Abstract
Persistent microorganisms in endodontically treated teeth produce volatile sulfur compounds (VSC) such as methyl mercaptan, hydrogen sulfide, and thioether. In this retrospective study, we evaluated the ex vivo immune response of peripheral blood mononuclear cells to sulfur compounds in 354 patients with systemic diseases. These systemic findings are correlated with semiquantitative values of a VSC indicator applied directly on endodontically treated teeth. Data elucidate the role of VSC in patients with immunologic diseases and the role of a semiquantitative chairside test, like the VSC indicator presented here, in correlation to IFNg and IL-10 sensitization in peripheral blood mononuclear cells. The association between ex vivo-stimulated cytokines and endodontically derived sulfur components is supported by the fact that the number of interferon gamma- and/or interleukin-10-positive sensitized patients declined significantly 3–8 months after extraction of the corresponding teeth.
Keywords: mercaptan, hydrogen sulfide, thioether, interferon gamma, interleukin 10
Background and problem
Endodontic failures in dentistry mostly result from bacterial infections with gram-negative anaerobic bacilli. In the field of modern dental endodontics, there is awareness of the problem of bacterial colonization in the tubules of root-filled teeth (RFT), and new methods to minimize these risks are constantly being developed.1 Even though X-rays of root canal treatment show no anomalies, these areas often contain bacteria and inflamed or necrotic tissue, proving that not all periradicular inflammations can be diagnosed via X-rays.2 The anaerobes most frequently isolated from primarily and secondarily infected root canals are sulfate-reducing bacteria, similar to those found in periodontal disease, including Porphyromonas gingivalis and Fusobacterium nucleatum. These bacteria are the main producers of methyl mercaptan, dimethylsulfide, and diethylsulfide (Merc/Thio).3 In the past, there was no process available (based on objective criteria) to reliably identify RFT by using the suspected outgas of Merc/Thio that were produced from bacterial degradation products and biogenic amines in the form of volatile sulfur compounds (VSC). The first aim of this study is to show a semiquantitative chairside test available to the dentist for this purpose. Further details of the development of the volatile sulfur compound indicator (VSCI) chairside kit are available on the website.4 This test can help a dentist make a decision as to whether RFT can be viewed as critical for a patient with immunological diseases due to high Merc/Thio content,5 even in the absence of signs of change on the X-rays of the root tip. The second aim of this study is to investigate the ex vivo immune response to sulfur compounds. In addition to the issues associated with the local quantities of Merc/Thio, this research also attempts to clarify their role in the immune system with data obtained by objective means.
Materials and methods
An indicator to objectify VSC on endodontic teeth
Pathogenic anaerobic bacteria in RFT produce toxic sulfurous compounds such as thiols, VSC, and mercaptan. There is ample literature pertaining to the fact that methyl mercaptan and hydrogen sulfur compounds could be a contributing factor in the immunological cascade of events leading to local tissue degradation. In this paper, we demonstrate that with a VSCI, a decision can be reached within minutes as to whether this tooth is releasing toxins that are possibly over-stimulating systemic immunological cascades. Instead of performing a microbiological analysis and evaluations of bacterial marker germs, the indicator verifies the presence of relevant bacterial metabolic products,5 especially since bacteriological examinations do not enable any conclusions to be made regarding clinically important bacterial metabolism. This is particularly important if the periodontal region is not yet showing clear signs of clinical changes.
The process in the mouth is simple: a sulcus swab is taken using a nonsterile paper tip or a small sponge. The sample from the sulcus fluid is added to a reagent mixture that triggers a change in color to yellow in the presence of sulfurous compounds (Figure 1). The VSCI detects the elevated discharge of bacterial toxins in the sulcus of suspect teeth based on five gradings (0= zero; 1= moderate; 2= evident; 3= clear; 4= strong; and 5= extremely strong), and it verifies the bacterial degradation products by a change in swab color (the color changes to yellow). The more intense the color change, the higher the concentration of sulfhydryls. Both H2S and other sulfhydryl compounds such as methyl mercaptan (CH3SH), dimethyl sulfide (CH3SCH3), and dimethyl disulfide (CH3SSCH3) are verified. These volatile sulfhydryl compounds are produced by anaerobic bacteria and fungi in the oral cavity.6
Figure 1.
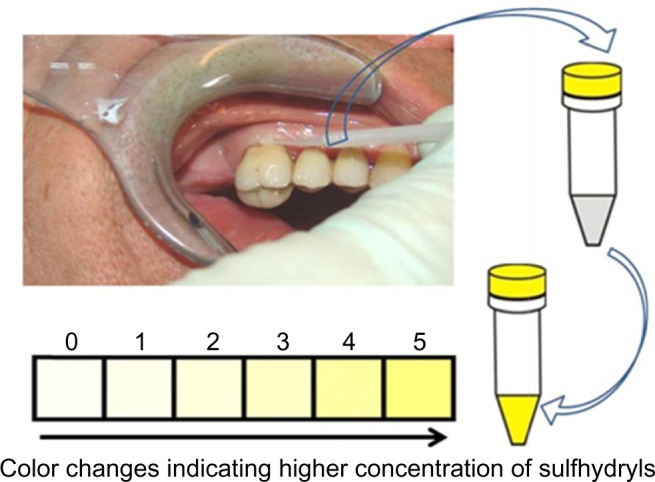
Procedure for semiquantitative analysis for presence of VSC.
Notes: Sulcus swab with a small sponge from the patient immersed in reagent mixture causes a change in color to yellow in the presence of sulfurous compounds produced as bacterial degradation products. The changed color can be classified based on six grading levels (0–5) for semiquantitative determination of VSCI.
Abbreviations: VSC, volatile sulfur compounds; VSCI, volatile sulfur compound indicator.
Systemic aspects of the VSCI test
In general medicine and in the prevention of disease, infections in the tooth and jaw are associated with a number of systemic diseases such as apoplexy, arthritis, myocardial infarction, hypertension, arteriosclerosis, infections of prosthetic limbs, the hematogenous spread of bacteria, cerebral abscesses, and Alzheimer’s disease.7–9 In light of these correlations, doctors and dentists are able to prescribe preventive dental treatment for patients, and they can also advise patients on these oral problems, even before there are clear symptoms of periodontal or X-ray changes, and before the patient’s health might be irreversibly damaged. A normal X-ray and the absence of visible apical inflammations on their own are not proof of the harmlessness of RFT.2,10–12 Our research focuses on gram-negative anaerobic bacteria. These form three types of toxins:
Exotoxins: these are released extracellularly as bacteria grow and migrate from a focus of inflammation to remote parts of the body, causing cell death.
Endotoxins: these are localized on the external membrane of the bacteria and are released as cells die. Through the activation of immune cells, especially macrophages, they induce local inflammation responses (interleukin [IL]-1, tumor necrosis factor [TNF]-a, and also interferon gamma [IFNg]) by nonspecific T-cell stimulation. These reactions contribute to periodontal inflammation, which all dentists are familiar with in terms of both diagnosis and treatment.
Nonprotein toxins: These are byproducts that result from bacterial metabolism, and they are also referred to as mercaptan in the form of hydrogen sulfide (H2S) – as in the example of methyl mercaptan (CH3SH) – and as thioether in the case of their organic sulfide compounds.
This article focuses on the immunological effects of the nonprotein toxins, mercaptan and thioether (Merc/Thio), and it examines their reactions in closer detail.
Cytokine analyses – specific effector cell typing to Merc/Thio
This study has adopted a laboratory-based effector cell typing process to detect Merc/Thio,13 so as to identify a possible correlation between VSC from RFT and the triggering of a chronic inflammation response. In the case of specific effector cell typing for Merc/Thio not caused by pathogens, isolated mononuclear cells (lymphocytes and monocytes) of patients were stimulated with a suitable dose of VSC, and after 24 hours, the cytokines IFNg and IL-10 were measured in the cell culture supernatant. IFNg and IL-10 were selected because of their indicator function for T-cell subsets. IFNg is a mainly specific cytokine of TH1 effector T-cells. IL-10 is expressed by regulatory T-cells and TH2 cells, the most important counter-regulating T-lymphocytes. Positive responses, as compared to a nonstimulated control specimen, are indicative of the existence of sensitization. In this case, inflammatory responses are to be expected in the body upon contact with VSC, especially since a type IV immune response to the modified proteins can be regarded as the cause of sensitization to Merc/Thio, and consequently as one cause of chronic inflammation. Serum samples were obtained by centrifugation of coagulated whole blood and stored at −80°C for further analysis. Cytokine concentrations were determined by commercially available enzyme-linked immunosorbent assays and IP-10 (EMD Millipore, Billerica, MA, USA). Analyses were performed according to the manufacturer’s instructions.
In 354 patients, peripheral blood mononuclear cells (PBMC) were isolated from 10 mL of venous heparinized whole blood by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. PBMC were resuspended in RPMI 1640 (Biochrom KH, Berlin, Germany), supplemented with 2 mM of L-glutamine (Sigma-Aldrich Co, St Louis, MO, USA) and 10% heat-inactivated autologous serum. PBMC at the concentration of 1×106/mL were seeded in flat-bottom 24-well microtiter plates (Nunclon, Wiesbaden, Germany) and stimulated with 500 ng/mL each of methanethiol (CH3SH, methyl mercaptan; Sigma-Aldrich Co., St Louis, MO, USA) and dimethyl sulfide (CH3SCH3, dimethylthioether; Sigma-Aldrich Co.). PBMC cultured in the absence of sulfur compounds served as control. Cells were cultured in a humidified incubator with 5% CO2 at 37°C for 24 hours. After collection of cell supernatants by centrifugation, secreted IFNg and IL-10 were determined using Human Cytokine Panel I (MPXHCYTO-60K; EMD Millipore), according to the manufacturer’s instructions. The Luminex® 200™ with xPonent® Software (Luminex, Austin, TX, USA) was used for detection. Finally, IFNg and IL-10 values were obtained by subtracting unstimulated control values from the sulfur compounds’ stimulated values. In addition, in preliminary investigation, trypan blue staining was performed to confirm that applied doses of sulfur compounds avoided cytotoxic effects during the 24 hours of stimulation.
Study cohort
A total of 354 patients were tested for sensitization as a result of exposure to Merc/Thio in our clinic with the following demographic data: average age, 58.3 years; range, 42–79 years; and sex (female/male), 196/58. The inclusion criteria for each patient included the presence of one or more RFT with semiquantitative VSCI measurement on each existing RFT. All test persons in the group had to be suffering from systemic diseases with immunological background, ie, tumors of the female organs or abdomen; fibromyalgia; rheumatoid arthritis; allergies; chronic fatigue syndrome; multiple sclerosis/Parkinson’s/neurodegenerative diseases; trigeminal neuralgia; thyroid/autoimmune diseases; vegetative complaints; cardiovascular disease; susceptibility to infections/weak immunity; or prostate tumors. Exclusion criteria included concomitant use of immunosuppressive treatments for these systemic diseases. Apical infections visible on X-ray, as well as inflammatory parodontopathies on the RFT, also served as exclusion criteria, as gingival bleeding precludes the acquisition of reliable VSCI measurements.
Statistics
Merc/Thio stimulation test results and cytokine levels were analyzed via Mann–Whitney U-test. A comparison of the positive or negative scores of the ex vivo stimulation test was performed using the chi-square test. A positive sensitization to Merc/Thio exposure corresponds to IFNg >0.3 IU/mL or IL-10 >10 IU/mL (upon the subtraction of basal values). P-values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS, version 19 (IBM Corporation, Armonk, NY, USA).
Results
Comparison of positive or negative Merc/Thio sensitization in the study cohort
Effector cell typing in the laboratory is used to detect derailed immune reactions caused by VSC in the patient’s serum. The degree of immunological sensitization is defined by the level of IFNg and IL-10 in terms of IU/mL. In 2011 and 2012, we had obtained serum from a total of 354 patients for Merc/Thio effector cell typing. When defining a positive result of the ex vivo stimulation assay as IFNg >0.3 IU/mL and/or IL-10 >10 IU/mL, 182 of the 354 patients examined (51%) scored positive, while 172 scored negative, as a result of exposure to Merc/Thio. Of the 354 individuals examined, 182 showed sensitization to IFNg and/or IL-10 as a result of exposure to Merc/Thio and 172 individuals showed no sensitization to IFNg and/or IL-10. Figure 2 shows a graphical representation of the distribution: one in two patients with RFT showed IFNg- and/or IL-10-based sensitization to Merc/Thio. The distribution of the IFNg/IL-10 constellations within the group of patients exhibiting sensitization indicates that 61 of the 182 patients are only sensitized to IFNg, and that 100 of the 182 patients are sensitized only to IL-10; 21 patients reacted to both concurrently.
Figure 2.
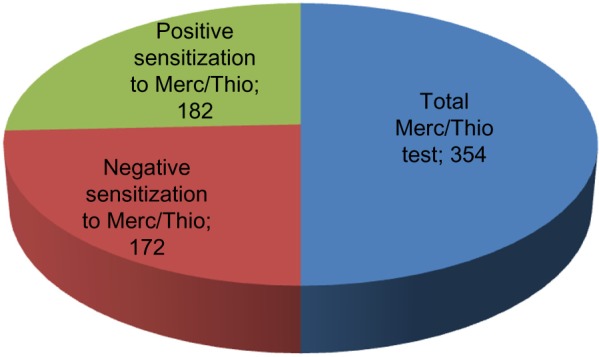
Distribution of study group based on patients’ sensitization to Merc/Thio exposure.
Notes: Positive sensitization is sensitization to IFNg and/or IL-10, and negative sensitization is no sensitization to IFNg and/or IL-10 as a result of exposure to Merc/Thio. IFNg and IL-10 levels estimated in ex vivo PBMC cultures and serum samples from patients.
Abbreviations: Merc/Thio, mercaptan and thioether; PBMC, peripheral blood mononuclear cells; IFNg, interferon gamma.
Local toxin measurements with a VSCI
In order to measure and compare the total toxin levels among individuals in the sensitized and nonsensitized groups, the local toxin values obtained from the VSCI were totaled for each of the 354 patients. Thus, if a sensitized patient had four RFT with VSCI values of 0, 3, 2, and 3, respectively, his load of 8 was added to the total VSCI values obtained from the 182 sensitized patients. The individual VSCI values from the group of nonsensitized patients (n=172) were evaluated in the same way. VSCI values represent a grading system, and are thus not defined by units (see Figure 3).
Figure 3.
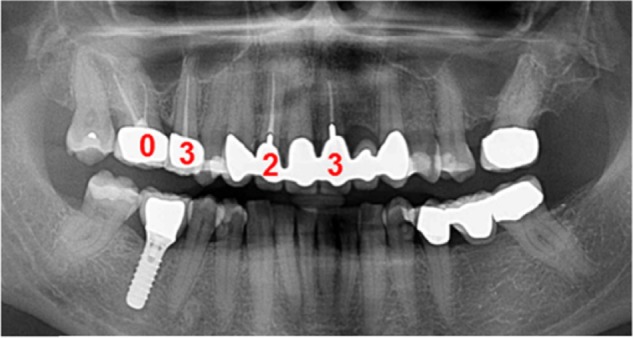
Calculation of total VSCI value in a representative patient with four RFTs.
Notes: VSCI values obtained after semiquantitative analysis were 0, 3, 2, and 3, respectively, in four RFTs. The total VSCI value equals 8 corresponding to total toxin levels in this patient.
Abbreviations: RFTs, root-filled teeth; VSCI, volatile sulfur compound indicator.
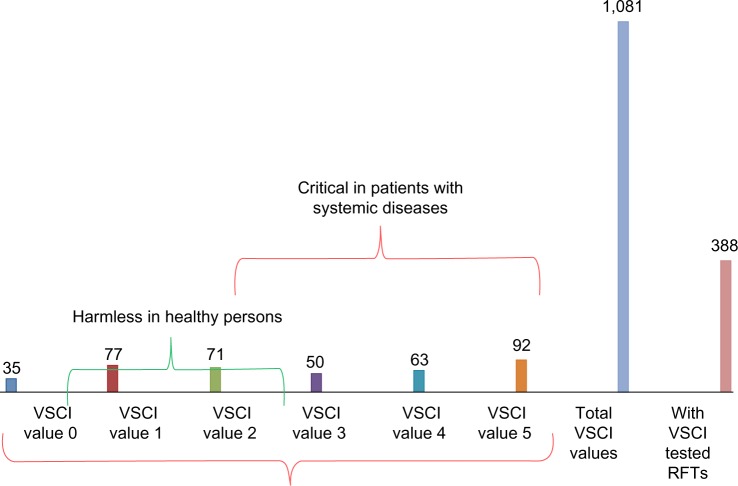
From the 354 examined patients, a total of 388 RFT were semiquantitatively measured for VSC with the VSCI test, and these values were summated in the manner explained earlier. The results were divided into six levels from 0 to 5 according to the VSCI grading scale in Figure 1: measurements of 0, 1, and 2 are assessed as not requiring treatment and are not relevant to healthy persons. In the case of systemic diseases, however, local toxin measurements at levels 2–5 should be examined in connection with systemic sensitization as a result of exposure to Merc/Thio. The assessment of the intensity distribution of the VSCI values across the 388 tested RFT represented in Table 1 reveals that 277 of these teeth showed local VSC values at levels from 2–5, amounting to ~78%.
Table 1.
Distribution of total VSCI values according to IFNg/IL-10 constellations in the positive-sensitization group
| Group | Description of group | Number of patients | VSCI values | Validation quotient |
|---|---|---|---|---|
| 1 | IFNg positive/IL-10 negative | 61 | 389 | 6.38 |
| 2 | IL-10 positive/IFNg negative | 100 | 542 | 5.42 |
| 3 | IFNg positive/IL-10 positive | 21 | 119 | 5.67 |
Notes: The total VSCI values before extraction of RFT are reported. Group 1 represents patients with unbalanced TH1-dominant sensitization; group 2 corresponds to patients with latent (balanced) sensitization; and group 3 includes patients who have partially balanced TH1-dominant sensitization. A positive sensitization consists of patients sensitized by exposure to Merc/Thio and the degree of immunological sensitization is defined by the level of IFNg and IL-10, where IFNg is >0.3 IU/mL and/or IL-10 is >10 IU/mL.
Abbreviations: Merc/Thio, mercaptan and thioether; RFT, root-filled teeth; VSCI, volatile sulfur compound indicator; IFNg, interferon gamma.
Comparison of VSCI values and immunological Merc/Thio sensitization
For the group of patients with positive sensitization to Merc/Thio (n=182), there was a total VSCI value of 1,050. The mean value for IFNg was 1.26 IU/mL (standard deviation [SD] ±2.23), and for IL-10, the mean value was 44.08 IU/mL (SD ±51.53) with a P-value <0.0386 for the group with negative non-sensitization; this is considered as statistically significant. For the group of 172 patients who were not sensitized by exposure to Merc/Thio, there was a total VSCI value of 35. Table 2 shows the comparison of the VSCI values for patients sensitized to IFNg/IL-10 with the VSCI total for nonsensitized patients. Overall, 182 sensitized patients with a total VSCI value of 1,050 showed a validation quotient of 5.77 (ratio of 1,050 VSCI value:182 Merc/Thio sensitized patients), while 172 nonsensitized patients with a total VSCI value of 35 showed a corresponding validation quotient of 0.2 (ratio of 35 VSCI value:172 non-Merc/Thio-sensitized patients).
Table 2.
Comparison of IFNg/IL-10 sensitization levels and total VSCI values in the study cohort (n=354)
| Reaction to Merc/Thio Sensitization assay
|
Comparison of VSCI values
|
Immunological response
|
|||
|---|---|---|---|---|---|
| Group | Number of patients | Total VSCI values | Validation quotient | IFNg (IU/mL) | IL-10 (IU/mL) |
| Positive sensitization | 182 | 1,050 | 5.77 | 1.26±2.23 (mean ± SD) | 44.08±51.53 (mean ± SD) |
| Negative sensitization | 172 | 35 | 0.20 | All <0.3 | All <10 |
| P-value (t-test) | <0.0386 | ||||
Notes: Positive sensitization consists of patients sensitized by exposure to Merc/Thio. Negative sensitization consists of patients not sensitized by exposure to Merc/Thio.
Abbreviations: Merc/Thio, mercaptan and thioether; SD, standard deviation; VSCI, volatile sulfur compound indicator; IFNg, interferon gamma.
Distribution of VSCI values in three IFNg/IL-10 constellations in Merc/Thio sensitization assay
The test investigates the immunological sensitization to Merc/Thio. A positive result makes it likely that protein degradation products are present in the patient’s blood and that these contribute to local or systemic inflammation. In principle, there are four constellations of the test results:
Negative = no sensitization;
IFNg positive/IL-10 negative = unbalanced TH1-dominant sensitization;
IFNg positive/IL-10 positive = partially balanced TH1-dominant sensitization; and
IFNg negative/IL-10 positive = latent (balanced) sensitization.
The Interpretation of the four possible constellations to obtain the results of the Merc/Thio stimulation test can be:
Ignorance: IL-10 and IFNg negative – ie, the immune system does not detect the substances. No reliable exclusion of an overstimulated immune system, but inflammation associated with Merc/Thio is unlikely, for example: IFNg-stimulated, 0.2 IU/mL (<0.3 IU/mL normal level); IL-10-stimulated, 7.8 IU/mL (<10 IU/mL normal level). Interpretation: no indication of cellular sensitization to the tested protein degradation products. It is therefore unlikely that these substances are the cause of a local or systemic inflammation response related to the immune system.
Inflammation (TH1): IFNg positive, IL-10 weak or not traceable. Indication of overstimulation of the immune system and proof that antigen-specific TH1 cells, which may trigger inflammation with Merc/Thio, are present: IFNg-stimulated, 3.2 IU/mL (<0.3 IU/mL normal level); IL-10-stimulated, 7.8 IU/mL (<10 IU/mL normal level). Interpretation: the findings indicate a TH1-dominant cellular cytokine response to the protein degradation products, Merc/Thio. This finding may therefore be indicative of an associated local or systemic inflammation in response to these substances.
Overstimulation of the immune system, as well as sensitization and the absence of TH1 cells, contradict the current existence of an inflammatory process involving Merc/Thio. Nevertheless, the possibility of immune dysregulation is possible: IFNg-stimulated, <0.1 IU/mL (<0.3 IU/mL normal level); IL-10-stimulated, 230 IU/mL (<10 IU/mL normal level). Interpretation: although the clear triggering of the TH2 cytokine, IL-10, by the tested protein degradation products suggests the existence of cellular sensitization, the absence of an IFNg response (TH1) means that an associated current local or systemic inflammation response is rather unlikely. A control test after 2–3 weeks should be recommended.
The indication that overstimulation of the immune system and sensitization occurred together with the presence of TH1 cells suggests that the current presence of an inflammatory process is possible with only a partial counter-regulation by increased stimulation of IL-10: IFNg-stimulated. 2.8 IU/mL (<0.3 IU/mL normal level); IL-10-stimulated, 127.0 IU/mL (<10 IU/mL normal level). Interpretation: the cytokine synthesis induced by protein degradation products confirms the suspected presence of cellular sensitization. Even if the cytokine response is attributable to TH1 (IFNg) and TH2 (IL-10) cells, the significant level of IFNg may be the cause of a current local or systemic inflammatory response associated with these products (this represents only partially balanced sensitization).
Table 1 shows the breakdown of the total VSCI values for the three IFNg/IL-10 constellations: group 1 = unbalanced TH1-dominant sensitization; group 2 = latent (balanced) sensitization; group 3 = partially balanced TH1-dominant sensitization. The distribution of individuals sensitized to IFNg alone versus those sensitized to IL-10 alone showed a ratio of 61:100 in favor of IL-10, thus indicating a slight predominance of sensitization toward IL-10. When the local VSCI values for IFNg yielded a total VSCI value of 389 for 61 patients who had tested positive, the resulting quotient was 6.38; for IL-10, the local VSCI values produced a total VSCI value of 542 for 100 patients that were only sensitized to IL-10, with a quotient of 5.42. With respect to the 21 patients who tested positive for sensitivity to both IFNg and IL-10, the total VSCI value obtained was 119, with a quotient of 5.67.
Change in immunological sensitization to Merc/Thio after the extraction of RFT
As of March 2013, 14 of the 182 sensitized patients who were recruited from 2011/2012 had undergone extraction of all RFT, and they had achieved control of Merc/Thio sensitization in the VSC stimulation assay. The clinical pictures for these 14 patients were as follows: Hashimoto’s thyroiditis (n=3); hypothyroidism (n=1); rheumatoid arthritis (n=2); chronic fatigue syndrome (n=3); chronic sinusitis (n=2); cardiac arrhythmia (n=1); and trigeminal neuralgia (n=1). The postextraction group that exhibited persistent RFT also showed a mean IFNg sensitization of 1.45 IU/mL (SD ±1.83), and those with extracted RFT demonstrated a remaining mean IFNg sensitization of 0.38 (SD ±1.52); this was not statistically significant (P<0.509). Nevertheless, a comparison of the mean values of the total sensitization levels before and after the RFT extractions revealed a reduction in IFNg sensitization by a factor of 4 (1.45:0.38) (Figure 5).
Figure 5.
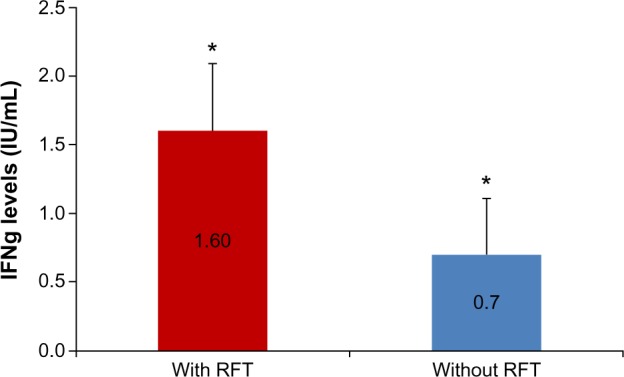
Mean IFNg levels before and after the extraction of RFT in patients with systemic diseases.
Notes: Comparison of mean IFNg levels before (with RFT) and after removal of RFT (without RFT). The study group is comprised of patients with systemic diseases (n=14) and error bars indicate standard error of mean. *2.3-fold change.
Abbreviations: RFT, root-filled teeth; IFNg, interferon gamma.
For IL-10, the postextraction group with persisting RFT exhibited a mean IL-10 sensitization of 63.71 IU/mL (SD ±65.3), and those with extracted RFT showed a remaining mean IL-10 sensitization level of 27.41 IU/mL (SD ±34.3); this was not statistically significant (P<0.133). Nevertheless, a comparison of the mean values obtained from the total of the sensitization indices before and after the extraction of RFT showed a reduction in IL-10 sensitization by a factor of 2.31 (63.71:27.41) (Figure 6).
Figure 6.
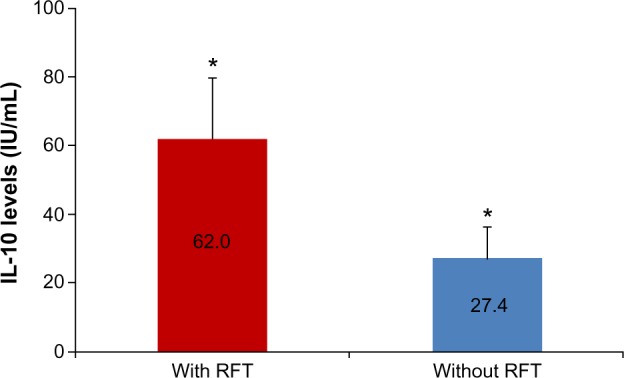
Mean IL-10 levels before and after the extraction of RFT in patients with systemic diseases.
Notes: Comparison of mean IL-10 levels before (with RFT) and after removal of RFT (without RFT). The study group is comprised of patients with systemic diseases (n=14) and error bars indicate standard error of mean. *2.3-fold change.
Abbreviation: RFT, root-filled teeth.
Discussion
The unforeseen complexity and breadth of the possible triggers of systemic diseases require new scientific structures and procedures. Systemic responses of the body manifest themselves as a set of interacting immunological symptoms with negative repercussions for the rest of the body. Other etiological factors are also involved in this systemic response – ie, genetic polymorphisms, epigenetic factors, functional modulations, environmental factors, and immunological engrams of the neuroendocrine immune system, which are triggered once and then work repeatedly in the same form, even without the presence of the primary trigger.14 It is likely that in an advanced stage of chronic exposure to the biogenic amines Merc/Thio, there will be a degree of disconnect in the normally homogenous homeostatic processes that occur between the superior regulatory systems, thus rendering the return to one’s normal physiological state difficult.15,16 We will discuss and interpret the acquired data from this perspective.
The aim of our study was to verify the correlation between the local oral toxin measurements on RFT with VSCI, and to determine the degree of sensitization noted in the immune system as a result of this exposure to Merc/Thio. Thus, we tried to determine the significance of the local discharges of Merc/Thio in RFT to assess the systemic impacts among patients with immunological diseases. Inflammatory cells – particularly lymphocytes – can be activated as a result of immunological sensitization, especially where there is an existing exposure to Merc/Thio; these cells can trigger both a local and a systemic immune response. To this extent, a positive result on the Merc/Thio ex vivo stimulation assay means that the protein degradation products that have been derived from RFT serve as an inflammatory trigger that is specific to a particular patient.
Merc/Thio sensitization assay correlated to local toxin measurement with VSCI
The results of the Merc/Thio sensitization tests show that more than 50% of the patients in our study group with RFT show inflammatory (via the overexpression of IFNg) or dampening (via the overexpression of IL-10) immune behavior. From this, a ratio of the VSCI intensity among sensitized and nonsensitized patients of 28.85 (5.77:0.2) was obtained. This means that the VSCI values measured locally are directly linked to the presence and intensity of systemic sensitization; in particular, higher VSCI values are associated with greater sensitization in the Merc/Thio assay. For patients with systemic diseases and locally positive VSCI values, the probability of suffering from immunological sensitization following exposure to dental Merc/Thio is therefore 25-fold higher. This factor has significant impacts on the diagnosis and treatment of all patients with symptoms that are based on immunological dysregulation.14,15 As such, our findings might be clinically relevant for treatment. The clinical diagnoses among our group of 182 patients with sensitization to Merc/Thio were severe systemic diseases. Given the correlation between Merc/Thio sensitization and the presence of certain conditions, it seems as though checking for potential immunological sensitization would be appropriate for all patients with dental RFT, as this can aid in the detection of systemic issues; this is also important because this group showed elevated local toxin values on existing RFT as well.
However, the data listed above (in “Distribution of VSCI values in three IFNg/IL-10 constellations in Merc/Thio sensitization assay”) showed that locally overexpressed VSCI values cannot be clearly assigned to one of the three possible IFNg/IL-10 constellations; the corresponding VSCI values yield roughly the same ratio. Therefore, it is not possible to make any statements about whether the local VSC of Merc/Thio has a stimulating impact on the immune system of patients with RFT via IFNg or a dampening impact via IL-10. As such, local VSCI measurements do not provide any information about the nature of the systemic sensitization resulting from exposure to the protein degradation products, Merc/Thio. The level of the toxins to be measured locally does not enable one to determine the specific degree of immunological interference.
In contrast, our data elucidate that there is a continuous reduction in the IFNg inflammatory response in the systems that are specific to each patient’s clinical picture following the removal of RFT. Overall, it was established that the removal of RFT had been successful. However, it should be noted that if the remaining IFNg inflammatory response in patients with these immunological diseases is only a quarter (25%) of the value recorded with persisting RFT, this is a measure of success. If, in these patients with these clinical pictures, the IL-10 response of the system is only 50% of the value with the RFT intact, success is also achieved. Therefore, it was established that the RFT treatment had been successful. However, in cases where patients with the clinical pictures described above yielded IL-10 values that were 50% of those recorded with the RFT intact, this is also a measure of success when compared to the administration of other immune-regulating drugs. In summary, it can thus be stated that both the reduction of IFNg sensitization by a factor of 4 and IL-10 sensitization by a factor of 2.3 following extraction of the RFT also provide an indicator of possible therapeutic interventions in patients with toxin-producing RFT and immunological systemic diseases.
A clinical case: extraction of RFT with high VSCI scores and monitoring the development of Merc/Thio sensitization
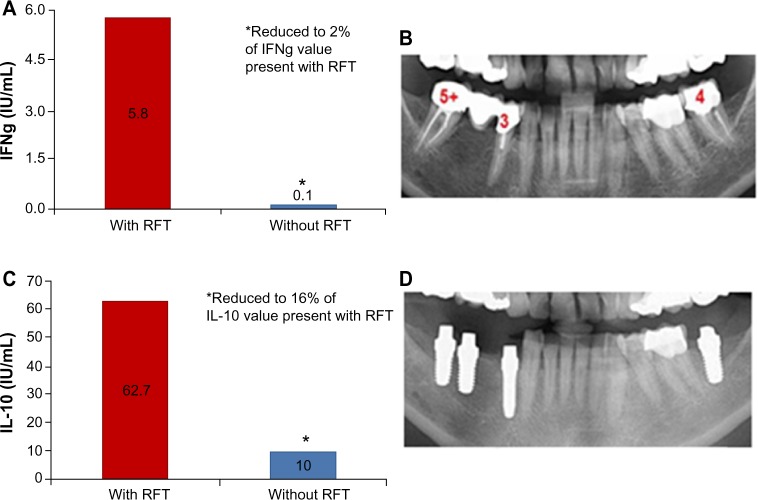
The following case documents the integrated procedure of measuring local toxins with VSCI and the relevance of these measurements to systemic sensitization; the risk of an immunological trigger was determined from elevated IL-10 and IFNg values, and the monitoring of treatment was also performed. The patient (Ms SH; 60 years of age) described her medical history as follows: Hashimoto’s thyroiditis for about 6 years; rheumatoid arthritis for about 3 years; pigment abnormalities all over her body; and various allergies (eg, to methyl methacrylate). Figure 7 shows the initial X-ray findings with the VSCI values of the three RFT, and the lower X-ray is taken following both the extraction of the RFT and their replacement with nontoxic ceramic implants.
Figure 7.
An example of the clinical sample tested for IFNg and IL-10 sensitization to Merc/Thio exposure before and after removal of RFT.
Notes: (A) and (C) IFNg and IL-10 levels in the clinical sample before removal of RFT (with RFT) and after removal of RFT (without RFT). (B) X-rays show initial findings with the VSCI values of three RFT and (D) the replacement of three RFT with ceramic implants in this patient.
Abbreviations: Merc/Thio, mercaptan and thioether; RFT, root-filled teeth; VSCI, volatile sulfur compound indicator; IFNg, interferon gamma.
The comparison of Merc/Thio sensitization of this patient before and after the replacement of three RFT with ceramic implants demonstrated a good success rate in relation to the desired treatment objective of stabilizing immunity. Following removal of the RFT, IFNg expression had been reduced down to 2% of the value obtained prior to the restoration, and IL-10 expression was reduced to 6% of the pre-extraction level. The values for both IFNg and IL-10 were below the reference values from the control test (Figure 7). As a result, conditions for the successful treatment of the immunological symptoms of Hashimoto’s thyroiditis, rheumatoid arthritis, skin efflorescence, and allergic reactions in this patient were established.
Limitations of the study
As the number of our cases is limited in the presented study, a subsequent multicenter prospective study is required to fully elucidate the role of VSC in patients with immunologic diseases, the role of a semiquantitative chairside test like the VSCI presented here, and the role of the laboratory effector cell typing on IFNg/IL-10 in disease progression. The data do not indicate that a patient with immunologic disease should not be provided with endodontic treatment.
Conclusion
Our data suggest that endodontically treated teeth/RFT could add an extra dimension to the development of systemic diseases and to the maintenance of good health. The probability of existing levels of Merc/Thio resulting in immunological sensitization – either via the promotion of inflammation by IFNg or via immunological dampening by IL-10 – is approximately 25-times higher in patients with RFT with a positive local toxin measurement (as per the VSCI) when compared to patients not exposed to these toxins. From an integrative and holistic perspective, the local VSCI measurement is therefore an additional meaningful parameter for the pathogenetic weighting of RFT. The VSCI thus enables dentists and doctors to preserve teeth without having to face the risk that toxic bacteria will spread throughout the body. Effector cell typing for IFNg/IL-10 seems to be a key laboratory parameter for assessing the systemic interactions of RFT. Assessing immunological triggers among individuals like VSCI-positive RFT patients already presenting with systemic diseases might be an additional therapeutic aspect in initiating the remission of a systemic disease. The challenge posed by these discoveries is the need to raise awareness of the possible induction of immunological diseases by RFT throughout the medical and dental community. As the number of our cases is limited in the presented study, a subsequent multicenter prospective study is required to fully elucidate the role of VSC in patients with immunologic diseases and the role of a semiquantitative chairside test like the VSCI presented here. The diagnostic value and the role of novel laboratory effector cell typing on IFNg/IL-10 in disease progression needs to be verified in further studies. However, our data do not indicate that a patient with immunologic disease should not be provided with endodontic treatment.
Figure 4.
Distribution of 388 RFTs according to VSCI grading scale.
Abbreviations: RFTs, root-filled teeth; VSCI, volatile sulfur compound indicator.
Acknowledgments
English-language editing of this manuscript was provided by Journal Prep.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Claesson R, Edlund MB, Persson S, Carlsson J. Production of volatile sulfur compounds by various Fusobacterium species. Oral Microbiol Immunol. 1990;5(3):137–142. doi: 10.1111/j.1399-302x.1990.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin lM, Skribner JE, Gaengler P. Factors associated with endodontic treatment failures. J Endod. 1992;18:625–627. doi: 10.1016/S0099-2399(06)81335-X. [DOI] [PubMed] [Google Scholar]
- 3.Jung IY, Choi BK, Kum KY, et al. Molecular epidemiology and association of putative pathogens in root canal infection. J Endod. 2000;26:599–604. doi: 10.1097/00004770-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 4. http://www.orotox.de [homepage on the Internet] Munich: OroTox; 2012. Available from: http://www.orotox.de. Accessed. [Google Scholar]
- 5.Tansy MF, Kendall FM, Fantasia J, Landin WE, Oberly SR, Sherman W. Acute and subchronic toxicity studies of rats exposed to vapors of methyl mercaptan and other reduced-sulfur compounds. J Toxicol Environ Health. 1981;8:71–88. doi: 10.1080/15287398109530051. [DOI] [PubMed] [Google Scholar]
- 6.Lechner J. Multi-dimensional system diagnosis of the root-filled tooth. ZWR-Das deutsche Zahnärzteblatt. 2012;121:640–644. German. [Google Scholar]
- 7.Nagaoka S, Miyazaki Y, Liu HJ, Iwamoto Y, Kitano M, Kawagoe M. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J Endod. 1995;21(2):70–73. doi: 10.1016/S0099-2399(06)81098-8. [DOI] [PubMed] [Google Scholar]
- 8.Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol 2000. 2011;55:87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen-sulfide toxicity. Crit Rev Toxicol. 1984;13(1):25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 10.Hannah RS, Roth SH. Chronic exposure to low concentrations of hydrogen sulfide produces abnormal growth in developing cerebellar Purkinje cells. Neurosci Lett. 1991;122:225–228. doi: 10.1016/0304-3940(91)90864-p. [DOI] [PubMed] [Google Scholar]
- 11.Blum JY, Michailesco P, Abadie MJ. An evaluation of the bactericidal effect of the Nd:YAP laser. J Endod. 1997;23:583–585. doi: 10.1016/S0099-2399(06)81127-1. [DOI] [PubMed] [Google Scholar]
- 12.Moritz A, Doertbudak O, Gutknecht N, Goharkhay K, Schoop U, Sperr W. Nd:YAG laser irradiation of infected root canals in combination with microbiological examinations. J Am Dent Assoc. 1997;128:1525–1530. doi: 10.14219/jada.archive.1997.0092. [DOI] [PubMed] [Google Scholar]
- 13.Institute for Medical Diagnostics [homepage on the Internet] Berlin: [Accessed February 20, 2015]. Available from: http://www.imd-berlin.de/labor/fachbereiche/immunologie.html. [Google Scholar]
- 14.Kirkpatrick CJ, Fuchs S, Peters K, Brochhausen C, Hermanns MI, Unger RE. Visions for regenerative medicine: interface between scientific fact and science fiction. Artif Organs. 2006;30:822–827. doi: 10.1111/j.1525-1594.2006.00306.x. [DOI] [PubMed] [Google Scholar]
- 15.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–1116. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Straub RH, Herfarth H, Falk W, Andus T, Schölmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J Neuroimmunol. 2002;126:116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]