Abstract
In evaluating disease changes it is critical to have measurements that are sensitive, specific and reliable. Cognitive decline, particularly in the context of Alzheimer’s disease (AD), is an area that has attracted a large number of recent studies, and as such the proposed biomarkers used in these investigations need to be validated. In this review we highlight studies with important implications about the role of imaging biomarkers in cognitive decline and dementia as well as in distinguishing preclinical dementia, prior to evidence of cognitive decline. Structural changes determined on magnetic resonance imaging (MRI), both cross-sectional and longitudinal provide early prediction of dementia, particularly when combined with other measures. Molecular imaging using PET and SPECT tracers quantify the presence or activity of receptors, transporters, enzymes, metabolic pathways and proteins. The newest developments in molecular imaging will be described and methods compared. Distinguishing features of imaging biomarkers among dementias and the spectrum of preclinical AD, MCI and AD will be described. Appropriate use criteria for amyloid PET will be delineated. While these efforts are still in the early phase of development, there is great promise for further development in structural MRI and PET technologies.
Clinical Use of Biomarkers in Cognitive Decline
There has been a steadily growing number of studies examining cognitive decline in the elderly. Many of these studies have had a small number of enrollees. It is becoming increasingly important to determine which studies and methods have achieved sufficient sensitivity and specificity that they can guide diagnostic or therapeutic decisions. The papers included in this review were based on Pubmed searches for the terms FDG and dementia, amyloid PET, florbetapir, florbetaben, flutemetamol, PiB, FPCIT, ioflupane, preclinical dementia and MRI, as well as through consultation with experts in the field. Studies with autopsy confirmation of imaging findings were given preference for inclusion.
While CSF studies have shown that decreased amyloid concentrations, increased tau or increased tau/amyloid concentrations are reliable biomarkers for detecting the AD pathophysiological process (1,2) we will focus on imaging biomarkers. We will highlight those studies with sufficient power to make meaningful conclusions concerning the role of imaging biomarkers in cognitive decline and dementia. In addition we will highlight studies and methods that distinguish preclinical dementia, prior to any evidence of cognitive decline but after pathological brain changes have occurred. In medicine, biomarkers refer to measurable characteristics that reflect the presence and severity of a disease process. Validation of a biomarker entails quantifying the measurement’s sensitivity, specificity, prior probability, positive predictive value and negative predictive value (3). In the case of AD, the Consensus Report of the Working Group on Molecular and Biochemical Markers of Alzheimer’s Disease recommended that a particular measurement should detect a fundamental aspect of neuropathology and be confirmed in postmortem cases (4). Further, the sensitivity should be >80% for detecting AD the specificity should be >80% for distinguishing other dementias. In the subsequent fifteen years since this report was issued, the major focus of molecular and structural imaging for dementia has been on Alzheimer-type dementia (AD), frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB). These three types of dementia differ in presentation, prognosis, etiology and response to therapeutics, although clinical overlap is not uncommon, and thus the need for biomarkers is apparent (5–9). Additionally, cognitive impairment is a relatively late feature of the pathophysiology of AD, which has important implications for developing therapies intended to slow or stop progression of neurodegeneration.
Traditionally, the clinical work up of dementia has focused on clinical assessment, neuropsychological testing, and exclusion of other etiologies. The National Institutes of Aging (NIA) and the Alzheimer’s Association have issued new diagnostic criteria for AD and mild cognitive impairment (MCI) and now suggest that the use of biomarkers and neuroimaging can enhance diagnostic confidence (7, 10). Specific definitions for stages of preclinical AD were introduced as well (11). Preclinical AD Stage I was defined as asymptomatic cerebral amyloidosis (the presence of increased amyloid binding on positron emission tomography (PET) scan or low amyloid concentrations on lumbar puncture (LP)). Stage II was defined as Stage I plus downstream neurodegeneration (the presence of elevated tau on LP, abnormal 2-deoxy-2-[18F]fluoro-D-glucose (FDG) metabolism on PET scan or abnormal volumetric loss on structural magnetic resonance imaging (MRI) scan). Stage III was defined as Stage II with the addition of subtle cognitive decline (9). An important concept introduced in these guidelines is the AD pathophysiological process (e.g. amyloid deposition in the brain), which includes preclinical disease prior to AD dementia. There are, however several important exceptions to this progression that have been reported. Jack et al. (12) have described suspected non-AD pathology (SNAP) subjects who had normal amyloid PET imaging but abnormal neurodegeneration biomarker studies. In addition, longitudinal ADNI data (2) found that different neuroinjury biomarkers differed in classifying subjects as abnormal.
Autopsy studies have demonstrated that the accuracy of clinical diagnosis for AD is approximately 70–80% (13–16). In addition to limitation in accurate diagnosis, reliance on clinical assessment alone may not be optimal for clinical trials for therapies that slow or prevent the progression of dementia because some of the preclinical AD pathophysiological processes appear to precede clinical manifestations of dementia by many years (17–18). Biomarkers for the AD pathophysiological process could be used to select participants in clinical trials as well as to monitor response to therapies. It is important to note that these recent guidelines issued by the NIA and Alzheimer’s Association regarding imaging and CSF biomarkers thus far have been primarily limited to research applications, although some studies (19) discuss the clinical diagnosis of AD with biomarker support.
Structural Biomarkers
Very mild Alzheimer’s disease (AD) or mild cognitive impairment (MCI) are characterized by magnetic resonance imaging (MRI) volumetric decreases in medial temporal lobe structures, including the hippocampus (20), where hippocampal volume (HCV) is correlated with beta-amyloid (Aβ)-associated memory decline (21–22). Subjects with MCI who show abnormalities in MRI and/or CSF biomarkers are at greater risk for cognitive decline and progression to AD than subjects without these abnormalities (23–24). See also references 25–26 for review articles. Furthermore, it has been suggested that the rate of hippocampal atrophy in MCI predicts the rate of conversion to AD. Several groups have found that small hippocampal atrophy rates led to slower transitions to AD, while fast conversion to AD was characterized by high hippocampal atrophy rate (27–30). Presymptomatic individuals who eventually converted to AD also had a profile of reduced cortical thickness and accelerated hippocampal atrophy rates (31–32). It is important to note that MRI measurements require careful use of computerized image processing methods, especially to detect rates of regional atrophy
The sensitivity for detecting within-subject changes in structure is quite high. In one study, predictive prognosis of MR images obtained at one time point versus combining single-time-point measures with 1 year change measures were compared, with the latter providing significantly improved discrimination in prediction of AD conversion (33). Comparative sensitivity for detection of longitudinal atrophy changes identified entorhinal cortex and inferior temporal cortex as regions with greatest sensitivity (34), potentially providing enough power to detect treatment induced change (34).
In addition to stand-alone prediction of AD, MRI has been used to augment CSF biomarkers. CSF markers of increased tau, decreased amyloid, and increased tau/amyloid ratio predict progression to AD (see 1, 2 for reviews). In MCI subjects who were abnormal on both CSF and MRI measures, versus only one measure, there was a 4 times higher risk to progress to AD within less than 2 years (35–36). A recent study using the NIA-AA definition of preclinical AD found that in a one year follow up study the rates were significantly different across the preclinical stages (37). The rate in stage 0 was 5%, Stage I (amyloidosis only) was 11%, Stage II (including structural MRI abnormalities) was 21% and Stage III, with the addition of cognitive change was 43% (37). Thus, adding structural MRI to amyloid alone improved the prediction of progression. Combining gray matter structural volumes, diffusion tensor imaging and CSF protein biomarkers yielded 91% accuracy, 85% sensitivity, and 96% specificity in predicting the conversion of MCI to AD (38).
The most recent frontier in neuroimaging prediction is conversion to MCI in non-symptomatic individuals (pre-symptomatic). Several studies of normal controls found that the volume of restricted parts of the hippocampus (the CA1 and subiculum) were more closely associated than total HCV with conversion to MCI (39–42). Similarly using localized components analysis to identify 7 independent patterns of hippocampal atrophy Carmichael et al (29) found that multiple measures of localized hippocampal atrophy were significantly associated with CSF amyloid concentrations while total hippocampal volume was not. Recently, early structural abnormalities in the neocortex have also aroused growing interest. Decreased gray matter volume in the parietal lobe, especially in the angular gyrus (30) and in prefrontal cortex (43) were described in advance of development of MCI. A previously determined AD-like pattern applied to asymptomatic individuals predicted conversion to MCI (44). Notably atrophy in these preselected regions preceded loss of hippocampal volume, was detectable more than 10 years before clinical onset of the disease and correlated with CSF Aβ42/tau ratio and amyloid load measured by Pittsburgh Compound B (PiB) binding (44–45).
fMRI Biomarkers
Because fMRI is significantly less established as a potential clinical tool we refer the reader to review articles on the significant literature on fMRI measures as potential biomarkers in preclinical dementia. These include task-based changes in hippocampal activity (primarily during encoding (46), especially hippocampal hyperactivation early in the course of the disease process (47) and alterations in resting state functional connectivity (reviewed in 48).
PET and SPECT Biomarkers
Molecular imaging uses tracers whose in vivo uptake patterns and kinetics indicate and quantify the presence or activity of specific biochemical processes including receptors, transporters, enzymes and metabolic pathways. Currently, positron emission tomography (PET) and single photon emission computed tomography (SPECT) which both use radiolabeled tracers are the primary molecular imaging techniques employed for imaging in dementia in humans. PET has higher spatial and temporal resolution and is more easily quantified than SPECT and will be the primary focus in this review.
Molecular imaging has established utility for neuroimaging in dementia, particularly AD (49–50). The glucose analogue 2-deoxy-2-[18F]fluoro-D-glucose (FDG), 11C- and 18F-labeled tracers that bind fibrillary beta-amyloid (Aβ), and the dopamine transporter ligand [123I]FPCIT (also known as ioflupane) will be discussed in this section as biomarkers for specific dementias. [18F]FDG has been evaluated in each of these types of dementia, while Aβ imaging has focused primarily on AD. FPCIT has been used primarily to differentiate dementia with Lewy bodies (DLB) from AD. Other tracers and targets such as PET agents for tau imaging (51–53) are under investigation, but there is currently not enough evidence to support their use as clinical imaging biomarkers in dementia and cognitive impairment.
Pathologic analysis of brain tissue obtained at autopsy is considered the gold standard for establishing the sensitivity, specificity and accuracy of biomarkers in dementia. There are several considerations unique to PET and SPECT biomarkers for dementia. The methods used for image acquisition, reconstruction and analysis can affect the diagnostic performance of these imaging modalities, particularly when quantitative data analysis is performed. Because of spatial resolution limitations of PET and SPECT, brain atrophy can artifactually decrease measured tracer uptake and can be a potential confound to visual and quantitative analysis. Correction for atrophy can be performed based on anatomic imaging with CT or MRI.
Alzheimer’s disease (AD)
1) [18F]FDG
[18F]FDG-PET is the most widely used PET tracer in the United States for both oncologic and dementia imaging, and the regional uptake and retention of the PET tracer FDG in the brain can provide a quantitative measure of brain glucose metabolism. Numerous studies have demonstrated progressively decreasing brain uptake of FDG in AD patients over time, thought to reflect neuronal injury and loss occurring predominantly in the parieto-temporal, frontal and posterior cingulate cortices. Currently, FDG-PET studies are reimbursed by the Centers for Medicare and Medicaid Services (CMS) for differentiating suspected AD from FTD. The clinical interpretation of FDG-PET studies for the diagnosis of dementia can be performed by qualitative visual analysis of the relative levels of FDG uptake in relevant regions of the brain. Quantitative analysis of regional FDG uptake can also be performed through comparison with normative databases, and there is data suggesting that this type of analysis can improve diagnostic accuracy, particularly with less experienced readers (54–55).
The sensitivity of FDG-PET for the diagnosis of early AD is approximately 90% although the specificity for distinguishing AD from other types of dementia is lower (71–73%) in studies that used autopsy confirmation as the reference standard (55–56). There is also data supporting the use of FDG-PET to predict which healthy individuals will develop MCI and which individuals with MCI will progress to clinical AD (57–58). Some studies suggest that FDG may be a better marker for progressive cognitive decline compared to amyloid imaging and CSF measures of Aβ levels (59). There is also growing evidence that abnormal brain accumulation of tracers targeting Aβ occurs before changes in FDG uptake (17, 60).
A few studies have examined the ability of FDG to discriminate patients with AD from those with FTD or DLB. In FTD, the typical pattern of FDG hypometabolism predominantly involves the anterior aspects of the frontal and temporal lobes, often asymmetrically. In studies of subjects with AD and FTD, high specificities have been reported (93–98%) with more variable sensitivities (53–95%) (61–63). Some of this variation is likely due to differences in patient population, methods and reference standard (pathologic confirmation versus clinical diagnosis). In a study of 31 patients with autopsy-confirmed AD and 14 with FTD, FDG-PET was more accurate than clinical assessment and differentiated AD from FTD with a specificity of 98% and sensitivity of 86% (55). The pattern of glucose hypometabolism is similar in AD and DLB, but occipital hypometabolism typically is present in DLB but not in AD, which can be used to distinguish these dementias. In studies of subjects with AD and DLB, the reported sensitivities and specificities are variable with ranges of values of 75 –83% and 72–93%, respectively (64–65).
2) Amyloid imaging
Abnormal homeostasis and aggregation of beta-amyloid (Aβ) is a hallmark of the pathologic diagnosis of AD and is thought to play a central role in the pathogenesis of AD (66–67). The deposition of Aβ in the brain appears to precede the development of AD by up to 10–15 years (18, 68). A number of small molecule PET tracers suitable for measuring fibrillary Aβ in the living human brain have been developed over the past decade. One of the first was the PET tracer [11C]Pittsburgh compound B (PiB), and this tracer has been used extensively for research in subjects with AD and other dementias. More recently, several 18F-labeled amyloid imaging agents have been developed and evaluated for Aβ imaging including florbetapir (AV-45), (69) flutemetamol, (70) florbetaben,(71), and NAV4694 (also known as AZD4694 (72). These tracers are better suited to routine clinical use due to the longer half-life of F-18 compared to C-11 (110 min vs. 20 min). These tracers are similar in terms of mechanism of action by binding to the fibrillary form of the Aβ protein that is present in neuritic amyloid plaques (73).
The most rigorous evaluations of the correlation between imaging findings and pathologic confirmation of AD at autopsy are currently available for florbetapir, flutemetamol, and florbetaben, all of which have been approved by the FDA for use in patients with cognitive impairment. Comparison with autopsy results demonstrated that positive florbetapir-PET, flutemetamol-PET and florbetaben-PET studies corresponded to moderate or frequent Aβ plaques on neuropathology. The properties of these tracers are summarized in Table 1, and similar high median sensitivities and specificities for the detection of Aβ plaques were observed with all 3 tracers. However, there was substantial variability in the performance between readers, emphasizing the need for reader training prior to clinical interpretation. In addition to reader error, other factors including brain atrophy, patient movement during the scan, and image acquisition too soon or too late after tracer injection could lead to false negative and false positive amyloid-PET studies. The tracer NAV4694 is currently in late phase clinical trials and appears to have similar diagnostic properties based on the available published data (72). Small studies comparing the brain uptake of PiB and Aβ plaques on histopathologic analysis have yielded mixed results, and sensitivity and specificity measurements cannot be provided based on this limited data. Test-retest measures of SUVR with [11C]PiB, [18F]florbetapir, [18F]florbetaben and [18F]flutametamol have shown good test-retest reliability with average differences of on the order of 1–6% between repeated imaging sessions (74–77).
Table 1.
Comparison of FDA-approved fluorine-18 PET tracers for amyloid in patients with cognitive impairment. These data are from the prescribing information for each tracer. The range of sensitivities and specificities include in-person training and electronic training. Median sensitivities and specificities are based on 5 readers except in the case of in-person training for florbetaben which was based on 3 readers (91–92).
| Generic Name | Trade Name | Manufacturer | Specificity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|
| Median | Individual | Median | Individual | |||
| Florbetapir | Amyvid | Eli Lilly | 82–92 | 69–95 | 95 | 90–100 |
| Florbetaben | Neuracq | Piramal | 96–98 | 90–100 | 77–80 | 47–83 |
| Flutemetamol | Vizamyl | GE | 88–93 | 81–93 | 84–88 | 44–92 |
Amyloid-PET is not currently reimbursed by the CMS, which limits the widespread availability for routine clinical use. Coverage with evidence development (CED) to provide reimbursement for amyloid-PET as part of clinical research studies is planned but not currently implemented at the time of the writing of this article. CED is expected to provide reimbursement for amyloid-PET studies performed for patients that are enrolled in a clinical trial and/or registry that will provide outcomes data needed for CMS to reconsider the coverage decision for amyloid-PET.
With this class of tracers moving from the research to the clinical setting, their proper use will require both referring health care providers and imaging physicians to understand which patient populations will benefit from β-amyloid imaging as well as the implications of both positive and negative imaging studies. For florbetapir, florbetaben, and flutemtamol, a negative study (no abnormally increased cortical tracer uptake) is inconsistent with the diagnosis of dementia due to AD but does not exclude other dementias or neurological disorders that are not associated with β-amyloid pathology. In contrast, a positive study with florbetapir indicates the presence of abnormal levels of amyloid but does not by itself establish the diagnosis of AD dementia. As with PiB, positive florbetapir PET studies occurs in 20–30% of cognitively normal older people, (78), the percentage expected for any reliable amyloid biomarker based on autopsy reports of amyloid plaques, and consistent with preclinical AD. Additionally, Aβ deposition has been reported in DLB, and AD pathology can potentially coexist with neurological conditions causing cognitive decline. Because abnormal amyloid PET and CSF studies are currently the earliest known phenotypic marker of the AD pathophysiological process and appear to precede clinically detectable cognitive decline, these agents may be particularly useful if disease-modifying therapies become available.
In January 2013, a joint report from the Society of Nuclear Medicine and Molecular Imaging and Alzheimer’s Association issued appropriate use criteria for amyloid PET (79). The recommendations are based primarily on a literature review combined with expert consensus. These guidelines consider amyloid PET to be appropriate in certain clinical scenarios for individuals who meet the following criteria:
Objectively confirmed cognitive complaint
Possible etiologies include AD but the diagnosis remains uncertain after full evaluation by a dementia expert
Diagnostic certainty and management are expected to be affected by the amyloid PET results
The following clinical scenarios are considered appropriate by these guidelines for the clinical use of amyloid PET for individuals who meet the above criteria:
Progressive or persistent unexplained MCI
Atypical course or mixed presentation in patients meeting the core clinical criteria for possible AD
Early age of onset of progressive dementia, typically less than 65 years of age
The following scenarios are considered inappropriate for the clinical use of amyloid PET:
Probable AD with typical age of onset
To assess severity of dementia
Positive family history, presence of APOE_4 or suspected carrier of autosomal dominant as the sole reason for amyloid PET
No objective confirmation of cognitive impairment on clinical exam
Asymptomatic individuals
Legal, insurance coverage, employment screening and other non-medical uses
Frontotemporal dementia (FTD)
1) [18F]FDG-PET
[18F]FDG has shown utility in distinguishing AD from FTD based on different patterns of decreased regional brain glucose metabolism. Unlike AD, the brain regions with the most marked relative decreased in [18F]FDG uptake are in the frontal and/or anterior temporal cortices in FTD. Overall, studies of subjects with AD and FTD, high specificities have been reported (93–98%) with more variable sensitivities (53–95%) (61–63). The largest study assessing the ability of [18F]FDG to distinguish AD (n=31) from FTD (n=14) with pathologic confirmation found sensitivity of 86% and specificity of 98% (62).
2) Amyloid agents
There is currently insufficient data to define the role of amyloid imaging agents as a biomarker to distinguish FTD from AD, although the different pathophysiologies and several small studies suggest that Aβ imaging may be useful to distinguish FTD from AD. Together, these studies demonstrate that 11–25% of patients with clinically diagnosed FTD have abnormally increased cortical Aβ deposition as measured with [11C]PIB or [18F]florbetaben (80–82). None of these studies had autopsy confirmation, and the significance of the Aβ deposition in the FTD subjects is unclear. This result may be due to incorrect clinical diagnosis of FTD with AD as the actual cause of dementia, but alternatively a small percentage of patients with FTD and abnormal cortical Aβ deposition may have co-morbid AD pathophysiology.
Dementia with Lewy bodies (DLB)
1) [18F]FDG-PET
[18F]FDG has shown utility in distinguishing AD from DLB based on different patterns of decreased regional brain glucose metabolism (83–84). The pattern of decreased brain [18F]FDG uptake in DLB is similar to AD with the exception of involvement of occipital cortex, particularly the primary visual cortex, in DLB but not AD. In studies of subjects with AD and DLB, the reported sensitivities and specificities are variable with ranges of values of 75 –83% and 72–93%, respectively (64–65, 83). In a study combining both clinical and histopathologic confirmation of diagnosis, [18F]FDG-PET was found to have a 90% sensitivity and 80% specificity for distinguishing AD from DLB (84).
2) Dopamine transporter (DAT) imaging
The SPECT agent [123I]FPCIT (ioflupane) has been used to discriminate DLB from other dementias based on the loss of dopaminergic neurons which in turn leads to decreased DAT density in the striatum. This agent has also been used to study the loss of dopaminergic neurons in Parkinson’s disease and related syndromes and is approved for clinical use in Europe and the U.S. to distinguish Parkinsonian syndromes from essential tremor (85). A 2007 multicenter trial in Europe with 326 subjects demonstrated that FPCIT has a sensitivity of 78% and specificity of 90% for distinguishing DLB from other dementias, primarily AD, using clinical diagnosis as the reference standard (86). A smaller retrospective study (n=44) demonstrated lower sensitivity (63%) but higher specificity (100%) based on consensus diagnosis after 12 month follow up as the reference standard (87). A small prospective study that included 20 patients with dementia and pathologic analysis at autopsy, FPCIT was 88% specific and 100% specific for differentiating DLB from other dementias compared to lower values of 75% and 44%, respectively, based on initial clinical diagnosis (88).
3) Amyloid agents
There is insufficient data to use amyloid imaging agents to distinguish DLB from AD. The available data suggests that Aβ deposition occurs frequently in DLB and may correlate with cognitive deficits (89–90).
Summary
In recent years there have been rapid changes in imaging of patients with suspected cognitive decline. Most striking have been the emergence of amyloid imaging methods that detect increased brain amyloid binding with high sensitivity and specificity. While negative scans are helpful in ruling out cases of suspected Alzheimer’s disease, a positive scan is more complicated to interpret, as other diseases besides AD have increased amyloid, and increased amyloid binding also appears in cognitively normal elderly, years before clinically symptomatic disease occurs. A joint report from the Society of Nuclear Medicine and Molecular Imaging and Alzheimer’s Association has issued appropriate use criteria for amyloid PET. Other imaging modalities that may help to distinguish among etiologies for cognitive decline include FDG-PET to distinguish both FTD and DLB from AD. Structural markers augment the diagnosis among dementing disorders but as of yet are not diagnostic by themselves. All of these methods now have reliability and repeatability measures and thus have an objective basis in aiding in diagnosis. Further, improvements in imaging technology have now pushed the frontier back for distinguishing presymptomatic AD from MCI. While these efforts are still in the early phase of development, there is great promise for further development in structural MRI and PET technologies.
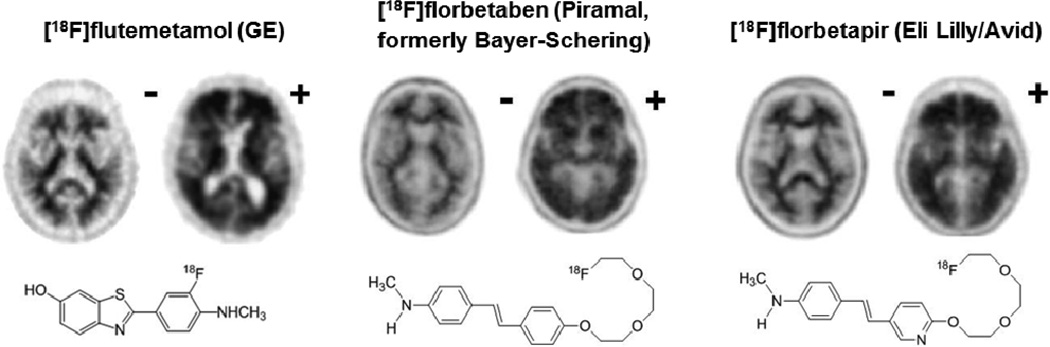
Figure 1.
The three amyloid-binding ligands currently approved by the FDA and representative PET scan images (adapted from 93). The minus (−) sign indicates a negative scan while a plus sign (+) indicates a positive scan for amyloid-containing neuritic plaques. Table 1 shows the corresponding diagnostic performance characteristics for each ligand.
Acknowledgments
Supported by NIMH K24 MHO79510 (YIS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Dr. Sheline reports no biomedical financial interests or potential conflicts of interest. Dr. McConathy has acted as a consultant and member of the speakers bureau for Eli Lilly/Avid Radiopharmaceuticals, as a consultant for GE Healthcare, and as a consultant for Siemens Healthcare.
References
- 1.Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2014 May 2; doi: 10.1016/j.jalz.2014.02.004. pii: S1552-5260(14)00066-1. [Epub ahead of print] Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF Tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013 Nov;126(5):659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tevak Z, Kondratovich M, Mansfield E. US FDA and Personalized Medicine: In vitro Diagnostic Regulatory Perspective. Personalized Medicine. 2010;7(5):517–530. doi: 10.2217/pme.10.53. [DOI] [PubMed] [Google Scholar]
- 4.The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer's Disease Neurobiol Aging.”. Neurobiol Aging. 1998 May-Jun;19(3):285. Review. Erratum. [PubMed] [Google Scholar]
- 5.Grand JH, Caspar S, Macdonald SW. Clinical features and multidisciplinary approaches to dementia care. J Multidiscip Healthc. 2011;4:125–147. doi: 10.2147/JMDH.S17773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 Suppl):417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 7.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien J, Ames D, Burns AS. In: 4th ed. Dementia, editor. London: Hodder Arnold; 2010. [Google Scholar]
- 10.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. PMCID: 3220946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Annals of neurology. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W. Clinico-neuropathological correlation of Alzheimer's disease in a community-based case series. J Am Geriatr Soc. 1999;47(5):564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 14.Ranginwala NA, Hynan LS, Weiner MF, White CL., 3rd Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry. 2008;16(5):384–388. doi: 10.1097/JGP.0b013e3181629971. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC. Practice parameter: Diagnosis of dementia (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 16.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers. J Neuropathol Exp Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, et al. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(Pt 4):1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vos SJ, van Rossum IA, Verhey F, Knol DL, Soininen H, Wahlund L, et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. American Academy of Neurology. 2013;80(12):1124–1132. doi: 10.1212/WNL.0b013e318288690c. [DOI] [PubMed] [Google Scholar]
- 25.Henry MS, Passmore AP, Todd S, McGuinness B, Craig D, Johnston JA. The Development of Effective Biomarkers for Alzheimer's Disease: A Review. Int J Geriatr Psychiatry. 2013;28:331–340. doi: 10.1002/gps.3829. [DOI] [PubMed] [Google Scholar]
- 26.Risacher SL, Saykin AJ. Neuroimaging and Other Biomarkers for Alzheimer's Disease: The Changing Landscape of Early Detection. Annu Rev Clin Psychol. 2013;9:621–648. doi: 10.1146/annurev-clinpsy-050212-185535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung KK, Bartlett JW, Barnes J, Manning EN, Ourselin S, Fox NC. Cerebral atrophy in mild cognitive impairment and Alzheimer disease: Rates and acceleration. Neurology. 2013 Feb 12;80(7):648–654. doi: 10.1212/WNL.0b013e318281ccd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald KE, Bartlett JW, Leung KK, Ourselin S, Barnes J ADNI investigators. The value of hippocampal and temporal horn volumes and rates of change in predicting future conversion to AD. Alzheimer Dis Assoc Disord. 2013 Apr-Jun;27(2):168–173. doi: 10.1097/WAD.0b013e318260a79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmichael O, Xie J, Fletcher E, Singh B, DeCarli C Alzheimer's Disease Neuroimaging, I. Localized hippocampus measures are associated with Alzheimer pathology and cognition independent of total hippocampal volume. Neurobiol Aging. 2012;33(6):1124 e1131–1141 e1131. doi: 10.1016/j.neurobiolaging.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westman E, Muehlboeck JS, Simmons A. Combining MRI and CSF measures for classification of Alzheimer's disease and prediction of mild cognitive impairment conversion. Neuroimage. 2012;62(1):229–238. doi: 10.1016/j.neuroimage.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 31.Sabuncu MR, Desikan RS, Sepulcre J, Yeo BT, Liu H, Schmansky NJ, et al. The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch Neurol. 2011 Aug;68(8):1040–1048. doi: 10.1001/archneurol.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011 Apr 19;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEvoy LK, Holland D, Hagler DJ, Jr, Fennema-Notestine C, Brewer JB, Dale AM. Mild Cognitive Impairment: Baseline and Longitudinal Structural MR Imaging Measures Improve Predictive Prognosis. Radiology. 2011;259(3):834–843. doi: 10.1148/radiol.11101975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland D, Brewer JB, Hagler DJ, Fennema-Notestine C, Dale AM. Subregional neuroanatomical change as a biomarker for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(49):20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouwman FH, Schoonenboom SN, van der Flier WM, van Elk EJ, Kok A, Barkhof F, et al. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging. 2007;28(7):1070–1074. doi: 10.1016/j.neurobiolaging.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73(4):294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. PMCID: 3348848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douaud G, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Smith S. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. The Journal of Neuroscience. 2013;33(5):2147–2155. doi: 10.1523/JNEUROSCI.4437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging. 2010;31:1099–1106. doi: 10.1016/j.neurobiolaging.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazarczyk MJ, Hof PR, Bouras C, Giannakopoulos P. Preclinical Alzheimer disease: identification of cases at risk among cognitively intact older individuals. BMC Med. 2012;10:127. doi: 10.1186/1741-7015-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maruszak A, Thuret S. Why looking at the whole hippocampus is not enough—a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Frontiers in cellular neuroscience. 2014;8:95. doi: 10.3389/fncel.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 43.Burgmans S, van Boxtel MP, Smeets F, Vuurman EF, Gronenschild EH, Verhey FR, Uylings HB, Jolles J. Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiol Aging. 2009;30:1413–1419. doi: 10.1016/j.neurobiolaging.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-b associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhatwal JP, Sperling RA. Functional MRI of mnemonic networks across the spectrum of normal aging, mild cognitive impairment, and Alzheimer's disease. Journal of Alzheimer's Disease. 2012;31:S155–S167. doi: 10.3233/JAD-2012-120730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putcha D, Brickhouse M, O'Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. The Journal of Neuroscience. 2011;31(48):17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biological psychiatry. 2013;74(5):340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, et al. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9(7):560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 50.Small GW, Bookheimer SY, Thompson PM, Cole GM, Huang SC, Kepe V, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161–172. doi: 10.1016/S1474-4422(08)70019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia CF, Shankle WR, Lerner AJ, Su M, Elizarov A, Kolb HC. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. Journal of Alzheimer's Disease. 2014;38(1):171–184. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- 52.Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, Mu F, Sinha A, Su H, Szardenings AK, Walsh JC, Wang E, Yu C, Zhang W, Zhao T, Kolb HC. [18F] T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimer's & Dementia. 2013;9(6):666–676. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Harada R, Okamura N, Furumoto S, Tago T, Maruyama M, Higuchi M, Yoshikawa T, Arai H, Iwata R, Kudo Y, Yanai K. Comparison of the binding characteristics of [18F] THK-523 and other amyloid imaging tracers to Alzheimer’s disease pathology. European journal of nuclear medicine and molecular imaging. 2013;40(1):125–132. doi: 10.1007/s00259-012-2261-2. [DOI] [PubMed] [Google Scholar]
- 54.Coleman RE. Positron emission tomography diagnosis of Alzheimer's disease. Neuroimaging Clin N Am. 2005;15(4):837–846. doi: 10.1016/j.nic.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 56.Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69(9):871–877. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- 57.Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosconi L, Brys M, Glodzik-Sobanska L, De Santi S, Rusinek H, de Leon MJ. Early detection of Alzheimer's disease using neuroimaging. Exp Gerontol. 2007;42(1–2):129–138. doi: 10.1016/j.exger.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73(15):1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lowe VJ, Kemp BJ, Jack CR, Jr, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50(6):878–886. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dukart J, Mueller K, Horstmann A, Barthel H, Moller HE, Villringer A, et al. Combined evaluation of FDG-PET and MRI improves detection and differentiation of dementia. PLoS One. 2011;6(3):e18111. doi: 10.1371/journal.pone.0018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain. 2007;130(Pt 10):2616–2635. doi: 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- 63.Panegyres PK, Rogers JM, McCarthy M, Campbell A, Wu JS. Fluorodeoxyglucose-positron emission tomography in the differential diagnosis of early-onset dementia: a prospective, community-based study. BMC Neurol. 2009;9:41. doi: 10.1186/1471-2377-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50(10):1638–1645. doi: 10.2967/jnumed.109.065870. [DOI] [PubMed] [Google Scholar]
- 65.Kono AK, Ishii K, Sofue K, Miyamoto N, Sakamoto S, Mori E. Fully automatic differential diagnosis system for dementia with Lewy bodies and Alzheimer's disease using FDG-PET and 3D-SSP. Eur J Nucl Med Mol Imaging. 2007;34(9):1490–1497. doi: 10.1007/s00259-007-0380-y. [DOI] [PubMed] [Google Scholar]
- 66.Tarawneh R, Holtzman DM. Biomarkers in translational research of Alzheimer's disease. Neuropharmacology. 2010;59(4–5):310–322. doi: 10.1016/j.neuropharm.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finder VH. Alzheimer's disease: a general introduction and pathomechanism. J Alzheimers Dis. 2010;22(Suppl 3):5–19. doi: 10.3233/JAD-2010-100975. [DOI] [PubMed] [Google Scholar]
- 68.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir) J Nucl Med. 2010;51(6):913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 71.Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, et al. Cerebral amyloid-beta PET with florbetaben ((18)F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10(5):424–435. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- 72.Cselenyi Z, Jonhagen ME, Forsberg A, Halldin C, Julin P, Schou M, et al. Clinical validation of 18F-AZD4694, an amyloid-beta-specific PET radioligand. J Nucl Med. 2012;53(3):415–424. doi: 10.2967/jnumed.111.094029. [DOI] [PubMed] [Google Scholar]
- 73.Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009;50(11):1887–1894. doi: 10.2967/jnumed.109.065284. PMCID: 3065020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM. Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53(3):378–384. doi: 10.2967/jnumed.111.090340. Epub 2012/02/15. doi: 10.2967/jnumed.111.090340. PubMed PMID: 22331215. [DOI] [PubMed] [Google Scholar]
- 75.Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Meltzer CC, Schimmel K, Tsopelas ND, DeKosky ST, Price JC. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46(12):1959–1972. Epub 2005/12/07. PubMed PMID: 16330558. [PubMed] [Google Scholar]
- 76.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks DJ. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Annals of neurology. 2010;68(3):319–329. doi: 10.1002/ana.22068. Epub 2010/08/06. doi: 10.1002/ana.22068. PubMed PMID: 20687209. [DOI] [PubMed] [Google Scholar]
- 77.Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G, O'Keefe G, Ackerman U, Tochon-Danguy H, Chan JG, Reininger CB, Fels L, Putz B, Rohde B, Masters CL, Rowe CC. Amyloid Imaging with 18F-Florbetaben in Alzheimer Disease and Other Dementias. J Nucl Med. 2011 doi: 10.2967/jnumed.111.089730. PubMed PMID: 21764791. [DOI] [PubMed] [Google Scholar]
- 78.Pontecorvo MJ, Mintun MA. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer's disease. Alzheimers Res Ther. 2011;3(2):11. doi: 10.1186/alzrt70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson KA, et al. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. J Nucl Med. 2013 doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Engler H, Santillo AF, Wang SX, Lindau M, Savitcheva I, Nordberg A, et al. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2008;35(1):100–106. doi: 10.1007/s00259-007-0523-1. [DOI] [PubMed] [Google Scholar]
- 81.Rabinovici GD, Furst AJ, O'Neil JP, Racine CA, Mormino EC, Baker SL, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68(15):1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 82.Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G, et al. Amyloid Imaging with 18F-Florbetaben in Alzheimer Disease and Other Dementias. J Nucl Med. 2011 doi: 10.2967/jnumed.111.089730. [DOI] [PubMed] [Google Scholar]
- 83.Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50(3):358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 84.Albin RL, Minoshima S, D'Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluorodeoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47(2):462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- 85.Hoyte RM, Lin SS, Christman DR, Atkins HL, Hauser W, Wolf AP. Organic radiopharmaceuticals labeled with short-lived nuclides. 3. 18F-labeled phenylalanines. J Nucl Med. 1971;12(6):280–286. [PubMed] [Google Scholar]
- 86.McKeith I, O'Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305–313. doi: 10.1016/S1474-4422(07)70057-1. [DOI] [PubMed] [Google Scholar]
- 87.O'Brien JT, McKeith IG, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194(1):34–39. doi: 10.1192/bjp.bp.108.052050. [DOI] [PubMed] [Google Scholar]
- 88.Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176–1181. doi: 10.1136/jnnp.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25(15):2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.FDA prescribing information Amyvid. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202008s020lbl.pdf.
- 92.FDA prescribing information Vizamyl. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203137s000lbl.pdf.
- 93.Rowe CC, Pejoska S, Mulligan RS, Jones G, Chan JG, Svensson S, Cselényi Z, Masters CL, Villemagne VL. Head-to-head comparison of 11C-PiB and 18F-AZD4694 (NAV4694) for β-amyloid imaging in aging and dementia. J Nucl Med. 2013 Jun;54(6):880–886. doi: 10.2967/jnumed.112.114785. [DOI] [PubMed] [Google Scholar]