Abstract
Healthy aging is associated with a decline in basic perceptual abilities, as well as higher-level cognitive functions such as working memory. In a recent perceptual training study using moving sweeps of Gabor stimuli, Berry et al. (2010) observed that older adults significantly improved discrimination abilities on the most challenging perceptual tasks that presented paired sweeps at rapid rates of 5 & 10 Hz. Berry et al. further showed that this perceptual training engendered transfer-of-benefit to an untrained working memory task. Here, we investigated the neural underpinnings of the improvements in these perceptual tasks, as assessed by event-related potential (ERP) recordings. Early visual ERP components time-locked to stimulus onset were compared pre- and post- training, as well as relative to a no-contact control group. The visual N1 and N2 components were significantly enhanced after training, and the N1 change correlated with improvements in perceptual discrimination on the task. Further, the change observed for the N1 and N2 was associated with the rapidity of the perceptual challenge; the visual N1 (120–150 ms) was enhanced post-training for 10 Hz sweep pairs, while the N2 (240–280 ms) was enhanced for the 5 Hz sweep pairs. We speculate that these observed post-training neural enhancements reflect improvements by older adults in the allocation of attention that is required to accurately dissociate perceptually overlapping stimuli when presented in rapid sequence.
Keywords: aging, visual perception, cognitive training, perceptual learning, working memory, transfer of benefit, ERP
Introduction
It is well documented that even healthy aging is associated with a decline in cognitive abilities in the domains of perception, attention and working memory (WM) (Craik & Salthouse, 2000; Schneider & Pichora-Fuller, 2000). Aspects of age-related declines in WM have been related to primary deficits in perception (Craik & Salthouse, 2000; Wigfield et al., 1994), although studies that successfully parse between perception and WM impairments are scarce. Furthermore, while studies have shown perceptual learning to occur in both younger (Ball & Sekuler, 1987; Schoups et al., 1995) and older adults (Fahle and Daum, 1997; Alain et al., 2001), there is little evidence of transfer of benefits to other cognitive functions such as WM. Using ERP recordings, we investigate the sensory cortical plasticity underlying perceptual learning in healthy aging, and assess how this relates to behavioral gains in perceptual discrimination on the task itself and concomitant gains in WM on an untrained task.
In a recent study from our lab, Berry et al. (2010) demonstrated that visual perceptual abilities of older adults can be enhanced by adaptive perceptual discrimination training. Participants trained on discriminations of sweeping Gabor patterns that involved distinguishing the sweep directions of two sweeps (inward contraction vs. outward expansion) presented rapidly as a pair of stimuli. This SweepSeeker training was adaptive to performance accuracy, thus presenting more challenging sweep rates immediately after successful performance and slowed sweep rates after failed performance. Berry et al. evaluated post vs. pre-training perceptual improvements using a non-adaptive assessment that presented sweep pairs at fixed rates of 2.5 Hz, 5 Hz and 10 Hz (the fixed-speed test), and found significant benefit only on the most rapidly presented challenging sweep rates of 5 Hz and 10 Hz. This result supported previous evidence of perceptual learning in older adults with challenging discrimination practice (Fahle & Daum, 1997). Uniquely, Berry et al. also showed that this training benefitted performance on an untrained perceptual task and an untrained WM delayed-recognition task that both used dot motion kinematogram stimuli.
Thus, the Berry et al. (2010) study highlighted the benefits of challenging perceptual learning in aging on perception abilities, as well as higher order cognitive function such as WM. To follow up on these findings, here we explore the neural mechanisms by which older individuals learned to better perform on these challenging perceptual discriminations of sweep pairs presented at such rapid rates of 5 Hz and 10 Hz in the fixed-speed test.
The fixed-speed perceptual assessment presented single sweep (ss) and double sweep (ds) gabor patch stimuli in separate blocks (Figure 1a). In either block, stimuli were randomized to appear at three different stimulus durations of 50 ms, 100 ms or 200 ms. In the ds block these durations respectively corresponded to presentation rates of 10 Hz, 5 Hz and 2.5 Hz, as the inter-stimulus intervals (isi) matched the stimulus durations. Henceforth, these stimuli are abbreviated as ss50, ss100, ss200 and ds50, ds100, ds200. For both single or double sweep trials, participants made speeded discriminations on whether each presented sweep was expanding or contracting. The fixed-speed assessment was performed at two sessions, T1 and T2 that were 3–5 weeks apart, by two experimental groups consisting of 15 older adults who trained on ten hours of visual sweep discrimination and 15 older adults who engaged in no training. Ten hours of training was chosen as a feasible training dose for participants for which there would be high probability of full compliance over the 3–5 weeks of training with 40 minutes of training per session. Also, based on prior studies in the literature, we estimated that approximately ten hours on a training module would engender sufficient learning, and training-related benefits may plateau (Green and Bavelier, 2003, Dahlin et al., 2008, Li et al., 2008).
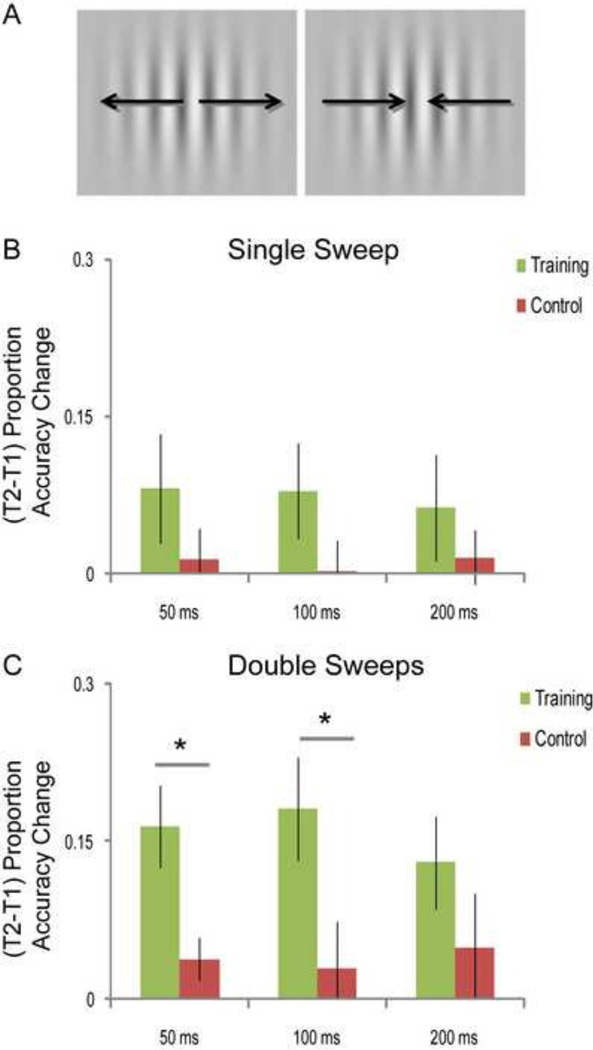
Figure 1. Behavioral Performance.
A. An example of the double sweep stimulus; the first sweep shown (left) is expanding and the second sweep (right) is contracting. B. (T2-T1) change in proportion correct accuracies on single sweep stimuli (ss50, ss100 and ss200) for the training group (green) and control group (red). No significant performance differences were observed between groups. C. (T2-T1) change in proportion correct accuracies on double sweep stimuli (ds50, ds100 and ds200) for the training (green) and control (red) group. The training group showed significantly greater accuracies at T2 relative to T1 for the ds50 and ds100 stimuli, compared to the control group.
On the fixed speed test, Berry et al. (2010) observed that performance accuracy significantly improved for the challenging ds50 and ds100 stimuli post-training. Here, we analyzed the ERP responses to these stimuli to specifically assess the training-related neural correlates of these behavioral effects. We hypothesized that neuroplasticity in visual processing would be observed for the ds50 and ds100 stimuli, in accordance with the performance improvements that were previously reported. Also, that this plasticity would emerge at different time points in the visual processing stream, i.e. earlier for the ds50 stimulus, which requires more rapid adjustments in attention than ds100, as the second stimulus in the double sweep sequence appears at 100 ms (for ds50) versus 200 ms (for ds100).
Additionally, neurobehavioral correlations were pursued to assess the strength of the relationship between the underlying neuroplasticity metrics and changes in behavioral performance observed in the fixed-speed sweeps assessment, as well as in the independent WM assessment, also performed at T1 and T2 (see Berry et al., 2010 for a detailed analysis of the WM performance changes as well as effects of training on WM neural encoding).
Results
Behavioral Performance
Proportion correct accuracies and response times to ss and ds stimuli at both T1 and T2 assessment sessions are shown in Table 1 and 2, and the (T2-T1) change in accuracy in Fig. 1b–c. After confirming Levene’s test for homogeneous variances across groups, differences in baseline performance measures at T1 were analyzed in 3-factor ANOVAs with a between-subject factor of group (training vs. control) and two within-subject factors of sweep type (ss vs. ds) and sweep speed (50 ms, 100 ms and 200 ms). There were no overall group differences in baseline performance (p>0.9). Baseline accuracy for ss stimuli was approximately 20% greater than for ds stimuli (F(1,28)=107.82, p<0.0001) and there was also a main effect of sweep speed with greater accuracy for 200 ms > 100 ms > 50 ms stimuli (F(2,56)=209.59, p<0.0001); there were no group interactions with these measures.
Table 1.
Proportion correct accuracies (mean ± s.e.m) for single sweep (ss50, ss100 and ss200) and double sweep (ds50, ds100, ds200) stimuli.
| Sweeps | Group | 50 ms | 100 ms | 200 ms | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| Single | Training | .66 ± .04 | .74 ± .04 | .86 ± .05 | .94 ± .02 | .92 ± .05 | .98 ± .01 |
| Control | .66 ± .04 | .68 ± .05 | .84 ± .04 | .84 ± .03 | .91 ± .03 | .93 ± .01 | |
| Double | Training | .32 ± .02 | .48 ± .03 | .66 ± .05 | .84 ± .02 | .78 ± .05 | .91 ± .01 |
| Control | .36 ± .04 | .40 ± .04 | .66 ± .06 | .69 ± .03 | .79 ± .05 | .40 ± .04 | |
Table 2.
Response times in milliseconds (mean ± s.e.m) for single sweep (ss50, ss100 and ss200) and double sweep (ds50, ds100, ds200) stimuli.
| Sweeps | Group | 50 ms | 100 ms | 200 ms | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| Single | Training | 772 ± 53 | 843 ± 49 | 585 ± 38 | 647 ± 43 | 478 ± 35 | 509 ± 37 |
| Control | 842 ± 62 | 805 ± 63 | 653 ± 41 | 674 ± 41 | 540 ± 40 | 555 ± 25 | |
| Double | Training | 1668 ± 115 | 1630 ± 137 | 1358 ± 81 | 1256 ± 102 | 1244 ± 116 | 1039 ± 72 |
| Control | 1724 ± 150 | 1552 ± 90 | 1349 ± 61 | 1356 ± 74 | 1217 ± 64 | 1156 ± 51 | |
We found that T2 session accuracies did not conform to the Levene’s test of homogenous variances across groups (Levene’s p<0.05 for ss100, ss200, ds100 and ds200 T2 performance), hence improvements in accuracy with training were analyzed using the Mann Whitney U non-parametric test. These tests showed significant session accuracy improvements in the trained vs. control participants only for the ds50 (p=0.003) and ds100 (p=0.03) stimuli but not for others (p>0.05). These results were previously briefly described in Berry et al. (2010).
Reaction time measures all conformed to the Levene’s test and hence were compared using a 4-factor ANOVA with one between-subject factor of group (training vs. control) and three within-subject factors of session (T1 vs. T2), sweep type (ss vs. ds) and sweep speed (50 ms, 100 ms and 200 ms). No group × session interaction (p>0.8) nor any further interactions between group, session, sweep type and sweep speed emerged, revealing that performance improvements were confined to accuracy, and were not the result of speed-accuracy tradeoffs.
As Berry et al. (2010) also conducted a WM assessment in addition to the fixed-speed test to assess training transfer effects, we share those results here. For the delayed recognition WM task, the (T2-T1) change in mean proportion correct accuracies for trained individuals was 0.08 ± 0.02, while there was a smaller change observed for the control group, 0.003 ± 0.03. The group × session interaction was significant (F(1,28)=4.29, p=0.05) and post-hoc t-tests showed that the proportion correct WM performance significantly improved only in the training group (training: p=0.0007, control: p=0.92).
Event-related Potential (ERP) Responses
ERP analysis focused on the two conditions that exhibited significant performance enhancement (ds50 and ds100) to assess the neural processing modifications underlying these specific performance accuracy gains. The ERP processing components of interest were the visual P1, N1 and the N2, time-locked to the onset of the first sweep in the double sweep sequence. The visual P1 and N1 reflect early sensory processing in visual cortices and are modulated by top-down attention control (Hillyard and Anllo-Vento, 1998). The N2 is known to reflect an early decision process, which controls response selection in sensory discrimination tasks, and localizes to the region of the anterior cingulate cortex (Ritter et al., 1979; Towey et al., 1980; van Veen and Carter, 2002; Carretié et al., 2004; Gajewski et al., 2008; Folstein and Van Petten 2008). Components later than 300 ms post-stimulus onset were not analyzed as it becomes more and more difficult to distinguish the contribution of sensory, decisionmaking and response selection processes at these latencies.
The ERP components were analyzed for 28 (instead of 30) participants, 14 in each group; EEGs from 1 participant in both training and control group had to be excluded due to problems with EEG data acquisition, which caused neural data for entire blocks to be missing in one of the sessions. The P1 and N1 component amplitudes were quantified over 15 occipital sites (as average of three clusters: left and right hemisphere and midline; 6 electrodes in each hemisphere and 3 at midline: O1/2, PO3/4, PO7/8, P3/4, P5/6, P7/8, POz, Oz, Iz) in the 70–90 ms and 120–150 ms peak latency range for P1 and N1, respectively. The N2 component peaked at central electrode sites, a known scalp distribution for this component (reviewed in Folstein and Van Petten, 2008), and was quantified over 15 central sites (as average of three clusters: left and right hemisphere and midline; 6 in each hemisphere and 3 at midline: FC1/2, FC3/4, C1/2, C3/4, CP1/2, CP3/4, FCz, Cz, CPz) in the 240–280 ms peak latency range. These amplitude data were all subjected to 4-way ANOVA with a between-subject factor of group (training vs. control) and 3 within-subject factors of ERP component (P1, N1, N2), session (T1 vs. T2) and sweep type (ds50 vs. ds100).
The 4-way ANOVA showed a main effect of group (F(1,26)=5.77, p=0.02), a group × session interaction (F(1,26)=5.41, p=0.03), a group × ERP component interaction (F(2,52)=3.87, p=0.027) as well as a group × session × ERP component interaction (F(2,52)=3.43, p=0.04). Follow-up showed that it was driven by the N1 and N2 components that were differentially modulated across sessions and groups, but not the P1 component. Thus, for greater sensitivity, this 4-way group × session × ERP component × sweep type ANOVA was repeated with only the N1 and N2 components in the ERP factor. This ANOVA also showed a main effect of group (F(1,26)=7.63, p=0.01), a group × session interaction (F(1,26)=8.44, p=0.007), a group × ERP component interaction (F(1,26)=4.78, p=0.04), but also a 4-way group × session × ERP component × sweep type interaction (F(1,26)=4.64, p=0.04). This 4-way interaction suggested a differential modulation of the N1 and N2 components for the ds50 vs. ds100 stimuli with training.
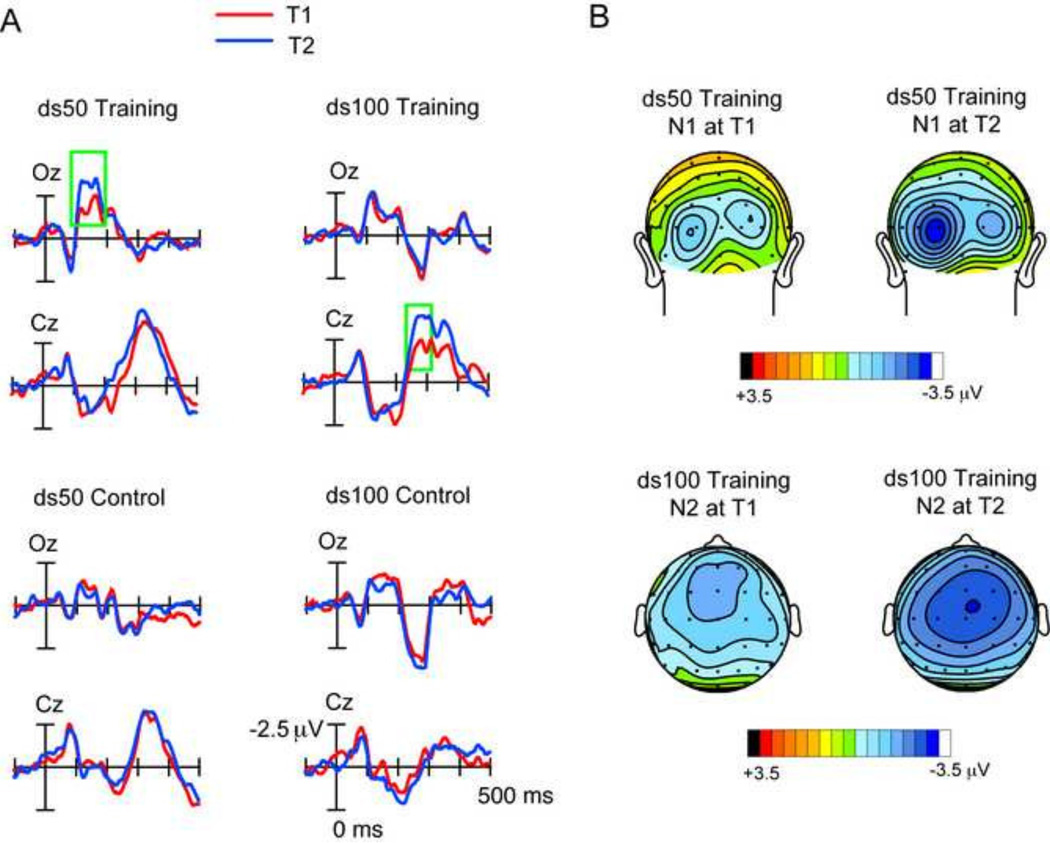
To further parse the 4-way interaction, we conducted separate group × session 2-way ANOVAs on each stimuli i.e. ds50 and ds100, and for each component, i.e. N1 and N2. For the ds50, the N1 response showed a significant group × session interaction (F(1,26)=7.97, p=0.009) and post-hoc tests confirmed that this result was driven by a significant N1 amplitude enhancement in the training (p=0.02) but not control (p=0.25) group. The ds50 N2 response showed no group × session interaction (p=0.49), although there was a main effect of group (p=0.04) and main effect of session (p=0.01). For the ds100 stimulus, the N1 response did not yield significant effects (group: p=0.8, session: p=0.2, group × session, p=0.07). In contrast, the ds100 N2 response was significantly enhanced by training, as revealed by a significant group × session interaction (F(1,26)=4.78, p=0.04), which was further confirmed in post-hoc tests (training group: p=0.03, control group: p=0.43). Together, these results showed that sensory processing related to the very rapid ds50 stimuli was specifically enhanced by training in the N1 latency range, while the less rapid ds100 stimuli were enhanced during N2 processing. Figure 2a shows ERPs at occipital Oz and central Cz sites that are exemplars for N1 and N2 processing, respectively. Neural responses are shown for the ds50 and ds100 stimuli (left and right) and in the training and control group (top and bottom). The scalp topographies of the ds50 N1 enhancement with training and the ds100 N2 enhancement with training are shown in Fig 2b.
Figure 2. ERP Responses.
A. Top: T1 (red) versus T2 (blue) ERPs time-locked to the stimulus onset of ds50 (left) and ds100 (right) at occipital Oz and central Cz sites. Training group and control group ERPs are on top and bottom, respectively. Negative potentials are plotted above the horizontal and the scale for all ERP time series are depicted in the right bottom-most plot. B. Topography maps for the ds50 N1 ERP peak amplitude in the training group showing enhancement at T2 relative to the T1 session (top row). Topography maps for the ds100 N2 peak amplitude in the training group showing enhancement at T2 relative to T1 (bottom row).
The (T2-T1) difference in P1, N1 and N2 session amplitudes are shown for the training and control group in Table 3 for all stimuli in the fixed-speed test, along with the p values of the group t-tests on these (T2-T1) difference amplitudes. These data further confirm that there were no other significant group differences than the ds50 N1 and ds100 N2 results analyzed above.
Table 3.
(T2-T1) ERP amplitude difference in µV (mean ± s.e.m) for single and double sweep stimuli (ss50, ss100, ss200, ds50, ds100, ds200) in the training and control group. The group comparison t-test p values are depicted alongside, with the two significant enhancements of N1 for ds50 stimuli and N2 for ds100 stimuli shown in bold.
| P1 component (T2-T1) µV | |||||||||
| Sweeps | 50 ms | 100 ms | 200 ms | ||||||
| Training | Control | p | Training | Control | p | Training | Control | p | |
| Single | −0.59 ± 0.25 | −0.28 ± 0.51 | 0.6 | −0.31 ± 0.28 | 0.68 ± 0.90 | 0.3 | 0.24 ± 0.39 | 0.24 ± 0.37 | 0.9 |
| Double | 0.33 ± 0.33 | −0.09 ± 0.52 | 0.5 | 0.14 ± 0.31 | 0.38 ± 0.44 | 0.6 | 0.07 ± 0.43 | 0.11 ± 0.68 | 0.9 |
| N1 component (T2-T1) µV | |||||||||
| Sweeps | 50 ms | 100 ms | 200 ms | ||||||
| Training | Control | p | Training | Control | p | Training | Control | p | |
| Single | −0.45 ± 0.46 | 0.29 ± 0.71 | 0.4 | −0.31 ± 0.40 | 1.35 ± 1.07 | 0.2 | −0.18 ± 0.35 | 0.88 ± 0.41 | 0.06 |
| Double | −1.04 ± 0.38 | 0.41 ± 0.34 | 0.009 | −0.23 ± 0.32 | 1.00 ± 0.54 | 0.07 | −0.15 ± 0.37 | 0.96 ± 0.55 | 0.1 |
| N2 component (T2-T1) µV | |||||||||
| Sweeps | 50 ms | 100 ms | 200 ms | ||||||
| Training | Control | p | Training | Control | p | Training | Control | p | |
| Single | 0.57 ± 0.45 | 1.26 ± 0.76 | 0.4 | 0.26 ± 0.47 | 0.14 ± 0.82 | 0.9 | 0.40 ± 0.65 | 0.27 ± 0.53 | 0.9 |
| Double | −1.11 ± 0.54 | −0.67 ± 0.36 | 0.5 | −1.14 ± 0.47 | 0.46 ± 0.56 | 0.04 | −0.99 ± 0.53 | 1.11 ± 1.32 | 0.2 |
Neurobehavioral Correlations
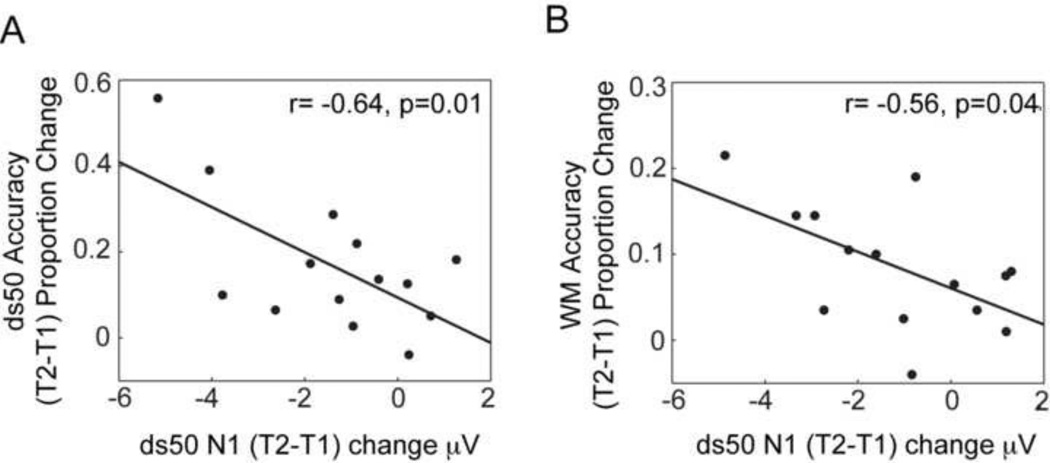
To probe the relationship between the visual ds50 N1 and ds100 N2 modulations in the training group and the training-related accuracy improvements on the perceptual task itself and the untrained WM task, we conducted Pearson’s product-moment neurobehavioral correlations. The (T2-T1) N1 amplitude enhancement observed for ds50 stimuli was significantly correlated with the improvement in ds50 accuracies (r(12)=−0.64, p=0.01), i.e. more negative N1 amplitudes post-training associated with improved ds50 performance (Fig. 3a). Moreover, this ds50 N1 amplitude change was also significantly correlated with the improvement in WM delayed recognition performance (r= −0.56, p=0.04), again with greater (more negative) N1 amplitudes posttraining corresponding to greater WM accuracy (Fig. 3b). The ds50 N1 change did not correlate with the N1 modulation observed during WM encoding by Berry et al. (2010) (p>0.5). The ds100 N2 modulation did not correlate with training-related improvements in ds100 accuracy (p>0.1) or with the observed WM accuracy gains (p>0.7), or the N1 modulation in the WM task (p>0.6). It has been suggested that there is a dynamic shift between much reduced activation of visual association cortical areas and heightened activity in the anterior cingulate cortex during the N2 (van Veen and Carter, 2002, Carretié et al., 2004). These different cortical source activations during the N2 may be differentially activated in different individuals potentially generating variability in the scalp component measurements, one possible reason why N2 correlations with behavior were not observed.
Figure 3. Neurobehavioral Correlations.
A. Significant correlation between the ds50 N1 ERP amplitude enhancement from T1 to T2 and performance measures: the ds50 accuracy improvement (A) and the working memory (WM) accuracy improvement (B).
Discussion
This study adds to the growing evidence that aging brains are capable of training-related neuroplasticity; while there are many studies that demonstrate effects of training on aging cognition there are only a few studies that have elucidated underlying neural modulations with training (Erickson et al., 2007, Dahlin et al., 2008, Berry et al., 2010, Engvig et al., 2012, Gajewski and Falkenstein, 2012, Anguera et al., 2013, Anderson et al., 2013, Mishra et al., 2013). Neuroplasticity in these studies is evidenced in varied brain regions, ranging from sharpening of subcortical processing (Anderson et al., 2013), modulations in sensory processing (Berry et al., 2010; Mishra et al., 2013), striatal activations (Dahlin et al., 2008) and frontal cortex based changes (Erickson et al., 2007, Engvig et al., 2012, Gajewski and Falkenstein, 2012, Anguera et al., 2013). Of these, studies that have incorporated EEG recordings show training-related temporal modulations early in stimulus processing, i.e. 0–250 ms post-stimulus onset (Berry et al., 2010, Mishra et al., 2013), as well as at mid-latency time ranges of 250–500 ms that invoke frontal and parietal plasticity (Gajewski and Falkenstein, 2012, Anguera et al., 2013). Thus, there is no specific constraint on neuroplasticity in aging either with respect to the timing of the responses or involved brain regions. In general, the variety of spatial and temporal neural activations are governed by the components and complexity of the cognitive training task.
Using ERP recordings, we show neuroplasticity in sensory processing that underlies learning on a perceptually challenging task. Accuracy improvements observed, specifically on the short duration double sweep stimuli (ds50 and ds100), mapped onto specific ERP component modulations. The visual N1 was selectively enhanced post-training for ds50 stimuli, while N2 processing was enhanced with training for ds100 stimuli. Further, neurobehavioral correlations showed a significant relationship between the training-related early N1 enhancement and post-training improvements in ds50 performance accuracy. Moreover, the visual N1 enhancement also correlated with improvements on an untrained WM delayed recognition assessment, previously characterized by Berry et al. (2010). These results suggest that early sensory neuroplasticity, such as during visual N1 processing may underlie both enhanced perception and higher cognitive function such as WM; it would interesting to corroborate these findings in future studies with larger sample sizes and/or with transcranial electrical stimulation methods (e.g. Sadeh et al., 2011, Zanto et al., 2011) that investigate how activity disruptions during N1 processing latencies influence perceptual and WM performance.
Both ds50 and ds100 are very challenging perceptual tasks, as evidenced by the low pre-training accuracies of 30–60% relative to all other assessed stimuli. These stimuli essentially appear to be overlapping to the untrained individual, and yet training results in significant accuracy improvements. The underlying neuroplasticity observed in the N1 and N2 ERP components may suggest a common neural mechanism that engenders these perceptual performance improvements. Of note, no such neural modulations were observed for the other relatively easier to discriminate stimuli, i.e. single sweeps and the slowest rate 2.5 Hz double sweeps (ds200). For ds200, the second sweep appears at 400 ms after the first sweep onset, presumably well after the sensory processing of the first sweep is complete. Thus, the N1 and N2 modulations are specific to the most challenging perceptually overlapping conditions. Further, the time of occurrence of the selective N1 enhancement in the case of ds50 stimuli and the selective N2 enhancement in the case of ds100 stimuli closely coincided with the presentation of the second sweep in the double sweep sequence. For ds50 the second sweep onsets at 100 ms and the N1 enhancement occurs at 120–150 ms, and for ds100 the second sweep is presented at 200 ms with N2 enhancement following shortly at 240–280 ms. It is possible that the rapid occurrence of the second sweep creates the demand on neural processing to distinguish the two almost overlapping sweeps, and that the N1 and N2 effects reflect successfully meeting these demands. Greater attentional allocation at the time of occurrence of the second sweep is one potential mechanism that could enable neural and consequent behavioral distinction of the first and second sweep. The timing of differential enhancement effects, at the N1 for ds50 and at the N2 for ds100, seems to reflect the response of the system to the challenge created by the timing of the representation of the second sweep entering the visual processing stream, and thus the time when interferences with the ongoing processing of the first sweep occurs. It thus makes sense that the ds100 task results in enhancement of more central attentional processes, since it occurs at a later stage in processing when more central processes are being engaged. Indeed, both the N1 and N2 ERP components are known to be enhanced by attention (Hillyard et al., 1998, Mishra et al., 2012, N1 reviewed in Hillyard and Anllo-Vento, 1998, N2 reviewed in Folstein and Van Petten, 2008) and thus, may be the substrates of increased selective attention allocation with training. In fact, the N2 has also been previously shown to be modulated during processing of nearly overlapping sequential visual stimuli in the attention blink paradigm (Sergent et al., 2005). Lastly, the neurobehavioral correlations between the N1 enhancement and ds50 perceptual performance improvements, as well as with WM gains, may also be supported by the same selective attention mechanism. If perceptual training leads to enhanced allocation of selective attention to challenging sensory stimuli, then such a general cognitive control mechanism would also benefit stimulus encoding on an untrained cognitive assessment such as the WM task.
It is worth noting that the perceptual training we employed showed cognitive transfer to an untrained working memory task as demonstrated by Berry et al. (2010), and we further showed neurobehavioral correlations with N1 plasticity using this task. This cognitive generalization of the perceptual training is in contrast to many other studies that have found very specific effects of training confined to specific visual features as well as stimulus location in the visual field (e.g., Karni & Sagi, 1991, Shiu & Pashler, 1992, Crist et al., 1997, reviewed in Fahle, 2005). The training we implemented differs from paradigms used in these other studies in two important respects, which have been found to robustly drive neuroplasticity (Merzenich et al., 1991, Merzenich and deCharms, 1996, Mishra et al., 2013). (1) The training was adaptive on a trial-by-trial basis in response to the participant’s performance, such that shorter stimulus durations were presented in response to good performance to enforce faster perceptual decisions, while stimuli appeared for longer times in response to poor performance. Many other perceptual studies did not employ such adaptive updating in their training approach. (2) Our training incorporated cycles of feedback and reward at multiple levels, ranging from positive and negative feedback on a trial-by-trial level, as well as cumulative block and session feedback. It has been found that feedback greatly accelerates learning (Fahle, 2004, Dobres and Watanabe, 2012). This feature was either not implemented or only implemented as negative reinforcement in the other perceptual training studies. Thus, we hypothesize that our performance-adaptive and feedback-reward driven training was responsible for the observed effects. Also of note, others have suggested that cognitive transfer occurs for tasks that invoke similar underlying neural processes or cortical activations (Fahle, 2005, Dahlin et al., 2008, Zelinski, 2009). In our task, individuals learned to discriminate moving gabor sweeps and the transfer task was also for moving stimuli, thus it is possible that the common feature of visual motion drove the training transfer; in fact visual N1 related plasticity was observed in both tasks suggesting a common underlying neural mechanism. Finally, age differences between studies may also account for differences in training outcomes. Prior training studies have conventionally tested young adult participants, who may already exhibit near peak performance with little room for improvement with training, while older adults with impaired perceptual/ cognitive abilities relative to their younger counterparts may benefit more from training (Karbach and Kray, 2009, Anguera et al., 2013).
One anomaly between the current findings and those of Berry et al. (2010) is that here we find enhancement of the N1 component to double sweep stimuli that correlates with the WM transfer-of-benefit. In contrast, Berry et al. found that the N1 ERP responses to the WM encoding stimuli were diminished by training and that this reduced N1 amplitude correlated with the WM gains. At first glance, these opposing modulations of the visual N1 seem inconsistent; also we did not find a significant correlation between these measures. However, these conflicting findings can be resolved by taking into consideration the differences in task demands. In the current study, the N1 enhancement underlay improvements on the perceptual challenge, possibly driven by enhanced attentional allocation at the time of challenge, i.e. when the second sweep is presented. On the other hand, the reduced N1 during WM encoding found by Berry et al. (2010) may be the result of reduced demands of an easier post-training WM task, which resulted in reduced attentional effort during encoding and thus a reduced N1 that correlated with WM behavioral gains. Using perceptual thresholding methods, Berry et al. further confirmed that the post-training WM encoding was indeed perceptually easier than pre-training encoding. WM encoding stimuli were dot motion kinematograms that had been perceptually thresholded in each participant pre-training. During the post-training assessment, participants performed the WM task on their original pre-training perceptual thresholds and showed WM gains. However, when their perceptual thresholds were separately evaluated post-training, these thresholds were also improved; but importantly there were no gains for WM evaluated at the new post-training perceptual thresholds compared to pre-training WM performance. Together, these results suggest that neural processing at the time of the N1 component can be flexibly modulated by increased or decreased attentional demands, which in turn correlates with behavioral gains. The N1 localizes to neural source generators in the ventral extrastriate cortex (Gomez Gonzalez et al., 1994, Di Russo et al., 2002) and indeed neural processing in these cortices has been shown to undergo both relative enhancements and reductions during attention conditions (Motter, 1993).
In summary, we demonstrate the neuroplastic mechanisms underlying perceptual training in healthy aging; additionally we show associations between early sensorineural changes and perceptual, as well as WM cognitive gains. To date, visual perceptual training has been shown to be beneficial for correcting visual weaknesses such as amblyopia (Astle et al., 2011, Levi and Li, 2009). Here we extend the usefulness of perceptual training by suggesting that it may also play a role in improving WM, especially since early sensory plasticity correlates with the transfer of benefit. Overall, these results are in line with other training studies that have emphasized perceptual training to be a valuable component of cognitive training in diverse neurocognitively impaired populations (Mahncke et al., 2006, Fisher et al., 2009, Vinogradov et al., 2012). The interpretation is that higher-fidelity perceptual representations in sensory cortices are then propagated through multiple levels of neural processing to enhance higher cognitive functions (Kumano and Uka, 2013); here we add to this by suggesting that attentional allocation precisely timed with the appearance of sensory challenge impacts the enhanced sensory neural representations.
Experimental Procedure
All human data collection procedures, including ethical research conduct, participant details, neuropsychological assessments, perceptual training and EEG data acquisition, are from Berry et al. (2010).
Ethical Statement
Participants were paid for their participation and gave written informed consent. The Committee on Human Research at the University of California, San Francisco, approved the EEG portion of the study. The cognitive training portion of the study received separate approval by an independent IRB review board (Independent Review Consulting Incorporated, Corte Madera, CA).
Participants
32 healthy older adults (age: mean ± s.d. (standard deviation) 71.93 ± 7.53, 18 females) were recruited; no participants recruited for this study engaged in any other PositScience or other cognitive training program during the course of this study. Participants were randomly assigned to control and training groups after consent. Ten participants, five in either training/ control group had sporadically engaged in some PositScience or other commercially available cognitive training in the past that was not related to the SweepSeeker training studied here, and had stopped any such activity prior to their first lab screening visit. Two of the enrolled participants did not complete the study because of unwillingness to participate in the final EEG session. Behavioral statistics reported reflect 30 participants, 15 of who completed 10 sessions of training and 15 serving as controls. EEG statistics reported reflect 28 participants, 14 each in the training and control group; 1 individual in either group could not be included due to loss of EEG data attributed to technical problems. Participants had 13–21 years of education (mean ± s.d. 17.24 ± 2.32 years) with no significant difference across groups (p=0.34). All participants had normal or corrected-to-normal vision, did not have a history of stroke, traumatic brain injury, psychiatric illness, or previous experience with visual cognitive training. No participants took psychotropic medications. Participants were characterized as cognitively normal using standard neuropsychological assessments conducted prior to study initiation.
All participants were from the San Francisco Bay Area and recruited using a database of research volunteers at Posit Science, which was previously compiled using local advertisements and mailings. Contraindications were screened for during a standardized phone interview. Participants were randomized to training or control groups after signing consent forms for participation in the study at Posit Science offices. Experimenters from the University of California, San Francisco who conducted the behavioral and EEG analysis were blinded until after data collection for the final group analysis was completed.
Neuropsychological Assessments
Baseline neuropsychological measures were collected for each participant to confirm that cognitive performance was within two standard deviations of the normative values for their age and education. Mini-mental state exam (MMSE) and NeuroTrax (Mindstreams) measures of global cognition, memory, executive function, attention, and information processing speed were completed by all participants. All MMSE scores were greater than 27 and there was no significant difference between training and control groups (p=0.87). NeuroTrax has been validated for use as an assessment for the detection of possible mild cognitive impairment (Doniger et al., 2005, Dwolatzky et al., 2003). There were no significant differences in Neurotrax measures across groups (p>0.59).
Perceptual Training
Participants in the training group completed 10 hours of visual cognitive training using the Sweep Seeker program (InSight, Posit Science). Sweep Seeker training is a stand-alone module in the Posit Science InSight software package. The perceptual training exercise was embedded in a block type game to encourage attention, provide feedback and rewards, and improve compliance for the 10 hours of training. Additionally, the software was designed to be easy to use, so that previous experience with computers would not limit the population that may benefit from such a cognitive training approach. Training took place in 40-minute sessions, 3–5 sessions/week for 3–5 weeks. Training occurred either in research offices (n=6) or at home (n=9) where computer equipment was provided to participants. Participants did not have the option of doing some training in home and some at the research offices. There were no location dependent differences in trained task performance measured by repeated measures ANOVA with factors location (home vs. office) and time (pre-training performance vs. post-training performance) as indicated by no location × session interaction (F(1,13)=0.89, p=0.36). The data from each training session was automatically uploaded to remote servers, providing a complete record of program usage (e.g., days trained, total training time) and progress (e.g., stimulus challenge level). Participants were phoned regularly to encourage compliance.
Each trial consisted of two sweeping Gabor pattern stimuli (sine wave patterns windowed by a 2D Gaussian) (Figure 1a). The patterns either expanded or contracted across a range of spatial frequencies (0.50 to 5.00 cycles per degree) and subtended 8 degrees of visual angle. The stimulus presentation time and ISI were adjusted together using an adaptive staircase algorithm (ZEST) (King-Smith et al., 1994). Differing colors and orientations of sweeps were varied across training conditions to promote engagement with training. These varying colors and orientations may facilitate generalization, especially to other tasks that rely on color/ orientation, but we did not assess such color/orientation based transfer in our studies. Vertical, horizontal, and diagonal orientations were utilized in distinct blocks. Steps were taken to assure that training conditions at home and in the office were standardized by calibrating stimuli to accurately specify chromaticities and relative luminances on home computers. Participants indicated the sequence of stimulus presentation by clicking on icons presented after each trial. All training was performed at the 85% correct level of the psychometric function estimated by the ZEST algorithm. Thresholds were calculated by taking the log mean of two randomly interleaved staircases.
Fixed-Speed Perceptual Assessment
This task was similar to the training task except that stimulus presentation parameters, specifically stimulus duration and ISI were non-adaptive to performance accuracy. Also unlike the training, assessment stimuli were gray and vertically oriented, i.e. did not vary in color and orientation. All stimuli subtended 8 degrees of visual angle at a 75 cm viewing distance and were centered at the fovea. The stimuli were presented on a black background of luminance level 0.32 cd/m2. Stimuli were presented through E-Prime software (Psychology Software Tools, Inc.) run on a Dell Optiplex GX620 and a ViewSonic G220fb CRT monitor.
Gabor pattern stimuli were presented on every trial either as a single sweep, which expands or contracts across a range of spatial frequencies (0.50 to 5.00 cycles per degree), or double sweeps, i.e. two sweeps presented sequentially. Both single and double sweep Gabor patterns were presented at three different stimuli durations of 50 ms, 100 ms and 200 ms. Incase of double sweeps, the inter-stimulus interval (ISI) and the second sweep duration matched the stimulus duration of the first sweep. So for 50 ms, 100 ms and 200 ms double sweeps, the second sweep onset was at 100 ms, 200 ms and 400 ms respectively, corresponding to double sweep presentation rates of 10 Hz, 5 Hz and 2.5 Hz. The three stimuli durations were randomly shuffled in the single and double sweep blocks. Single and double sweep blocks were separated. Participants performed 4 total blocks, 2 blocks each for single and double sweeps respectively, with block order counterbalanced across participants. 120 stimuli occurred in each block, 40 stimuli at each of three stimuli durations. Thus over 2 block repeats there were a total of 80 trials per stimulus type. On each trial in the single and double sweep blocks, participants discriminated expansion vs. contraction of single sweeps or of both sweeping stimuli for the double sweep sequence. For all data analyses, the six stimuli types are abbreviated as ss50, ss100, ss200 and ds50, ds100 and ds200, with ss and ds corresponding to single and double sweeps followed by the millisecond stimulus duration. Participants performed this fixed-speed assessment at the T1 and T2 sessions that were separated by 3–5 weeks; training group participants performed perceptual training in the interim period while the control group did not engage in any training.
Working Memory Assessment
Stimuli
The stimuli consisted of a circular aperture containing 290 dots (0.08°×0.08° each) that subtended 8° of visual angle at a 75 cm viewing distance and were centered at the fovea. This field of 290 spatially random gray scale dots moved with 100% coherence at an oblique angle at 10° per second. Stimuli were presented with a gray fixation cross in the center of the circular aperture with a black background of luminance level 0.32 cd/m2. All four sectors of the aperture were used (i.e. northeast, northwest, southeast, southwest) except the cardinal directions (up, down, left, right). The experimental stimuli consisted of 12 different directions of motion (3 per sector). Stimuli were presented through E-Prime software (Psychology Software Tools, Inc.) run on a Dell Optiplex GX620 and a ViewSonic G220fb CRT monitor.
Thresholding
Participants completed a motion thresholding test prior to the onset of the main experiment in order to minimize the effects of individual differences in discriminability. A staircase procedure (2° increments) required participants to determine whether two motion stimuli were moving in the same direction. The two 100% coherent motion stimuli were presented for 800 ms each and separated by 2000 ms. An angle of discrimination (the difference between two directions of motion) was selected for each participant as the largest angle at which discrimination performance was less than 100%.
Experimental Procedure
In a paradigm previously described (Berry et al., 2009, Mishra et al., 2013a), participants were presented with three different tasks randomized across six blocks, with two blocks per task. There were two delayed recognition WM tasks, one with no interference (NI) during the WM delay and one with an interrupting stimulus (IS) during the WM delay that required attention. The third task instructed participants to passively view the stimuli (PV). The WM task required participants to encode a dot motion stimulus and retain its direction of motion in WM for 7 seconds, after which a match/non-match probe was presented with the non-match direction of motion calibrated as per each participant’s perceptual threshold. This WM experiment was performed at T1 and T2 and Berry et al. (2010) showed that T2 performance significantly exceeded T1 on the NI WM task.
EEG Data Acquisition
Participants sat in an armchair in a dark, sound-attenuated room for the behavioral assessments with simultaneous neural recordings. Electrophysiological signals were recorded with an ActiveTwo BioSemi 64-channel Ag-AgCl active electrode EEG acquisition system in conjunction with ActiView software (BioSemi). Signals were amplified and digitized at 1024 Hz with a 24-bit resolution. All electrode offsets were between ±20 mV. Anti-aliasing filters were used during data acquisition. Precise markers of stimulus presentation were acquired using a photodiode.
The three-dimensional coordinates of each electrode and of three fiducial landmarks (the left and right preauricular points and the nasion) were determined by means of a BrainSight (Rogue Research) spatial digitizer. The mean Cartesian coordinates for each site were averaged across all subjects and used for topographic mapping.
Behavioral Analysis
Behavioral performance for single sweeps was analyzed as proportion correct accuracy and response times in milliseconds, calculated separately for ss50, ss100 and ss200 stimuli. For ds50, ds100 and ds200 stimuli, proportion correct accuracy was based on correctly discriminating both sweeps in the double sweep sequence, and double sweep response times were the total time taken to respond to the first and second sweep. WM accuracy data for all participants were directly obtained from Berry et al. (2010) as cue-probe match/non-match correct discriminations on the NI WM task.
All behavioral data were first tested for homegeneity of variances across groups using the Levene’s test, and if they passed the test (p>0.05), the data were analyzed in repeated measures ANOVAs with a between-subject factor of group (training vs. control group) and a within-subject factor of assessment session (T1 vs. T2). Specifically, group × session interactions were of main interest to uncover modulations of behavior as a result of training. Post-hoc analyses consisted of within group T1 vs. T2 two-tailed paired t tests. If as per the Levene’s test homogeneity of variances was not observed (p<0.05), then those data were compared using non-parametric statistics, specifically the Mann Whitney U test; this was only found to be true for the accuracy data for the T2 session.
ERP Analysis
Raw EEG data were digitally re-referenced off-line to the average of the left and right mastoids. Eye artifacts were removed through independent component analyses by excluding components consistent with topographies for blinks and eye movements and the electrooculogram time series. Data were high-pass filtered at 0.1 Hz to exclude ultraslow DC drifts. This preprocessing was conducted in the Matlab (Mathworks) EEGLab toolbox (Swartz Center for Computational Neuroscience, UC San Diego). Further data analyses were performed using custom ERPSS software (Event-Related Potential Software System; UC San Diego). Signals were averaged in 500 ms epochs with a 100 ms prestimulus interval. The averages were digitally low-pass filtered with a Gaussian finite impulse function (3 dB attenuation at 46 Hz) to remove high-frequency noise produced by muscle movements and external electrical sources. Epochs that exceeded a voltage threshold of ±75 µV were rejected.
Components of interest were quantified in the 0–300 ms ERPs over distinct electrode sets that corresponded to sites at which component peak amplitudes were maximal. Specifically, the early visual N1 component was quantified at its peak latency 120–150 ms post-stimulus onset at fifteen occipital sites (6 channels in each hemisphere: O1/2, PO3/4, PO7/8, P3/4, P5/6, P7/8; and 3 midline channels: POz, Oz, Iz). The N2 processing component was quantified 240–280 ms post-stimulus onset at 15 frontocentral sites (6 in each hemisphere: FC1/2, FC3/4, C1/2, C3/4, CP1/2, CP3/4; and 3 midline channels: FCz, Cz, CPz). All ERP data were first confirmed to pass the Levene’s test for homogeneity of variances between groups. Then statistical analyses for ERP components used repeated measures ANOVAs with a Greenhouse–Geisser correction applied when appropriate. Repeated measures ANOVAs had a between-subject factor of group (trained vs. control group) and a within-subject factor of assessment session (T1 vs. T2). Specifically, group × session interactions were of main interest to uncover modulations of neural processing as a result of training. Post-hoc analyses consisted of within group T1 vs. T2 two-tailed paired t tests.
This ERP component analysis was additionally confirmed by conducting running point-wise two-tailed paired t tests of within group T1 & T2 session data at all scalp electrode sites. In this analysis, a significant difference is considered if at least 10 consecutive data points meet the 0.05 alpha criterion and is a suitable alternative to Bonferroni correction for multiple comparisons (Guthrie and Buchwald, 1991; Murray et al., 2001; Molholm et al., 2002). This analysis did not yield any new effects other than the components of interest described above.
Neurobehavioral correlations
Neural-behavioral correlations were used to evaluate the impact of training-related changes in sweep neural processing with changes in performance accuracy on the sweep assessment as well as the WM assessment. Correlations were two-tailed Pearson’s product-moment correlations.
HIGHLIGHTS.
Perceptual training benefits sensory discrimination for challenging visual stimuli.
Early visual processing is enhanced by perceptual training in healthy aging.
Neuroplasticity in the visual N1 ERP component correlates with perceptual gains.
Visual N1 neuroplasticity correlates with improvements in untrained working memory.
Early visual processing changes underlie transfer-of-benefit to working memory.
Acknowledgements
This work was supported by the National Institute of Health grants 5R01AG040333 (AG), 5R24TW007988-05 subaward VUMC38412 (JM), and Posit Science Corporation. We thank Anne Berry for collecting the data, and Luigi Maccotta, Aneesha Nilakantan and Rabeea Abbas for help with data analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, McDonald KL, Ostroff JM, Schneider B. Age-related changes in detecting a mistuned harmonic. J. Acoust. Soc. Am. 2001;109:2211–2216. doi: 10.1121/1.1367243. [DOI] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4357–4362. doi: 10.1073/pnas.1213555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera Ja, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley a. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle AT, Webb BS, McGraw PV. Can perceptual learning be used to treat amblyopia beyond the critical period of visual development? Ophthalmic Physiol. Opt. 2011;31:564–573. doi: 10.1111/j.1475-1313.2011.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Res. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Rutman AM, Clapp WC, Gazzaley A. Practice-related improvement in working memory is modulated by changes in processing external interference. J. Neurophysiol. 2009;102:1779–1789. doi: 10.1152/jn.00179.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The Influence of Perceptual Training on Working Memory in Older Adults. PLoS One. 2010;5:e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Hinojosa Ja, Martín-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Hum. Brain Mapp. 2004;22:290–299. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F, Salthouse T. Handbook of Aging and Cognition. second ed. Mahwah, New Jersey; 2000. [Google Scholar]
- Crist RE, Kapadia MK, Westheimer G, Gilbert CD. Perceptual learning of spatial localization: specificity for orientation, position, and context. J. Neurophysiol. 1997;78:2889–2894. doi: 10.1152/jn.1997.78.6.2889. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dobres J, Watanabe T. Response feedback triggers long-term consolidation of perceptual learning independently of performance gains. J. Vis. 2012;12:9. doi: 10.1167/12.8.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Zucker DM, Schweiger A, Dwolatzky T, Chertkow H, et al. Towards practical cognitive assessment for detection of early dementia: a 30-minute computerized battery discriminates as well as longer testing. Curr. Alzheimer Res. 2005;2:117–124. doi: 10.2174/1567205053585792. [DOI] [PubMed] [Google Scholar]
- Dwolatzky T, Whitehead V, Doniger GM, Simon ES, Schweiger A, et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:1–12. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol. Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Fahle M, Daum I. Visual learning and memory as functions of age. Neuropsychologia. 1997;35:1583–1589. doi: 10.1016/s0028-3932(97)00069-9. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: a case for early selection. J. Vis. 2004;4:879–890. doi: 10.1167/4.10.4. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: specificity versus generalization. Curr. Opin. Neurobiol. 2005;15:154–160. doi: 10.1016/j.conb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Stoerig P, Falkenstein M. ERP--correlates of response selection in a response conflict paradigm. Brain Res. 2008;1189:127–134. doi: 10.1016/j.brainres.2007.10.076. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Training-induced improvement of response selection and error detection in aging assessed by task switching: effects of cognitive, physical, and relaxation training. Front. Hum. Neurosci. 2012;6:130. doi: 10.3389/fnhum.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Gonzalez CM, Clark VP, Fan S, Luck SJ, Hillyard SA. Sources of attention-sensitive visual event-related potentials. Brain Topogr. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electro- physiological and neuroimaging evidence. Philos. Trans. R. Soc. Lond. B. Biol Sci. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. U.S. A. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach J, Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Dev. Sci. 2009;12:978–990. doi: 10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE, Grigsby SS, Vingrys AJ, Benes SC, Supowit A. Efficient and unbiased modifications of the QUEST threshold method: theory, simulations, experimental evaluation and practical implementation. Vision Res. 1994;34:885–912. doi: 10.1016/0042-6989(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Kumano H, Uka T. Neuronal mechanisms of visual perceptual learning. Behav. Brain Res. 2013;249:75–80. doi: 10.1016/j.bbr.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res. 2009;49:2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Schmiedek F, Huxhold O, Röcke C, Smith J, Lindenberger U. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol. Aging. 2008;23:731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, et al. Memory enhancement in healthy older adults using a brain plasticity-based training paradigm: A randomized, controlled study. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, deCharms R. Neural representations, experience, and change. In: Llinas R, Churchland P, editors. The Mind-Brain Continuum. Boston: The MIT Press; 1996. pp. 61–81. [Google Scholar]
- Merzenich MM, Recanzone GH, Jenkins W. How the brain functionally rewires itself. In: Arbib M, Robinson JA, editors. Natural and Artificial Parallel Computations. New York: MIT Press; 1991. [Google Scholar]
- Mishra J, Anguera JA, Ziegler D, Gazzaley A. Chapter 14 - A Cognitive Framework for Understanding and Improving Interference Resolution in the Brain. In: Merzenich MM, Nahum M, Van Vleet TM, editors. Progress in Brain Research. Elsevier; 2013. pp. 351–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Zanto T, Nilakantan A, Gazzaley A. Comparable mechanisms of working memory interference by auditory and visual motion in youth and aging. Neuropsychologia. 2013a;51:1896–1906. doi: 10.1016/j.neuropsychologia.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Martínez A, Schroeder CE, Hillyard SA. Spatial attention boosts short-latency neural responses in human visual cortex. Neuroimage. 2012;59:1968–1978. doi: 10.1016/j.neuroimage.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res. Cogn. Brain Res. 2002;14:115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J. Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Murray MM, Foxe JJ, Higgins BA, Javitt DC, Schroeder CE. Visuo-spatial neural response interactions in early cortical processing during a simple reaction time task: a high-density electrical mapping study. Neuropsychologia. 2001;39:828–844. doi: 10.1016/s0028-3932(01)00004-5. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HG, Friedman D. A brain event related to the making of a sensory discrimination. Science. 1979;203:1358–1361. doi: 10.1126/science.424760. [DOI] [PubMed] [Google Scholar]
- Russo F Di, Sereno MI, Pitzalis S, Hillyard SA. Cortical Sources of the Early Components of the Visual Evoked Potential. Hum. Brain Mapp. 2001;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh B, Pitcher D, Brandman T, Eisen A, Thaler A, Yovel G. Stimulation of category-selective brain areas modulates ERP to their preferred categories. Curr. Biol. 2011;21:1894–1899. doi: 10.1016/j.cub.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Schoups AA, Vogels R, Orban GA. Human perceptual learning in identifying the oblique orientation: retinotopy, orientation specificity and monocularity. J. Physiol. 1995;483(Pt 3):797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Schneider B, Pichora-Fuller M. Implications of perceptual deterioration of cognitive aging research. In: Craik F, Salthouse T, Mahwah N, editors. The Handbook of Aging and Cognition. New Jersey: Lawrence Erlbaum Associates; 2000. pp. 155–219. [Google Scholar]
- Shiu L-P, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept. Psychophys. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- Towey J, Rist F, Hakerem G, Ruchkin DS, Sutton S. N250 latency and decision time. Bull. Psychon. Soc. 1980;15:365–368. [Google Scholar]
- Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Villers-Sidani E. Cognitive Training for Impaired Neural Systems in Neuropsychiatric Illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigfield R, Gilbert R, Fleming PJ. SIDS: risk reduction measures. Early Hum. Dev. 1994;38:161–164. doi: 10.1016/0378-3782(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM. Far transfer in cognitive training of older adults. Restor. Neurol. Neurosci. 2009;27:455–471. doi: 10.3233/RNN-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]