Abstract
Background
Plasma amyloid β (Aβ) peptides levels have been examined as a low-cost, accessible marker for risk of incident Alzheimer’s disease (AD) and dementia, but results have varied between studies. We reassessed these associations in one of the largest, prospective, community-based studies to date.
Methods
A total of 2189 dementia-free, Framingham Study participants over age 60 years (mean age 72±8; 56% women) had plasma Aβ1-42 and Aβ1-40 measured and were followed prospectively (mean 7.6±3.0 years) for dementia/AD.
Results
Increased plasma Aβ1-42 levels were associated with lower risk of dementia (Hazard ratios: Aβ1-42 HR=0.80 [0.71–0.90], p<0.001; Aβ1-42/Aβ1-40 ratio HR=0.86 [0.76–0.98], p=0.027) and AD (Aβ1-42 HR=0.79 [0.69–0.90], p<0.001; Aβ1-42/Aβ1-40 ratio HR=0.83 [0.72–0.96], p=0.012).
Conclusion
Our results suggest that lower plasma Aβ levels are associated with risk of incident AD and dementia. They encourage further evaluation of plasma Aβ levels as a biomarker for risk of developing clinical AD and dementia.
Keywords: Aβ peptides, plasma biomarker, incident Alzheimer’s disease, incident dementia, Framingham heart study, epidemiology, meta-analysis
1 Background
Dementia is a major public health problem with worldwide prevalence expected to reach 115.4 million persons by 2050, representing an enormous societal and financial burden to affected individuals, their families and the health care systems they will need to access [1]. Dementia due to Alzheimer’s Disease (AD) represents 50 to 70% of all dementia and thus, successfully targeting this pathophysiology should help diminish the burden of dementia. Therapeutic trials have, so far, had very limited success, likely because brain damage builds up during a long preclinical phase [2] so that treatment starting at the time of the clinical dementia diagnosis comes when those lesions are already irreversible. Historically, visualization ofAD neuropathology was only possible through post-mortem examination of the brain, allowing a definite diagnosis. Recently, several biomarkers have become available and allow assessment of the activity of AD pathophysiological processes in living patients, before the onset of clinical dementia [3].
Among these hallmark pathophysiological processes, abnormal production and aggregation of amyloid β (Aβ) isoforms (mainly Aβ42 and Aβ40) in the brain is one of the earliest, beginning several decades before onset of clinical symptoms [4] and triggering a cascade of events leading to synaptic loss, neuronal death and clinical dementia [5]. Thus, following the activity of the amyloid process in asymptomatic subjects could be useful to select those persons at higher risk of developing AD, for inclusion in clinical trials of putative treatments for AD and in the future, for preventive interventions [6].
Currently, Aβ peptide concentrations are most frequently detected in body fluids, by measuring Aβ levels in the cerebrospinal fluid (CSF), or through brain imaging of amyloid deposition. CSF levels fall in parallel with increased brain deposition as AD pathology accumulates and thus, can help refine the differential diagnosis in subjects with clinical dementia [7,8] and identify healthy elderly and individuals with mild cognitive impairment (MCI) who are at high risk of developing AD in the future [9–11]. But lumbar puncture is invasive and amyloid imaging expensive, restricting their wide-spread or frequent use in large populations of asymptomatic subjects.
Given their greater accessibility, there is considerable interest in examining whether circulating Aβ levels correlate with AD risk. A recent meta-analysis by Koyama et al and subsequent studies suggest such an association [12–15] but high inter-assay variability, differences in study design and follow-up time have led to significant heterogeneity and conflicting results [14, 16].
In order to help clarify this matter, we measured plasma Aβ1-42 and Aβ1-40 in a prospective, community-based, cohort under ongoing surveillance for AD, using a validated, commercially available amyloid assay. Our main objective was to assess longitudinal associations between plasma Aβ peptides levels and risk of incident dementia and AD in our study. Our secondary objective was to update the meta-analysis published by Koyama et al [14].
2 Methods
2.1. Study Sample
The Framingham Heart Study (FHS) is an ongoing community-based prospective cohort study of cardiovascular disease and its risk factors. It was initiated in 1948 with the enrollment of 5209 women and men aged 28 to 74 years (Original Cohort) [17]. Original cohort participants are reassessed biennially at a comprehensive core examination and have been examined 31 times to date [18]. In 1971, offspring of the original cohort and the spouses of these offspring (n = 5124, age 5–70 years, 3548 biological offspring, 1576 offspring spouses) were enrolled in the Framingham Offspring Cohort [19]. They have been examined every 4 to 8 years since, 9 times to date, for a core examination [20]. In addition, both cohorts have been under ongoing surveillance for cognitive decline and dementia since 1975.
A total of 4,039 participants who attended the 23rd Original cohort examination (1992–1996, n=772) or the 7th Offspring examination (1998–2001, n=3267) had plasma Aβ1-42 and Aβ1-40 measured. We excluded participants aged < 60 years (n=1532) as they were unlikely to develop late-onset Alzheimer’s disease, and to be more consistent with other studies. We also excluded participants with prevalent dementia (n=42) or with no follow-up (n=276), yielding a subsample of 2189 participants for longitudinal assessment of dementia and Alzheimer’s disease risks related to plasma Aβ concentrations.
The study protocol was approved by the Institutional Review Board of the Boston University Medical Center and all participants provided written informed consent.
2.2. Plasma Aβ Assessment
EDTA plasma specimens used for the Aβ analyses were drawn into K3-EDTA evacuated specimen tubes, in the early afternoon in a supine nonfasting state for Original cohort, and in the morning in a supine fasting state for Offspring cohort. Specimen tubes were centrifuged for 30 minutes at 1850g at 4 degrees Celsius. Plasma was then separated from cells after centrifugation and placed at −80 degrees Celsius, within 90 minutes of venipuncture. The original aliquots consisted of 2mL of plasma in 3mL cryogenic storage vials for Original cohort samples and 700 μL of plasma in 1mL cryogenic storage vials for Offspring cohort samples. All specimens were stored at −80 degrees Celsius until they were aliquoted in March 2012 to be frozen and shipped for the assay. Therefore, specimens were thawed once prior to Aβ measurement. New aliquots consisted of 150 μL of plasma in 0.5 mL cryogenic storage vials. All samples were analyzed at the Department of molecular pharmacology and experimental therapeutics of the Mayo Clinic, Jacksonville, FL, from June to August 2012. Quantification of Aβ isoforms in plasma was performed using INNO-BIA plasma Aβ forms assays (Innogenetics, Ghent, Belgium), which is a multiplex microsphere-based Luminex xMAP technique that allows simultaneous analysis of Aβ1-40 and Aβ1-42 [21]. Measurements were done in duplicate in a randomly selected sample representing 9% of all samples. Intra-assay coefficients of variations (CV) for Aβ1-40 and Aβ1-42 were 3.2% and 2.6% and inter-assay CVs were 10.5% and 7.6%, respectively. Analysis of 146 phantom samples showed intraclass correlation coefficients of 0.916 and 0.943 and CV of 4.8% and 3.5%, respectively.
2.3. Dementia and Alzheimer’s Disease Diagnosis
We screened participants at each examination for possible cognitive decline through a number of mechanisms, including an administration of the Folstein Mini-Mental Status Examination (MMSE) [22], Briefly, a MMSE score below the education-specific cutoff score (which ranged from ≤26 in college-educated persons to ≤23 in persons with less than a high school education), a decline of 3 or more points between two successive administrations, or a decline of more than 5 points compared with any previous examination prompted further in-depth testing. Other mechanisms included referral by FHS staff and physicians at regular clinic exams, by self, family or primary care physician, referral following health updates or ancillary studies by other FHS working groups, and referral based on performance on two neuropsychological test batteries administered 6 years apart to most participants as part of an independent initiative [20, 23]. Participants with a stroke diagnosis were also examined at 6, 12 and 24 months for possible cognitive decline. Persons “flagged” as having possible MCI or otherwise being at risk for developing dementia underwent a more detailed neuropsychological evaluation, including the Logical Memory test from the Wechsler Memory Scale, the Similarities test from the Wechsler Adult Intelligence Scale, the Trail-Making Tests A and B, Boston Naming Test, Controlled Word Association Test, Hooper Visual Organization Test, Clock Drawing Test, and Wide Range Achievement Test. A neurological examination was also performed, blinded from the neuropsychological evaluation results. If the neuropsychological testing or neurological evaluation suggested a decline in cognitive function, and other sources of data could not clarify if the person had MCI or AD, we administered a structured family interview and all persons were assigned a Clinical Dementia Rating [24] scale score. We then determined whether each person fulfilled criteria for a diagnosis of dementia, the probable date of onset and type of dementia at a consensus review conducted by a panel comprising at least one behavioral neurologist and one neuropsychologist. The panel reviewed all available records including examinations by FHS investigators, hospital and nursing home records, data from structured family interviews, imaging and when available, autopsy data. Participants with dementia met criteria outlined in the Fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria [25], and were required to have symptoms for at least 6 months. Participants with AD met National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria for definite, probable or possible AD [26]. For the present analyses, data for incident dementia obtained till December 2012 were used.
2.4. Statistical Analysis
We log-transformed plasma Aβ levels (Aβ1-42, Aβ1-40) in order to account for the skewness of their distributions. Plasma Aβ ratio was computed as [Aβ1-42/Aβ1-40], and then log- transformed. Plasma Aβ levels were either studied as a continuous variable and in that case were also standardized to reflect risk associated with a difference of 1 standard deviation (SD) in baseline levels, or as quartiles.
Longitudinal associations of plasma Aβ levels with risk of incident dementia and AD were assessed using Cox regression models. In the dementia analysis, participants were followed from the baseline examination until they developed dementia, died or had been followed for 10-years. Persons whose cognitive status at 10 years after baseline was not known were censored at the time they were last documented to be free of dementia (n=960). In the AD analysis, additional right censoring at time of other dementia diagnosis was performed. Successive adjustment strategies were used, namely: adjustment for age and gender (Model A); further adjustment for education, APOE ε4 status (model B); and for prevalent hypertension, diabetes and cardiovascular disease, current smoking, total cholesterol levels and being in the top sex-specific quartile of waist-hip ratio (model C). In threshold models, the survival without AD was also estimated from model A within each quartile of plasma Aβ levels. We also performed adjustment for creatinine levels in a sensitivity analysis, as data were not available in all participants (n=2018). We computed Spearman correlation coefficients of creatinine levels with plasma Aβ concentrations, and a Cox regression model adjusted for age, gender and creatinine levels (model A+). We could not adjust for platelet counts as these had not been measured at the same examinations as plasma Aβ levels.
We then examined whether plasma Aβ concentrations improved AD risk prediction when added to models with age, gender, education and APOEε4 by computing continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) [27, 28]. Those measures and their confidence intervals were estimated using bootstrap to account for the risk of overfitting, and interpreted by comparing their values to simulated data [29], Briefly, we used IDI≈0.004 and NRI≈0.16, IDI≈0.024 and NRI≈0.40 and IDI≈0.06 and NRI≈0.62 to characterize small, moderate, and large improvements, respectively. Finally, we updated the results of the meta-analysis published by Koyama et al [14]. Using the same selection of prospective cohorts, we first extracted hazard ratios of incident AD or dementia and their 95% confidence intervals from minimally adjusted models comparing lowest versus highest quantiles (quartiles or tertiles) of plasma Aβ1-42 levels, plasma Aβ1-40 levels or plasma Aβ1-42/Aβ1-40 ratio. In addition to our results and those published by Shah et al [15], and Lui et al [12], we contacted three previously published studies, including Shah et al, that had measured plasma Aβ levels, to obtain unpublished hazard ratios [13, 15, 30]. Lastly, we performed fixed- and random-effect meta-analyses, and evaluated heterogeneity using the I2 measure.
Cox regression analyses were performed using Statistical Analysis System software version 9.2 (SAS Institute, Cary, NC). Meta-analyses were performed using R version 3.02 and the metafor package [32]; the latter were also used to create survival curves and forest plots.
3 Results
After up to 10 years of follow-up (mean time ± standard deviation: 7.6 ± 3.0 years; median time: 9 years), 237 participants were diagnosed with dementia (194 had AD). The main characteristics of the study population are summarized in table 1.
Table 1.
Characteristics of the study population at baseline
| Population at risk for dementia (n=2189) | |
|---|---|
| General characteristics | |
| Age, years, mean (standard deviation) | 72.16 (7.57) |
| Women, % (N) | 56.28 (1232) |
| Education, % (N) | |
| No high school degree | 12.84 (276) |
| High school degree | 34.74 (747) |
| Some college | 26.65 (573) |
| College graduate | 25.77 (554) |
| APOE ε4 genotype : ≥ 1 APOE ε4 allele, % | 21.51 (465) |
| Confounders | |
| Hypertension, % (N) | 61.17 (1359) |
| Cardiovascular disease, % (N) | 24.17 (529) |
| Smoking, % (N) | 7.59 (166) |
| Total cholesterol, mg/dL, mean (standard deviation) | 201.19 (36.63) |
| Diabetes, % (N) | 15.12 (327) |
| Plasma Aβ levels | |
| Aβ1-42 levels (pg/mL)* | 44.0 (37.5–50.8) |
| Aβ1-40 levels (pg/mL)* | 163.6 (142.7–185.4) |
| Aβ1-42/Aβ1-40 ratio* | 0.268 (0.234–0.308) |
mean (standard deviation) are presented unless otherwise stated
Those variables were log-transformed in the analyses. Thus, the median (interquartile range) is used instead of mean (standard deviation)
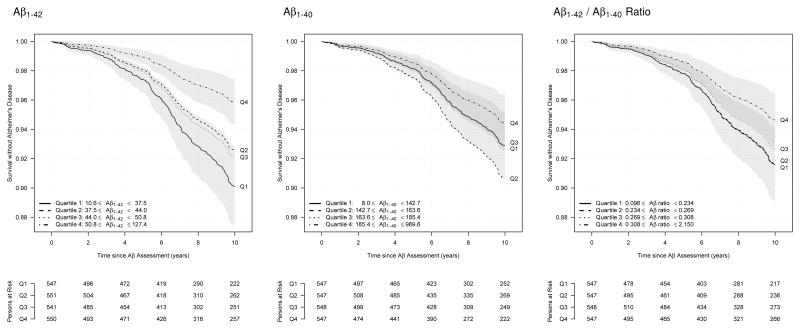
Longitudinal associations between plasma Aβ levels and risk of incident AD and dementia are presented in table 2. Increased plasma Aβ1-42 levels were associated with lower risks of incident AD and dementia after adjustment for age and gender. An increase of one standard deviation of plasma Aβ1-42 levels was associated with a decrease of 21% in incident AD risk (hazard ratio (HR)=0.79; 95% confidence interval (CI) [0.69–0.90]; p=3.00 × 10−04) and 20% in incident dementia risk (HR=0.80; 95% CI [0.71–0.90]; p=2.00 × 10−04). Risks of AD by quartiles of plasma Aβ1-42 levels are presented in table 2 and figure 1. Compared to participants from the fourth quartile (highest levels), participants from the first quartile of plasma Aβ1-42 had a greater than two-fold risk of AD (HR=2.46; 95% CI [1.60–3.78]) and dementia (HR=2.20; 95% CI [1.51–3.20]). Similar, although weaker, associations were observed for plasma Aβ1-42/Aβ1-40 ratio. No statistically significant association was observed for plasma Aβ1-40 levels.
Table 2.
Association of plasma Aβ levels with risk of incident dementia and Alzheimer’s disease
| Continuous (per SD) | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| HR | 95% CI | P | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | ||
| Model A* | |||||||||||
| Aβ1-40 | AD | 0.93 | [0.81–1.06] | 2.59×10−01 | 1.29 | [0.85–1.96] | 1.71 | [1.17–2.51] | 1.28 | [0.84–1.96] | Ref. |
| Dementia | 0.91 | [0.81–1.03] | 1.26×10−01 | 1.50 | [1.03–2.19] | 1.58 | [1.10–2.27] | 1.48 | [1.01–2.16] | Ref. | |
| Aβ1-42 | AD | 0.79 | [0.69–0.90] | 3.00×10−04 | 2.46 | [1.60–3.78] | 1.82 | [1.18–2.81] | 1.94 | [1.26–3.00] | Ref. |
| Dementia | 0.80 | [0.71–0.90] | 2.00×10−04 | 2.20 | [1.51–3.20] | 1.52 | [1.03–2.24] | 1.66 | [1.13–2.44] | Ref. | |
| Aβ1-42/Aβ1-40 ratio | AD | 0.83 | [0.72–0.96] | 1.23×10−02 | 1.59 | [1.06–2.39] | 1.58 | [1.05–2.38] | 1.38 | [0.90–2.12] | Ref. |
| Dementia | 0.86 | [0.76–0.98] | 2.73×10−02 | 1.52 | [1.05–2.20] | 1.48 | [1.02–2.14] | 1.39 | [0.95–2.04] | Ref. | |
| Model B† | |||||||||||
| Aβ1-40 | AD | 0.93 | [0.81–1.05] | 2.51×10−01 | 1.30 | [0.85–1.99] | 1.78 | [1.21–2.62] | 1.33 | [0.86–2.04] | Ref. |
| Dementia | 0.92 | [0.82–1.03] | 1.65×10−01 | 1.45 | [0.99–2.12] | 1.64 | [1.14–2.36] | 1.53 | [1.04–2.24] | Ref. | |
| Aβ1-42 | AD | 0.79 | [0.69–0.90] | 4.00×10−04 | 2.36 | [1.53–3.64] | 1.81 | [1.17–2.81] | 1.86 | [1.20–2.87] | Ref. |
| Dementia | 0.81 | [0.72–0.91] | 6.00×10−04 | 2.06 | [1.41–3.01] | 1.53 | [1.04–2.26] | 1.63 | [1.11–2.40] | Ref. | |
| Aβ1-42/Aβ1-40 ratio | AD | 0.84 | [0.72–0.97] | 1.55×10−02 | 1.58 | [1.05–2.38] | 1.47 | [0.98–2.22] | 1.29 | [0.84–1.99] | Ref. |
| Dementia | 0.87 | [0.77–1.00] | 4.28×10−02 | 1.47 | [1.01–2.12] | 1.38 | [0.95–2.00] | 1.32 | [0.90–1.93] | Ref. | |
| Model C‡ | |||||||||||
| Aβ1-40 | AD | 0.92 | [0.81–1.05] | 2.28×10−01 | 1.30 | [0.84–2.01] | 1.88 | [1.27–2.78] | 1.29 | [0.83–2.00] | Ref. |
| Dementia | 0.92 | [0.81–1.03] | 1.44×10−01 | 1.44 | [0.97–2.13] | 1.73 | [1.20–2.51] | 1.49 | [1.01–2.20] | Ref. | |
| Aβ1-42 | AD | 0.79 | [0.68–0.90] | 5.00×10−04 | 2.31 | [1.49–3.58] | 1.67 | [1.06–2.63] | 1.75 | [1.12–2.75] | Ref. |
| Dementia | 0.81 | [0.72–0.92] | 1.20×10−03 | 1.95 | [1.32–2.87] | 1.40 | [0.94–2.09] | 1.53 | [1.03–2.27] | Ref. | |
| Aβ1-42/Aβ1-40 ratio | AD | 0.85 | [0.73–0.98] | 2.72×10−02 | 1.59 | [1.05–2.40] | 1.38 | [0.91–2.09] | 1.27 | [0.81–1.99] | Ref. |
| Dementia | 0.89 | [0.78–1.02] | 8.57×10−02 | 1.44 | [0.99–2.10] | 1.27 | [0.87–1.85] | 1.29 | [0.86–1.92] | Ref. | |
Abbreviation: AD, Alzheimer’s disease; HR, Hazard ratio; SD, Standard deviation; 95% CI, 95% Confidence interval; Ref., Reference
NOTE. Hazard ratios, confidence intervals and P-value were evaluated in Cox regression models
Model A: adjusted for age and gender
Model B: adjusted for age, gender, education and APOE ε4 status
Model C: adjusted for age, gender, education, APOE ε4 status, prevalent hypertension, diabetes and cardiovascular disease, current smoking, total cholesterol levels, and being in the top sex-specific quartile of waist-hip ratio
Figure 1.
Survival without Alzheimer’s disease according to quartiles of plasma Aβ1-42, Aβ1-40 and Aβ1-42/Aβ1-40 ratio
NOTES. Survival is derived from a Cox model adjusted for age and gender. 95% confidence intervals are represented for first and fourth quartiles.
Associations between plasma Aβ1-42 levels and incident AD and dementia remained significant after additional adjustment for APOE ε4 status, education, hypertension, diabetes, cardiovascular disease, current smoking, total cholesterol levels and waist-hip ratio, and hazard ratios remained stable (see table 2). Similarly, associations between plasma Aβ1-42/Aβ1-40 ratio and incident AD remained significant and hazard ratios remained stable. Conversely, associations between plasma Aβ1-42/Aβ1-40 ratio and incident dementia became non-significant in Model C. Plasma Aβ levels presented low correlation with creatinine levels, with Spearman coefficients of 0.09, 0.19 and −0.07 for Aβ1-42, Aβ1-40 and Aβ1-42/Aβ1-40 ratio, respectively. Adjustment for creatinine levels in addition to age and gender did not modify the estimation of hazard ratios (see supplementary Table).
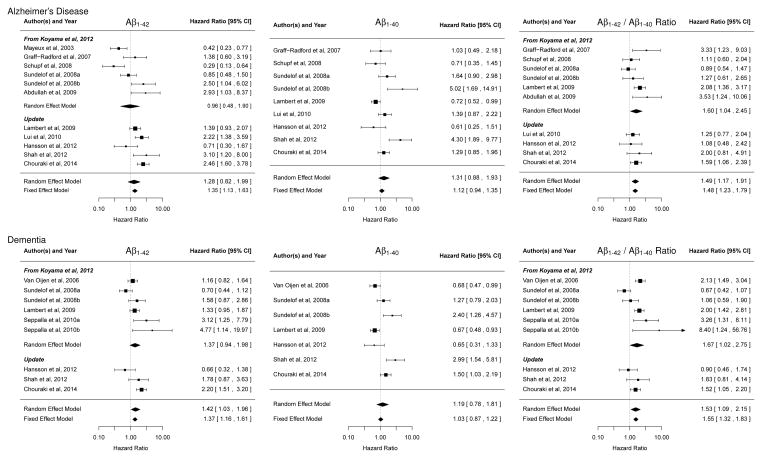
Improvement of AD risk prediction related to plasma Aβ concentrations is illustrated in table 3. Addition of plasma Aβ1-42 concentrations to a model including age, gender, education and APOEε4 status significantly improved AD risk prediction, but the magnitude of this improvement was small to moderate, with a continuous IDI of 0.0105 (95% CI=[0.0036–0.0184]), and a continuous NRI of 0.2592 (95% CI=[0.1152–0.3980]). Updated meta-analyses of reported associations of plasma Aβ1-42 levels, plasma Aβ1-40 levels and plasma Aβ1-42/Aβ1-40 ratio and incident AD and dementia are shown in figure 2. Despite high heterogeneity among the cohorts, combining our data with published results from Lui et al and Shah et al, and unpublished results from Shah et al, Hansson et al, and Lambert et al, confirmed the associations between plasma Aβ1-42/Aβ1-40 ratio and incident AD and dementia, with random-effect pooled HRs of 1.49 (95% CI=[1.17–1.91]; p=1.39 × 10−03; I2=36.71%) and 1.53 (95% CI=[1.09–2.15]; p=1.38 × 10−02; I2=70.74), respectively. The association of plasma Aβ1-42 levels and incident AD remained non-significant (pooled HR=1.28; 95% CI=[0.82–1.99]; p=2.79 × 10−01; I2=80.86%), and became significant with incident dementia (pooled HR=1.42; 95% CI=[1.03–1.96]; p=3.35 × 10−02; I2=68.42%), when compared with the previous meta-analysis. The associations of plasma Aβ1-40 levels and incident AD and incident dementia were non-significant (pooled HR=1.31; 95% CI=[0.88–1.93]; p=1.79 × 10−01; I2=74.13% and pooled HR=1.19; 95% CI=[0.78–1.81]; p=4.27 × 10−01; I2=82.33%, respectively).
Table 3.
Alzheimer’s disease risk prediction improvement of plasma Aβ1-42 and Aβ1-40 levels
| Aβ1-40 – Continuous (SD) | Aβ1-40 – Q4 vs Q123 | Aβ1-42 – Continuous (SD) | Aβ1-42 – Q4 vs Q123 | |
|---|---|---|---|---|
| Continuous | ||||
| IDI [95% CI] | 0.0015 [−0.0011–0.0043] | 0.0062 [−0.0005–0.0130] | 0.0105 [0.0036–0.0184] | 0.0046 [−0.0032–0.0127] |
| NRI [95% CI] | 0.1632 [0.0154–0.2976] | 0.0338 [−0.0929–0.1594] | 0.2592 [0.1152–0.3980] | 0.1298 [0.0198–0.2437] |
Abbreviations: SD, Standard deviation; Q4, 4th quartile; Q123, combined 1st, 2nd and 3rd quartiles, IDI, Integrated discrimination improvement; NRI, Net reclassification improvement; 95% CI, 95% Confidence interval
NOTE. Improvement was evaluated when adding the measure of interest in a Cox regression model of risk of incident Alzheimer’s disease adjusted for age, gender, education and APOEε4
Figure 2.
Meta-analysis of plasma Aβ1-42 levels, plasma Aβ1-40 levels and Aβ1-42/Aβ1-40 ratio and risk of incident Alzheimer’s disease and dementia (updated from Koyama et al, 2012)
4 Discussion
This study represents one of the largest prospective studies of plasma Aβ levels and risk of incident AD and dementia to date. We found a significant association between low plasma Aβ1-42 levels and Aβ1-42/Aβ1-40 ratio and higher risk of incident AD and dementia. Combining our results with the existing literature confirmed the associations of plasma Aβ1-42/Aβ1-40 ratio with incident AD and dementia, despite high heterogeneity.
Low plasma Aβ1-42 levels were associated with higher risk of incident AD and dementia in the Framingham cohorts. Conversely, we found no significant association using plasma Aβ1-40 levels. Association of plasma Aβ levels with risk of AD and dementia has been debated, due to contradictory results ranging from protective to deleterious, including absence of associations [12–16]. Our results add a new piece of evidence in favor of this association, allowing better estimation of the direction of this association. Aβ42 peptides are suspected to be more prone to aggregate and to exert deleterious effects on neurons, than Aβ40 peptides [33]. The significant association we found between plasma Aβ1-42/Aβ1-40 ratio and incident AD and dementia, although weaker than with plasma Aβ1-42, supports the hypothesis that AD results from an imbalance between Aβ1-42 and Aβ1-40 peptides. The direction of the observed associations is compatible with the hypothesis that late-onset AD results from impaired Aβ clearance from the brain, as opposed to autosomal dominant or Down syndrome forms of AD, in which a relative overproduction of Aβ1-42 peptides seems to be involved [34]. Furthermore, the observed associations were noted over a long period of follow-up and consistent with the theory that alterations in Aβ peptides are an early event in the pathophysiology of AD [2]. Part of the Aβ pool in plasma could come from platelets [35], and whether plasma Aβ can reflect the aggregation of Aβ in the brain is still debated. In favor of the latter are recent reports of associations of low plasma Aβ1-42/Aβ1-40 ratio with increased amyloid brain uptake, as measured by Pittsburgh compound B (PiB) PET scan [36–38]. Brain PiB PET was not available in FHS at the time of this study and we could not adjust for platelet counts as they had been measured at different exams. Moreover, given that the vast majority of dementia cases were Alzheimer’s disease in FHS, it is difficult to assess the specificity of plasma Aβ levels to AD. In the meta-analysis, effect sizes tended to be higher with dementia than AD, but as the set of studies used differed and the confidence intervals greatly overlapped, further studies are needed to answer this question, especially since Aβ levels are much more likely to reflect the mechanistic pathway for AD than other dementias.
Updated meta-analysis confirmed the associations of plasma Aβ1-42/Aβ1-40 ratio with incident AD and dementia, and association of plasma Aβ1-42 levels with incident dementia became significant. Despite these improved results, high heterogeneity was important in all meta-analyses. Differences in blood sampling (fasting versus non fasting, time of the day, tube used for collecting blood) and storage protocols may account for a substantial portion of the observed heterogeneity [16]. Moreover, assays may differ in their capacity to capture Aβ peptides, and most of them can only detect free, circulating monomers, which represent less than 50% of all circulating Aβ peptides [39]. Finally, sample size, follow-up time and analysis strategies may also generate differences in association estimates. Furthermore, other studies of interest could not be included in this meta-analysis as they did not report risk of AD or dementia according to quantiles of plasma Aβ levels [40–42], In 274 non-demented participants from the Cardiovascular Health Study, Lopez et al reported high plasma Aβ levels and ratio at baseline in those with incident dementia after 4.5 years of follow-up, but those results became not significant after multivariable adjustment [41]. In 585 participants from the Vienna Transdanube Aging study, Blasko et al reported higher levels of plasma Aβ42 in cognitively normal participants who converted to MCI or AD after 2.5 years of follow-up compared to participants who remained cognitively stable [40]. In the Alzheimer’s Disease Neuroimaging Initiative, no significant association between plasma Aβ levels or ratio and convertion to MCI/AD was reported [42], Therefore, there is a need for standardized protocols for blood sampling and storage, improved assays and collaborative efforts of analysis, re-analysis and meta-analysis to tackle the heterogeneity problems that have hampered the study of plasma Aβ peptides so far.
Study of net reclassification improvement and integrated discrimination improvement showed a modest improvement in prediction of AD risk over the integrative predictive value of age, gender, education, and APOEε4. These results suggest that plasma Aβ concentrations could be used as a biomarker for risk prediction of AD and dementia, more likely as part of a panel of relevant biomarkers. A study has recently observed that using plasma Aβ concentrations in combination with other plasma biomarkers improved prediction of neocortical Aβ burden over age, APOEε genotype and Clinical Dementia Rate Scale sum of boxes [43].
Our study has several strengths: with more than 2000 participants, it is one of the largest studies of plasma Aβ levels to date. The Framingham Heart Study is a prospective cohort, with a long period of follow-up, and, as a single center study, had homogeneous and standardized procedures for dementia assessment. We used a commercially available assay of plasma Aβ levels, which, we hope, will facilitate reproducibility of our results and meta-analysis. Finally, we tried to account for possible confounding factors in our statistical analysis.
The main limitations include the differences in blood sampling conditions between the Original and the Offspring cohorts, which could confound the real association between plasma Aβ levels and risk of AD and dementia, the largely European sample, which limits generalizability to other ethnicities, and the lack of repeated measures of plasma Aβ concentrations. Aβ aggregation is a slow and dynamic process and repeated measures may better estimate the risk of incident AD as well as the best timing for preventive intervention [44].
Overall, these results suggest that lower plasma Aβ42 and Aβ40 levels precede and are associated with risk of incident AD and dementia. They encourage further evaluation of plasma amyloid β levels as a potential biomarker for preclinical AD and risk of developing clinical AD and dementia.
Supplementary Material
Research in context.
1. Systematic review
A low-cost, accessible, and efficient biomarker for incipient Alzheimer’s disease is needed. We found that low plasma Aβ1-42 levels are associated with high risk of incident Alzheimer’s disease and dementia in 2189 participants over age 60 that were followed prospectively for up to 10 years in the Framingham Heart Study. We also meta-analyzed our results with data from prior publications.
Interpretation
This work confirms the suspected associations between plasma Aβ1-42 levels and risk of incident AD and dementia. Plasma Aβ levels could represent a useful biomarker for preclinical Alzheimer’s disease and dementia.
3. Future directions
Further studies are needed to confirm these associations, and to evaluate the added value of plasma Aβ levels in terms of risk prediction, whether alone, or as part of a biomarker panel. This will require further standardization of procedures for samples collection and conservation, plasma Aβ assays, study design and analyses.
Acknowledgments
This work was supported by the dedication of the Framingham Heart Study participants.
Funding/Support
Framingham Heart Study
This work received support from the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract no. N01-HC-25195) and grants from the National Institute of Neurological Disorders and Stroke (NS17950) and the National Institute of Aging (AG08122, AG16495 and AG033193).
Honolulu Asia Aging Study
This work was supported by the National Institutes of Health (National Institute on Aging contract NO1-AG-4-2149, Cooperative Agreements 5U01AG017155-09 and 5U01AG019349-08, and the Intramural Research Program of the National Institutes of Health and with resources at the Veterans Affairs Pacific Islands Health Care System). The information contained in this article does not necessarily reflect the position or the policy of the US government, and no official endorsement should be inferred.
Three City Study
The Three-City Study was performed as part of a collaboration between the Institut National de la Santé et de la Recherche Médicale (INSERM), the Victor Segalen-Bordeaux II University, and Sanofi- Synthelabo. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C Study was also funded by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, MGEN, Institut de la Longevité, Agence Francaise de Securité Sanitaire des Produits de Santé, the Aquitaine and Bourgogne Regional Councils, Fondation de France, and the joint French Ministry of Research/INSERM “Cohortes et collections de données biologiques” program. Lille Génopole received an unconditional grant from Eisai. This work was additionally funded by the CNRS, the Nord Pas-de-Calais Regional Council, the European Regional Development Fund, and grants from INSERM-DHOS-INCA (Project A08037ECS) and the European Community’s cNEUPRO programme (contract LSHM-CT-2007-037950).
Prospective Population Study of Women and Gerontological (PPSW) and Geriatric Population (H70) Studies
This work was supported by the Swedish Research Council; Swedish Brain Power; ALF funding for medical training and research; the Torsten and Ragnar Söderberg Foundation; Hans-Gabriel & Alice Trolle-Wachtmeisters foundation for medical research; State University of New York Research Foundation.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke; the National Heart, Lung and Blood Institute; the National Institute of Aging; or the National Institutes of Health.
Conflict of Interest Disclosures
None reported.
Role of the Sponsor
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization and Alzheimer’s Disease International. Dementia: A Public Health Priority. 2012. [Google Scholar]
- 2.Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry MS, Passmore AP, Todd S, McGuinness B, Craig D, Johnston JA. The development of effective biomarkers for Alzheimer’s disease: a review. Int J Geriatr Psychiatry. 2013;28:331–340. doi: 10.1002/gps.3829. [DOI] [PubMed] [Google Scholar]
- 4.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science (80-) 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 10.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund L-O, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 11.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lui JK, Laws SM, Li Q-X, Villemagne VL, Ames D, Brown B, et al. Plasma amyloid-beta as a biomarker in Alzheimer’s disease: the AIBL study of aging. J Alzheimers Dis. 2010;20:1233–1242. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- 13.Hansson O, Stomrud E, Vanmechelen E, Östling S, Gustafson DR, Zetterberg H, et al. Evaluation of plasma Aβ as predictor of Alzheimer’s disease in older individuals without dementia: a population-based study. J Alzheimers Dis. 2012;28:231–238. doi: 10.3233/JAD-2011-111418. [DOI] [PubMed] [Google Scholar]
- 14.Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma Amyloid-β as a Predictor of Dementia and Cognitive Decline: A Systematic Review and Meta-analysis. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah NS, Vidal J-S, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements - a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res Ther. 2013;5:8. doi: 10.1186/alzrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DAWBER TR, MEADORS GF, MOORE F. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farmer ME, White LR, Kittner SJ, Kaplan E, Moes E, McNamara P, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60:1023–1040. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 19.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 20.Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, et al. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp Aging Res. 2004;30:333–358. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 21.Blennow K, De Meyer G, Hansson O, Minthon L, Wallin A, Zetterberg H, et al. Evolution of Abeta42 and Abeta40 levels and Abeta42/Abeta40 ratio in plasma during progression of Alzheimer’s disease: a multicenter assessment. J Nutr Health Aging. 2009;13:205–208. doi: 10.1007/s12603-009-0059-0. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Au R, Seshadri S, Knox K, Beiser A, Himali JJ, Cabral HJ, et al. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9:673–686. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 25.American Psychiatric Association and American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 1994. [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert J-C, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, et al. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009;73:847–853. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 32.Viechtbauer W. Conducting Meta-Analyses in R with the metafor. Package Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 33.Jarrett JT, Berger EP, Lansbury P. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 34.Lambert J-C, Amouyel P. Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21:295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Inestrosa NC, Ross GS, Fernandez HL. Platelets are the primary source of amyloid beta-peptide in human blood. Biochem Biophys Res Commun. 1995;213:96–103. doi: 10.1006/bbrc.1995.2103. [DOI] [PubMed] [Google Scholar]
- 36.Devanand DP, Schupf N, Stern Y, Parsey R, Pelton GH, Mehta P, et al. Plasma Aβ and PET PiB binding are inversely related in mild cognitive impairment. Neurology. 2011;77:125–131. doi: 10.1212/WNL.0b013e318224afb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rembach A, Watt AD, Wilson WJ, Villemagne VL, Burnham SC, Ellis KA, et al. Plasma Amyloid-β Levels are Significantly Associated with a Transition Toward Alzheimer’s Disease as Measured by Cognitive Decline and Change in Neocortical Amyloid Burden. J Alzheimers Dis. 2013 doi: 10.3233/JAD-131802. [DOI] [PubMed] [Google Scholar]
- 38.Swaminathan S, Risacher SL, Yoder KK, West JD, Shen L, Kim S, et al. Association of plasma and cortical amyloid beta is modulated by APOE ε4 status. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesini P, Pérez-Grijalba V, Monleón I, Boada M, Tárraga L, Martínez-Lage P, et al. Reliable Measurements of the β-Amyloid Pool in Blood Could Help in the Early Diagnosis of AD. Int J Alzheimers Dis. 2012;2012:604141. doi: 10.1155/2012/604141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasko I, Jellinger K, Kemmler G, Krampla W, Jungwirth S, Wichart I, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging. 2008;29:1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toledo JB, Vanderstichele H, Figurski M, Aisen PS, Petersen RC, Weiner MW, et al. Factors affecting Aβ plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol. 2011;122:401–413. doi: 10.1007/s00401-011-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnham SC, Faux NG, Wilson W, Laws SM, Ames D, Bedo J, et al. A blood-based predictor for neocortical Aβ burden in Alzheimer’s disease: results from the AIBL study. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.40. [DOI] [PubMed] [Google Scholar]
- 44.Rissman RA, Trojanowski JQ, Shaw LM, Aisen PS. Longitudinal plasma amyloid beta as a biomarker of Alzheimer’s disease. J Neural Transm. 2012;119:843–850. doi: 10.1007/s00702-012-0772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.