Abstract
Event-related potentials (ERPs) have been proposed as biomarkers capable of reflecting individual differences in neural processing not necessarily detectable at the behavioral level. However, the role of ERPs in developmental research could be hampered by current methodological approaches to quantification. ERPs are extracted as an average waveform over many trials, however, actual amplitudes would be misrepresented by an average if there was high trial-to-trial variability in signal latency. Low signal temporal consistency is thought to be a characteristic of immature neural systems, although consistency is not routinely measured in ERP research. The present study examined the differential contributions of signal strength and temporal consistency across trials in the error-related negativity (ERN) in 6-year-old children, as well as the developmental changes that occur in these measures. The 234 children were assessed annually in kindergarten, 1st, and 2nd grade. At all assessments signal strength and temporal consistency were highly correlated with the average ERN amplitude, and were not correlated with each other. Consistent with previous findings, ERN deflections in the averaged waveform increased with age. This was found to be a function of developmental increases in signal temporal consistency, whereas signal strength showed a significant decline across this time period. Additionally, average ERN amplitudes showed low-to-moderate stability across the three assessments whereas signal strength was highly stable. In contrast, signal temporal consistency did not evidence rank order stability across these ages. Signal strength appears to reflect a stable individual trait whereas developmental changes in temporal consistency may be experientially influenced.
Keywords: Intra-individual variability, ERN, Developmental Change
Introduction
Neural processes have received increasing attention in the study of individual differences in affective and cognitive systems that may underlie risk for adverse behavioral outcomes. Several researchers have proposed that event-related potentials (ERPs) may have endophenotypic properties, reflecting core individual traits that are thought to be stable across time (Hajcak, 2012; Iacono & Malone, 2011; Olvet & Hajcak, 2008). If supported, ERPs would have enormous potential in research elucidating developmental mechanisms associated with adverse outcomes. However, the majority of research supporting the association between ERPs and specific psychological profiles has been conducted in adolescent and adult samples. As researchers increasingly seek to assess ERPs in younger children, careful consideration must be paid to whether these components are developmentally invariant, particularly during phases of rapid neural change (Davies, Segalowitz, & Gavin, 2004). In this study, we focus specifically on the developmental characteristics of the error-related negativity (ERN) in a sample of children assessed annually in kindergarten, 1st and 2nd grades.
The ERN is typically measured in speeded response tasks where erroneous responses are likely to occur even when participants are aware of the correct stimulus-response rules. Typically within 100ms following an error, an ERN signal is generated that reflects the evaluation of the effectiveness of current behavior, allowing for the rapid recruitment of additional cognitive resources in pursuit of improving future task performance (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993; Scheffers & Coles, 2000; Wessel, 2012). Consistent with its perceived role in monitoring performance, ERN deflections increase as a function of the motivation for accuracy (Gehring et al., 1993; Hajcak, Moser, Yeung, & Simons, 2005; Kim, Iwaki, Uno, & Fujita, 2005).
Larger ERN deflections have been reported in association with better executive functioning (Larson & Clayson, 2011) and academic outcomes (Hirsh & Inzlicht, 2010). Larger ERN deflections have also been observed among individuals with increased sensitivity to errors and negative feedback such as those with clinical syndromes related to anxiety and depressive disorders (Gehring, Himle, & Nisenson, 2000; Hajcak, McDonald, & Simons, 2003; Tucker, Luu, Frishkoff, Quiring, & Poulsen, 2003) as well as non-clinical personality traits such as high negative affect and trait anxiety (Boksem, Tops, Wester, Meijman, & Lorist, 2006; Hajcak, McDonald, & Simons, 2004). Larger ERN deflections have also been observed among individuals shown to learn faster in response to negative reinforcement (i.e. avoid a negative consequence) than positive reinforcement (Frank, Woroch, & Curran, 2005). Conversely, muted ERN amplitudes have been found among individuals characterized by low sensitivity to negative feedback such as those with clinical syndromes on the externalizing spectrum (Franken, van Strien, Franzek, & van de Wetering, 2007; Hall, Bernat, & Patrick, 2007; Liotti, Pliszka, Perez, Kothmann, & Woldorff, 2005).
Development of the ERN
An initial cross-sectional study of individuals from age 7 to 18 indicated that ERNs were not readily apparent in grand averaged waveforms until early adolescence (Davies et al., 2004). This appeared to result from individual ERNs in children being less easily identified and considerably more variable across individuals, resulting in a muted waveform when individual participants were averaged together. Additional cross-sectional studies similarly show a clear linear increase in grand-averaged ERN amplitudes into adulthood, suggesting that variability among individuals reduces across development (Davies et al., 2004; Kim, Iwaki, Imashioya, Uno, & Fujita, 2007; Wiersema, van der Meere, & Roeyers, 2007). More recent research has successfully documented the presence of an ERN signal in children under the age of 8, although developmental differences are often observed. One study identified an ERN signal in children ages 5-7, but unlike adults, these children’s ERN deflections did not increase when they were told to focus on accuracy (Torpey, Hajcak, & Klein, 2009). However, another study with children 7 - 8 years old reported that the ERN signal was greater among participants who performed the task while being watched by a friend, suggesting that children may show the same association between ERN amplitude and motivation, but differ developmentally in the contextual factors capable of motivating them (Kim et al., 2005). In a study of anxiety symptoms, the expected association between greater ERN amplitude and symptom severity was detected in young adolescents (ages 11-13) but not in pre-adolescent children (ages 8-10) (Meyer, Weinberg, Klein, & Hajcak, 2012). The studies reported to date leave open many questions about whether the ERN reflects the same psychological constructs in children it is believed to index in adults, as well as whether experimental conditions must be developmentally adapted in order to improve ERN detection. However, the pattern of inconsistent evidence for the ERN in children may be, at least in part, due to the approach of assessing amplitude in averaged waveforms that may not accurately reflect the neural activity on any individual trial. In other words, the association between ERN amplitude and individual differences in young children may be confounded by measurement constraints.
Potential Impact of Trial-to-Trial Variability on the ERN
Averaging waveforms across repeated trials is common practice in efforts to amplify signal, assumed to be consistent across trials, while eliminating noise, assumed to be random across trials. This process, however, relies upon the validity of the assumption that the signal occurs in a temporally consistent place in each trial. For a response-locked component such as the ERN, variability in the temporal relationship between error recognition and motor response could occur as a function of variable cognitive processing speed as well as more global neural inefficiency in efferent motoric responding. If this assumption is violated such that there is variability in signal latency relative to the response, the averaging process will result in a muted waveform that is not representative of the actual signal amplitude in any trial. Traditional measures of ERN amplitude will thus be a function of both the signal strength in each trial and the temporal consistency of the signal across trials.
Intra-individual variability, previously presumed to be noise, has received increasing attention as a valid indicator of the functional state of behavioral systems. Behavioral variability, typically measured as the standard deviation of response times, is thought to reflect inefficiencies in neural systems (Segalowitz & Segalowitz, 1993). Children routinely exhibit greater levels of intra-individual variability in behavioral measures, such as reaction time, when compared with adults, and increased behavioral variability is thought to reflect developmentally immature neural systems (Aggarwal & Lillystone, 2000; Hultsch & MacDonald, 2004; Li et al., 2004). It is possible that the greater variability in reaction time observed in children reflects greater variability in neural signaling that also hampers detection of the ERN signal. Both the ERN and behavioral variability have been associated with dopaminergic networks (Bäckman, Nyberg, Lindenberger, Li, & Farde, 2006; Holroyd & Coles, 2002; Li & Lindenberger 1999), and developmental changes observed in ERN amplitude as well as behavioral variability mirror the developmental trajectory of prefrontal dopamine (DA) receptors (Tarazi & Baldessarini, 2000). In addition, developmental changes in myelination are thought to increase the efficiency of the transmission of neural signals resulting in decreased behavioral variability and improved cognitive capabilities (Nagy, Westerberg, & Klingberg, 2004; Schmithorst, Wilke, Dardzinski, & Holland, 2005).
Although no research to date has directly examined ERN amplitude in children as a function of neural maturation, some research has been conducted in elderly populations. Neural declines in advanced aging may in some ways mirror the developmental inclines in neural systems seen in childhood. Advanced aging is characterized by a decrease in frontal DA receptors, an increase in behavioral variability, anda muting of ERN deflections (Bäckman et al., 2006; Band & Kok, 2000; Falkenstein, Hoormann, & Hohnsbein, 2001; Hultsch, Macdonald, & Dixon, 2002;). Similarly, decreased white matter integrity has been linked to age-related cognitive decline (Charlton et al., 2006; Madden et al., 2009; Sullivan & Pfefferbaum, 2006), as well as increased behavioral variability (Bunce et al., 2007; Fjell, Westlye, Amlien, & Walhovd, 2011; Ullén, Forsman, Blom, Karabanov, & Madison, 2008). At least one study has reported an association between reduced white matter integrity and decreased ERN deflections in a sample of healthy adults (Westlye, Walhovd, Bjornerud, Due-Tonnessen, & Fjell, 2009). Interestingly, one study reported that decreases in the average ERN amplitude seen in old age were due not only to a general decrease in signal strength, but also to an increase in the variability of the ERN signal (Kolev, Beste, Falkenstein, & Yordanova, 2009).
Although researchers have speculated that the developmental increase in average ERN amplitude through adolescence may be due to an increase in signal temporal consistency in addition to an increase in average signal strength (Segalowitz & Dywan, 2009), this hypothesis remains largely unexplored. Evidence suggests that behavioral variability is associated with lower ERN amplitudes in children ages 7 - 9 years (Richardson, Anderson, Reid, & Fox, 2011). Thus it is possible that behavioral variability commonly seen in children is associated with trial-to-trial variability in signal latency that could underlie the muted ERN waveforms also observed in children.
Time-Frequency Decomposition
Evidence is increasing that signal temporal consistency specifically is associated with a range of cognitive processes such as successful memory encoding (Fell, Ludowig, Rosburg, Axmacher, & Elger, 2008; Klimesch et al., 2004) and memory retrieval (Schack & Klimesch, 2002). However, to our knowledge no study has assessed the developmental changes of signal temporal consistency or the association between changes in temporal consistency and changes in the amplitude of averaged waveforms. To examine this, we sought to decompose the ERN signal into separate components reflecting signal strength and signal temporal consistency across trials. Researchers have postulated that the ERN signal results from an increase in phase-locked activity in the theta (4-8 Hz) waveband (Luu, Tucker, & Makeig, 2004; Trujillo & Allen, 2007). In this model, signal strength in each trial is estimated using sine waves with frequencies in the theta range. The sine waves that describe each trial can differ in their power and in their phase offset. The power of the sine waves that describe a trial is defined by the amplitude of the sine waves and indicates the strength of the signal in a given trial. The phase offset of the sine waves indicates the temporal location of the peaks and troughs of the sine waves in a given trial. This approach allows for an ERN signal to be decomposed into signal strength, computed as the average theta power across trials, and temporal consistency, computed as the phase coherence in the theta waveband across trials. Combined, these two components explain the majority of the variance of the ERN (Cavanagh, Zambrano-Vazquez, & Allen, 2012). By examining these components separately, their respective contributions to changes in the average ERN waveform over time can be assessed.
The Present Study
As reviewed above, researchers are increasingly interested in assessing the ERN in younger children with the presumption that this component will serve as a biomarker of individual differences that may be in effect prior to the development of full behavioral syndromes. The ability of the ERN to serve in this role is predicated on several qualities that have, to date, not been fully assessed. First, we examine whether there are developmental changes in the ERN by conducting 3 annual assessments with children from kindergarten through 2nd grade. To our knowledge, longitudinal assessments of the ERN have not been conducted in this age range. We hypothesize that the amplitude of the ERN deflection will increase within individuals over this range, and that behavioral variability will decrease. Furthermore, we hypothesize that significant increases in signal strength and temporal consistency will occur across this time period, accounting in part for the increase in amplitude in the average signal. Second, we examine whether signal temporal consistency is associated with behavioral variability. Given the wealth of evidence of sexually dimorphic effects in neural processes (see Cahill, 2014), we include sex in each of these models in order to determine whether males and females differ in any respect of developmental maturation.
Finally, in addition to the changes occurring as a function of ongoing brain development, we further examine whether the ERN possess trait-like properties by examining individuals’ rank-order stability within the sample. The ERN has shown high test-retest reliability (r = .74) over a two-week period in a sample of undergraduate students (Olvet & Hajcak, 2009) and (r = .62) over the course of 3-6 weeks in middle adolescence (Segalowitz et al., 2010). Moderate rank-order stability (r = .65) of the ERN has also been found amongst college students over the course of 1-2 years (Weinberg & Hajcak, 2011). However, the stability of the ERN in younger samples is unknown. High stability across the 3 assessments would provide strong support for the ERN as an early emerging trait-like factor. In contrast, low stability would indicate that children can undergo differential developmental trajectories during this developmental range and suggest that the ERN does not possess strong trait-like properties until later.
Methods
Participants
Data for this study were drawn from a larger study of children’s socioemotional development in conjunction with a school-based preventive intervention, the full details of which can be found in [BLINDED]. Briefly, teachers were asked to respond to a brief behavioral screening questionnaire in the fall of the kindergarten year for all students in the class. Children identified as having behavioral risk (assessed through teacher report of aggressive or oppositional behavioral symptoms; n = 207) were recruited, and half were randomly assigned to participate in the intervention component of the study. An additional n = 132 children with no symptoms were recruited as comparisons. Because the focus of the present study is on normative developmental progression, only data from the n = 234 (65% male) children who were not exposed to the intervention condition, and who had at least one ERP assessment are included. Of these 234 participants 70% were African American, 20% Hispanic, 9% Caucasian, and 1% Asian. Once pooled, this sample of children (which included both children identified as having behavioral risk and comparison children), filled the full range of both externalizing and internalizing symptoms, with normal distributions at the sample level (see BLINDED for more details).
Children were assessed longitudinally in the fall of their kindergarten year (K), spring of their 1st grade year (G1), and spring of their 2nd grade year (G2). At first assessment, children ranged in age from 5.18 to 7.47 years (M = 6.10, SD = .39). As with any longitudinal study, participant attrition resulted in a smaller sample size at G2 (n = 163) compared to K. Participants who did not participate at all three assessments did not differ from those with complete data at the G2 assessment with regard to behavioral variability or ERN amplitude at the initial assessment (ps> .29).
Procedures
Physiological assessments took place in a recreational vehicle that was retrofitted as a mobile laboratory. The mobile laboratory was driven to the school allowing for testing during the school day. Research assistants (RAs) escorted the child to the vehicle where they explained the task to the child, who was then asked to give verbal assent. RAs then affixed autonomic and EEG electrodes, and seated the children in front of a computer monitor where they completed a Go/No-go task (described below) followed by an emotion induction task. Only EEG data from the Go/No-go task at the K, G1, and G2 assessments are examined in the present study.
Go/No-go
The Go/No-go task was a modified version of a program developed by M. Lewis and J. Stieben (Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006). At the start of each trial a stimulus consisting of either a cartoon character (K) or acomputer-generated human face (G1 and G2) was presented. Participants were instructed to press a button on a response box after the presentation of every stimulus (Go trial) unless the newly presented stimulus matched the immediately preceding stimulus (No-go trial). The task lasted approximately 10 minutes and consisted of 225 Go trials interspersed with 105 No-go trials. Prior to beginning the task research staff explained the game to the child using full-page color illustrations. The child then engaged in a short practice round to familiarize him/her with the rate of stimulus presentation and to establish task difficulty. In order to ensure an appropriate number of error trials for analyses task difficulty was standardized across all participants. Children with an error rate between 40% and 60% at the end of the practice session began the full game. Children with a higher error rate were given the practice session again until they were able to improve their performance. Children with a lower error rate were also re-administered the practice session with a faster stimulus presentation rate in order to make the task more challenging. At the kindergarten assessment all children began the task at the slowest presentation rate, which was 900ms. During the game an adaptive algorithm made dynamic adjustments to the inter-stimulus interval to help maintain the targeted error rate for the duration of the task.
Prior to beginning the task, children were informed that they would be winning points and that if they earned enough points they would be given a prize that consisted of a gift bag filled with stickers and small toys. Points were awarded for correct responses and subtracted for incorrect responses. Because of the age of the sample, points were presented visually rather than numerically. At slightly variable intervals of approximately 10 trials, cumulative point total was shown with a thermometer bar alongside a cartoon character who was smiling and giving a “thumbs up” or frowning and giving a “thumbs down” depending on the participant’s performance since the previous feedback.
The task consisted of three blocks that lasted approximately 2.5 minutes each. A 30s break was given between each block. During the 1st and 3rd block, participants earned more points for correct responses than they lost for incorrect responses. During the second block the scoring algorithm was changed such that they lost points, a manipulation designed to examine hypotheses related to the role of affect in cognitive processing (in the 3rd block all points are earned back and all children won the prize). However, because that manipulation was unrelated to the developmental questions examined here, the analyses presented below only make use of data from the error trials extracted from the 1st and 3rd blocks. Notably, the pattern of results is identical when examining the error trials from all three blocks.
EEG Recording
EEG data were recorded from a BioSemi ActiveTwo system (BioSemi, Amsterdam, Netherlands) with 32 standard extended 10-20 scalp channels using sintered-silver electrodes. To detect and later correct ocular artifacts, additional electrodes were placed on the left and right suborbital ridge under the pupil and 1 cm outside the left and right lateral canthi. The analog signal was digitized according to the BioSemi zero-reference principle (the voltage at each site is quantified relative to the common mode sense and driven right leg loop) at 1024 Hz and online low-pass filtered at 512 Hz. The recording was made from DC amplifiers with a gain of one using 24-bit A-D conversion, and filtered offline with a 1-30 Hz bandpass in Brain Vision Analyzer. As a substitute for impedance measures quantifying signal quality, electrode offsets were maintained below 50 μV.
EEG Post-Processing
Data were post-processed using Brain Vision Analyzer 2.0. The time-series for each electrode was re-referenced to the average of all sites and bandpass filtered between 1 and 30 Hz. No-go trial errors of commission were segmented from −800ms to 800ms with respect to button presses indicating the erroneous responses. Corrections for eye blinks were made using the Gratton and Coles algorithm (Gratton, Coles, & Donchin, 1983) and data were baseline corrected relative to the average voltage in the window −600 to −400ms prior to the incorrect response so that the baseline would capture a period prior to stimulus onset. Segments containing a voltage step greater than 100μV between sampling points or data outside the range of −75μV to 75μV were marked as artifact and removed.
Additionally, assessments where fewer than 6 No-go error trials remained after artifact rejection were excluded from analyses (loss of 36 assessments from 32 participants). Finally, assessments where error rates exceeded 60% were deemed as indicative of participants’ disengagement from the task and were excluded from analysis (loss of 16 assessments from 16 different participants). Clean, processed data from the Fz electrode (total of 494 assessments from 234 participants) were exported to MATLAB and analyzed using custom written software that extracted signal strength and temporal consistency as described below.
Time-Frequency Decomposition
Following procedures described by Mallat (2008), the spectral properties of Fz voltage fluctuations during each trial were described using a set of complex Morlet wavelets given by
| (1) |
wheret is time, f is frequency, σtis the standard deviation in time domain around frequency f and α is a scaling factor typically set to 4.7 (see also Cavanagh et al., 2012). Wavelets with a fixed width of 750 ms, corresponding to between 3 and 6 wave cycles for the frequencies of interest, were generated in 1 Hz frequency steps and were convolved with the No-go error of commission trial data, one trial at a time, to yield Wk(t,f). The resultant wavelet transformation, represented in Cartesian coordinates asWk(t,f) = a + bi (where i is the square root of −1), can alternatively be described in Polar coordinates as a function of magnitude and phase offset as shown in Equations 2 and 3.
| (2) |
| (3) |
These representations of neural activity were subsequently used to calculate average signal strength and signal temporal consistency across trials (described below).
Measures
ERN Amplitude
ERN amplitude was measured for each child at each assessment (K, G1, G2) using a peak-to-peak method. First, a composite waveform was obtained for each participant by averaging the waveforms from artifact-free No-go error trials. ERN amplitude was then derived from the average waveform as the difference in μV between the most negative peak in the 0-100ms post-response window and the most positive peak in the −100-0ms pre-response window.
Average Signal Strength
Using the wavelet transformations described above, average signal strength at each assessment was defined as the average spectral power in the theta waveband (4-8 Hz) from 0 to 100ms following erroneous responses on No-go trials. Specifically,
| (4) |
where n is the number of trials, t is time in ms, f is frequency in Hz, and c is a scaling factor such that the result equals the average power at each time point from 0 to 100ms at 4-8Hz.
Signal Temporal Consistency
Similarly, signal temporal consistency at each assessment was defined as the extent of phase coherence in the theta waveband from 0 to 100ms post-response.
| (5) |
Behavioral Variability
Behavioral variability at each assessment was measured as the within-person standard deviation of response times on Go trials in which the child responded within a 100 ms and 1000 ms post-stimulus window. Given increasing accuracy with age, behavioral variability was indicated by responses to, on average M = 150.31 (SD = 34.78; range = 14 – 209) Go trials at the K assessment, M = 196.59 (SD = 35.01, range = 70 – 252) trials at the G1 assessment, and M = 217.17 (SD = 24.01, range = 113-254) trials at the G2 assessment.
Data Analysis
Our main interest was to describe and examine (a) age-related changes and sex differences in ERN amplitude, signal strength and signal temporal consistency, (b) the association between behavioral variability and signal temporal consistency, and (c) the extent of stability in between-person differences in these measures across this age range.
Age-related Changes in ERN Measures
To examine age-related changes and sex differences in development, multilevel models of change (Singer & Willett, 2003) were fit to the repeated measures of ERN amplitude, average signal strength, signal temporal consistency, and behavioral variability. Specifically, the model of change was specified as
where Ytj is observed outcome (e.g., ERN amplitude, average signal strength) at assessmentt for participant j, β0j is a person-specific intercept indicating level of the outcome at age 6 years,β1j is a person-specific linear rate of age-related change, and etj are assessment-specific residuals. Person-specific intercepts and rates of change are, in turn, modeled as
| (6) |
where γs are sample-level parameters indicating the expected score for the prototypical 6 year old child (γ00), the average rate of age-related change (γ10) the extent of sex differences in those expected scores (γ01), and rates of change (γ11), and residual between-child differences unrelated to sex, u0j and u1j, that may be correlated but are uncorrelated with assessment specific residuals etj. Sex was coded such that males = 1 and females = −1. Following usual trimming practices, random effects were trimmed to obtain the best fitting error structure. In the present context this meant constraining the between-child variance in rates of age-related change to zero when the parameter did not contribute significantly to model fit. Follow-up analyses were used to examine if and how age-related changes in the typically examined ERN amplitude may be mediated by changes in signal temporal consistency using the Sobel (1982) method (see Krull & MacKinnon, 2001). All models were fit using SAS 9.3 (proc mixed, Littell, Milliken, Stoup, & Wolfinger, 1996) using minimum variance quadratic unbiased estimation (Rao, 1972) and treating incomplete data as missing at random (Little & Rubin, 1987). Maintaining conservative evaluation with respect to the sample size, observations more than 3 standard deviations away from the sample mean within a given assessments (i.e. 3 or 4 observations) were excluded from each analysis (results from analyses with or without the outliers removed do not differ). Significance of effects was evaluated at α = .05.
Associations among ERN Amplitude, Signal Components and Behavioral
Variability
Pairwise correlations between concurrently measured behavioral variability and ERN amplitude, average signal strength, and signal temporal consistency were conducted for each assessment to measure sample level associations of between-person differences. Due to data missing for individual assessment points either due to attrition or exclusion of artifactual data the sample sizes for these correlations range from n = 106 to n = 199.
Trait-like properties of ERN
Rank order stability across years was examined using pairwise Pearson’s correlations between the ERN amplitude, average signal strength, signal temporal consistency, and behavioral variability scores obtained at K, G1, and G2.
Results
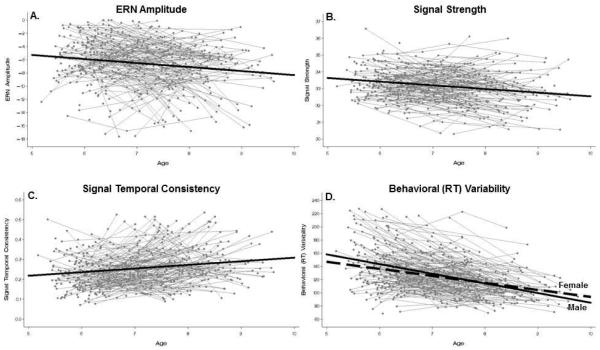
Descriptive statistics for all measures are given in Table 1. Average amplitude of ERN deflections in μV increased across the three assessments (from −6.00 to −7.35). The effect sizes for year-to-year change in the sample-level averages were small (d = .18 and d = .20 respectively). Highlighting the importance of development time-scale, these sample-level changes accumulate to what is a moderate effect size when looking at change from K to G2 (d = .39). Average behavioral variability decreased across the three assessments (from 138.33 to 109.13). Notably, the sample also became more homogenous with respect to the dispersion of children’s behavioral variability (between-child variance going from 34.19 to 21.51). Effect sizes for year-to-year change were moderate (d = .33 and d = .68 respectively), accumulating into a large effect when looking at change from K to G2 (d = 1.12). Average signal temporal consistency increased across the three assessments (from .23 to .28) with effect sizes for year-to-year change in the sample-level averages being in the small to moderate range (d = .38, d = .22 respectively) and accumulating to d = .56 for K to G2. Signal strength decreased across the three assessments (from 33.34 to 32.88) with year-to-year effect sizes (d = .20, d = .27 respectively) comparable to the other ERN metrics, accumulating to d = .45 for K to G2.
Table 1.
Sample-level descriptives across Kindergarten, Grade 1, and Grade 2.
| Assessment | Age | ERN Amplitude |
Signal Strength |
Temporal Consistency |
Behavioral Variability |
|---|---|---|---|---|---|
| K (n = 218) |
6.10 (.39) | −6.00 (3.41) | 33.34 (0.88) | .23 (.08) | 138.33 (34.19) |
| G1 (n = 172) |
7.20 (.41) | −6.62 (3.61) | 33.16 (1.02) | .26 (.09) | 126.96 (26.38) |
| G2 (n = 162) |
8.27 (.56) | −7.35 (3.58) | 32.88 (1.09) | .28 (.10) | 109.13 (21.51) |
Note. Empirical sample-level means (standard deviations in parentheses). K = Kindergarten, G1 = Grade 1, G2 = Grade 2. ns vary as all children did not participate or provide usable data at all waves. Participants with incomplete data did not significantly differ on the outcomes of interest from those with complete data.
Age-related Changes in ERN Measures and Behavioral Variability
Results from the multilevel models of change examining age-related change and sex differences are given in Table 2 and illustrated in Figure 1. The pattern seen in the sample-level averages noted above was reflected in the formal models of age-related change, with significant age-related changes apparent in all four measures of neural activity and behavior. For example, the prototypical trajectory for ERN amplitude was characterized by a deflection of γ00= −5.90 (p< .05) at age 6 years, which increased in negativity at a rate of γ10= −0.61 (p< .001) units per year. Similarly, the prototypical trajectory of signal strength declined at a rate of γ10= −0.22 (p< .001) per year. In contrast, signal temporal consistency increased from γ00= 0.24 (p< .001) at a rate of γ10= 0.02 (p< .001) units per year. No main effects for sex or sex × age interactions were apparent for any of the three ERN measures (ps> .10). Behavioral variability exhibited age-related decline at an average rate of γ10= −12.64 (p< .001) units per year. Sex differences in levels of behavioral variability did not significantly differ (γ01= 3.55, p= .11; boys not significantly higher than girls) around an average level at age 6 (γ00= 139.76, p< .001). However, boys showed significantly faster decline over time than girls (γ11= −2.00, p= .05).
Table 2.
Results from multilevel models of change examining developmental changes and sex differences in ERN Amplitude, Average Signal Strength, Signal Temporal Consistency and Behavioral Variability.
| ERN Amplitude |
Average Signal Strength |
Signal Temporal Consistency |
Behavioral Variability |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
| Fixed Effects | ||||||||
| Intercept, γ00 | −5.90* | (0.25) | 33.40* | (0.07) | 0.24* | (0.01) | 139.76* | (2.19) |
| Sex, γ01 | −0.11 | (0.25) | −0.03 | (0.07) | −0.01 | (0.01) | 3.55 | (2.19) |
| Age, γ10 | −0.61* | (0.16) | −0.22* | (0.03) | 0.02* | (0.004) | −12.64* | (1.01) |
| Sex*Age, γ11 | −0.01 | (0.16) | 0.04 | (0.03) | 0.007 | (0.004) | −2.00* | (1.01) |
| Random Effects | ||||||||
| Variance, intercept σ2u0 | 1.98* | (0.75) | 0.65* | (0.08) | 0.0005 | (0.0005) | 587.48* | (93.44) |
| Variance, Age σ2u1 | -- | -- | -- | -- | -- | -- | 27.24 | (32.10) |
| Cov intercept, Age σu0u1 | -- | -- | -- | -- | -- | -- | −122.56* | (42.92) |
| Residual variance | 10.40* | (0.89) | 0.32* | (0.03) | 0.0082* | (0.0007) | 402.53* | (50.03) |
| −2LL | 2596.6 | 1245.5 | −890.8 | 5116.5 | ||||
= p < .05. −2LL = −2 Log Likelihood.Analyses based on between 485 and 552 Go-/No-go trials nested within 234 children.
Figure 1.
Plots of ERN amplitude(A), signal components (B, C), and behavioral variability (D) across time. Bold black lines illustrate the prototypical trajectory in each measure over time. Data points and trajectories are combined across males and females for models with no significant effect of Sex. Prototypical male and prototypical female lines are included for behavioral variability to illustrate the significant Age x Sex interaction effect for that variable.
Signal Temporal Consistency as a Mediator of Developmental Changes in ERN Amplitude
Given that signal temporal consistency increased with age, the hypothesis that increases in ERN amplitudes were mediated by increases in signal temporal consistency was directly examined. A mediational model for signal strength was not examined because the hypothesis that signal strength would increase with age was not supported. In order to formally test the mediational effect, signal temporal consistency (person-centered) was added to the model of change (Eq. 6) as a time-varying predictor (γ20) of ERN amplitude.
Results of the mediation model are presented in Table 3. Increases in signal temporal consistency over time significantly predicted more negative ERN amplitudes (γ20 = −12.64, p< .001). Sobel’s z-test (Sobel, 1982) revealed that changes in signal temporal consistency significantly mediated the association between age and ERN amplitude (z = −3.4, p < .001), and accounted for 38% of the total effect of age on ERN amplitude.
Table 3.
Results from multilevel model of change examining extent to which association between Age and ERN Amplitude is due to developmental changes in Signal Temporal Consistency.
| ERN Amplitude |
||
|---|---|---|
| Parameter | Estimate | SE |
| Fixed Effects | ||
| Intercept, γ00 | −6.11* | (0.24) |
| Sex, γ01 | −0.20 | (0.24) |
| Age, γ10 | −0.38* | (0.15) |
| Signal temporal consistency, γ20 | −12.64* | (2.21) |
| Sex*Age, γ11 | 0.09 | (0.15) |
| Random Effects | ||
| Variance, intercept σ2u0 | 3.11* | (0.72) |
| Variance, signal temporal consistency σ2u1 | 136.80* | (79.32) |
| Cov intercept, temporal consistency σu0u1 | 9.33 | (5.74) |
| Residual variance | 7.21* | (0.80) |
| −2LL | 2495.5 | |
Notes. Signal temporal consistency entered as a time-varying person-centered variable; age centered at 6 years; Sex coded males = 1 and females = −1.
= p<.05. −2LL = −2 Log Likelihood.Analyses based on 482 Go-/No-go trials nested within 226 children.
Associations among ERN Amplitude, Signal Temporal Consistency and Behavioral Variability
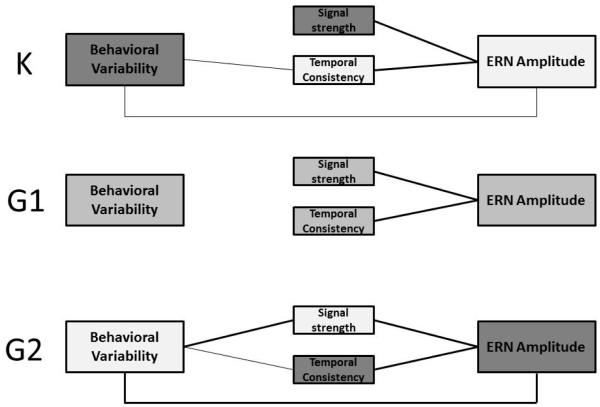
Pearson’s Correlations among behavioral variability and ERN amplitude, average signal strength, and signal temporal consistency are reported in Table 4 and illustrated in Figure 2. As expected, more negative ERN deflections were associated with greater signal strength and greater temporal consistency at each assessment. Signal strength and temporal consistency did not correlate with each other, consistent with the notion that each contributes independently to ERN amplitude.
Table 4.
Pairwise Pearson Correlations Amongst Measures.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. K ERN Amp | -- | ||||||||||
| 2. G1 ERN Amp | .11 | -- | |||||||||
| 3. G2 ERN Amp | .18* | .23* | -- | ||||||||
|
|
|||||||||||
| 4. K Signal Strength | −.29* | −.32* | −.17 t | -- | |||||||
| 5. G1 Signal Strength | −.19* | −.37* | −.23* | .62* | -- | ||||||
| 6. G2 Signal Strength | −.15 t | −.28* | −.36* | .56* | .70* | -- | |||||
|
|
|||||||||||
| 7. K Temporal Consistency |
−.43* | .10 | −.06 | .11 | .02 | −.01 | -- | ||||
| 8. G1 Temporal Consistency |
−.05 | −.22* | .02 | .01 | −.04 | .05 | .08 | -- | |||
| 9. G2 Temporal Consistency |
−.09 | −.06 | −.37* | .05 | .02 | .06 | .03 | .09 | -- | ||
|
|
|||||||||||
| 10. K Behavioral Variability |
.13t | −.04 | −.03 | .00 | .00 | .02 | −.15* | −.17* | −.13 | -- | |
| 11. G1 Behavioral Variability |
.09 | .08 | .08 | −.08 | −.08 | −.09 | −.14 t | −.07 | −.04 | .52* | -- |
| 12. G2 Behavioral Variability |
.22* | .13 | .26* | −.11 | −.16 t | −.25* | −.03 | −.12 | −.15t | .44* | .50* |
Note. p < .05,
p< .10. ns for each cell range from 106 to 199.
Figure 2.
Concurrent associations among ERN amplitude, signal components, and behavioral variability. Bold lines reflect significant correlations whereas thin lines reflect associations at the trend level. Developmental changes in average levels across the 3 years are depicted by changes in shading where darker boxes represent higher values.
The hypothesized relation between lower ERN amplitude and greater behavioral variability was r = .13, p = .06, at K, behavioral variability at K was significantly associated with lower signal temporal consistency (r = −.15, p = .03), which in turn was associated with less negative ERN amplitudes (r = −.43, p< .001). At G1, behavioral variability was not associated with any of the ERN measures. At G2, there was a significant association between greater behavioral variability and less negative ERN amplitudes as hypothesized (r = .26, p = .002). Greater behavioral variability at G2 was correlated r = −.15, p = .07, with lower signal temporal consistency and r = −.25, p = .003 with lower signal strength.
Rank Order Stability of ERN metrics and Behavioral Variability
Rank order stability coefficients among the K, G1, and G2 assessments are presented in Table 4. As expected, rank order stability in behavioral variability was moderate across all 3 assessments (rs = .44 to .52, ps< .001). ERN amplitude measured at K (r = .18, p = .06) and at G1 (r = .23, p = .02) were both positively correlated with ERN amplitude at G2, somewhat suggestive of stability. Contrary to expectations however, amplitude measured at K did not significantly correlate with amplitude measured at G1 (r = .11, p = .21). Average signal strength showed high stability across all time points (rs = .56 to .70, ps< .001). In contrast, rank order of signal temporal consistency at any one assessment was not associated with rank order at other assessments (rs = .03 - .09, ps> .30).
Discussion
The present study characterized the ERN signal in terms of both signal strength and temporal consistency and examined how these aspects of the ERN changed over 3 years during childhood. Although the ERN has been well studied in adults, far less is known about the presence of the ERN in children younger than age 8, and a lack of longitudinal data leaves much unknown about the stability of the ERN over developmental time. The longitudinal design of the present study allowed for the investigation of the relation of within-person changes in behavioral variability and neural activity underlying performance monitoring as well as the stability of between-person differences in this age range. Consistent with cross-sectional studies, ERN amplitude became increasingly negative with age (Davies et al., 2004; Wiersema et al., 2007). Although ERN amplitudes are related an individual’s confidence in their performance and thus increase with learning (see Walsh & Anderson, 2012), it is unlikely that the effects observed in this study are a function of repeated experience with the task across assessments. The task was designed to minimize learning effects as task rules were made explicit through visual instructions and practice trials prior to the start of ERP recording. Further, task difficulty was adjusted over time to account for increases in skill. Most importantly, the decomposition of the ERN into elements of signal strength and temporal consistency suggests that the increasingly negative deflections are not a function of greater signal strength but rather an increase in temporal consistency of the neural signal over this time period, supporting the conjecture that these changes are a result of developmental maturation. Results also indicate that these two distinct components of the ERN signal show different levels of rank-order stability during this developmental period.
Within-Person Changes in the ERN
To our knowledge, this is the first study to examine how signal strength and signal temporal consistency contribute to ERN amplitude during childhood. In particular, this study examines children from approximately age 6- through age 8- years, when the ERN signal is thought to be emerging. Difficulty in identifying a clear ERN signal on grand averaged waveforms is thought to result from greater inter-individual variability in this age range. The current study further suggests that ERN amplitude in younger children may be affected by greater levels of intra-individual variability in the temporal consistency of the neural process under investigation. Low levels of signal temporal consistency could explain why some children fail to show an ERN signal in their average waveform despite having the ability to behaviorally exhibit performance monitoring capabilities. Furthermore, the pattern of findings presented here indicates that only age-related increases in signal temporal consistency accounted for the increased ERN amplitudes, as signal strength showed an unpredicted decline across the three assessments.
The age-related changes in signal temporal consistency and average signal strength may be evidence of increasingly efficient functioning of neural systems underlying performance monitoring. Neurons transmit information with signals encoded in action potentials that affect the polarization of post-synaptic neurons. Post-synaptic neurons integrate signals from numerous sources and fire an action potential only if the threshold potential is reached. The effect of a single action potential on a post-synaptic neuron, however, will diminish with time. Therefore, for a neuron to reach threshold potential it must receive signals from many action potentials in a short temporal window. This highlights the importance of timing of the receipt of a signal, as information encoded in a signal with imprecise timing could be lost. A neural system characterized by low temporal precision would process information less efficiently due to information loss during transmission (Barlow, 1961). However, an inefficient neural system could maximize functionality by compensating for information loss by increasing the number of signals that are sent. Thus, an equally functional but less efficient neural system could be differentiated by greater activity and less temporal precision. Viewed through this lens, the increases in signal temporal consistency found in the present study could be evidence of maturing neural systems that are able to more efficiently monitor performance. Thus signal strength may decline as temporal precision increases, allowing the system to achieve the same level of function with less energy expended. This suggests that, at least in this age range, amplitude extracted from the average waveform may not accurately reflect capacity as it is unduly affected by low temporal consistency.
Some previous studies support the supposition that signal strength serves a compensatory role early in development when temporal consistency is low. Results from a previous study investigating age-related changes in the dynamics of auditory perception across childhood found a decrease in average signal strength and an increase in signal temporal consistency similar to the changes reported in the current study (Müller, Gruber, Klimesch, & Lindenberger, 2009). This pattern of EEG findings could also be considered consistent with developmental data from neuroimaging. Neuroimaging studies lack the temporal precision to differentiate between signal strength and trial-to-trial temporal consistency, but studies often find that children exhibit more diffuse fMRI activation patterns than adults, even in the absence of performance differences. This could reflect less efficient neural systems that require a greater number of resources to process the same amount of information (Casey, Giedd, & Thomas, 2000; Durston et al., 2006).
Although some studies have reported differences in ERN amplitude between males and females (Davies et al., 2004; Larson, South, & Clayson, 2011), no significant sex differences were observed here. Our results are consistent with the lack of sex differences in other ERN research with pre-pubertal participants (Davies et al., 2004; Torpey, Hajack, Kim, Kujawa, & Klein, 2012). The current findings thus extend support for a lack of sex differences in ERN amplitude as well as the signal strength and temporal consistency components of amplitude, and the rate of developmental change at these ages. However, sex differences did emerge in the developmental progression of behavioral variability. Boys on average showed more rapid declines in variability across the 3 assessments.
Stability in Between-Person Differences in ERN
Despite evidence of significant within-person changes, between-person stability in relative rank could still be maintained if developmental changes occurred in a homogeneous manner (i.e. all children change in a similar fashion). Previous studies have found the ERN to be a stable trait-like measure in adulthood and evidence of stability in younger children would suggest that the ERN is a strong biomarker of individual differences that could be inferred regardless of the assessment age (Olvet & Hajcak, 2009; Segalowitz et al., 2010). In this sample, however, evidence was inconsistent for the rank-order stability of the ERN across time. Both K and G1 ERN amplitudes were modestly correlated with G2 ERN amplitude suggestive of some degree of stability. Unexpectedly, amplitude measured a K did not correlate significantly with amplitude measured at G1. It is not clear what explains this lack of relation, but the finding reduces confidence in the trait-like stability of ERN amplitude at this age. However, despite low stability in the ERN amplitude, evidence of high stability was found across all three assessments for average signal strength. The highly trait-like nature of average signal strength suggests that it may be a better measure of trait-like individual differences in younger children than the more traditionally measured average amplitude. In contrast, signal temporal consistency showed no rank-order stability over this age range. Thus the relatively modest stability seen in the average amplitude may occur because amplitude contains one highly stable component (signal strength) and one unstable component (signal temporal consistency). Future research is needed to identify the experiential factors contributing to developmental changes in temporal consistency as well as when individual differences in temporal consistency stabilize and become trait-like.
The lack of correlation between the K and G1 amplitude measures, despite both being correlated with G2, is unexpected and warrants caution in interpretation. The G1 assessment is further anomalous in that this was the only time point during which behavioral variability showed no correlation with average amplitude. Although it is unclear what mechanisms underlie this pattern, it is possible to speculate on a developmental explanation. It is possible that the age range captured in this study spans a tumultuous transitional phase in neural organization as temporal consistency increasingly accounts for ERN amplitude. At young ages, individual differences in neural processing may be driven primarily by signal strength as individuals are near a developmental floor on signal temporal consistency. As children age, they will presumably approach a developmental ceiling at which further individual increases in consistency are unlikely. Near asymptote, temporal consistency would become more trait-like. The rate of this transitional process of decreasing signal strength and increasing signal temporal consistency may be highly variable between persons leading to instability in rank when measured at a time when the system is still in flux. It is possible that although individuals differ in their developmental rate, individuals may still arrive at a somewhat stable point within the distribution. As an example, pubertal development is characterized by a nonlinear progression between uniform beginning and endpoints. Although all individuals start at the same biologically pre-pubertal state and end at full sexual maturity, individual differences in timing and duration of the transition lead to temporary fluctuations in rank order in a variety of attributes (Marceau, Ram, Houts, Grimm, & Susman, 2011). Because of this, height measured in childhood correlates more strongly with adult height than height measured in adolescence, despite adolescence being a more proximal time point (see Wohwill, 1980 for discussion). Similarly, differences in timing and rate of development during this period of rampant neural change could temporarily obscure the appearance of trait-like stability in ERN amplitude. Far more research is needed to identify the factors contributing to the developmental parameters of this change process as well as its predictive validity for psychological outcomes.
Association between Neural and Behavioral Variability
Consistent with previous findings, the present study showed that behavioral variability decreased as children moved from K through G2, thought to reflect increased efficiencies in neural processing (Eckert & Eichorn, 1977; Segalowitz & Segalowitz, 1993). Despite the significant degree of within-person change, between-person stability for behavioral variability was comparable to that reported in adults (Flehmig, Steinborn, Langer, Scholz, & Westhoff, 2007; Saville et al., 2011). The rate of change was observed to be somewhat faster in boys than in girls in this sample.
Higher levels of behavioral variability were associated with less negative ERN amplitudes at K and G2, with no association observed at G1. The finding that behavioral variability also does not correlate with ERN amplitude at G1 further supports the notion that the developmental flux in the neural systems at this stage may be temporarily obscuring the presence of otherwise stable associations. Behavioral variability was, as expected, correlated with signal temporal consistency at K and G2. At the G2 assessment, a correlation also emerged between behavioral variability and signal strength. The emergent correlation between signal strength and behavioral variability may reflect the developmental changes in the distribution of behavioral variability. As the children aged, behavioral variability declined in both level and variance, presumably reflecting the effects of maturation over that 2.5 year window. High behavioral variability at K could be the result of many factors including inefficiencies in any neuronal system involved in completing the task (e.g. stimulus processing, response selection, and motor execution). As these various systems mature, behavioral variability that remains high at G2 may be a more specific indication of serious performance monitoring deficits as reflected by low error-related signal power in addition to low signal temporal consistency.
Limitations
Although this sample size of this study is relatively large compared to previous studies assessing development of ERN signaling in youth, the present study covers a comparably limited age range (Davies et al., 2004; Wiersema et al., 2007). Future studies will need to be conducted to determine if the age-related changes in neural signaling underlying performance monitoring found in children in this study are general trends throughout childhood or if these changes are limited to a narrow developmental time frame. Assessments for this study were also conducted at fairly long intervals and thus provide a coarse depiction of the timing of developmental changes and the short term stability of these metrics.
The participants in the current study were predominantly African American and resided in an urban, low socioeconomic status (SES) region, which could affect the generalizability of the study’s findings. Socioeconomic factors have been shown to influence neural development (Bradley & Corwyn 2002). Although this may temper comparability of developmental timing of neural changes with other samples, the developmental change itself is presumed to be characteristically human and thus generally consistent across all potential samples. Future replications with samples from different demographic strata should examine whether the developmental trends reported here occur earlier or proceed more rapidly in higher-resource populations, as well as what types of contextual and experiential factors affect this developmental timing.
The present study also oversampled for aggression and half of the participants were reported to have at least some problem behaviors at the start of schooling. Both externalizing and internalizing disorders have been linked to differences in ERN amplitude and the greater range of these symptoms in the present sample compared with typical community-recruited samples in studies of normative development may have contributed to greater variability across participants. Using a sample that covered the full symptom range, and thus reducing concern about restriction of range present in more homogeneous samples, provided for a relatively stronger test of stability. However, this must also be taken into account when comparing findings to different samples. For instance, externalizing disorders have been characterized by relative developmental immaturity and thus the timing of the transitions in signal strength and temporal consistency reported here may occur at even earlier ages in higher SES samples. Given the interest of using the ERN to obtain better understanding of the developmental risk for psychopathological outcomes, it is especially critical to track the developmental progression of this ERP in high-risk samples, keeping in mind that both the characteristics of the ERN and how those characteristics develop over time could differ between groups.
Summary
Overall, this study demonstrates the potential utility of decomposing the ERN into two independent components: average signal strength and temporal consistency across trials, when examining the ERN in children. The increase in ERN amplitude observed as children age appears to be due to an increase in trial-to-trial temporal consistency and occurs despite a decline in signal strength over this time period. Moreover, signal strength demonstrated high rank-order stability over time, suggesting that this measure may be particularly useful for studying trait-like differences associated with the ERN amplitude. Conversely, signal temporal consistency did not demonstrate stability over time and therefore may be more sensitive to experiential or situational influence. Although more work is needed to understand the developmental mechanisms underlying changes in neural processing over time, our results indicate the value of considering both signal strength and temporal consistency when examining the ERN in younger children.
Research Highlights.
We extracted signal strength and signal variability on a trial-to-trial basis, both of which correlated with the ERN measured as an average across trials, but did not correlate with each other.
Across 3 annual assessments from an average age of 6 to age 8, signal strength showed high inter-individual stability whereas temporal consistency did not.
Across the 3 years, average ERN amplitude increased, consistent with previous developmental research.
The increase in average ERN amplitude was mediated by an increase in signal temporal consistency. Contrary to predictions, signal strength declined over this period.
Brain development during this period may be characterized by a shift from a more resource-intensive allocation of signal strength toward a more efficient pattern of temporal consistency.
Acknowledgments
Funding for this research was provided by awards from: The Pennsylvania Department of Health, The Social Science Research Institute at the Pennsylvania State University, and the National Science Foundation SES-1150844 to the last author; T32 DA017629 to the first author; R01-HD076994, R24-HD041025 to the second author; and Institute of Education Sciences R305B090007 to the third author. The authors wish to thank Heather Wadlinger, and Liza Oakes for their contributions to data processing, as well as Jennifer Ford for her exceptional project management. The authors also would like to acknowledge Mark Greenberg, Karen Bierman, Robert Nix, Michelle Jetha, and Michael Coccia for their roles in designing, executing, overseeing, and managing the project from which these data are drawn.
Footnotes
Author Note. David DuPuis, Interdepartmental Graduate Program in Neuroscience, Department of Human Development and Family Studies, The Pennsylvania State University; Nilam Ram, Department of Human Development and Family Studies, The Pennsylvania State University; Cynthia J. Willner, Department of Human Development and Family Studies, The Pennsylvania State University; Sarah Karalunas, Department of Psychiatry, Oregon Health & Science University; Sidney J. Segalowitz, Department of Psychology, Brock University, Lisa Gatzke-Kopp, Department of Human Development and Family Studies, The Pennsylvania State University.
Contributor Information
Sarah Karalunas, Oregon Health & Science University.
Sidney J. Segalowitz, Brock University
Lisa M. Gatzke-Kopp, The Pennsylvania State University
References
- Aggarwal A, Lillystone D. A follow-up pilot study of objective measures in children with attention deficit hyperactivity disorder. Journal of Paediatric Child Health. 2000;36:134–138. doi: 10.1046/j.1440-1754.2000.00464.x. PMID 10760011. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neuroscience & Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. PMID 16901542. [DOI] [PubMed] [Google Scholar]
- Band GP, Kok A. Age effects on response monitoring in a mental-rotation task. Biological Psychology. 2000;51:201–221. doi: 10.1016/s0301-0511(99)00038-1. PMID 10686366. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Possible principles underlying the transformations of sensory messages. In: Rosenblith WA, editor. Sensory Communications. M.I.T. Press; Boston: 1961. pp. 217–234. [Google Scholar]
- Boksem MA, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. PMID 16784728. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. DOI: http://dx.doi.org/10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, Christensen H, Dear K, Wen W, Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60-64 years. Neuropsychologia. 2007;45:2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. PMID 17382358. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. PMID 11035225. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. PMID 22091878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. PMID 16434657. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. PMID 15148003. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. PMID 16445387. [DOI] [PubMed] [Google Scholar]
- Eckert HM, Eichorn DH. Developmental variability in reaction-time. Child Development. 1977;48:452–458. DOI: http://dx.doi.org/10.2307/1128638. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography & Clinincal Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. PMID 1712280. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Experimental Brain Research. 2001;138:258–262. doi: 10.1007/s002210100712. PMID 11417467. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Rosburg T, Axmacher N, Elger CE. Phase-locking within human mediotemporal lobe predicts memory formation. Neuroimage. 2008;43:410–419. doi: 10.1016/j.neuroimage.2008.07.021. PMID 18703147. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, Walhovd KB. Reduced white matter integrity is related to cognitive instability. Journal of Neuroscience. 2011;31:18060–18072. doi: 10.1523/JNEUROSCI.4735-11.2011. PMID 22159119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flehmig HC, Steinborn M, Langer R, Scholz A, Westhoff K. Assessing intraindividual variability in sustained attention: Reliability, relation to speed and accuracy, and practice effects. Psychology Science. 2007;49:132–149. [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47:495–501. doi: 10.1016/j.neuron.2005.06.020. PMID 16102533. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biological Psychology. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. PMID 17196732. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Greenberg MT, Fortunato CK, Coccia MA. Aggression as an equifinal outcome of distinct neurocognitive and neuroaffective processes. Developmental Psychopathology. 2012;24:985–1002. doi: 10.1017/S0954579412000491. PMID 22781867. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error-detection and compensation. Psychological Science. 1993;4:385–390. DOI: http://dx.doi.org/10.1111/j.1467-9280.1993.tb00586.x. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. DOI: http://dx.doi.org/10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephaolography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science. 2012;21:101–106. [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. PMID 14602356. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain & Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. PMID 15518935. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. PMID 15787852. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. PMID 17470258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh JB, Inzlicht M. Error-related negativity predicts academic performance. Psychophysiology. 2010;47:192–196. doi: 10.1111/j.1469-8986.2009.00877.x. PMID 19674391. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychology Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. PMID 12374324. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. Journal of Gerontolgy. Series B, Psychological Sciences and Social Sciences. 2002;57:P101–115. doi: 10.1093/geronb/57.2.p101. PMID 11867658. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS. Intraindividual variability in performance as a theoretical window onto cognitive aging. In: Dixon R, Backman L, Nilsson L-G, editors. New Frontiers in Cognitive Aging. Oxford University Press; New York: 2004. pp. 65–88. DOI: DOI:10.1093/acprof:oso/9780198525691.003.0004. [Google Scholar]
- Iacono WG, Malone SM. Developmental endophenotypes: Indexing genetic risk for substance abuse with the P300 brain event-related potential. Child Development Perspectives. 2011;5:239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Imashioya H, Uno H, Fujita T. Error-related negativity in a visual go/no-go task: children vs. adults. Developmental Neuropsychology. 2007;31:181–191. doi: 10.1080/87565640701190775. PMID 17488215. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-related negativity in children: Effect of an observer. Developmental Neuropsychology. 2005;28:871–883. doi: 10.1207/s15326942dn2803_7. PMID 16266253. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Research. Cognitive Brain Research. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. PMID 15062867. [DOI] [PubMed] [Google Scholar]
- Kolev V, Beste C, Falkenstein M, Yordanova J. Error-related oscillations: Effects of aging on neural systems for behavioral monitoring. Journal of Psychophysiology. 2009;23:216–223. DOI 10.1027/0269-8803.23.4.216. [Google Scholar]
- Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivariate Behavioral Research. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE. The relationship between cognitive performance and electrophysiological indices of performance monitoring. Cognitive Affective and Behavioral Neuroscience. 2011;11:159–171. doi: 10.3758/s13415-010-0018-6. PMID 2126465. [DOI] [PubMed] [Google Scholar]
- Larson MJ, South M, Clayson PE. Sex differences in error-related performance monitoring. Neuroreport. 2011;22:44–48. doi: 10.1097/WNR.0b013e3283427403. PMID 21304441. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18:430–443. doi: 10.1162/089892906775990633. PMID 16513007. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Nilsson L-G, Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Hogrefe & Huber Publishers; Ashland, OH: 1999. pp. 103–146. [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. PMID 15016286. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. PMID 15871602. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stoup WW, Wolfinger RD. SAS system for mixed models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. Wiley; New York: 1987. [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. PMID 15261861. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of Cognitive Neuroscience. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. PMID 18564054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat S. A wavelet tour of signal processing. Academic Press; New York, NY: 2008. [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology. 2011;47:1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. PMID 22308177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V, Gruber W, Klimesch W, Lindenberger U. Lifespan differences in cortical dynamics of auditory perception. Developmental Science. 2009;12:839–853. doi: 10.1111/j.1467-7687.2009.00834.x. PMID 19840040. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. PMID 15453975. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. PMID 18694617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Research. 2009;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. PMID 19501071. [DOI] [PubMed] [Google Scholar]
- Rao CR. Estimation of variance and covariance components in linear models. Journal of the American Statistical Association. 1972;67:112–115. DOI: 10.1080/01621459.1972.10481212. [Google Scholar]
- Richardson C, Anderson M, Reid CL, Fox AM. Neural indicators of error processing and intraindividual variability in reaction time in 7 and 9 year-olds. Developmental Psychobiology. 2011;53:256–265. doi: 10.1002/dev.20518. PMID 21400488. [DOI] [PubMed] [Google Scholar]
- Saville CWN, Pawling R, Trullinger M, Daley D, Intriligator J, Klein C. On the stability of instability: Optimising the reliability of intra-subject variability of reaction times. Personality and Individual Differences. 2011;51:148–153. DOI: http://dx.doi.org/10.1016/j.paid.2011.03.034. [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neuroscience Letters. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. PMID 12361852. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology and Human Perceptual Performance. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. PMID 10696610. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Human Brain Mapping. 2005;26:139–147. doi: 10.1002/hbm.20149. PMID 15858815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz NS, Segalowitz SJ. Skilled performance, practice, and the differentiation of speed-up from automatization effects: Evidence from second language word recognition. Applied Psycholinguistics. 1993;14:369–385. DOI: http://dx.doi.org/10.1017/S0142716400010845. [Google Scholar]
- Segalowitz SJ, Dywan J. Individual differences and developmental change in the ERN response: Implications for models of ACC function. Psychology Research. 2009;73:857–870. doi: 10.1007/s00426-008-0193-z. PMID 19023593. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Murphy TI, Homan D, Chantziantoniou DK, Khan S. Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology. 2010;47:260–270. doi: 10.1111/j.1469-8986.2009.00942.x. PMID 20030755. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological Methodology. 1982;13:290–312. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. PMID 16887187. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. PMID 10708903. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2012;54:139–150. doi: 10.1002/dev.20590. PMID 21815136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology. 2009;34:749–761. doi: 10.1080/87565640903265103. PMID 20183731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clinincal Neurophysiology. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. PMID 17223380. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. Journal of Abnormal Psychology. 2003;112:667–678. doi: 10.1037/0021-843X.112.4.667. PMID 14674878. [DOI] [PubMed] [Google Scholar]
- Ullén F, Forsman L, Blom O, Karabanov A, Madison G. Intelligence and variability in a simple timing task share neural substrates in the prefrontal white matter. Journal of Neuroscience. 2008;16:4238–4243. doi: 10.1523/JNEUROSCI.0825-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MM, Anderson JR. Learning from experience: Event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neuroscience and Biobehavioral Reviews. 2012;36:1870–1884. doi: 10.1016/j.neubiorev.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test-retest reliability of error-related brain activity. Psychophysiology. 2011;48:1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. PMID 21496055. [DOI] [PubMed] [Google Scholar]
- Wessel JR. Error awareness and the error-related negativity: Evaluating the first decade of evidence. Frontiers in Human Neuroscience. 2012;6:88. doi: 10.3389/fnhum.2012.00088. PMID 22529791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Bjornerud A, Due-Tonnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus--A study combining diffusion tensor imaging and electrophysiology in healthy adults. Cerebral Cortex. 2009;19:293–304. doi: 10.1093/cercor/bhn084. PMID 18502729. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45:1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. PMID 17303199. [DOI] [PubMed] [Google Scholar]
- Wohwill JF. Cognitive development in childhood. In: Brim OG, Kagan J, editors. Constancy and Change in Human Development. Harvard University Press; Cambridge, MA: 1980. pp. 359–444. [Google Scholar]