Abstract
Lambert-Eaton myasthenic syndrome (LEMS) is an autoimmune disease that disrupts the normally reliable neurotransmission at the neuromuscular junction (NMJ). This disruption is thought to result from an autoantibody-mediated removal of a subset of the P/Q-type Ca2+ channels involved with neurotransmitter release. With less neurotransmitter release at the NMJ, LEMS patients experience debilitating muscle weakness. The underlying cause of LEMS in slightly more than half of all patients is small-cell lung cancer, and cancer therapy is the priority for these patients. In the remaining cases, the cause of LEMS is unknown and these patients often rely on symptomatic treatment options as there is no cure. However, current symptomatic treatment options, such as 3,4-diaminopyridine (3,4-DAP), can have significant dose-limiting side effects; thus, additional treatment approaches would benefit LEMS patients. Recent studies introduced a novel Ca2+ channel agonist (GV-58) as a potential therapeutic alternative for LEMS. Additionally, this work has shown that GV-58 and 3,4-DAP interact in a supra-additive manner to completely restore the magnitude of neurotransmitter release at the NMJs of a LEMS mouse model. In this review, we discuss synaptic mechanisms for reliability at the NMJ and how these mechanisms are disrupted in LEMS. We then discuss the current treatment options for LEMS patients, while also considering recent work demonstrating the therapeutic potential of GV-58 alone and in combination with 3,4-DAP.
Keywords: Neuromuscular junction, Ca2+ channels, Lambert-Eaton myasthenic syndrome, Neurotransmitter release, Presynaptic
Introduction
The neuromuscular junction (NMJ) is a strong, reliable synapse that consistently brings the postsynaptic muscle fiber to threshold. The large presynaptic terminal of the NMJ contains hundreds of individual neurotransmitter release sites, or active zones, where synaptic vesicle docking and fusion occur to mediate the release of neurotransmitter [1]. The reliability of the NMJ is due to the large safety margin for neurotransmitter release, meaning that an excess of neurotransmitter-containing vesicles fuse in response to each presynaptic action potential [2]. This excess neurotransmitter release ensures that the postsynaptic muscle cell is depolarized beyond what is required to reach threshold and initiate muscle contraction, even during periods of high frequency activity.
Although the NMJ is a reliable synapse, a number of disorders are associated with a disruption in the normally dependable communication at this synapse. One such disorder is Lambert-Eaton myasthenic syndrome (LEMS), an autoimmune disease characterized by a loss of a fraction of the presynaptic P/Q-type Ca2+ channels at the NMJ [3–5]. These presynaptic P/Q-type Ca2+ channels normally open in response to presynaptic action potential activity and allow the influx of Ca2+ ions that trigger synaptic vesicle fusion and neurotransmitter release. The LEMS-induced reduction in the number of presynaptic P/Q-type Ca2+ channels causes a decrease in the amount of action potential-evoked neurotransmitter release at the NMJ. Reduced neurotransmitter release leads to less effective initiation of muscle contraction, and as a result, patients with LEMS experience debilitating muscle weakness [6]. There is no cure for LEMS, but multiple symptomatic treatment approaches have been tested and some are currently in clinical use [7,8]. In this review, we will discuss the various properties of the NMJ leading to its reliability, and how the alteration of these properties in LEMS leads to the observed pathology. We will also discuss the currently available treatment options for LEMS and then consider novel treatment approaches that have been proposed recently for LEMS and other disorders of the NMJ.
The NMJ as a reliable synapse
The NMJ is a large model synapse that has been studied extensively for decades, especially with respect to presynaptic properties of neurotransmitter release [9]. This synapse has been especially valuable for studying the properties of neurotransmitter release for several reasons: it is a peripheral synapse that is easily accessible, it is a very large synapse that is amenable to experimental study, and since there is only one presynaptic terminal per postsynaptic muscle fiber, it has been relatively easy to interpret experimental results. As previously mentioned, one of the hallmarks of the NMJ is its strength and reliability. This synapse releases more chemical neurotransmitter than is required to bring the postsynaptic muscle cell to threshold (it is strong), and it can do this repeatedly (it is reliable) during short periods of high frequency activity (bursts of 5–10 action potentials at 50–100 Hz in “fast” muscles [10]). Although the NMJ has been the focus of numerous studies of neurotransmitter release, the exact mechanisms by which the NMJ achieves strength and reliability are not completely understood.
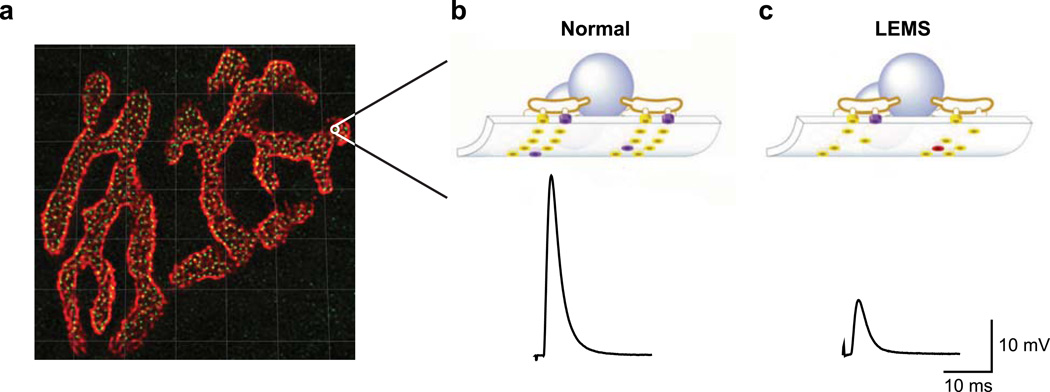
The mammalian NMJ is composed of hundreds of small, spatially isolated neurotransmitter release sites, or active zones (Fig. 1a, b). Within each active zone there is a single row of ~2–3 docked synaptic vesicles between two double rows of intramembranous particles [11], a portion of which are thought to be the P/Q-type Ca2+ channels required for neurotransmitter release. The estimated length of each punctate active zone is between ~80 nm (determined by measurements of the double row of intramembranous particles observed in electron microscopy [11,12]) and ~250 nm (determined by confocal imaging of the active zone protein bassoon [13]). The larger measurement made with confocal imaging of bassoon immunoreactivity may simply be at the limit of light microscopy resolution, or this larger measurement may reflect the possibility that the bassoon proteins in the active zone encompass a larger area as compared to the space occupied by the intramembranous particles of the active zone. Each small active zone is separated from each of its neighboring active zones by approximately 500 nm [13]. At the mouse NMJ, there are ~850 active zones per NMJ (Fig. 1a) [13,14], but despite the large number of active zones, only ~100 vesicles are released with each action potential stimulus [15]. This means that the average probability of release per active zone is only ~0.12, and the average probability of release per vesicle (assuming 2 vesicles per active zone) is only ~0.06. Therefore, each individual vesicle has a very low probability of release during any one action potential, but since there are thousands of vesicles ready to be released in each NMJ, the total number of vesicles released per action potential is easily sufficient to bring the postsynaptic muscle cell to threshold. Furthermore, the low probability of release for any one vesicle means that significant depression will not occur during brief periods of high frequency activity. Therefore, we view the NMJ as a strong and reliable synapse constructed of thousands of unreliable single-vesicle release sites [16,17], with each of these unreliable singlevesicle release sites being composed of a single vesicle docked at the active zone and a small number of closely associated Ca2+ channels.
Fig. 1.
Active zone organization in healthy and LEMS model NMJs. a A single mouse NMJ showing postsynaptic acetylcholine receptors (red) and presynaptic active zone neurotransmitter release sites (green). Grid lines = 5 µm. b Schematic of an individual active zone (top) along with a sample average end plate potential (EPP; a measure of the strength of neurotransmitter release) trace (bottom) from a healthy mouse NMJ. c Schematic of an individual active zone (top) along with a sample average EPP trace from a LEMS model mouse NMJ (bottom). Schematics of active zones (b, c) include synaptic vesicles (blue spheres), P/Qtype Ca2+ channels (purple cylinders), other Ca2+ channel subtypes (red cylinders) and active zone proteins other than Ca2+ channels (yellow cylinders). a Modified from Ref. [17]; b,c modified from [28; Copyright 2003 National Academy of Sciences, USA]
The specific mechanisms that contribute to a low average probability of release per vesicle at the mammalian NMJ are unclear, but some possibilities can be hypothesized based upon studies performed using other synaptic preparations. In the frog NMJ, for example, fast Ca2+ imaging studies suggest that a low probability of release per single vesicle arises from both a low probability of opening for any one Ca2+ channel during an action potential, and a low probability that a vesicle will be released even when a nearby Ca2+ channel does open [18]. Additionally, this work suggests that there are relatively few Ca2+ channels per active zone, with an average of ~1 Ca2+ channel per vesicle [18]. Therefore, at the frog NMJ, any individual vesicle would rarely be exposed to the Ca2+ ion flux from a nearby open Ca2+ channel, and vesicle fusion would rarely occur even in the event that a nearby channel did open. Considering that the mouse and frog NMJs are similar with respect to their rigid active zone organization, the mouse NMJ may have properties similar to those observed in the frog NMJ.
LEMS effects on the NMJ
LEMS is an autoimmune disease that attacks the neuromuscular active zone. The incidence of LEMS is estimated to be only about 0.48 per million [19], making LEMS a relatively rare disease. Despite its rarity, LEMS is well-known as a classic paraneoplastic disorder. About 50–60% of LEMS cases are paraneoplastic, most commonly observed in older individuals who have been long-term cigarette smokers and have developed a small-cell lung cancer tumor [20]. The remaining LEMS patients tend to be younger, do not have a history of smoking, and do not have an underlying tumor. In these patients, the cause of LEMS is unknown [21]. LEMS patients most commonly experience debilitating muscle weakness, but can also suffer from autonomic symptoms and reduced tendon reflexes [22]. In both tumor-associated LEMS and the idiopathic form of LEMS, the clinical symptoms are generally attributed to an autoantibody-mediated reduction in the number of P/Q-type voltage-gated Ca2+ channels at the presynaptic terminal of the NMJ [4,23]. In the case of tumor-associated LEMS, P/Q-type Ca2+ channels are expressed by the small cell lung cancer cells, and antibodies to P/Q-type Ca2+ channels are produced as part of the patients’ immune response to the tumor [24]. The reduction in the number of presynaptic P/Q-type Ca2+ channels leads to a reduction in the Ca2+ influx that occurs in response to a presynaptic action potential. With decreased presynaptic Ca2+ influx, the probability of synaptic vesicle fusion is also lowered, resulting in less neurotransmitter (acetylcholine) release per presynaptic action potential (Fig. 1c). Because less neurotransmitter is released per action potential, each presynaptic action potential is less likely to trigger a postsynaptic muscle contraction. The NMJ may attempt to compensate for the loss of P/Q-type Ca2+ channels in LEMS in part by upregulating the expression of other Ca2+ channel subtypes [25,26], but this compensation is not sufficient to restore normal levels of neurotransmitter release [27]. The upregulation of other Ca2+ channel subunits may be insufficient because there are simply fewer Ca2+ channels per active zone relative to healthy conditions. Another possibility is that the other Ca2+ channel subtypes may not be located in the same positions relative to docked synaptic vesicles as the P/Q-type Ca2+ channels in healthy active zones [28]. If the Ca2+ channels are located farther away from docked synaptic vesicles, then they will not be able to contribute the same levels of Ca2+ ions that are normally required to trigger the release of a synaptic vesicle. In addition to causing less neurotransmitter release due to a reduced Ca2+ influx, disruptions in the overall structure of the presynaptic release site caused by Ca2+ channel removal may also contribute to the reduction in neurotransmitter release. Freeze-fracture electron microscopy has shown a disruption in the structure of individual active zones in LEMS model mouse NMJs [12]. Furthermore, previous studies have demonstrated that the interaction between presynaptic Ca2+ channels and laminin β2 is critical for the maintenance of active zone organization [29,30]. Since the structural organization of active zones is important to synapse function [31–34], it seems plausible that disruption of active zone organization due to autoimmune attack of active zone proteins could be partly responsible for the deficits in neurotransmitter release in LEMS.
Although antibodies to P/Q-type Ca2+ channels are present in most LEMS patients and are generally considered responsible for the symptoms of LEMS, antibodies to other critical proteins involved in neurotransmitter release have also been detected in LEMS patients. These include antibodies to other Ca2+ channel subtypes, such as N-type and L-type channels [5,23,35,36]. Antibodies to these other Ca2+ channel subtypes could contribute to the autonomic symptoms observed in some LEMS patients [37]. In addition, other presynaptic proteins can also be targeted by LEMS antibodies, including synaptotagmin and M1 muscarinic acetylcholine receptors [38]. Synaptotagmin is considered to be the Ca2+ sensor for fast, synchronous neurotransmitter release at most synapses [39]. M1 muscarinic acetylcholine receptors are located on the presynaptic terminal of the NMJ and have been shown to be involved in the modulation of neurotransmitter release [40]. Interestingly, some LEMS patients are seronegative for P/Q-type Ca2+ channel antibodies. Despite not having a detectable level of P/Q-type Ca2+ channel antibodies, these seronegative patients have a similar clinical presentation as seropositive LEMS patients [26,41]. The symptoms in seronegative LEMS patients may be caused by antibodies to other presynaptic proteins such as synaptotagmin or M1 muscarinic receptors. Another possibility is that these patients may have P/Q-type Ca2+ channel antibodies at levels below what is detectable with diagnostic assays.
LEMS is diagnosed using a combination of clinical symptoms, electrophysiological measurements, and tests to determine antibody levels [22]. The most prominent symptom in LEMS patients is muscle weakness of the proximal limbs, although the weakness does eventually progress distally [22]. LEMS patients also commonly report symptoms associated with autonomic dysfunction, which can include dry mouth, erectile dysfunction in men and constipation [22,42]. It is also common for LEMS patients to present with reduced tendon reflexes [6,22]. In terms of electrophysiological measurements to diagnose LEMS, repetitive nerve stimulation (RNS) is often used [43]. The results of the RNS test in LEMS patients show a decrease in the compound muscle action potential (CMAP) amplitude during low frequency stimulation (2–5 Hz), and an increase in CMAP amplitude during post-exercise stimulation or during high frequency stimulation [44]. Lastly, antibody panels to test for the presence of antibodies to Ca2+ channels can be used in the diagnosis of LEMS [23].
Treatment options for LEMS
There is no cure for LEMS and few treatment options are available to alleviate the symptoms. If LEMS is present along with small-cell lung cancer, then cancer therapy is the priority. In LEMS patients with an associated tumor, successful treatment of the tumor also alleviates the symptoms of LEMS [45,46]. The simultaneous alleviation of LEMS is presumably due to the fact that the removal/reduction of the tumor has removed the underlying cause of LEMS in these patients. If LEMS presents without an associated tumor, or if LEMS symptoms are not alleviated during or following cancer treatment, then treatment can include drugs that alleviate the symptoms associated with LEMS and/or immunomodulatory therapies. As discussed below, immunosuppression may only be recommended for those patients in whom symptomatic treatment is not effective [47].
3,4-DAP as a symptomatic treatment option
Symptomatic treatment strategies that increase neurotransmitter release have emerged as the primary therapeutic approach [7,8,47–49]. The most common symptomatic treatment option is 3,4-diaminopyridine (3,4-DAP; Fig. 2a), a voltage-gated K+ channel inhibitor that broadens the presynaptic action potential [50]. The broadening of the presynaptic action potential allows more voltage-gated Ca2+ channels to open, thus increasing Ca2+ entry into the nerve terminal during each action potential. 3,4-DAP can be used alone or, less commonly, in combination with an acetylcholinesterase inhibitor. Although 3,4-DAP increases neurotransmitter release, there are dose-limiting side effects, such as paresthesia, difficulty sleeping, and, paradoxically, fatigue and deterioration in muscle strength that prevent adequate symptomatic relief for many LEMS patients [47,51–54]. The latter two may be due to reported effects on axonal K+ channels that limit firing frequencies [55], and/or reduction in activity-dependent facilitation caused by 3,4-DAP [56]. Thus, 3,4-DAP indirectly increases Ca2+ entry into the nerve terminal, but due to dose-dependent limitations used to reduce the risk of serious side-effects (3,4-DAP doses < 80 mg/day) [47], 3,4-DAP often does not provide enough symptomatic relief for patients to return to normal activity [51].
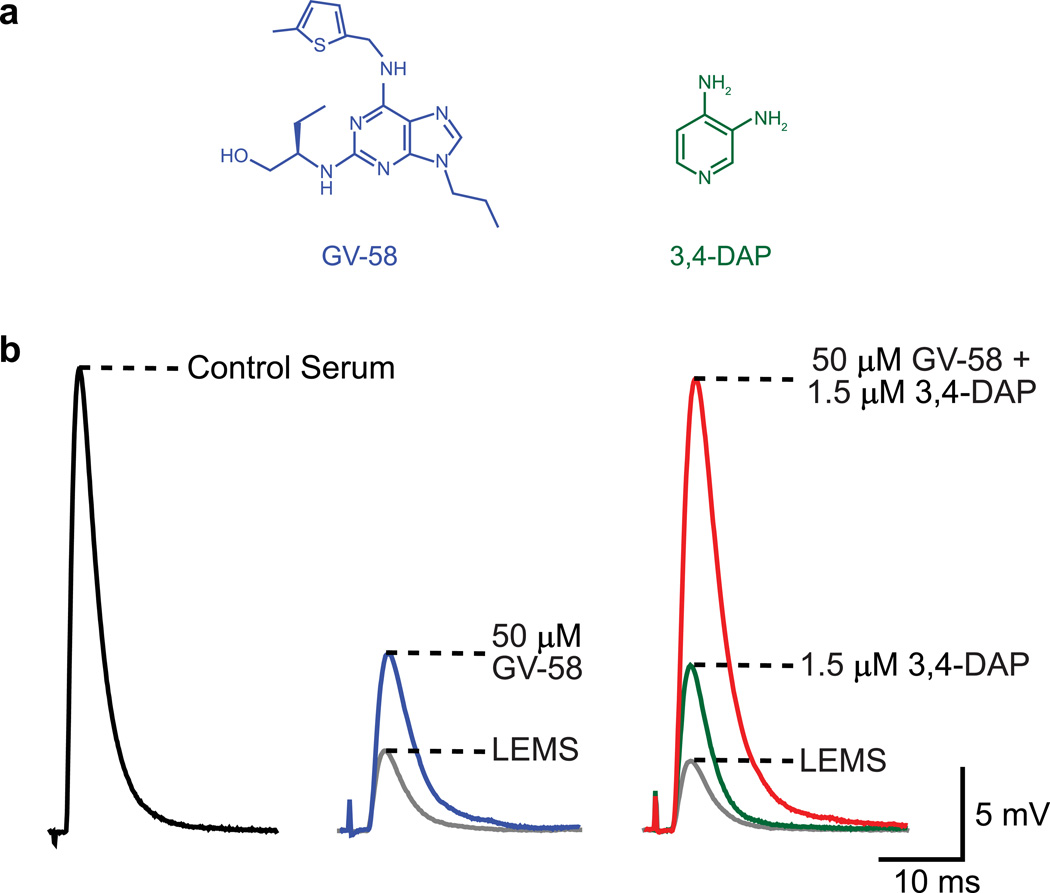
Fig. 2.
The combined application of GV-58 and 3,4-DAP completely restores the magnitude of neurotransmitter release at LEMS model mouse NMJs. a Structure of GV-58 (left, blue) and 3,4-DAP (right, green). b Sample EPP traces from a NMJ of a control serum-treated mouse (quantal content = 107.5 ± 3.6; left), from a LEMS model NMJ before (quantal content = 26.7 ± 1.4) and after application of 50 µM GV-58 (quantal content = 48.4 ± 2.7; middle), and from a LEMS model NMJ before drug application, following application of 1.5 µM 3,4-DAP (quantal content = 49.0 ± 4.4) and following application of 50 µM GV-58 plus 1.5 µM 3,4-DAP (quantal content = 105.1 ± 4.0; right) [70]. a Modified from Ref. [15]; b modified from Ref. [70]
Immunomodulatory therapy
If patients do not receive enough relief from 3,4-DAP, the next line of treatment usually involves some form of immunomodulatory therapy. Immunosuppressive drugs are usually given, with the combination of prednisolone and azathioprine being the most common. Studies have reported that LEMS patients show significant improvement following the combination treatment of prednisolone and azathioprine [57,58]. The combined prednisolone and azathioprine treatment strategy received support from a study that compared the effectiveness of the combined prednisolone plus azathioprine treatment versus prednisolone alone in treating myasthenia gravis [59]. In this study, adding azathioprine to the treatment protocol resulted in fewer side effects and a lower number of treatment failures [59]. Additionally, there is evidence that LEMS patients taking high doses of prednisolone alone only experienced a mild to moderate improvement that did not persist following a reduction in dose [60,61], lending further support to the use of the combined prednisolone and azathioprine treatment. However, immunosuppressants have generally not been favored, as side effects may be severe and include leukopenia, liver dysfunction, nausea, vomiting, and hair loss [47].
Two other immunomodulatory treatment options for LEMS include intravenous immunoglobulin (IVIg) and plasma exchange. A randomized, cross-over trial has reported significant improvements that last for several weeks in LEMS patients treated with IVIg [62]. Multiple case reports have also supported the potential benefits of using IVIg to treat LEMS [63–65]. The beneficial effects of IVIg treatment in LEMS patients seems to result from the decline in circulating Ca2+ channel antibodies [62], although the exact mechanism by which IVIg reduces antibody titer remains unknown. Plasma exchange is another treatment approach that temporarily reduces Ca2+ channel antibody titer. Plasma exchange induces a rapid improvement in LEMS symptoms that diminishes after approximately six weeks [57]. Other treatment options are available, and more comprehensive discussions of the available therapeutic approaches for LEMS can be found elsewhere [7,8,22,66].
Ca2+ channel agonists as a possible future treatment approach
Considering that 3,4-DAP is not completely effective for all LEMS patients due to dose-limiting side effects, the development of alternative symptomatic treatment options that are as effective or even superior to 3,4-DAP would be greatly beneficial to the LEMS patient population. As 3,4-DAP indirectly increases the Ca2+ influx by prolonging the action potential duration and therefore increasing the number of Ca2+ channels that open during an action potential, a more direct approach to increase Ca2+ influx might be beneficial. An obvious target for an alternative treatment approach is the population of remaining presynaptic Ca2+ channels. An agonist that would increase Ca2+ influx only through Ca2+ channels that are already open in response to an action potential depolarization would be optimal. Recently, such a Ca2+ channel agonist was developed and evaluated for effects on neurotransmitter release in LEMS model mouse NMJs [15,67]. This Ca2+ channel agonist, termed GV-58 (Fig. 2a), was synthesized as a novel analog of (R)-roscovitine, an inhibitor of cyclin-dependent kinases that previously has been shown to have Ca2+ channel activity [68]. Specifically, (R)-roscovitine increases the mean open time of Ca2+ channels, thus increasing Ca2+ influx [69]. Liang et al. (2012) strategically synthesized novel analogs of (R)-roscovitine with the goal of creating a compound with specificity as a Ca2+ channel agonist. GV-58 was generated as part of this lead optimization and was shown to have a significantly reduced potency as a Cdk antagonist and an increased efficacy and potency as an agonist of P/Q-type (Cav2.1) and N-type (Cav2.2) Ca2+ channels [15,67]. When evaluated in a LEMS passive transfer model mouse NMJ, GV-58 significantly increased neurotransmitter release and also partially restored short-term plasticity characteristics [15].
As discussed earlier, we hypothesize that there are two low probability steps in the neurotransmitter release process at the mammalian NMJ: the low probability that any one Ca2+ channel will open during an action potential and the low probability that a vesicle will fuse even when a nearby Ca2+ channel does open. 3,4-DAP increases neurotransmitter release by increasing the probability that any one Ca2+ channel will open during an action potential, and GV-58 increases neurotransmitter release by increasing the probability that a vesicle will fuse when a nearby channel does open (by keeping the channels open longer and increasing Ca2+ influx). Considering these mechanisms, we hypothesized that 3,4-DAP and GV-58 would have a supra-additive interaction to greatly increase neurotransmitter release at LEMS NMJs. In fact, Tarr et al. (2014) evaluated the combined application of these two compounds in LEMS model mouse NMJs. When given alone, GV-58 increased neurotransmitter release to a similar extent as 3,4-DAP. Strikingly, the combined application of GV-58 and 3,4-DAP acted supra-linearly to completely restore the magnitude of neurotransmitter release to the level of control NMJs (Fig. 2b), and GV-58 plus 3,4-DAP also elicited a nearly complete restoration of short-term plasticity characteristics in LEMS model NMJs [70]. In summary, while the use of GV-58 alone might improve muscle contraction to the same extent as 3,4-DAP alone, the combined application of both compounds seems to have the greatest potential to restore muscle contraction to normal levels.
The incomplete restoration of short-term plasticity characteristics at LEMS model mouse NMJs after exposure to a combination of 3,4-DAP and GV-58 suggests that LEMS-induced disruption of the presynaptic active zone structure may have a lingering effect on synaptic function. In the normal mouse NMJ, there is very little, if any, facilitation during short high frequency trains of action potentials [15,25,71,72]. Conversely, in the LEMS model mouse NMJ, there is a large amount of facilitation throughout the same train of action potentials [15,25]. The LEMS-induced attack on the presynaptic active zones of the NMJ clearly causes an increase in facilitation, but the potential mechanisms that underlie this increase in facilitation depend in part upon the normal organization of the active zones. This facilitation could result from a simple reduction in the overall influx of Ca2+ per action potential, which in turn would result from a decrease in the number of Ca2+ channels around any one vesicle. This situation would suggest that many Ca2+ channels normally contribute Ca2+ ions to the release of a single vesicle. If Ca2+ channel-vesicle stoichiometry is closer to one-to-one, then simply reducing the number of Ca2+ channels would likely not account for the observed increase in facilitation during trains. In this situation, a more likely explanation is a change in the organization of the active zone. This could occur if newly inserted Ca2+ channels are positioned further away from vesicles (outside the double row of intramembranous particles) [28], or if the LEMS-induced removal of Ca2+ channels actually disassembles the active zones and completely alters the normal spatial relationship between presynaptic Ca2+ channels and docked synaptic vesicles. In light of these possible mechanisms, a complete restoration of short-term plasticity characteristics is unlikely, and the best outcome may only be a nearly complete restoration. These lingering effects on short-term synaptic plasticity are likely not as important as the complete restoration of the magnitude of transmitter released that is obtained by the combined use of 3,4-DAP and GV-58.
In summary, the development of a combined use of a Ca2+ channel agonist (like GV-58) with 3,4-DAP shows promise as a possible alternative treatment option for LEMS. A combined treatment approach may allow the use of lower doses of each compound, thus significantly reducing the likelihood of side effects and increasing functional recovery of the NMJ. Further studies on the specificity of GV-58 will be required. GV-58 has been shown to have no agonist activity on Cav1.3 (L-type) channels [15], but its activity on other Ca2+ channel subtypes, and on other voltage-gated channels such as Na+ and K+ channels, has yet to be determined. Nonetheless, GV-58 shows promise as a potential future therapeutic for LEMS patients, especially in combination with the current common treatment 3,4-DAP.
Conclusions
An understanding of the mechanisms that control neurotransmitter release at the NMJ aids in our understanding of neuromuscular function both in healthy and disease conditions. This information is also important in the development of therapeutic approaches that might be helpful in the management of neuromuscular diseases such as LEMS. In particular, the recent development of novel Ca2+ channel agonists may prove helpful in developing new treatment approaches for LEMS and other neuromuscular disorders. The potential usefulness of these novel Ca2+ channel agonists for patients will depend of the outcome of further pre-clinical testing.
Acknowledgements
We thank A.E. Homan for critically reading the manuscript. This work was supported by the ARCS Foundation scholarship (to T.B.T.) and grants from the National Science Foundation (0844604 and 1249546 to S.D.M.), the National Institutes of Health (GM067082 to P.W.), the Muscular Dystrophy Association (295271 to S.D.M.) and the University of Pittsburgh Central Research Development Fund (to S.D.M).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- 2.Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 3.Lambert EH, Eaton LM, Rooke ED. Defect of neuromuscular conduction associated with malignant neoplasms. Am J Physiol. 1956;187:612–613. [Google Scholar]
- 4.Vincent A, Lang B, Newsom-Davis J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert-Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 1989;12:496–502. doi: 10.1016/0166-2236(89)90109-4. [DOI] [PubMed] [Google Scholar]
- 5.Meriney SD, Hulsizer SC, Lennon VA, Grinnell AD. Lambert-Eaton myasthenic syndrome immunoglobulins react with multiple types of calcium channels in small-cell lung carcinoma. Ann Neurol. 1996;40:739–749. doi: 10.1002/ana.410400510. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain. 1988;111:577–596. doi: 10.1093/brain/111.3.577. [DOI] [PubMed] [Google Scholar]
- 7.Maddison P. Treatment in Lambert-Eaton myasthenic syndrome. Ann N Y Acad Sci. 2012;1275:78–84. doi: 10.1111/j.1749-6632.2012.06769.x. [DOI] [PubMed] [Google Scholar]
- 8.van Sonderen A, Wirtz PW, Verschuuren JJ, Titulaer MJ. Paraneoplastic syndromes of the neuromuscular junction: therapeutic options in myasthenia gravis, lambert-eaton myasthenic syndrome, and neuromyotonia. Curr Treat Options Neurol. 2013;15:224–239. doi: 10.1007/s11940-012-0213-6. [DOI] [PubMed] [Google Scholar]
- 9.Katz B. The release of neural transmitter substances. Liverpool: Liverpool Univ. Press; 1969. [Google Scholar]
- 10.Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- 11.Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J Comp Neurol. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci U S A. 1983;80:7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz R, Cano R, Casanas JJ, Gaffield MA, Betz WJ, Tabares L. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci. 2011;31:2000–2008. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarr TB, Malick W, Liang M, Valdomir G, Frasso M, Lacomis D, Reddel SW, Garcia-Ocano A, Wipf P, Meriney SD. Evaluation of a novel calcium channel agonist for therapeutic potential in Lambert-Eaton myasthenic syndrome. J Neurosci. 2013;33:10559–10567. doi: 10.1523/JNEUROSCI.4629-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarr TB, Dittrich M, Meriney SD. Are unreliable release mechanisms conserved from NMJ to CNS? Trends Neurosci. 2013;36:14–22. doi: 10.1016/j.tins.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meriney SD, Dittrich M. Organization and function of transmitter release sites at the neuromuscular junction. J Physiol. 2013;591:3159–3165. doi: 10.1113/jphysiol.2012.248625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. J Neurosci. 2011;31:11268–11281. doi: 10.1523/JNEUROSCI.1394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirtz PW, Nijnuis MG, Sotodeh M, Willems LN, Brahim JJ, Putter H, Wintzen AR, Verschuuren JJ Dutch Myasthenia Study G. The epidemiology of myasthenia gravis, Lambert-Eaton myasthenic syndrome and their associated tumours in the northern part of the province of South Holland. J Neurol. 2003;250:698–701. doi: 10.1007/s00415-003-1063-7. [DOI] [PubMed] [Google Scholar]
- 20.Titulaer MJ, Maddison P, Sont JK, Wirtz PW, Hilton-Jones D, Klooster R, Willcox N, Potman M, Sillevis Smitt PA, Kuks JB, Roep BO, Vincent A, van der Maarel SM, van Dijk JG, Lang B, Verschuuren JJ. Clinical Dutch-English Lambert-Eaton Myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol. 2011;29:902–908. doi: 10.1200/JCO.2010.32.0440. [DOI] [PubMed] [Google Scholar]
- 21.Titulaer MJ, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: tumor versus nontumor forms. Ann N Y Acad Sci. 2008;1132:129–134. doi: 10.1196/annals.1405.030. [DOI] [PubMed] [Google Scholar]
- 22.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- 23.Lennon VA, Kryzer TJ, Griesmann GE, O'Suilleabhain PE, Windebank AJ, Woppmann A, Miljanich GP, Lambert EH. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med. 1995;332:1467–1474. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- 24.Roberts A, Perera S, Lang B, Vincent A, Newsom-Davis J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature. 1985;317:737–739. doi: 10.1038/317737a0. [DOI] [PubMed] [Google Scholar]
- 25.Flink MT, Atchison WD. Passive transfer of Lambert-Eaton syndrome to mice induces dihydropyridine sensitivity of neuromuscular transmission. J Physiol. 2002;543:567–576. doi: 10.1113/jphysiol.2002.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh SJ, Hatanaka Y, Claussen GC, Sher E. Electrophysiological differences in seropositive and seronegative Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2007;35:178–183. doi: 10.1002/mus.20672. [DOI] [PubMed] [Google Scholar]
- 27.Smith DO, Conklin MW, Jensen PJ, Atchison WD. Decreased calcium currents in motor nerve terminals of mice with Lambert-Eaton myasthenic syndrome. J Physiol. 1995;487:115–123. doi: 10.1113/jphysiol.1995.sp020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci U S A. 2003;100:3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin beta2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci. 2011;31:512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holderith N, Lorincz A, Katona G, Rozsa B, Kulik A, Watanabe M, Nusser Z. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci. 2012;15:988–997. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng J, He L, Zheng H, Xue L, Luo F, Shin W, Sun T, Kuner T, Yue DT, Wu LG. Calcium-channel number critically influences synaptic strength and plasticity at the active zone. Nat Neurosci. 2012;15:998–1006. doi: 10.1038/nn.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.tom Dieck S, Specht D, Strenzke N, Hida Y, Krishnamoorthy V, Schmidt KF, Inoue E, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Hagiwara A, Brandstatter JH, Lowel S, Gollisch T, Ohtsuka T, Moser T. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2012;32:12192–12203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahidullah M, Le Marchand SJ, Fei H, Zhang J, Pandey UB, Dalva MB, Pasinelli P, Levitan IB. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. J Neurosci. 2013;33:19590–19598. doi: 10.1523/JNEUROSCI.3396-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston I, Lang B, Leys K, Newsom-Davis J. Heterogeneity of calcium channel autoantibodies detected using a small-cell lung cancer line derived from a Lambert-Eaton myasthenic syndrome patient. Neurology. 1994;44:334–338. doi: 10.1212/wnl.44.2.334. [DOI] [PubMed] [Google Scholar]
- 36.Motomura M, Lang B, Johnston I, Palace J, Vincent A, Newsom-Davis J. Incidence of serum anti-P/O-type and anti-N-type calcium channel autoantibodies in the Lambert-Eaton myasthenic syndrome. J Neurol Sci. 1997;147:35–42. doi: 10.1016/s0022-510x(96)05303-8. [DOI] [PubMed] [Google Scholar]
- 37.Waterman SA, Lang B, Newsom-Davis J. Effect of Lambert-Eaton myasthenic syndrome antibodies on autonomic neurons in the mouse. Ann Neurol. 1997;42:147–156. doi: 10.1002/ana.410420204. [DOI] [PubMed] [Google Scholar]
- 38.Takamori M. Lambert–Eaton myasthenic syndrome: Search for alternative autoimmune targets and possible compensatory mechanisms based on presynaptic calcium homeostasis. J Neuroimmunol. 2008;201–202:145–152. doi: 10.1016/j.jneuroim.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Südhof TC. Neurotransmitter Release: The Last Millisecond in the Life of a Synaptic Vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santafe MM, Lanuza MA, Garcia N, Tomas J. Muscarinic autoreceptors modulate transmitter release through protein kinase C and protein kinase A in the rat motor nerve terminal. Eur J Neurosci. 2006;23:2048–2056. doi: 10.1111/j.1460-9568.2006.04753.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakao YK, Motomura M, Fukudome T, Fukuda T, Shiraishi H, Yoshimura T, Tsujihata M, Eguchi K. Seronegative Lambert-Eaton myasthenic syndrome: study of 110 Japanese patients. Neurology. 2002;59:1773–1775. doi: 10.1212/01.wnl.0000037485.56217.5f. [DOI] [PubMed] [Google Scholar]
- 42.O'Suilleabhain P, Low PA, Lennon VA. Autonomic dysfunction in the Lambert-Eaton myasthenic syndrome: serologic and clinical correlates. Neurology. 1998;50:88–93. doi: 10.1212/wnl.50.1.88. [DOI] [PubMed] [Google Scholar]
- 43.Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2001;24:1239–1247. doi: 10.1002/mus.1140. [DOI] [PubMed] [Google Scholar]
- 44.Oh SJ, Kurokawa K, Claussen GC, Ryan HF., Jr Electrophysiological diagnostic criteria of Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2005;32:515–520. doi: 10.1002/mus.20389. [DOI] [PubMed] [Google Scholar]
- 45.Mason WP, Graus F, Lang B, Honnorat J, Delattre JY, Valldeoriola F, Antoine JC, Rosenblum MK, Rosenfeld MR, Newsom-Davis J, Posner JB, Dalmau J. Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert-Eaton myasthenic syndrome. Brain. 1997;120:1279–1300. doi: 10.1093/brain/120.8.1279. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Bruzzi J, Rodriguez-Garza VP, Komaki RR. Lambert-eaton myasthenic syndrome in a patient with small-cell lung cancer. Clin Lung Cancer. 2006;7:282–284. doi: 10.3816/CLC.2006.n.008. [DOI] [PubMed] [Google Scholar]
- 47.Lindquist S, Stangel M. Update on treatment options for Lambert-Eaton myasthenic syndrome: focus on use of amifampridine. Neuropsychiatr Dis Treat. 2011;7:341–349. doi: 10.2147/NDT.S10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verschuuren JJ, Wirtz PW, Titulaer MJ, Willems LN, van Gerven J. Available treatment options for the management of Lambert-Eaton myasthenic syndrome. Expert Opin Pharmacother. 2006;7:1323–1336. doi: 10.1517/14656566.7.10.1323. [DOI] [PubMed] [Google Scholar]
- 49.Keogh M, Sedehizadeh S, Maddison P. Treatment for Lambert-Eaton myasthenic syndrome. Cochrane Database Syst Rev. 2011;2 doi: 10.1002/14651858.CD003279.pub3. CD003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirsch GE, Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978;22:507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedehizadeh S, Keogh M, Maddison P. The use of aminopyridines in neurological disorders. Clin Neuropharmacol. 2012;35:191–200. doi: 10.1097/WNF.0b013e31825a68c5. [DOI] [PubMed] [Google Scholar]
- 52.Wirtz PW, Verschuuren JJ, van Dijk JG, de Kam ML, Schoemaker RC, van Hasselt JG, Titulaer MJ, Tjaden UR, den Hartigh J, van Gerven JM. Efficacy of 3,4-diaminopyridine and pyridostigmine in the treatment of Lambert-Eaton myasthenic syndrome: a randomized, double-blind, placebo-controlled, crossover study. Clin Pharmacol Ther. 2009;86:44–48. doi: 10.1038/clpt.2009.35. [DOI] [PubMed] [Google Scholar]
- 53.Oh SJ, Claussen GG, Hatanaka Y, Morgan MB. 3,4-Diaminopyridine is more effective than placebo in a randomized, double-blind, cross-over drug study in LEMS. Muscle Nerve. 2009;40:795–800. doi: 10.1002/mus.21422. [DOI] [PubMed] [Google Scholar]
- 54.Sanders DB, Massey JM, Sanders LL, Edwards LJ. A randomized trial of 3,4-diaminopyridine in Lambert-Eaton myasthenic syndrome. Neurology. 2000;54:603–607. doi: 10.1212/wnl.54.3.603. [DOI] [PubMed] [Google Scholar]
- 55.Miralles F, Solsona C. 3,4-Diaminopyridine-induced impairment in frog motor nerve terminal response to high frequency stimulation. Brain Res. 1998;789:239–244. doi: 10.1016/s0006-8993(97)01516-3. [DOI] [PubMed] [Google Scholar]
- 56.Thomsen RH, Wilson DF. Effects of 4-aminopyridine and 3,4-diaminopyridine on transmitter release at the neuromuscular junction. J Pharmacol Exp Ther. 1983;227:260–265. [PubMed] [Google Scholar]
- 57.Newsom-Davis J, Murray NM. Plasma exchange and immunosuppressive drug treatment in the Lambert-Eaton myasthenic syndrome. Neurology. 1984;34:480–485. doi: 10.1212/wnl.34.4.480. [DOI] [PubMed] [Google Scholar]
- 58.Maddison P, Lang B, Mills K, Newsom-Davis J. Long term outcome in Lambert-Eaton myasthenic syndrome without lung cancer. J Neurol Neurosurg Psychiatry. 2001;70:212–217. doi: 10.1136/jnnp.70.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palace J, Newsom-Davis J, Lecky B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology. 1998;50:1778–1783. doi: 10.1212/wnl.50.6.1778. [DOI] [PubMed] [Google Scholar]
- 60.Tim RW, Massey JM, Sanders DB. Lambert-Eaton myasthenic syndrome (LEMS). Clinical and electrodiagnostic features and response to therapy in 59 patients. Ann N Y Acad Sci. 1998;841:823–826. doi: 10.1111/j.1749-6632.1998.tb11024.x. [DOI] [PubMed] [Google Scholar]
- 61.Tim RW, Massey JM, Sanders DB. Lambert-Eaton myasthenic syndrome: electrodiagnostic findings and response to treatment. Neurology. 2000;54:2176–2178. doi: 10.1212/wnl.54.11.2176. [DOI] [PubMed] [Google Scholar]
- 62.Bain PG, Motomura M, Newsom-Davis J, Misbah SA, Chapel HM, Lee ML, Vincent A, Lang B. Effects of intravenous immunoglobulin on muscle weakness and calcium-channel autoantibodies in the Lambert-Eaton myasthenic syndrome. Neurology. 1996;47:678–683. doi: 10.1212/wnl.47.3.678. [DOI] [PubMed] [Google Scholar]
- 63.Bird SJ. Clinical and electrophysiologic improvement in Lambert-Eaton syndrome with intravenous immunoglobulin therapy. Neurology. 1992;42:1422–1423. doi: 10.1212/wnl.42.7.1422. [DOI] [PubMed] [Google Scholar]
- 64.Takano H, Tanaka M, Koike R, Nagai H, Arakawa M, Tsuji S. Effect of intravenous immunoglobulin in Lambert-Eaton myasthenic syndrome with small-cell lung cancer: correlation with the titer of anti-voltage-gated calcium channel antibody. Muscle Nerve. 1994;17:1073–1075. doi: 10.1002/mus.880170919. [DOI] [PubMed] [Google Scholar]
- 65.Muchnik S, Losavio AS, Vidal A, Cura L, Mazia C. Long-term follow-up of Lambert- Eaton syndrome treated with intravenous immunoglobulin. Muscle Nerve. 1997;20:674–678. doi: 10.1002/(sici)1097-4598(199706)20:6<674::aid-mus3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 66.Antoine JC, Camdessanche JP. Treatment options in paraneoplastic disorders of the peripheral nervous system. Curr Treat Options Neurol. 2013;15:210–223. doi: 10.1007/s11940-012-0210-9. [DOI] [PubMed] [Google Scholar]
- 67.Liang M, Tarr TB, Bravo-Altamirano K, Valdomir G, Rensch G, Swanson L, DeStefino NR, Mazzarisi CM, Olszewski RA, Wilson GM, Meriney SD, Wipf P. Synthesis and Biological Evaluation of a Selective N- and P/Q-Type Calcium Channel Agonist. ACS Med Chem Lett. 2012;3:985–990. doi: 10.1021/ml3002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan Z, Chi P, Bibb JA, Ryan TA, Greengard P. Roscovitine: a novel regulator of P/Qtype calcium channels and transmitter release in central neurons. J Physiol. 2002;540:761–770. doi: 10.1113/jphysiol.2001.013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeStefino NR, Pilato AA, Dittrich M, Cherry SV, Cho S, Stiles JR, Meriney SD. (R)-roscovitine prolongs the mean open time of unitary N-type calcium channel currents. Neuroscience. 2010;167:838–849. doi: 10.1016/j.neuroscience.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tarr TB, Lacomis D, Reddel SW, Liang M, Valdomir G, Frasso M, Wipf P, Meriney SD. Complete reversal of Lambert-Eaton myasthenic syndrome synaptic impairment by the combined use of a K+ channel blocker and a Ca2+ channel agonist. J Physiol. 2014;592:3687–3696. doi: 10.1113/jphysiol.2014.276493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorlochter M, Irintchev A, Brinkers M, Wernig A. Effects of enhanced activity on synaptic transmission in mouse extensor digitorum longus muscle. J Physiol. 1991;436:283–292. doi: 10.1113/jphysiol.1991.sp018550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marce M, Griffin JW, Rich MM, Sumner CJ. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci. 2009;29:842–851. doi: 10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]