxiety, anger, and depression (Nolen-Hoeksema et al., 2008; Thomsen et al., 2003).
Despite suggestive research on the role of rumination and sleep quality, it remains unclear whether rumination fully and consistently accounts for a role of depressed mood on sleep and perceived health. Importantly, rumination appears to be a relatively stable trait, even in the presence of fluctuating mood (Nolen-Hoeksema, 2000; Nolen-Hoeksema et al., 2008). The primary goal of the present research was to examine the degree to which depressed mood at baseline predicts sleep quality and self-reported health approximately two months later and the extent to which ruminative tendencies uniquely account for this association in a sample of healthy young adults.
Method
Procedure
College students from a large university in Pennsylvania were recruited from classes as part of a larger survey study on connections between emotion, stress, pain, coping, and health behaviors. All procedures were approved by the Institutional Review Board administrative office of the affiliated institution. After obtaining informed consent, all participants were sent an online link via email to complete questionnaire measures online using Qualtrics software (2012 version; Provo, UT, USA) at baseline (September 2012, henceforth “Time 1”) and approximately two months later (December 2012, henceforth “Time 2”). Participants were offered 0.5% of their total grade of extra credit for completing the Time 1 survey and additional extra credit (0.5 – 1.0 % of their total grade) for participation in the Time 2 survey.
Participants
We excluded individuals who reported chronic pain at either Time 1 or Time 2 in order to avoid issues with depressed mood, sleep, or other key study variables that are often confounded with chronic pain (chronic pain has been shown to exacerbate negative mood by interfering with cognitive processes, e.g., Gaskin et al., 1992; Shackman et al., 2011). Chronic pain was determined by participants answering “yes” to the question “Have you experienced or are currently experiencing chronic pain-- pain that persists or comes and goes over 6 months or more?” In order to maximize variability and statistical power, no other inclusionary or exclusionary criteria (e.g., age, gender, socioeconomic status, or substance abuse) were utilized.
In order to model temporal mediation, we used only data from participants who completed both surveys. Of those without chronic pain, 368 participants took the survey at Time 1, whereas 165 took the survey at both time points. To check for potential sampling bias between those who took the survey at Time 1 versus those who took it at both Time 1 and Time 2, we compared means of these two samples using paired samples t-tests on age, gender, and all total scores on the Pittsburgh Sleep Quality Index, Ruminative Response Scale, Center for Epidemiological Studies-Depression Scale, and the State Trait Anxiety Inventory. There were no significant differences between the means in the two samples on any of the variables analyzed. A small subset of the 165 participants (n = 9) were missing questions on the Pittsburgh Sleep Quality Index, which appeared to be missing at random. They were retained as long as they had completed enough questions to form 70% of the subscale, which resulted in the loss of no additional participants.
Measures
Depressed mood
The Centers for Epidemiological Studies-Depression scale (Radloff, 1977) measures facets of depressed mood over the past week. This scale was completed by participants at both Time 1 and Time 2. The total scale consists of 20 items answered on a scale of 0 (“rarely or none, less than 1 day”) to 3 (“most of the time, 5–7 days”). Overall scores range from 0 to 60, with higher scores indicating a greater frequency of depressed mood. A score of 16 or greater is typically considered indicative of clinical levels of depression in non-clinical samples (Radloff, 1977). This scale has high internal consistency in the general population (.85) and in psychiatric populations (0.90), and high test-retest reliability, with correlations ranging from 0.51 to 0.67 (tested over two to eight weeks) and .32 and .54 (tested over 3 months to one year) (Hann et al., 1999; Radloff, 1977)
Anxiety
The Trait Anxiety Inventory (taken from the larger State-Trait Anxiety Inventory; Spielberger et al., 1983) measures stable, trait-like tendencies to perceive situations as fear-provoking. This scale was completed by participants at Time 1 only. The trait portion of the scale consists of 20 questions, with items on a scale of 1 (“almost never”) to 4 (“almost always”). Scores range from 20 to 80, with higher scores indicating great levels of anxiety. The scale has high internal consistency (0.89) and high test-retest reliability (0.88) among multiple time points (Spielberger et al., 1983).
Rumination
The Ruminative Response Scale (taken from the larger Response Styles Questionnaire; Nolen-Hoeksema & Morrow, 1991) measures the trait-like tendency to exhibit repetitive and non-constructive negative thoughts. This scale was completed by participants at both Time 1 and Time 2. It consists of a 22-item scale, answered on a scale of 1 (“never”) to 4 (“always”), and asks participants to report on what they generally do. Scores range from 22 to 88, with higher scores indicating a greater frequency of ruminative tendencies. The scale exhibited high internal consistency (0.90) and a high test-retest correlation (0.67) (Butler & Nolen-Hoeksema, 1994; Mika et al., 2013; Nolen-Hoeksema & Morrow, 1991).
Sleep quality
The widely-used 19-item Pittsburgh Sleep Quality Index (Buysse et al., 1989) was used to measure total sleep quality during the past month. This scale was completed by participants at both Time 1 and Time 2. It consists of seven subscales (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction) which are summed together to determine a comprehensive sleep quality index measure ranging from 0 to 21, with higher scores indicating poorer sleep quality. Scores of 5 or greater on the overall scale are indicative of poor sleep quality (Buysse et al., 2008; Buysse et al., 1989). The scale has high internal consistency (0.83) and a high test-retest correlation (0.85) (Buysse et al., 1989).
Self-reported health
Participants’ ratings of health were obtained via the Self-Reported Health Status scale (Ware & Sherbourne, 1992). Participants were asked, “In general, would you say your health is: 1-Excellent, 2-Very good, 3-Good, 4-Fair, 5-Poor”), which is an item taken from the Self-Reported Health Status scale used to assess general self-rated health. This item was completed by participants at both Time 1 and Time 2. This single-item assessment of health is a widely used measure and is highly predictive of other measures of health, including morbidity and mortality (Bosch, 2014).
Statistical Analyses
The Center for Epidemiological Studies-Depression Scale and State-Trait Anxiety Inventory, both from Time 1, were utilized to examine if these stable trait-like tendencies predicted other trait-like measures of rumination and recent sleep and self-reported health, as measured by the Ruminative Response Scale, Pittsburgh Sleep Quality Index, and Self-Reported Health Status scale, all at Time 2. SPSS 21.0 software (Armonk, NY: IBM Corp) was used for all descriptive analyses and correlations, and follow-up structural equation modeling was conducted using AMOS 20.0 software (Chicago: SPSS).
In evaluating the adequacy of all models, we primarily considered three fit indices: the root mean square error of approximation (RMSEA; Steiger & Lind, 1980) as an important estimate of how well the model fit the population’s covariance matrix (Hooper et al., 2008); the comparative fit index (CFI; Bentler, 1990), which performs well even with small sample sizes; and the Tucker-Lewis index (TLI; Tucker & Lewis, 1973). Based on stringent recommendations, both a CFI and TLI value of 0.90 or greater is considered to indicate good fit and values of 0.95 or greater to represent excellent fit (Hu & Bentler, 1998; West, 2012). The RMSEA estimate is considered to indicate good fit to the data at values of 0.10 or less, with values less than 0.06 representing excellent fit (Byrne, 2001; Hu & Bentler, 1998). The chi-square (χ2) statistic is also provided; ideally this statistic is non-significant in the model, but it is highly influenced by sample size (Hooper et al., 2008; West, 2012) and is therefore provided primarily to aid in model interpretability and to enable comparisons between models, as is recommended by multiple sources (Hayduk, 2007; Kline, 2005).
Results
Descriptives, Correlations, and Missing Data
The final sample of 165 students was 64.2% female (n = 106) with a mean age of 20.38 (SD = 1.06 years). Because gender was not correlated with any of the measured variables and based on theoretical considerations regarding the lack of gender differences in depression in college students (Butler & Nolen-Hoeksema, 1994), gender was not added as a predictor of any variable. Age was also not added as a predictor due to its lack of correlation with all key variables and the homogeneity of the age of the sample.
Correlations, means, and standard deviations of key study variables are reported in Table 1. All variables were within the acceptable range for skewness and kurtosis, signifying normal distribution. Anxiety, depressed mood, and rumination were all associated with total sleep quality as determined by the total Pittsburgh Sleep Quality Index (r = .37, p < .001; r = .35, p < .001; r = .38, p < .001, respectively) and self-reported health (r = .43, p < .001; r = .43, p < .001; r = .36, p < .001, respectively). The mean score on the total Center for Epidemiological Studies-Depression Scale was 14.06 (±10.09), with 28.5% of participants (n = 47) scoring a 16 or higher on the this scale, which is suggestive of clinical depression. The mean score on the State-Trait Anxiety Inventory was 40.61 (±10.05). The mean score on the total Pittsburgh Sleep Quality Index was 8.96 (± 2.44), with 87.3% (n = 144) of the participants having poor sleep quality, indicated by a score greater than 5 on the total Pittsburgh Sleep Quality Index.
Table 1.
Descriptive Statistics and Bivariate Correlations between Measured Variables
| M | SD | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|
| 1. Anxiety, T1 | 40.61 | 10.05 | -- | ||||
| 2. Depressed mood, T1 | 14.06 | 10.09 | .73** | -- | |||
| 3. Rumination, T2 | 35.77 | 12.05 | .58** | .66** | -- | ||
| 4. Sleep qualitya, T2 | 8.96 | 2.44 | .37** | .35** | .38** | -- | |
| 5. Self-reported health, T2 | 2.09 | 0.76 | .43** | .43** | .36** | .29** | -- |
Note: N = 165.
Abbreviations: T1 = Time 1; T2 = Time 2
Correlation is significant at the p < 0.01 level (2-tailed)
higher scores are indicative of worse sleep quality
Measurement Models
Measurement models of latent variables were created for the each of the trait rumination, anxiety, depressed mood, and sleep quality measures to ensure the best model fit (Little et al., 2002). The depressed mood variable (at Time 1) was parceled into the four predetermined subscales of the Center for Epidemiological Studies-Depression Scale: depressed mood, absence of pleasure, somatic manifestations of depression, and lack of interpersonal contact (Radloff, 1977). The chi-square of the depressed mood measurement model was χ2 (2, N = 165) = 6.06, p = .05; RMSEA = .11 (.01 – .22), TLI = .92, and CFI = .98, which demonstrates a good fit to the data.
Due to the relative homogeneity and single factor structure of the scale items trait rumination (at Time 2) was made into a latent variable by randomly parceling the individual scale items into three factors through the use of a random number generator. This latent variable was freed to correlated with anxiety (at Time 1) to ensure model identification. The chi-square of the anxiety and trait rumination measurement model was χ2 (8, N = 165) = 13.15, p = .11; RMSEA = .06 (.00 – .12), TLI = .97, and CFI = 1.0, which demonstrates a good fit to the data.
The latent overall sleep quality dependent variable (at Time 2) was parceled into four factors (subjective sleep quality, sleep latency, sleep duration, and daytime dysfunction) based on those subscales. Because only eight of the 165 participants, or 4.8%, used sleeping medication “once or twice a week” or “three or more times a week,” the sleep medication subscale was excluded from the sleep measurement model for statistical analyses. The subscales of habitual sleep efficiency and sleep disturbances were excluded due to their low factor loadings on the total sleep quality factor. The total score on the Ruminative Response Scale was quite strongly correlated with the daytime dysfunction subscale of the Pittsburgh Sleep Quality Index (r = .414, p < .001), but it was also strongly correlated with the sleep duration (r = .194, p = .013), subjective sleep quality (r = .233, p = .003), and sleep latency subscales (r = .213, p = .006). The chi-square of the sleep quality measurement model was χ2 (2, N = 165) = 2.31, p = .32; RMSEA = .03 (.00 – .16), TLI = .98, and CFI = .99, which demonstrates a good fit to the data. The error term of the Center for Epidemiological Studies-Depression Scale subscale “somatic manifestations of depression” and the residual of the total sleep quality latent variable were correlated (r = .38, p < .001) after examination of modification indices indicated that this would improve model fit and on the basis that a particular connection between these constructs is meaningful.
Structural Mediation Model
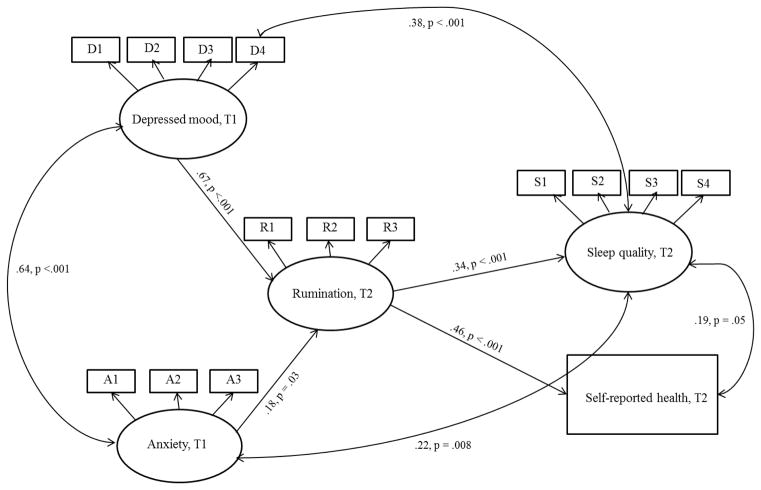
To better examine predictive pathways, a structural model examined whether the latent variable of rumination at Time 2 mediated the relationship between the latent variable of depressed mood at Time 1 and the measured variable of self-reported health at Time 2 and the latent variable of sleep quality at Time 2 (See Figure 1). This model was a good fit to the data; χ2 (83, N = 165) = 157.19, p < .001; RMSEA = .07 (.06 – .09), TLI = .93, CFI = .95 (Hooper et al., 2008; Steiger, 2007). The paths from depressed mood to rumination (β = .67, p < .001), anxiety to rumination (β = .18, p = .03), rumination to sleep (β = .34, p < .001), and rumination to self-reported health (β = .46, p < .001) were all significant. When the coefficients of the paths from depressed mood to rumination (β = .67) and rumination to sleep quality (β = .34) were multiplied, the mediated effect was β = .23. When the coefficients of the paths from depressed mood to rumination (β = .67) and rumination to self-reported health (β = .46) were multiplied, the mediated effect was β = .31. Using the joint significance test (see MacKinnon et al., 2002), rumination significantly mediated both the relationship between depressed mood and sleep quality and depressed mood and self-reported health. The direct paths from depressed mood to sleep and self-reported health were non-significant in the presence of the rumination mediator at the p < .05 level and were therefore removed from the final model.
Figure 1.
Structural Mediation Model
Abbreviations: T1 = Time 1 (baseline); T2 = Time 2. Structural mediation model of rumination as a mediator between anxiety and depressed mood and sleep quality and self-reported health. Unidirectional arrows between variables represent regression coefficients and bidirectional arrows represent correlation coefficients. For simplicity of presentation, error variances are not shown. (In the final model above, direct paths from depressed mood to sleep quality and anxiety and depressed mood to self-reported health were tested but were not significant or even marginally significant at the p < .05 level and were therefore removed.)
The final mediation-focused model (as described above and as shown in Figure 1) was also run without anxiety in the model to determine if rumination held as a mediator of the effects of depressed mood on sleep quality and self-reported health when the effects of anxiety were not controlled. This model was an acceptable fit to the data; χ2 (50, N = 165) = 103.08, p < .001; RMSEA = .08 (.06 – .10), TLI = .91, CFI = .94. The paths from depressed mood to rumination (β = .78, p < .001), and rumination to sleep (β = .35, p < .001) and rumination to self-reported health (β = .46, p < .001) were all still significant.
An additional alternative model was examined to confirm our expectation that a direct pathways model (in contrast to the mediation model tested above) was a significantly worse fit to the data. In this model, direct paths were specified between depressed mood on both outcomes (sleep quality and self-reported health), anxiety on both outcomes, and rumination on both outcomes. In this model, rumination predicted sleep quality (β = .18, p = .05) and self-reported health (β = .30, p < .001). Depressed mood did not directly predict sleep quality (β = −.10, p = .44) or self-reported health (β = .34, p = .29). Anxiety directly predicted sleep quality (β = .39, p = .002), but not self-reported health (β = .15, p = .13). The overall fit of this direct pathways model was a significantly poorer fit to the data [χ2 (82, N = 165) = 275.70, p < .001; RMSEA = .12 (.11 – .14), TLI = .82, CFI = .88] than the aforementioned mediational model based on a chi-square difference test that was conducted by examining the di erence of the χ2 values and the difference of the degrees of freedom of the two models in question.
Discussion
Overall, this study supports the hypothesis that rumination accounts for the association between depressed mood and overall sleep quality as well as self-reported health, at least among relatively healthy young adults. Importantly, in these analyses measurements of the trait-like tendency of depressed mood did not directly affect sleep measured two months later, but instead related to rumination, which in turn predicted recent sleep quality and self-reported health. These findings were obtained controlling for anxiety, but were comparable whether or not anxiety was controlled. These findings suggest that rumination may be an important and unique cognitive mechanism by which stable negative mood patterns lead to negative health behaviors like impaired sleep and poorer self-reported health.
These analyses were conducted in a population of relatively healthy young people. However, in this sample 29% of participants’ scores on the Center for Epidemiological Studies-Depression Scale suggested clinical depression, and 87% of participants’ scores on the total Pittsburgh Sleep Quality Index indicated poor overall sleep quality. Therefore, even in a population of relatively healthy college students (young adults without chronic pain), a number of individuals appeared to suffer from depressed mood, with even more suffering from impaired sleep. This phenomenon can be explained by the perseverative cognition hypothesis (Brosschot et al., 2006); in keeping with that hypothesis and related hypotheses about how rumination extends the reach of psychological stress on health (Gerin et al., 2012), rumination appears to be one of the mechanisms by which depressed mood can sustain the physiological activation that can impair sleep quality. College students face some unique psychosocial stressors, and young adults are not as strong at emotional regulation as older adults (Carstensen et al., 2003; Charles et al., 2009); therefore, college students and young adults in general may be at a particular risk for negative health outcomes caused by rumination.
Potential Biological and Behavioral Mechanisms
Although rumination explained the relationship between depressed mood and sleep quality and self-reported health in the present study, the question of how rumination leads to these negative health outcomes remains. The biological mechanisms by which rumination may lead to impaired sleep may involve the dysregulation of the neuro-endocrine-immune system. Depression and rumination have been linked to a hyperactivation of the hypothalamic-pituitary-adrenal axis (HPA axis; Pariante & Lightman, 2008), which can result in increased arousal and over-activation of acute phase proteins such as C-reactive protein (CRP; Pariante & Lightman, 2008), and pro-inflammatory cytokine production, particularly the cytokines tumor necrosis factor-alpha (TNF-α; Dowlati et al., 2010; Pariante & Lightman, 2008), interleukin-6 (IL-6; Dowlati et al., 2010; Pariante & Lightman, 2008), and interleukin-1 beta (IL-1β; Maes et al., 1993). Interestingly, TNF-α, IL-6, IL-1β are also some of the cytokines most strongly associated with sleep disorders and conditions such as insomnia, fatigue, and drowsiness (Maes et al., 1993). For example, in a study by Motivala et al. (2005) compared to non-depressed controls, depressed patients showed significantly higher elevations in circulating serum nocturnal IL-6. These results suggest that inflammation, as measured by these pro-inflammatory cytokines, may be the cause or the result of depressed mood and impaired sleep.
Another potential biological mediator of the connection between rumination and sleep is neurological functioning. Sleep is strongly associated with affective processes in multiple ways, particularly in the encoding of emotional memories and the regulation of mood (Walker & van Der Helm, 2009). The prefrontal cortex brain region has been shown to play an important mechanistic role in the regulation of affective reactivity, and has weakened control over such emotional processes in the presence of sleep loss (Baglioni et al., 2010). Attenuated prefrontal cortex response may reflect inherent individual genetic differences moderating the relationship between depressed mood and sleep quality or mechanisms by which depressed mood can lead to impaired sleep quality.
Finally, another route by which rumination may result in impaired sleep quality is through the adoption of negative health behaviors, such as alcohol use or lack of proper exercise. College student populations may be particularly at risk for these negative health behaviors due to the unique academic pressures they face and/or the drinking cultures of many colleges and universities (Weitzman, 2004). While not the primary focus of the present research, it is worth noting that the majority of the participants in the current research (56%, n = 93) reported that they had gotten “drunk or very high from alcohol” three or more times in the past month. Negative mood and poor sleep are bi-directionally related to alcohol use among undergraduate students (Ham & Hope, 2003; Jean-Louis et al., 1998). Thus, the observed patterns of heavy drinking in the present sample may represent a specific causal mechanism between depressed mood and impaired sleep quality. While the drinking behaviors of college students do not always match those of broader adult populations (O’Malley, 2004), negative mood and impaired sleep in general may prompt negative health behaviors as a form of coping that perpetuates the cycle of depressed mood and poor physical health as well as academic, social, and behavioral outcomes.
It is important to acknowledge that the relationship between sleep quality and rumination is likely bidirectional in some cases (Harvey, 2001; Van Moffaert, 1994); rumination may cause or exacerbate poor sleep quality, but poor sleep quality may also cause or exacerbate ruminative tendencies related to sleep loss or impairment. Sleep has also been implicated in emotion regulation and affective memory encoding (Walker & van Der Helm, 2009); thus, it is possible that sleep plays a causal role in determining mood state. However, follow-up analyses with a similar mediation model to that used in the present analysis, except with sleep quality and self-reported health as the mediators and rumination as the outcome, revealed significantly worse (p <.0001) model fit [χ2 (82, N = 165) = 214.01, RMSEA = .10 (.08 – .12), CFI = .92, TLI = .88]. These results run counter to an argument for reverse variable causation, and further support the notion that rumination is a driving force behind impaired sleep quality instead of sleep quality driving rumination.
Limitations and Future Directions
While full mediation was demonstrated using data from two time points, the data were non-experimental and cannot be used to determine causal relationships. Further, rumination and sleep loss are likely occurring in rapid succession of each other (e.g., nightly rumination before falling asleep can lead to impaired sleep quality that same night), and it therefore would have been ideal to have additional time points or daily assessments in order to better model causal pathways. It is also important to consider that a third unmeasured variable-- such as stress reactivity, previous history of trauma, or social support-- could be accounting for the relationships we found between depressed mood, rumination, and health-related outcomes. Future investigation of inflammatory cytokine and neural mechanisms should also be conducted to better elucidate these potential causal mechanisms in the pathway from rumination to sleep quality. More objective measurements of sleep quality through the use of actigraphy or polysomnographic techniques would also be beneficial.
The college students utilized in this study offer limited generalizability to older populations, as many college students show different patterns of depressed mood as compared to adults (Weitzman, 2004). However, it is important to assess patterns of mood, thought, and sleep in college students due to the unique academic pressures, social environment, and critical period of psychological development they face. It is also critical to understand psychological mechanisms in a relatively healthy population before initiating investigations with clinical populations.
Concluding Remarks and Implications
The results of this study help elucidate the mechanisms by which depressed mood can lead to impaired sleep quality and self-reported health. Rumination appears to be an important and unique psychological mediator of the relationship between depressed mood, overall sleep quality, and health status. Sleep has broad implications for adolescent development, as it is critical for the maintenance of psychophysiological processes such as learning, memory, and emotional processing (Walker & van Der Helm, 2009). Young individuals with impaired sleep may be at risk for other poor health outcomes later in life, including an increased risk for mortality and morbidity, specifically metabolic disorders (Spiegel et al., 2005), substance abuse (Mednick et al., 2010), chronic pain (Smith & Haythornthwaite, 2004), suicide (Goldstein et al., 2008; Liu & Buysse, 2006), and dementia (McCurry & Ancoli-Israel, 2003). Future sleep improvement-based interventions for adolescents and young adults may benefit from first examining and treating patterns of negative or depressive mood or ruminative tendencies, as potential mechanisms that can lead to poor sleep outcomes. Mindfulness or cognitive-behavioral interventions that specifically target addressing intrusive thoughts may be particularly useful for those with ruminative tendencies.
Acknowledgments
This research was supported in part by a grant from The Pennsylvania State University Social Science Research Institute, Level 1 Funding and by the National Science Foundation under Grant No. DGE1255832.
Footnotes
The authors Danica C. Slavish and Jennifer E. Graham-Engeland declare no conflict of interest.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants included in the study.
References
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews. 2010;14:227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Archives of General Psychiatry. 1992;49:651. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bosch JA. The use of saliva markers in psychobiology: Mechanisms and methods. Diagnostics and Disorders. 2014;24:99–108. doi: 10.1159/000358864. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Verkuil B, Thayer JF. Conscious and unconscious perseverative cognition: Is a large part of prolonged physiological activity due to unconscious stress? Journal of Psychosomatic Research. 2010;69:407–416. doi: 10.1016/j.jpsychores.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Buboltz W, Brown F, Soper B. Sleep habits and patterns of college students: A preliminary study. Journal of American College Health. 2001;50:131–135. doi: 10.1080/07448480109596017. [DOI] [PubMed] [Google Scholar]
- Butler LD, Nolen-Hoeksema S. Gender differences in responses to depressed mood in a college sample. Sex Roles. 1994;30:331–346. [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine. 2008;4:563. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Structural equation modeling with AMOS. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. [Google Scholar]
- Carstensen LL, Fung HH, Charles ST. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motivation and Emotion. 2003;27:103–123. [Google Scholar]
- Charles ST, Piazza JR, Luong G, Almeida DM. Now you see it, now you don’t: Age differences in affective reactivity to social tensions. Psychology and Aging. 2009;24:645–653. doi: 10.1037/a0016673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Gaskin ME, Greene AF, Robinson ME, Geisser ME. Negative affect and the experience of chronic pain. Journal of Psychosomatic Research. 1992;36:707–713. doi: 10.1016/0022-3999(92)90128-o. [DOI] [PubMed] [Google Scholar]
- Gaultney JF. The prevalence of sleep disorders in college students: Impact on academic performance. Journal of American College Health. 2010;59:91–97. doi: 10.1080/07448481.2010.483708. [DOI] [PubMed] [Google Scholar]
- Gerin W, Zawadzki MJ, Brosschot JF, Thayer JF, Christenfeld NJ, Campbell TS, Smyth JM. Rumination as a mediator of chronic stress effects on hypertension: A causal model. International Journal of Hypertension. 2012 doi: 10.1155/2012/453465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein TR, Bridge JA, Brent DA. Sleep disturbance preceding completed suicide in adolescents. Journal of Consulting and Clinical Psychology. 2008;76:84. doi: 10.1037/0022-006X.76.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: The sleep heart health study. Sleep Medicine Reviews. 2006;29:1009. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Graham JE, Streitel KL. Sleep quality and acute pain severity among young adults with and without chronic pain: The role of biobehavioral factors. Journal of Behavioral Medicine. 2010;33:335–345. doi: 10.1007/s10865-010-9263-y. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Moulds ML. The impact of rumination on sleep quality following a stressful life event. Personality and Individual Differences. 2007;42:1151–1162. [Google Scholar]
- Ham LS, Hope DA. College students and problematic drinking: A review of the literature. Clinical Psychology Review. 2003;23:719–759. doi: 10.1016/s0272-7358(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression scale (CES-D) Journal of Psychosomatic Research. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Insomnia: Symptom or diagnosis? Clinical Psychology Review. 2001;21:1037–1059. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Hayduk L, Cummings GG, Boadu K, Pazderka-Robinson H, Boulianne S. Testing! Testing! One, two three – testing the theory in structural equation models. Personality and Individual Differences. 2007:841–850. [Google Scholar]
- Hicks RA, Fernandez C, Pellegrini RJ. Striking changes in the sleep satisfaction of university students over the last two decades. Perceptual and Motor Skills. 2001;93:660. doi: 10.2466/pms.2001.93.3.660. [DOI] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen M. Structural equation modelling: Guidelines for determining model fit. Articles. 2008;2 [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structural modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Jean-Louis G, Gizycki HV, Zizi F, Nunes J. Mood states and sleepiness in college students: Influences of age, sex, habitual sleep, and substance use. Perceptual and Motor Skills. 1998;87:507–512. doi: 10.2466/pms.1998.87.2.507. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: Central pathways to morbidity and mortality. Journal of Psychosomatic Research. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. New York, NY: The Guilford Press; 2005. [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Medicine Reviews. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C, Buerger C. Determinants of subjective quality of life in depressed patients: The role of self-esteem, response styles, and social support. Journal of Affective Disorders. 2005;86:205–213. doi: 10.1016/j.jad.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Little TD, Cunningham WA, Shahar G, Widaman KF. To parcel or not to parcel: Exploring the question, weighing the merits. Structural Equation Modeling. 2002;9:151–173. [Google Scholar]
- Liu X, Buysse DJ. Sleep and youth suicidal behavior: A neglected field. Current Opinion in Psychiatry. 2006;19:288–293. doi: 10.1097/01.yco.0000218600.40593.18. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin-1 beta : A putative mediator of HPA axis hyperactivity in major depression? American Journal of Psychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Ancoli-Israel S. Sleep dysfunction in Alzheimer’s disease and other dementias. Current Treatment Options in Neurology. 2003;5:261–272. doi: 10.1007/s11940-003-0017-9. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. European Journal of Pharmacology. 2013;716:106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Möller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, Lo JC, Santhi N, von Schantz M, Smith CP. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proceedings of the National Academy of Sciences. 2013;110:E1132–E1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosko S, Zetin M, Glen S, Garber D, Deantonio M, Sassin J, McAnich J, Warren S. Self-reported depressive symptomatology, mood ratings, and treatment outcome in sleep disorders patients. Journal of Clinical Psychology. 1989;45:51–60. doi: 10.1002/1097-4679(198901)45:1<51::aid-jclp2270450107>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosomatic Medicine. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Progress in Cardiovascular Diseases. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. 2008 Sleep in America Poll. Washington D.C: 2008. [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- O’Malley PM. Maturing out of problematic alcohol use. Alcohol Research and Health. 2004;28:202. [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. Journal of Applied Physiology. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Medicine Reviews. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Rosa RR, Bonnet MH, Kramer M. The relationship of sleep and anxiety in anxious subjects. Biological Psychology. 1983;16:119–126. doi: 10.1016/0301-0511(83)90058-3. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Keinan G, Daon K. Effects of stress on sleep: The moderating role of coping style. Health Psychology. 2004;23:542–545. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- Salovey P, Rothman AJ, Detweiler JB, Steward WT. Emotional states and physical health. American Psychologist. 2000;55:110. doi: 10.1037//0003-066x.55.1.110. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA. Rumination: Relationships with physical health. Innovations in Clinical Neuroscience. 2012;9:29–34. [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE. Social and economic burden of mood disorders. Biological Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain interrelate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medice Reviews. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. Journal of Applied Physiology. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Redwood City, CA: Mind Garden; 1983. [Google Scholar]
- Steiger JH, Lind JC. Statistically-based tests for the number of common factors. Paper presented at the Psychometric Society; Iowa City, Iowa. 1980. [Google Scholar]
- Takano K, Tanno Y. Self-rumination, self-reflection, and depression: Self-rumination counteracts the adaptive effect of self-reflection. Behaviour Research and Therapy. 2009;47:260–264. doi: 10.1016/j.brat.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Thomsen DK, Mehlsen MY, Olesen F, Hokland M, Viidik A, Avlund K, Zachariae R. Is there an association between rumination and self-reported physical health? A one-year follow-up in a young and an elderly sample. Journal of Behavioral Medicine. 2004;27:215–231. doi: 10.1023/b:jobm.0000028496.41492.34. [DOI] [PubMed] [Google Scholar]
- Thomsen DK, Yung Mehlsen M, Christensen S, Zachariae R. Rumination—relationship with negative mood and sleep quality. Personality and Individual Differences. 2003;34:1293–1301. [Google Scholar]
- Tsypes A, Aldao A, Mennin DS. Emotion dysregulation and sleep difficulties in generalized anxiety disorder. Journal of Anxiety Disorders. 2013;27:197–203. doi: 10.1016/j.janxdis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- Van Moffaert MMMP. Sleep disorders and depression: The ‘chicken and egg’ situation. Journal of Psychosomatic Research. 1994;38(Supplement 1):9–13. doi: 10.1016/0022-3999(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Walker MP, van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin. 2009;135:731. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item-short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Weitzman ER. Poor mental health, depression, and associations with alcohol consumption, harm, and abuse in a national sample of young adults in college. The Journal of Nervous and Mental Disease. 2004;192:269–277. doi: 10.1097/01.nmd.0000120885.17362.94. [DOI] [PubMed] [Google Scholar]
- West SG, Taylor AB, Wu W. Handbook of structural equation modeling. New York, NY: Guilford; 2012. Model fit and model selection in structural equation modeling; pp. 209–231. [Google Scholar]
- Zawadzki MJ, Graham JE, Gerin W. Rumination and anxiety mediate the effect of loneliness on depressed mood and sleep quality in college students. Health Psychology. 2013;32:212–222. doi: 10.1037/a0029007. [DOI] [PubMed] [Google Scholar]