Abstract
Objective
The objective was to determine how stimulation timing applied during reaching influenced neuroplasticity related to practice. Older adult participants were studied to increase relevance for stroke rehabilitation and aging.
Methods
Sixteen participants completed 3 sessions of a reaching intervention with 480 planar robotic movement trials. Sub-threshold, single-pulse transcranial magnetic stimulations (TMS) were delivered during the late reaction time (LRT) period, when muscle activity exceeded a threshold (EMG-triggered), or randomly. Assessments included motor evoked potentials (MEP), amplitude, and direction of supra-threshold TMS-evoked movements and were calculated as change scores from baseline.
Results
The direction of TMS-evoked movements significantly changed after reaching practice (p < 0.05), but was not significantly different between conditions. Movement amplitude changes were significantly different between conditions (p < 0.05), with significant increases following the LRT and random conditions. MEP for elbow extensors and flexors, and the shoulder muscle that opposed the practice movement were significantly different between conditions with positive changes following LRT, negative changes following EMG-triggered, and no changes following the random condition. Motor performance including movement time and peak velocity significantly improved following the training but did not differ between conditions.
Conclusions
The responsiveness of the motor cortex to stimulation was affected positively by stimulation during the late motor response period and negatively during the early movement period, when stimulation was combined with robotic reach practice.
Significance
The sensitivity of the activated motor cortex to additional stimulation is highly dynamic.
Keywords: Transcranial magnetic stimulation, motor control, neuroplasticity, reaching, aging, rehabilitation
Introduction
Neurorehabilitation efforts have focused on intense structured interventions to promote neuroplasticity because stroke is a leading cause of long-term disability worldwide. Robotic rehabilitation devices assist massed practice of upper extremity movement at high repetition rates (Lo et al., 2010, Conroy et al., 2011). They can also be used to change the learning environment, e.g., provide assistance or resistance to the motor task or train new mappings for movement to environmental effect (Krebs et al., 1998, Stein et al., 2004, MacClellan et al., 2005). Non-invasive brain stimulation such as transcranial magnetic stimulation (TMS) has been used to enhance neuroplasticity by modulating the neurophysiologic state and/or motor output (Cohen et al., 1998, Bütefisch et al., 2004, Kluger et al., 2007, Chen et al., 2009). There is an obvious potential synergy in combining TMS and repetitive motor practice using a robotic rehabilitation device.
A number of studies have demonstrated the potential to facilitate neuroplasticity with intense, repetitive training paradigms (Classen et al., 1998, Giacobbe et al., 2011). Classen et al., (1998) established that 30 minutes of brisk, repetitive practice of thumb movements in the direction opposite of the TMS-evoked movements changed the direction of the evoked movement. Similar work has been done at the wrist and elbow, but the greatest effects appear to be more distal (Krutky et al., 2007, Giacobbe et al., 2011). While these studies contributed to unveiling the principles of use-dependent plasticity for neurohabilitation, TMS also has been considered as a method to enhance neuroplasticity. Bütefisch et al., (2004) demonstrated the potential to increase training-dependent effects when TMS was applied to the motor cortex synchronously with some of the practiced thumb movements. This is therefore a proof-of-principle for facilitating neuroplasticity with intense motor training and TMS; however, this work has been largely isolated to a single degree-of-freedom in the distal upper extremity and the influence of the precise timing of stimulation has not been systematically investigated.
Extending this line of work to the upper extremity has begun through systematic steps to address important questions and has relied heavily on robotic training devices. First, TMS-evoked movements in the upper extremity as an outcome measure for neuroplasticity has been established (Jones-Lush et al., 2010, Lewis et al., 2012). Intense robotic reaching training facilitated plasticity in TMS-evoked upper extremity movements when reaches were practiced in a direction opposite of the initial evoked movement (Kantak et al., 2013). Further, TMS delivered at different times during practiced reaches modulated motor performance with improvements observed when TMS was delivered during the late reaction time (LRT) period (Massie et al., 2013b). A remaining question is how the timing of stimulation during intense reaching practice impacts the extent and type of neuroplasticity.
Applications of TMS could improve the efficacy of robotic reaching interventions, based on the rationale that a single TMS pulse delivered with precise timing in relation to the reaching movement will modulate the degree of neuroplasticity. We hypothesized that stimulation delivered during the LRT period would facilitate plasticity when compared to stimulation synchronized with muscle activity onset. The rationale was that spike-timing dependent plasticity is positive when presynaptic activity precedes postsynaptic (Stefan et al., 2000), while presynaptic activity following postsynaptic activity can result in long-term depression. The importance of stimulation timing delivered within 100 ms of movement onset has been demonstrated by Thabit et al., (2010), showing that pre-movement stimulation resulted in positive plasticity whereas stimulations that followed the activation of muscles had less effect. This time period (within 100 ms of movement onset) is a critical window of opportunity for cortical stimulation to influence voluntary movement, and comparing the pairing of stimulation with the onset of muscle activation (EMG triggered) with a late pre-movement period (150 ms prior to movement onset) has not been systematically studied. Because the optimization of timing applies to many types of motor rehabilitation, the results of this study will aid clinical researchers in the development of better therapeutic interventions that couple repetitive practice and other methods that affect brain activity, including non-invasive brain stimulation, virtual reality or other methods, with a goal of enhancing useful neuroplasticity for survivors of stroke.
Methods
Participants
Sixteen neurologically-intact participants (9 female; 7 male) were recruited for this study and provided informed consent in a protocol approved by the University of Maryland Institutional Review Board and the local Veterans Administration Research Committee. Participants ranged in age from 47–75 (mean 64.7 ± 8.7) years and were not taking medications known to affect cortical excitability. Further, they had no history of seizures, treatment with antiepileptic medication, implanted electronic devices, implanted metal in the head, or any other contraindications to TMS.
Experimental Setup
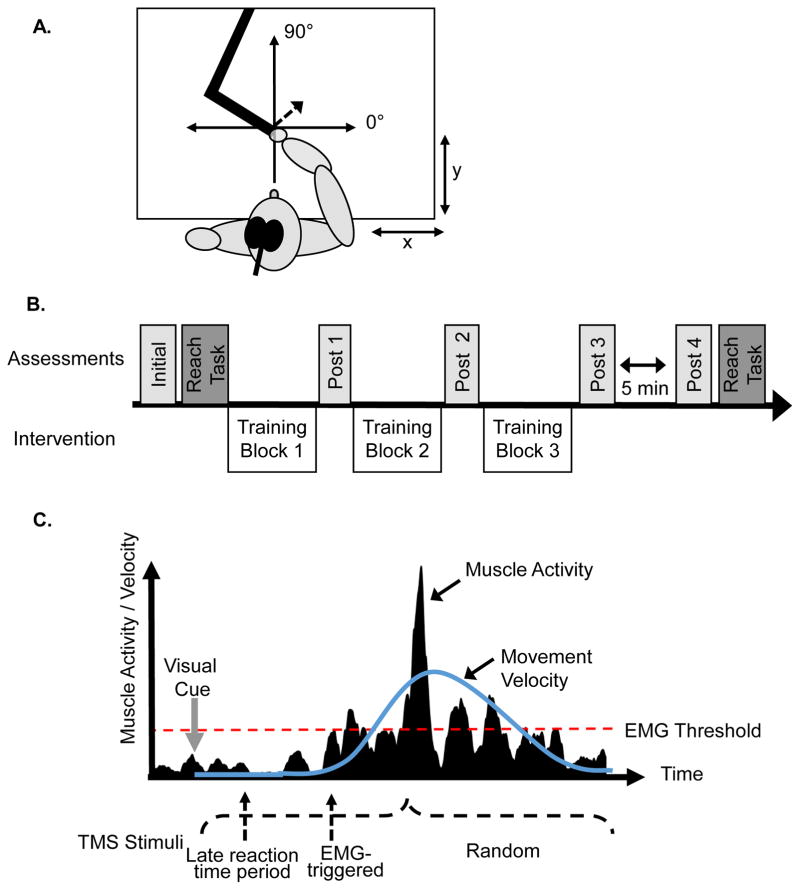
Participants completed 3 separate sessions with a minimum of 24 hours between sessions. The order of the visits was determined using a Latin square approach. The experimental protocol was the same for each visit except the timing of stimulation delivery during the training as described below. Surface electromyography (EMG) electrodes (B+L Engineering) were applied in bipolar montage to the muscle belly of biceps, triceps, anterior deltoid, and posterior deltoid muscles. Electrode placement was verified by confirming specificity of the EMG signal during voluntary contractions, and data were collected at 2000 Hz with a custom LabView program. Participants were comfortably seated at a planar rehabilitation robotic device (Interactive Motion Technologies, Inc., Cambridge, MA, USA) as depicted in Figure 1.A. The dominant hand and forearm rested in the cradle of the robot with the starting position of the hand at midline approximately 20cm from the edge of the table. This ensured consistent arm configuration within sessions and minimized differences between subjects. TMS coil placement over the contralateral primary motor cortex was guided with a stereotaxic device (Brainsight, Rogue Research, Montréal, Canada). We determined the movement hotspot as the location that elicited the largest TMS-evoked movements of the arm and hand as recorded by the movement of the robotic handle. The threshold was determined as the lowest intensity to elicit TMS-evoked movements of at least 1 mm in 5 of 10 consecutive stimulations. For participants whose threshold was above 100% of maximal stimulator output but in whom movements could be elicited at lower intensities, a movement threshold of 100% was used.
Figure 1.
A. The origin of the robot was individually determined with a mediolateral x axis and an anterior-poster y axis. The transcranial magnetic stimulation (TMS) coil was over the primary motor cortex. TMS stimulation was applied and each TMS evoked movement was recorded within the coordinate system as depicted by the dotted arrow. B. Participants completed an initial assessment and 4 post assessments. The 3 training blocks of 160 trials were separated by post assessments. A reaching task was also completed prior to and following the training blocks. C. Schematic of stimulation conditions. Stimulation during the late reaction time (LRT) was delivered prior to movement onset while the EMG-triggered was delivered when the muscle activity rose above a pre-determined threshold. The random condition was delivered in a predetermined range between visual cue and completion of movement.
Assessments were completed at 5 time points during each session (see initial, post 1, post 2, etc. in Figure 1.B). TMS was used to elicit movements and corresponding motor evoked potentials (MEP) as outcome measures using a stimulation intensity of 120% of the movement threshold. Ten stimuli were delivered at rest to record the movement amplitude and direction evoked by TMS stimulation (Magstim 200, Oxford, UK). The direction angle was calculated as a vector to the endpoint of the robot handle at the point of peak velocity (PV) (see Figure 1.A). The distance of the handle from the origin at that time point was calculated as a measure of the amplitude of the evoked movement. MEP data from the four muscles of interest were simultaneously collected with peak-to-peak amplitudes measured, then averaged across 10 trials. The muscles were grouped as agonists/antagonists based on the training direction (forward reaching had triceps and anterior deltoid as agonists and biceps and posterior deltoid as antagonists; vice versa for reaching backwards).
Reach Task
Participants performed a reaching task of 10 reaches in 180 degrees opposite to the TMS-evoked direction, which corresponded to the direction used for the training. The target was 10 cm from the home target (origin) and participants were instructed to reach as quickly as possible when the go cue turned green from red. The onset of the trials was the go cue and ended when the cursor arrived within a 1 cm radius of the target. Data collected from the robot were x, y coordinates of movement and the absolute velocity. Outcome variables included movement time (s), peak velocity (m/s), and time to peak velocity (s).
Training Blocks
Following the initial baseline assessment, participants completed 3 blocks of 160 trials of reaches in the direction 180 degrees opposite the TMS-evoked direction at the initial assessment. The cues were the same as described above and the robot provided 50 N of resistance during the movement and facilitated the passive return to the home circle. Subthreshold, single-pulse TMS (70% movement threshold) was delivered during every 2nd reach (240 stimulations total). The 3 conditions completed on separate visits were: LRT, EMG-triggered, and randomly (see Figure 1.C). The LRT condition delivered the stimulation approximately 150 ms prior to movement onset by calculating a fixed delay from the visual cue based on the reaction time during practiced movements (reaction time – 150 ms). Visual inspection of EMG during the training blocks ensured that no muscle activity was present at time of stimulation or the delay was adjusted accordingly. The EMG-triggered condition delivered stimulation when the root mean square (RMS) level of muscle activity exceeded an individually determined threshold that was approximately 0.1–0.2 mV. This ensured a sensitive threshold that reliably triggered the stimulation (error rate of less than 1%). The agonist muscle having the greatest amplitude change during the reach was used as the triggering muscle. The random condition delivered stimulation randomly at times between the visual cue and end of movement. A TMS assessment (Post 1 and 2) was delivered between each block of training and two, separated by 5 minutes of rest, were delivered at the completion of block 3 (Post 3 and 4; see Figure 1.B).
Data Analysis
The 10 TMS-evoked movements and MEP were measured and then averaged at each time-point. Change scores were calculated from the initial assessment for post-training (Post 1, Post 2, Post 3), and retention (Post 4) for outcomes related to TMS-evoked movements (direction, amplitude, and MEP). The amplitude data were calculated as a percent change from the initial assessment and the direction was the absolute change from initial assessment corrected for initial evoked direction such that all changes were between 0–180 degrees. MEP data were calculated as the change from initial assessment. Differences at baseline between conditions were analyzed using a one-way ANOVA for the movement threshold, and the direction and amplitude for the TMS-evoked movements. Mixed-effects models were used to compare the change scores with fixed effects of condition (3) and time (4), and subjects were entered as a random condition. We elected to not compute the interaction effect because data were calculated as change scores from baseline. Further, a priori, post-hoc analyses were performed to determine changes from baseline within each condition using the least-squares mean, i.e., were changes significantly different than zero which would indicate no change. Differences between conditions were analyzed with a one-way ANOVA corrected for multiple comparisons using Tukey’s adjustment if the condition effect was significant. The reaching task data were averaged from the 10 reaching movements and were analyzed with a repeated measures ANOVA with time (2) by condition (3) effects. Significance was set at p < 0.05.
Results
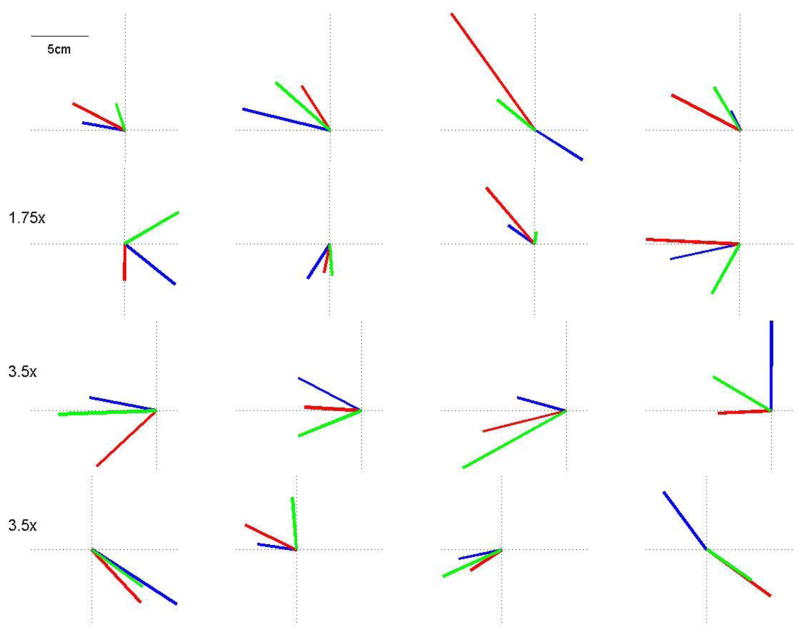
The movement thresholds at baseline were stable across conditions, F (2,47) = 0.013, p = 0.99, with an average of 78 ± 13% of maximum stimulator output. Additionally, no differences occurred between conditions at baseline for the amplitude, F (2,47) = 0.72, p = 0.49, and direction, F (2,47) = 0.4, p = 0.71, of the evoked movements. The averaged amplitude and directions from the initial assessment for each visit are plotted by subject in Figure 2. Four subjects had movement thresholds greater than 100% in at least 1 session, but some TMS-evoked movements were elicited at 100% and that stimulation strength was used in all measurements.
Figure 2.
Averaged mean amplitude and direction data at baseline for each participant and each visit. The late reaction time (LRT) condition was blue, the EMG-triggered was red, and the random condition was green. There were no differences between conditions for the baseline measures as the evoked movements were relatively stable across visits.
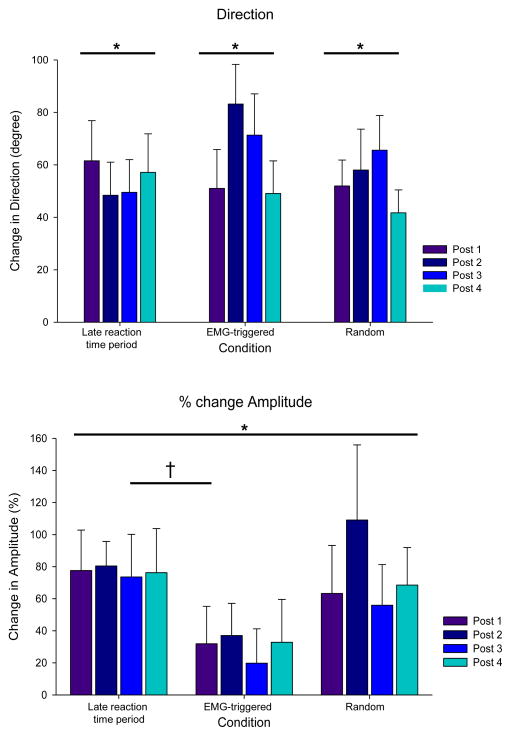
The mean group changes in direction and amplitude following each block of robotic training are depicted in Figure 3. Although there was not a main effect for condition, F(2,28) = 0.97, p = 0.39 or time, F(3,42) = 1.05, p =0.4, the TMS-evoked direction was significantly different from baseline following robotic reaching training with stimulation for each condition: LRT (t = 5.6, p < 0.0001); EMG-triggered (t = 6.6, p < 0.0001); and random (t = 5.6, p < 0.0001). The change in amplitude had a significant main effect for condition, F(2,30) = 3.75, p = 0.035, but not for time, F (3,45) = 0.68, p = 0.57. The mean amplitude change following the training with LRT stimulation was significantly larger than the amplitude change following the EMG-triggered condition (t = 2.5, p = 0.042). The amplitude changes in the random condition were not significantly different from the LRT (t = 0.37, p = 0.93) and the EMG-triggered conditions (t = 2.2, p = 0.093).
Figure 3.
A. Changes in the direction of TMS-evoked movements. There was a significant change in the direction of TMS-evoked movements in response to the reaching training but no differences between conditions. B. Changes in TMS evoked movement amplitude. There was a significant effect of condition on the percent amplitude change score (*, p < 0.05). There was a significant difference between the late reaction time (LRT) period and EMG-triggered condition (†, p = 0.04) but no other differences between conditions.
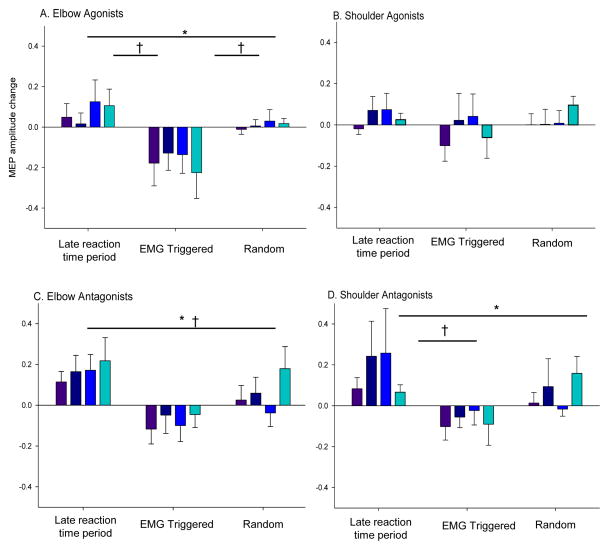
The MEP data collected simultaneously with the TMS-evoked movements are depicted in Figure 4 with the differences between conditions noted. There was a significant effect for condition in the elbow agonists F (2,29) = 12.2, p < 0.001, elbow antagonists, F (2,29) = 20.2, p < 0.001, shoulder antagonists, F (2,29) = 5.6, p = 0.0087, but not for shoulder agonists, F (2,29) = 1.3, p = 0.3. Although there was no significant effect (p > 0.05) for time between the change scores for any of the muscle groups, the decrease in the MEP amplitude of the elbow agonists following EMG-triggered stimulation/training was significantly different than zero (t = 3.53, p = 0.0014). The increase in amplitude following the LRT condition was not significant (t = 1.6, p = 0.12) nor was the change following random condition (t = 0.22 p = 0.82). The shoulder agonists did not change in amplitude following any of the conditions: LRT, (t = 1.2, p = 0.26); EMG-triggered condition, (t = 0.5, p = 0.6); random, (t = 0.6, p = 0.6). For the elbow antagonists, there was a significant increase in MEP amplitude following the LRT condition (t = 3.1, p = 0.005), and the changes following the EMG-triggered and random were not significant (p = 0.2, 0.4, respectively). There was a significant increase in MEP amplitude of the shoulder antagonists following training in LRT (t = 2.8, p = 0.009), but the changes were not significant following EMG-triggered (t = 1.2, p = 0.3), or random (t = 1.1, p = 0.3).
Figure 4.
Changes in MEP amplitude from the initial assessment. Data are plotted separately for elbow and shoulder agonists and antagonists. There were no changes in the shoulder agonists, but there was a significant effect of condition on the other change scores (*, p < 0.05) with significant differences between conditions (†, p < 0.05).
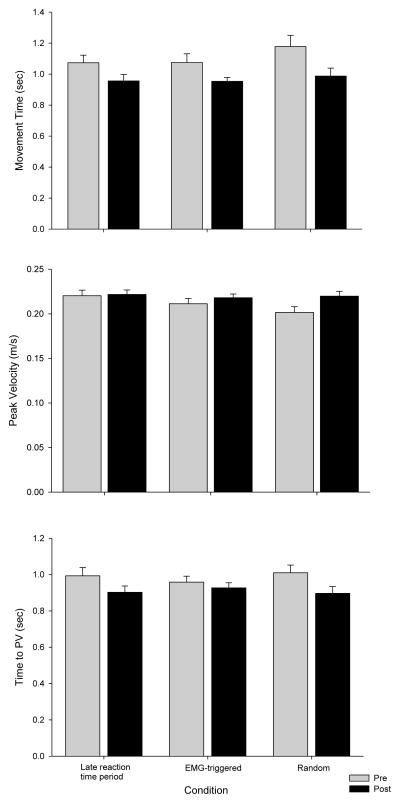
The motor performance on the reaching task completed before and after the reaching training are depicted in Figure 5. Participants had significantly decreased movement times following the training blocks F (1,13) = 21.1, p = 0.0005, but there was no difference between conditions, F (2,26) = 2.1, p = 0.14 and no time by condition interaction, F (2,26) = 0.57, p = 0.6. The peak velocity significantly increased with time F (1,13) = 7.4, p = 0.02, and between conditions, F (2,26) = 3.5, p = 0.047, but the interaction was not significant, F (2,26) = 2.4, p = 0.11. The time-to-peak velocity significantly decreased following training, F (1,13) = 16.8, p = 0.001, but there was no difference between conditions, F (2,26) = 0.1, p = 0.9.
Figure 5.
Motor performance outcomes are depicted for the practiced reaching movements before and after the intervention. Participants significantly decreased movement time, increased peak velocity, and decreased time to peak velocity following the intervention.
Discussion
This study demonstrated that the timing of stimulation during robotic reaching training modulated the type of motor system plasticity. Generally, motor output was greater following the LRT condition, while the reverse was true in the EMG-triggered condition. For example, antagonist MEP increased following LRT stimulation and the elbow agonist MEP amplitudes decreased following the EMG-triggered condition, yet no changes in MEP amplitude were observed following the random condition. The LRT condition also had larger movement amplitude changes compared to the EMG-triggered condition; however, these changes were not significantly different than the random condition. The random condition, serving as a control, likely had a combination of stimulation timing effects such that those coinciding with the LRT phase of movement had a stronger impact on evoked movement amplitude than those delivered after movement onset. Therefore, in support of our hypotheses, adjusting the timing of TMS in relation to movement onset during intense robotic reaching training led to distinct modulation of motor system plasticity. These results highlight a number of novel and important aspects of the study. First, this study focused on upper extremity goal-directed reaching whereas the majority of previous work has used the thumb or wrist as training models. Secondly, stimulation timing was precisely adjusted to coincide with important aspects of movement preparation and generation. Thirdly, we observed rapid changes early within the intervention that were maintained through a brief retention phase. Lastly, this study was conducted with older adults to address important issues related to developing this type of neurorehabilitation intervention.
Given the importance and potential benefit of exploiting concepts of use-dependent plasticity for neurorehabilitation, we focused on extending the early work of the thumb and wrist training models (Bütefisch et al., 2004, Krutky et al., 2007, Bütefisch et al., 2011) by implementing intense and repetitive goal-directed reaching using a planar robot that provided resistive forces. Early work established the rapid plasticity of M1 in response to repetitive motor practice with thumb movements (Classen et al., 1998, Bütefisch et al., 2004). More recently, Krutky and Perreault (2007) demonstrated that plasticity may be graded distal to proximal from training using the index finger, wrist, and elbow. No training, however, was conducted using the shoulder, which is considered the most proximal joint of the upper extremity and the primary driver of reaching in 2D (Karst et al., 1991). Our previous study established the rapid plasticity of participants engaged only in upper extremity reaching training, with direction changes in evoked movements but less change in amplitude (Kantak et al., 2013). This current study directly extends these results by combining precisely timed TMS during robotic reaching practice. The changes in direction following reaching training were similar between the studies; however, with stimulation and practice we also found significant increases in the amplitude of TMS-evoked movements, when calculated as a percent change score. We have provided initial evidence of modulating neuroplasticity using intense motor practice on a robotic rehabilitation device with precisely timed TMS.
In addition to demonstrating that combined motor practice with TMS influenced neuroplasticity, we found that the timing of stimulation had a strong effect. We delivered TMS during the reaction time before movement and triggered by EMG activity at the onset of movement, with the random condition serving as a control, and found that EMG-triggered led to decreased excitability of the elbow agonists and smaller changes in the evoked amplitude. Previous studies using the thumb (Bütefisch et al., 2004) and wrist (Bütefisch et al., 2011) training models essentially used an EMG-triggered protocol or randomly delivered stimulation. In the wrist model with the condition similar to EMG-triggered stimulation, stimulations were delivered with every second movement and MEP of the agonist muscle were measured. Interestingly, no significant differences in the MEP amplitude following training were observed, but there was a general trend for a reduction in amplitude. Our results are similar such that we observed a significant decrease in elbow agonist MEP amplitude following the EMG-triggered condition. Thabit et al., (2010) employed a movement-related cortical stimulation paradigm with stimulations either prior to or after movement and found that stimulation 50 ms prior to movement increased MEP amplitude. We observed similar results with stimulation 150ms prior to movement with significant increases in antagonist MEP amplitude. Previous research has demonstrated a general increase in M1 excitability and a decrease in inhibition during the 140ms prior to movement onset (Gilio et al., 2008) that is considered to facilitate the execution of the motor program. The extensive work by Churchland and colleagues has provided a foundation for how the movement preparation phase brings neural activity to an appropriate state to generate desired movement (Churchland et al., 2006, Churchland et al., 2007, Churchland et al., 2010). As such, the possibility exists that a single, sub-threshold stimulation to M1 during the LRT period had a priming effect and influenced specific muscle representations. The increase in elbow antagonist MEP may be due to the priming effect as these muscles were likely the primary muscles in the evoked movement at rest prior to the reaching intervention, i.e., we trained the reaching movements in 180 degrees opposite of the movement evoked at rest. In contrast, the stimulation delivered during the onset of muscle-activity likely supplemented activation of the active muscles, causing a negative feedback loop to down-regulate the excitability of these muscles for subsequent movements. Another possible explanation was that the relative strength of the stimulation may have differed between the LRT and EMG triggered conditions as the threshold became lower with the transition from preparation to action. However, the presence or amplitude of MEP was not different during the training conditions (data not shown) in the majority of the muscles. Systematically studying the effect of stimulation strength is an avenue for future studies to develop this type of intervention. We observed this decreased excitability most prominently in the muscles spanning the elbow joint. This may be due to co-activation of elbow muscles during reaching (Vandenberghe et al., 2010), and motor preparation and execution modulates those co-activations. Interestingly, we observed distinct differences in the direction of plasticity depending on the TMS condition with respect to the muscles acting as agonists vs. antagonists. This finding is supported by previous literature demonstrating that the combination of motor training and cortical stimulation can differentially alter muscle representations (Thabit et al., 2010, Massie et al., 2013a). Additional research is needed to fully reveal this relationship with respect to TMS evoked movements because similar changes in evoked directions were observed despite coupled agonist/antagonist MEP changes that differed between conditions. This suggests that TMS evoked movements are the result of the cumulative evoked muscle twitches of proximal musculature, and future research should include additional muscles of interest.
Our approach of probing timing-dependent plasticity in the motor cortex is also related to another model, paired associative plasticity, that shows strong timing dependence (Ziemann et al., 2004). The difference is that in our approach voluntary movement-related activity is paired with motor cortical stimulation, rather than sensory stimulation. While movement-related activity may be less precise in time, it also is dynamic and cannot be assumed to be constant over the reaction-time and movement period (Chen et al., 1998). These are important considerations for developing reaching interventions that use TMS because of the ability to differentially modulate the direction of plasticity depending on the timing of stimulation. For example, Gharabaghi et al., (2014) recently reported on the potential to use brain-state dependent stimulation within the context of coupling brain-machine interfaces with cortical stimulation. These findings demonstrate the potential to individually prescribe stimulation parameters depending on the desired direction of plasticity. The site of plasticity is also of interest for future studies because although it likely occurs in the cortex (Thabit et al., 2010), there is a possibility that the plasticity may occur at the level of the spinal cord (Taylor et al., 2009).
We observed a rapid plastic response to the planar robot training combined with stimulation. The lack of effects of time suggests that the initial rapid response was maintained throughout the course of the intervention and for a short time period following the training consistent with previous reports of rapidly developed plasticity sustained after intervention (Thabit et al., 2010). This may be, in part, regulated by homeostatic plasticity such that there is a limit to the degree of plasticity to prevent catastrophic excitability changes (Kuo et al., 2008). Although our retention phase was relatively short, additional research is required to demonstrate how plasticity is regulated over time with robotic reaching interventions to determine the most potent therapeutic applications. For example, future research should address the length of the retention phase in response to changing the length of the intervention. This can be completed within the single session time frame, i.e., the number of practiced reaches. Additionally, future research should determine what impacts consolidation over multiple sessions of reaching training with TMS.
In addition to demonstrating the impact of the stimulation timing, the results of the current study also highlight the ability to promote rapid plasticity in older adults. This is an important consideration because the majority of previous studies have used neurologically intact younger adults (approximate age of 25–30), and Rogasch et al., (2009) suggested that older adults had a reduced capacity for use-dependent plasticity with a motor training only paradigm, i.e., no stimulation during training. We had a similar range of participant ages (average age of 65), yet the TMS during the reaching is a novel component in the current study. The potential exists that TMS during motor training was the catalyst to overcome the limited plasticity observed during motor training only. The finding of enhanced plasticity when motor practice is combined with cortical stimulation has also been demonstrated with repetitive TMS (Koganemaru et al., 2010, Massie et al., 2013a). More definitive studies are needed and this represents an area of interest for future research. Another finding related to the older adult population was that we elicited TMS-evoked movements in all of our participants although the movement threshold varied substantially between participants. Interestingly, the evoked measures across baseline conditions were consistent as illustrated in Figure 2 suggesting that the TMS-evoked movements are relatively stable across time. These are important considerations to extend this line of work in survivors of stroke. Participants had improvements in motor performance over time with the robotic reaching intervention although there was no effect of condition. We postulate there was no difference between conditions because of the limited room for improvement in healthy older-adults, and these improvements were saturated with the effects of massed practice. Alternatively, practice-related changes in voluntary motor performance may have occurred that were not measured by our kinematic analysis. These could include shaping of the trial-to-trial variability of movement features such as trajectories or joint torques (Wu et al., 2014). We plan to measure such additional kinematic features in follow-up experiments and analyses as this work sheds light on the potential to use robotic interventions in older adults who may have age-related or impairment-related changes in reaching.
Conclusions
We demonstrated that the timing of TMS during robotic reaching training critically influences the type of plasticity with the most positive effects on movement amplitude observed when stimulation was delivered during the LRT phase. The consistency in directions between the evoked movement outcomes and the MEP further support the use of TMS-evoked movements as outcome measures, yet highlight the complex nature of these outcomes. Future work should focus on expanding these findings in clinically oriented populations such as survivors of stroke and for individuals experiencing age-related motor deficits.
Highlights.
Altering the timing of stimulation during a reaching intervention changes the direction and extent of plasticity.
Non-invasive brain stimulation may be a catalyst to promote plasticity in older adults.
Robotic reaching plus stimulation facilitated a rapid plastic response that was maintained during the intervention and for a short time period following the intervention.
Acknowledgments
This study was supported by USPHS NIH R01 HD061462. CLM and SSK were supported by NIDRR, UMANRRT program (H133P100014). The authors would like to thank the participants who volunteered for this study. We also thank Min Zhan, PhD, Jaime Lush, and Patti McCarthy, OTR for their assistance with data analysis, data acquisition, and participant recruitment.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bütefisch C, Heger R, Schicks W, Seitz R, Netz J. Hebbian-type stimulation during robot-assisted training in patients with stroke. Neurorehabil Neural Repair. 2011;25:645–655. doi: 10.1177/1545968311402507. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control. 2009;13:442–453. doi: 10.1123/mcj.13.4.442. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 1998;44:317–325. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron. 2010;68:387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol. 2006;96:3130–3146. doi: 10.1152/jn.00307.2006. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol. 2007;97:348–359. doi: 10.1152/jn.00808.2006. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Ziemann U, Chen R, Classen J, Hallett M, Gerloff C, et al. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:305–324. doi: 10.1097/00004691-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Conroy SS, Whitall J, Dipietro L, Jones-Lush LM, Zhan M, Finley MA, et al. Effect of gravity on robot-assisted motor training after chronic stroke: A randomized trial. Arch Phys Med Rehabil. 2011;92:1754–1761. doi: 10.1016/j.apmr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharabaghi A, Kraus D, Leao MT, Spuler M, Walter A, Bogdan M, et al. Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci. 2014;8:1–7. doi: 10.3389/fnhum.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe V, Volpe BT, Thickbroom GW, Fregni F, Pascual-Leone A, Krebs HI, et al. Reversal of TMS-induced motor twitch by training is associated with a reduction in excitability of the antagonist muscle. J Neuroeng Rehabil. 2011;8:1–8. doi: 10.1186/1743-0003-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilio F, Iacovelli E, Conte A, Frasca V, Gabriele M, Giacomelli E, et al. Asymmetric responses to repetitive transcranial magnetic stimulation (rTMS) over the left and right primary motor cortex in a patient with lateralized progressive limb-kinetic apraxia. Neurosci Lett. 2008;437:125–129. doi: 10.1016/j.neulet.2008.03.072. [DOI] [PubMed] [Google Scholar]
- Jones-Lush LM, Judkins TN, Wittenberg GF. Arm movement maps evoked by cortical magnetic stimulation in a robotic environment. Neuroscience. 2010;165:774–781. doi: 10.1016/j.neuroscience.2009.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Jones-Lush LM, Narayanan P, Judkins TN, Wittenberg GF. Rapid plasticity of motor corticospinal system with robotic reach training. Neuroscience. 2013;5:55–64. doi: 10.1016/j.neuroscience.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst GM, Hasan Z. Timing and magnitude of electromyographic activity for two-joint arm movements in different directions. J Neurophysiol. 1991;66:1594–1604. doi: 10.1152/jn.1991.66.5.1594. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Triggs WJ. Use of transcranial magnetic stimulation to influence behavior. Curr Neurol Neurosci Rep. 2007;7:491–497. doi: 10.1007/s11910-007-0076-5. [DOI] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Thabit MN, Ikkaku T, Shimada K, Kanematsu M, et al. Recovery of upper-limb function due to enhanced use-dependent plasticity in chronic stroke patients. Brain. 2010;133:3373–3384. doi: 10.1093/brain/awq193. [DOI] [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng. 1998;6:75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutky MA, Perreault EJ. Motor cortical measures of use-dependent plasticity are graded from distal to proximal in the human upper limb. J Neurophysiol. 2007;98:3230–3241. doi: 10.1152/jn.00750.2007. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, et al. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia. 2008;46:2122–2128. doi: 10.1016/j.neuropsychologia.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Vandal AC, McNair PJ. A method to monitor upper limb movement direction encoding in the corticomotor pathway. J Mot Behav. 2012;44:223–232. doi: 10.1080/00222895.2012.684081. [DOI] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. New Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacClellan LR, Bradham DD, Whitall J, Volpe B, Wilson PD, Ohlhoff J, et al. Robotic upper-limb neurorehabilitation in chronic stroke patients. J Rehabil Res Dev. 2005;42:717–722. doi: 10.1682/jrrd.2004.06.0068. [DOI] [PubMed] [Google Scholar]
- Massie CL, Tracy BL, Malcolm MP. Functional repetitive transcranial magnetic stimulation increases motor cortex excitability in survivors of stroke. Clin Neurophysiol. 2013a;124:371–378. doi: 10.1016/j.clinph.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Massie CL, Narayanan P, Kantak SS, Jones-Lush LM, Judkins TN, Wittenberg GF. Effects of motor cortical stimulation during planar reaching movement. J Rehabil Robotics. 2013b;1:42–53. [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol. 2009;107:1874–1883. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stein J, Krebs HI, Frontera WR, Fasoli SE, Hughes R, Hogan N. Comparison of two techniques of robot-aided upper limb exercise training after stroke. Am J Phys Med Rehabil. 2004;83:720–728. doi: 10.1097/01.phm.0000137313.14480.ce. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Martin PG. Voluntary motor output Is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci. 2009;29:11708–11716. doi: 10.1523/JNEUROSCI.2217-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabit MN, Ueki Y, Koganemaru S, Fawi G, Fukuyama H, Mima T. Movement-related cortical stimulation can induce human motor plasticity. J Neurosci. 2010;30:11529–11536. doi: 10.1523/JNEUROSCI.1829-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe A, Levin O, De Schutter J, Swinnen S, Jonkers I. Three-dimensional reaching tasks effect of reaching height and width on upper limb kinematics and muscle activity. Gait Posture. 2010;32:500–507. doi: 10.1016/j.gaitpost.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Wu HG, Miyamoto YR, Gonzalez Castro LN, Ölveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat Neurosci. 2014;17:312–321. doi: 10.1038/nn.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]