Abstract
Melanocortin receptors (MC3/4R) mediate most of the metabolic and cardiovascular actions of leptin.
Aim
here we tested if MC4R also contributes to leptin’s effects on respiratory function.
Methods
after control measurements, male Holtzman rats received daily microinjections of leptin, SHU9119 (MC3/4R antagonist) or SHU9119 combined with leptin infused into the brain lateral ventricle for 7 days. On the 6th day of treatment, tidal volume (VT), respiratory frequency (fR) and pulmonary ventilation (VE) were measured by whole-body plethysmography during normocapnia or hypercapnia (7% CO2). Baseline mean arterial pressure (MAP), heart rate (HR) and metabolic rate were also measured. VE, VT and fR were also measured in mice with leptin receptor deletion in the entire central nervous system (LepR/Nestin-cre) or only in proopiomelanocortin neurons (LepR/POMC-cre) and in MC4R knockout (MC4R−/−) and wild-type mice.
Results
leptin (5 μg/day) reduced body weight (~17%) and increased ventilatory response to hypercania, whereas SHU9119 (0.6 nmol/day) increased body weight (~18%) and reduced ventilatory responses compared to control-PBS group (Lep: 2119 ± 90 ml.min−1.kg−1and SHU9119: 997 ± 67 ml.min−1.kg−1, vs PBS: 1379 ± 91 ml.min−1.kg−1). MAP increased after leptin treatment (130 ± 2 mmHg) compared to PBS (106 ± 3 mmHg) or SHU9119 alone (109 ± 3 mmHg). SHU9119 prevented the effects of leptin on body weight, MAP (102 ± 3 mmHg) and ventilatory response to hypercania (1391 ±137 ml.min−1.kg−1). The ventilatory response to hypercania was attenuated in the LepR/Nestin-cre, LepR/POMC-cre and MC4R−/− mice.
Conclusion
these results suggest that central MC4R mediate the effects of leptin on respiratory response to hypercapnia.
Keywords: blood pressure, central chemoreception, hypercapnia, leptin, MC3/4 receptor, melanocortin system
Introduction
Previous studies suggest that leptin, an adipocyte derived cytokine, is an important modulator of respiration. In addition to its effects to reduce food intake and body weight and to increase sympathetic nervous system activity (SNA) and blood pressure (BP), central leptin administration also enhances baseline respiratory activity and chemorespiratory responses to carbon dioxide (CO2) (Bassi et al., 2012, Bassi et al., 2014, Hall et al., 2010, Inyushkina et al., 2010). However, the central mechanisms activated by leptin to facilitate respiratory responses are still unclear.
Previous studies have demonstrated an important role of the brain melanocortin system in mediating most of leptin’s actions on appetite, metabolism and cardiovascular function. Leptin depolarizes proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARC) inducing the release of alpha-melanocyte stimulating hormone (α-MSH) which, in turn, activates the melanocortin 3 and 4 receptors (MC3/4R) located in several hypothalamic nuclei as well as in other regions of the brain including the brainstem (Morton & Schwartz, 2011, Cowley et al., 2001). Deletion of leptin receptors specifically in POMC neurons abolished leptin’s ability to raise BP and to reduce fasting glucose and insulin levels (do Carmo et al., 2011). Moreover, pharmacological blockade of MC3/4R completely prevented the anorexic and antidiabetic effects of leptin (da Silva et al., 2004, da Silva et al., 2009). However, the role of brain MC3/4R in mediating the actions of leptin on respiratory function is not clear.
Support for a role of the brain melanocortin system in modulating ventilatory function comes from previous observations that obese agouti yellow mice, a model overexpressing the agouti protein which inhibits MC3/4R, exhibit attenuated ventilatory responses to CO2 (Polotsky et al. 2004). This finding is consistent with the possibility that MC3/4R may also be part of the mechanisms activated by the central chemoreflex that elicits respiratory responses to stimuli such as hypercapnia. Nevertheless, there have been no studies, to our knowledge, that evaluated the potential contribution of central MC3/4R on breathing.
To test the hypothesis that activation of brain MC3/4R is necessary for leptin to increase ventilatory responses to hypercapnia, we studied the effects of leptin on ventilation after chronically inhibiting MC3/4R using a pharmacological antagonist. We also examined the ventilatory responses to hypercapnia in mice with MC4R deficiency, leptin receptor deletion in the entire central nervous system (CNS), and in mice with leptin receptor deletion specifically in POMC neurons. The results suggest that central MC4R play a key role in leptin-induced increase in ventilatory response to hypercapnia.
METHODS
The experimental procedures and protocols of this study were approved by the Animal Experimentation Ethics Committee of the School of Dentistry of Araraquara, São Paulo State University, UNESP, Brazil and by the University of Mississippi Medical Center Institutional Animal Care and Use Committee, and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animals
Male 9-week-old Holtzman rats (n = 24) obtained from the colony of the São Paulo State University, Brazil, and male 20-week-old C57BL/6J wild-type (WT, n = 5), MC4R−/− (n = 5), LepR/POMC-cre (n = 3) and LepR/Nestin-cre (n = 3) mice were used. LepR/POMC-Cre mice were generated as previously described (do Carmo et al., 2011). LepR/Nestin-cre mice were generated by crossing Nestin-Cre mice (Jackson Laboratories, Bar Harbor, Maine) with LepRflox/flox mice (generously provided by Dr. Jeffrey Friedman, Rockefeller University, New York, NY). MC4R−/− and WT mice were purchased from the Jackson Laboratories. All animals were maintained on a 12 h–12 h light–dark cycle in a room with controlled temperature (23 ± 2 °C) and humidity (55 ± 10%). Standard rat chow and tap water were available ad libitum.
Intracerebroventricular cannula implantation
The rats were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg; Cristalia, Itapira, SP, Brazil) combined with xylazine (7 mg/kg; Agener União, Embu-Guaçu, SP, Brazil) and placed in a stereotaxic apparatus (Kopf, Tujunga, CA, USA). Under aseptic conditions a stainless steel cannula (10 × 0.7 mm) was implanted into the brain right lateral ventricle (LV) using the following stereotaxic coordinates: 0.4 mm caudal to bregma, 4.0 mm below the dura-mater and 1.6 mm to the right of the midline. The guide cannula was anchored to the skull with stainless steel screws and dental acrylic resin. A metal stylet was inserted to seal the guide cannula when not in use.
At the end of the surgery the rats received an intramuscular injection of benzylpenicillin (80.000 IUs) plus streptomycin (33 mg; Pentabiótico Veterinário – Pequeno Porte, Fort Dodge Saúde Animal Ltda., Campinas, Brazil (0.2 ml/rat) and a subcutaneous injection of analgesic/anti-inflamatory Ketoprofen 1% (Ketoflex, Mundo Animal, São Paulo, Brazil (0.03 ml/rat). The rats were allowed to recover for 7 days before accuracy of the cannula placement was tested by measuring the dipsogenic response (immediate drinking of at least 5 mL of water in 10 min) to a LV injection of angiotensin II (50 ng/1 μl).
Arterial catheter implantation
On the 6th day of treatment (one day before recording mean arterial pressure - MAP and heart rate - HR), the rats were anesthetized with ketamine and xylazine, as described above, and under aseptic conditions the femoral artery were isolated and cannulated with polyethylene tubes (PE-10 connected to a PE-50) filled with saline. The catheter were exteriorized between the scapulae and fixed on the back of the animal to allow recording of baseline MAP and HR in freely moving animals.
Daily i.c.v. microinjections
Leptin (5 μg/3 μl, purchased from the National Hormone & Peptide Program, CA, USA) and/or the melanocortin receptor antagonist SHU9119 (0.6 nmol/3 μl, Phoenix Pharmaceuticals, CA, USA) were administered into the LV of rats for 7 consecutive days. The doses of leptin and SHU9119 were chosen based on previous studies (Choi et al., 2003, Fendt et al, 2009, Inyushkin et al., 2009, Mark et al., 2009). The rats were divided into the following groups: 1) daily leptin treatment alone, 2) daily SHU9119 treatment alone; 3) daily SHU9119 treatment combined with leptin treatment (30 min interval between injections); and 4) Vehicle control group that received daily phosphate buffer saline injections (PBS, pH=7.4, 3 μl). The LV injections were administered at 24-h intervals for 7 days between 11:00 am and noon. The injections were made with a Hamilton syringe (5 μl capacity) coupled with a polyethylene tubing (PE-10) connected to a needle (0.3 mm in diameter and 12 mm in length). Each injection was administered into the LV over 30 s and the injector was left in place for additional 30 s to allow the drug to diffuse in the CSF, followed by removal of the injector and replacement of the stylet into the guide cannula.
Ventilation Measurements
Pulmonary ventilation (VE) was measured using whole-body plethysmography as previously described (Malan, 1973, Bassi et al., 2012). Briefly, rats and mice were acclimatized to the plethysmography chambers (Bonther, Ribeirao Preto, SP, Brazil), 5 L for rats and 1 L for mice, at room temperature (25° C) for ~40 minutes. The ports for gas exit or entrance in the chamber were closed to produce an internal constant volume. Chamber temperature was less than 1° C above room temperature (~25° C) with no significant variations over the period that all measurements were made (5 min). Rectal temperature, measured using a digital thermometer (Omron - MC-245, China) lubricated with vaseline, was constant and no significant changes were observed between groups. Signals of breathing frequency (fR, breathins.min−1) and tidal volume (VT, mV) were measured by changes in the pressure inside the chamber caused by inspiratory and expiratory temperature fluctuations using a spirometer (model ML141, AD Instruments, Colorado Springs, USA). Signals were analyzed using PowerLab data acquisition, AD Instruments, Colorado Springs, CO, USA. The system was calibrated with injections of 1 mL for rats or 0.2 mL for mice of room air with the animal inside the plethysmography chamber. VE was calculated as the product of fR and VT.
The tidal volume (VT) was calculated using the formula: , where:
PT is the pressure deflection (mV) associated with each VT,
PK is the pressure deflection (mV) associated with the calibration volume (VK) injection,
TB is the body core temperature (Kelvin degree),
TA is the air temperature in the animal chamber (Kelvin degree),
PB is the barometric pressure (mmHg),
PR is the water vapor pressure at Tc (mmHg),
PC is the vapor pressure of water vapor in the animal chamber (mmHg),
TR is the room temperature (Kelvin degree).
VE and VT are represented at the ambient barometric pressure, Tc, saturated with water vapor at this temperature (BTPS). The PC (the water vapor pressure in the animal chamber) was calculated indirectly by using an appropriate table (Dejours, P., 1981).
Measurements of metabolic rate
Oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory quotient (RQ = VCO2/VO2) were calculated using indirect calorimetry. Briefly, on the 6th day of LV treatment, the rats were acclimatized to the plethysmography chamber (5 L) at room temperature (25 °C) for ~30 minutes. The chamber was connected to a gas analyzer (model ML206, ADInstruments) to measure variations of O2 and CO2 inside the chamber during 1 minute.
Experimental protocol in rats
Rats were housed individually in standard metabolic cages for the duration of the study. Food and water intake were measured daily between 11:00 am and noon. Body weight and urinary excretion of Na+ and K+ were evaluated before starting and on day 7 of treatment. For the injections, each rat was removed from its home cage and while gently held, the stylet was removed and the injector inserted in the guide cannula. (see details of injections in Daily i.c.v. microinjections item). The rats were returned to their home cages following each injection. On the 6th day of LV injections, after receiving the injections, the rats were transferred to the plethysmography chamber for measurements of metabolic rate (VO2, VCO2 and RQ) and pulmonary ventilation under normocapnia and during central chemoreflex activation by hypercapnia (5 minutes breathing a gas mixture containing 21% O2 plus 7% CO2). To avoid the interference of the surgery, only after the end of the respiratory tests, the arterial catheter was implanted to record MAP and HR on the next day (7th day of treatment)
Experimental protocol in mice
The pulmonary ventilation protocol in mice was similar to the pulmonary ventilation protocol in rats described above. However, we did not instrument mice for LV treatment or cardiovascular measurements.
Statistical Analysis
The results are presented as mean ± SEM. Kolmogorov-Smirnov normality test and one-way or two-way ANOVA followed by Bonferroni’s post hoc test were used for comparisons using GraphPad Prism, San Diego, CA, USA. Statistical significance was accepted at the level of p < 0.05.
RESULTS
Metabolic and cardiorespiratory responses to daily LV leptin treatment
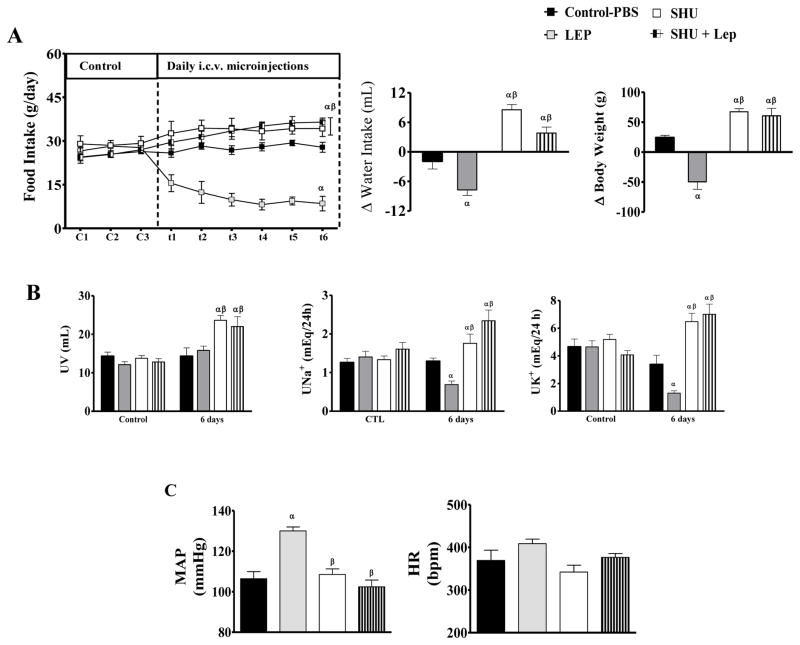
Chronic LV leptin treatment (5 μg/day) for 7 days reduced daily food intake, body weight and water intake with minor changes in urine volume (Figure 1A-B). Leptin also reduced urinary excretion of Na+ (0.68 ± 0.09 mEq/24 h, vs. PBS: 1.29 ± 0.08 mEq/24 h) and K+ (1.29 ± 0.16 mEq/24 h, vs. PBS: 3.37 ± 0.66 mEq/24 h), proportionally to the reduction in food intake (Figure 1B).
Figure 1.
(A) Daily food intake and changes in daily water intake and body weight. (B) Urinary volume (UV), Na+ (UNa+) and K+ (UK+) excretion during control period (C1-C3) and during LV treatment (t1-t6) with PBS (control), (LEP, 5 μg/day), SHU-9119 (SHU, 0.6 nmol/day) or SHU-9119 + leptin for 7 days. (C) Mean arterial pressure (MAP) and heart rate (HR) after LV treatments. n = 6/group. α = p<0.05 versus control-PBS group. β= p<0.05 versus leptin-treated group.
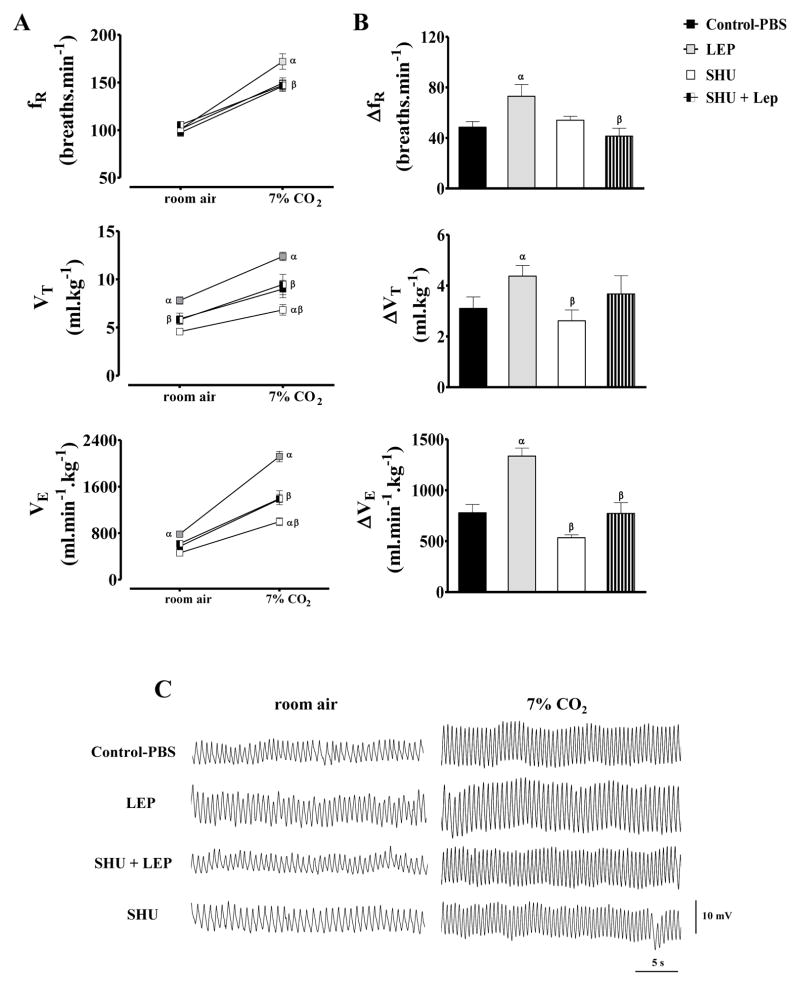
Under normocapnia (room air), leptin into the LV enhanced baseline VE (783±36 ml.min−1.kg−1, vs. control-PBS: 570±53 ml.min−1.kg−1) [F(6, 80) = 26.3; P < 0.0001] as a consequence of an increase in VT, whereas fR was not modified (Figure 2A). LV leptin treatment also enhanced 7% CO2-induced increase in VE (ΔVE = 1335±79 ml.min−1.kg−1, vs. PBS: 779±81 ml.min−1.kg−1) [F(3, 16) = 19.2; P < 0.0001] due to an increase in VT and fR (Figure 2B).
Figure 2.
(A) Respiratory frequency (fR), tidal volume (VT) and ventilation (VE) under normocapnia (room air) or hypercapnia (7% CO2), (B) changes (Δ) of fR, VT and VE produced by hypercapnia and (C) representative traces of rats breathing room air and 7% CO2 after receiving LV microinjections of PBS (control), (LEP, 5 μg/day), SHU-9119 (SHU, 0.6 nmol/day) or SHU-9119 + leptin for 6 days. n=6/group. α = p<0.05 versus control-PBS group. β = p<0.05 versus leptin-treated group.
Although VCO2 and VO2 were similar between leptin and PBS treated groups, leptin tended to reduce VCO2, which resulted in a significant reduction in RQ (0.73±0.02 ml.kg−1.min−1, vs. PBS: 0.86±0.04 ml.kg−1.min−1) (Table 1). In addition, leptin into the LV increased MAP (130±2 mmHg, vs. PBS: 106±2 mmHg) [F(6, 37) = 6.12; P < 0.0002] and also tended to increase HR (409±11 bpm, vs. PBS: 369±24 bpm) (Figure 2C).
Table 1.
VCO2, VO2, RQ, rectal temperature and body weight of rats treated with i.c.v. injections of PBS (control), leptin, SHU-9119 or SHU-9119 combined with leptin for 7 days.
| PBS | LEP | SHU | SHU + LEP | |
|---|---|---|---|---|
| VCO2 (ml.kg−1.min−1) | 12.9 ±1 | 10.9 ±0.7 | 12.7 ±0.5 | 11.3 ±0.5 |
| VO2 (ml.kg−1.min−1) | 15.1 ±1 | 15.1 ±0.6 | 13.8 ±0.6 | 13.7 ±0.3 |
| RQ (VCO2/VO2) | 0.86 ±0.04 | 0.73 ±0.02 α | 0.92 ±0.01 β | 0.83 ±0.04 β; |
| Rectal Temperature (°C) | 36.7 ±0.2 | 36.8 ±0.2 | 36.6 ±0.2 | 36.7 ±0.1 |
| Body Weight (g) | 342 ±8 | 286 ±9 α | 375 ±7 αβ | 386 ±12 αβ |
Values are means ±SE of metabolic rate (VCO2, CO2 consumption; VO2, oxygen consumption; RQ respiratory quotient) after 6 days of i.c.v. treatments with PBS (3 μl/day), leptin (5 μl/3 μl/day), SHU9119 (0.6 nmol/3 μl/day) or SHU9119+leptin (0.6 nmol/3 μl/day and 5 μg/3 μl/day, respectively). n = 6/group. α different from PBS and β different from leptin. ANOVA one-way (P < 0.05).
Metabolic and cardiorespiratory responses to daily LV treatment with SHU9119 alone or in combination with leptin
LV injections of the MC3/4R antagonist SHU9119 (0.6 nmol/day) increased daily food intake (36.5 ± 1.4 g, vs. PBS: 27.8 ± 1.7 g) and water intake (Δ+8.6 ±1.1 mL, vs. PBS: Δ −1.9 ± 1.6 mL). Body weight and urine volume increased proportionally to increases in food intake, which also explains the increased urinary Na+ and K+ excretion (Figure 1 A-B).
Baseline VE, VT and fR in normocapnia were not modified by SHU9119 treatment alone (Figure 2). During exposure to 7% CO2, the treament with SHU9119 reduced VE (997 ± 67 ml.min−1.kg−1, vs. PBS: 1379 ± 91 ml.min−1.kg−1) [F(6, 80) = 26.3; P < 0.01] and VT (6.83 ± 0.5 ml.kg−1 vs. PBS: 8.99 ± 0.89 ml.kg−1) [F(6, 80) = 15.4; P<0.05] (Figure 2-A). However, the increase (delta) of VE and VT produced by the exposure to 7% CO2 in rats treated with SHU9119 i.c.v. was not statistically different compared to control-PBS treatment (Figure 2-B). SHU9119 also did not alter fR, RQ, MAP or HR (Table 1 and Figure 2).
Although SHU9119 treatment alone for 7 days did not evoke marked changes in respiratory, metabolic or cardiovascular function, it abolished leptin’s effects to reduce food intake and body weight (Figure 1 and Table 1). In fact, rats treated with SHU9119 + leptin exhibited an increase of ~27% and ~13%, respectively, of food intake and body weight compared to control (Figure 1 and Table 1). Combined treatment, similar to SHU9119 alone, increased water intake and urine volume (Figure 1 A-B) and also increased urinary Na+ and K+ proportionally to the increases observed in food intake (Figure 1 A-B). SHU9119 abolished the increase in VE and VT produced by leptin in rats exposed to normocapnia or 7% CO2 (Figure 2-AB). SHU9119 also prevented the reduction in RQ and the increase in MAP evoked by leptin (Table 1, Figure 2C).
Baseline ventilation and ventilatory response to CO2 in LepR/Nestin-cre, LepR/POMC-cre and MC4R−/− mice
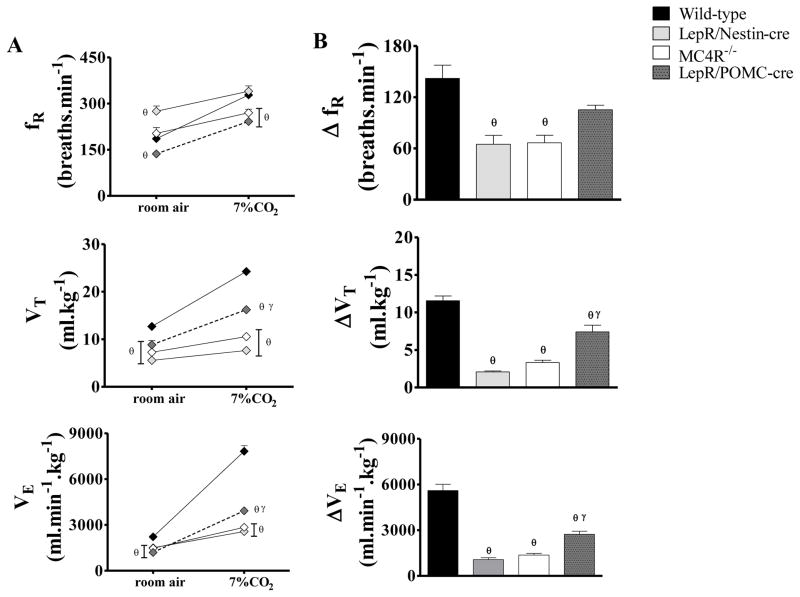
Baseline ventilation under normocapnia was reduced in LepR/Nestin-cre (1501 ± 105 ml.min−1.kg−1), LepR/POMC-cre (1197 ± 95 ml.min−1.kg−1) and MC4R−/− (1473 ± 124 ml.min−1.kg−1) compared to the wild-type (WT, 2221 ± 170 ml.min−1.kg−1) [F(3, 20)= 66.9; P < 0.0001]. LepR/Nestin-cre, LepR/POMC-cre and MC4R−/− mice had also reduced VT. LepR/POMC-cre mice also showed reduced fR, whereas LepR/Nestin-cre mice increased fR compared to WT (Figure 3A).
Figure 3.
(A) Respiratory frequency (fR), tidal volume (VT) and ventilation (VE) under normocapnia (room air) or hypercapnia (7% CO2) and (B) changes (Δ) of fR, VT and VE produced by hypercapnia in wild-type mice, in mice with leptin receptor deletion in the entire brain (LepR/Nestin-cre), in mice with leptin receptor deletion in POMC neurons (LepR/POMC-cre) and in mice with MC4R deficiency (MC4R−/−). n=3–5/group. θ = p<0.05 versus wild-type group and γ = p<0.05 versus LepR/Nestin-cre group.
The ventilation under 7% CO2 was markedly attenuated in LepR/Nestin-cre (2569 ± 215 ml.min−1.kg−1), LepR/POMC-cre (3923 ± 112 ml.min−1.kg−1) and MC4R-/- mice (2843 ± 24 ml.min−1.kg−1) compared to WT (7825 ± 373 ml.min−1.kg−1) [F(3, 20) = 66.9; P < 0.001] and this was mainly caused by a smaller increase in VT (Figure 3-A). LepR/POMC-cre and MC4R−/− mice also exhibited the smallest increase in fR under 7% CO2 compared to WT mice (Figure 3A). LepR/Nestin-cre mice presented fR similar to WT (Figure 3). The changes in ventilatory response during 7% CO2 were also attenuated when compared to WT values (Figure 3-B).
DISCUSSION
The present study demonstrates that chronic MC3/4R blockade with SHU9119 administered into the LV of rats reduced ventilatory responses to hypercapnia and abolished leptin-induced increase in baseline ventilation and in the respiratory response to central chemoreceptor activation. This suggests that the ventilatory effects of leptin depend on the activation of central MC3/4R. Moreover, the present results also showed that mice with leptin receptor deletion in the entire CNS or specifically in POMC neurons as well as mice with MC4R deficiency exhibit attenuated baseline and ventilatory responses to CO2, which reinforces the notion that leptin-induced improvement in ventilatory function is mediated by leptin’s direct actions on the CNS and requires functional brain melanocortin system including POMC-MC4R pathway.
The present results also corroborate previous studies demonstrating that chronic CNS MC3/4R blockade increases appetite, promotes rapid weight gain, abolishes leptin’s ability to raise BP (Kuo et al, 2003, da Silva et al., 2004) and to reduce RQ (Dubinion et al., 2010). LV leptin administration for 7 days decreased daily food intake and water intake and consequently body weight, without altering urine volume. However, as expected, due the marked reduction in food intake, daily urinary Na+ and K+ excretion were also reduced during leptin treatment. Similar to previous studies (Wang et al., 1999, Hogberg et al., 2006, Dubinion et al., 2010) our results also demonstrate that leptin decreases RQ due to a decreased VCO2. In spite of the reduction of VCO2, leptin improved the ventilatory response, which suggests that the effects of leptin on ventilation are due to a direct action on central mechanisms that control breathing and not to metabolic changes.
Previous studies have suggested that leptin facilitates ventilatory responses mainly by acting on brainstem areas involved in breathing control including the nucleus of solitary tract (NTS) (Inyushkina et al., 2010, Ciriello & Moreau 2013) and ventrolateral medulla (Bassi et al., 2012 and 2014). In the present study leptin was delivered into the LV and may have reached forebrain or hindbrain regions that contribute to breathing control. Leptin receptor deletion specifically in POMC neurons resulted in reduced ventilatory responses to hypercapnia, but given that POMC neurons are present in many areas of brain it is difficult to determine which groups of POMC neurons are most important in modulating ventilatory function and perhaps mediating, at least in part, leptin’s effects on breathing. Thus, additional studies are needed to address these questions.
Although an action of leptin in the forebrain to facilitate ventilatory responses is possible, these responses to chemical stimulation like hypercapnia and hypoxia result from an increased activity of the medullary neuronal network responsible for the generation and maintenance of the basic respiratory rhythm. However, since the 1960s and 1970s hypoxia or hypercapnia, as well as the electrical stimulation of the carotid sinus nerve, have been shown to activate the caudal hypothalamic region (Cross, 1963, Thomas, 1972) as well other areas of the brain like periaqueductal grey mesencephalic region (Lopes et al., 2014). Moreover, acid-sensing ion channels (ASICs) and transient receptor potential ankyrin subfamily member 1 (TRPA1), both described as a mediators of chemorespiratory responses can be found in the hypothalamus (Song et al., 2012, Pokorski et al., 2014). Elevated CO2 markedly increases c-Fos positive cells in PVN (Kc et al., 2002) and electrical stimulation of the PVN promotes an increase in ventilation (Duan et al., 1996, Yeh, 1997). Additionally, the disinhibition of neurons in the dorsomedial hypothalamus (DMH) promotes an increase in phrenic nerve activity (Mc Dowwall et al., 2007), while microinjection of gabazine into the perifornical region (PeF) in the hypothalamus, a region that express leptin receptors (Laque et al., 2013) promotes an increase of blood pressure, phrenic nerve discharge and firing rate of the chemosensitive retrotrapezoid neurons (Fortuna et al., 2009, Li & Nattie., 2013).
Therefore, with the present data it is not possible to determine the specific area of the brain where the melanocortin receptors activated by leptin to mediate ventilation are located.
Similar to a previous study (da Silva et al., 2004), our data demonstrate that leptin-mediated increase in BP is abolished by pre-treatment with SHU9119, which indicates the involvement of MC3/4R in mediating the actions of leptin on BP regulation. However, contrary to the previous study, we did not observe a reduction in BP or HR during SHU9119 treatment alone. One potential explanation for this difference may be the fact that in the present study we administered SHU9119 via daily LV bolus microinjections (0.6 nmol/day), whereas in the previous study SHU9119 was delivered continuously into the LV using osmotic minipumps at a rate of 1 nmol/h. Although SHU9119 treatment regimen used in the present study did not alter BP or HR, it produced the expected effects on appetite and it also affected ventilatory function.
In conclusion, the present results suggest that the brain melanocortin system, present including POMC neurons and MC3/4R, is a key component of the central mechanism activated by leptin to facilitate ventilatory responses. This study extends our previous findings and demonstrates that a functional brain melanocortin system is critical for leptin’s regulation of appetite, energy homeostasis and cardiovascular regulation and highlights yet another major important physiological function of the melanocortin system - modulation of ventilatory function.
Acknowledgments
Sources of research support: FAPESP (09/53205-3 and 09/54888-7), CNPq, Capes, NHLBI (PO1HL51971), and NIGMS (P20GM104357).
The authors work was supported by grants from FAPESP (09/53205-3 and 09/54888-7), CNPq, NIH (NHLBI PO1 HL51971 and NIGMS-P20GM104357).
Footnotes
CONFLICT OF INTEREST
We have no conflict of interest to disclose.
References
- Bassi M, Giusti H, Leite CM, Anselmo-Franci JA, do Carmo JM, da Silva AA, Hall JE, Colombari E, Glass ML. Central leptin replacement enhances chemorespiratory responses in leptin-deficient mice independent of changes in body weight. Pflügers Archiv European Journal of Physiology. 2012;464:145–153. doi: 10.1007/s00424-012-1111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi M, Furuya WI, Menani JV, Colombari DS, do Carmo JM, da Silva AA, Hall JE, Moreira TS, Wenker IC, Mulkey DK, Colombari E. Leptin into the ventrolateral medulla facilitates chemorespiratory response in leptin deficient (ob/ob) mice. Acta Physiol (Oxf) 2014;211(1):240–8. doi: 10.1111/apha.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Li CL, Page K, Westby A, Della-Fera MA, Lin J, Hartzell DL, Baile CA. Melanocortin receptors mediate leptin effects on feeding and body weight but not adipose apoptosis. Physiology & Behavior. 2003;79:795–801. doi: 10.1016/s0031-9384(03)00205-1. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Moreau JM. Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat. Neuroscience. 2013;229:88–99. doi: 10.1016/j.neuroscience.2012.10.065. [DOI] [PubMed] [Google Scholar]
- Cross BA, Silver IA. Unit activity in the hypothalamus and the sympathetic response to hypoxia and hypercapnia. Exp Neurol. 1963;7:375–393. doi: 10.1016/0014-4886(63)90019-0. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin3/4 receptors in mediating chronic cardiovascular, renal and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes. 2009;58(8):1749–1756. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejours P. Principle of Comparative Respiratory Physiology. 2. Elsevier; New York: 1981. [Google Scholar]
- do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan YF, Winters R, McCabe PM, Green EJ, Huang Y, Schneiderman N. Behavioral characteristics of defence and vigilance reactions elicited by electrical stimulation of the hypothalamus in rabbits. Behav Brain Res. 1996;81:33–41. doi: 10.1016/s0166-4328(96)00042-3. [DOI] [PubMed] [Google Scholar]
- Dubinion JH, da Silva AA, Hall JE. Enhanced blood pressure and appetite responses to chronic central melanocortin-3/4 receptor blockade in dietary-induced obesity. Hypertension. 2010;28(7):1466–1470. doi: 10.1097/HJH.0b013e328339f20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Burki H, Imobersteg S, Lingenhohl K, McAllister KH, Orain D, Uzunov DP, Chaperon F. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology. 2009;206(2):291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the re trotrapezoid nucleus by posterior hypothalamic stimulation. J Physiology. 2009;587(21):5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced Hypertension: Role of Sympathetic Nervous System, Leptin and Melanocortins. J Biological Chemistry. 2010;285(23):17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg H, Engblom L, Ekdahl A, Lidell V, Walum E, Alberts P. Temperature Dependence of O2 Consumption; Opposite Effects of Leptin and Etomoxir on Respiratory Quotient in Mice. Obesity. 2006;14:673–682. doi: 10.1038/oby.2006.76. [DOI] [PubMed] [Google Scholar]
- Inyushkin AN, Inyushkina EM, Merkulova NA. Respiratory Responses to Microinjections of Leptin into the Solitary Tract Nucleus. Neurosc Behav Physiol. 2009;39(3):231–240. doi: 10.1007/s11055-009-9124-8. [DOI] [PubMed] [Google Scholar]
- Inyushkina EM, Merkulova NA, Inyushkin AN. Mechanisms of the Respiratory Activity of Leptin at the Level of the Solitary Tract Nucleus. Neurosc Behav Physiol. 2010;40(7):707–713. doi: 10.1007/s11055-010-9316-2. [DOI] [PubMed] [Google Scholar]
- Kc P, Haxhiu MA, Trouth CO, Balan KV, Anderson WA, Mack SO. CO2-induced c-Fos expression in hypothalamic vasopressin containing neurons. Respiration Physiology. 2002;129:289–296. doi: 10.1016/s0034-5687(01)00321-8. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, da Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41:768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- Laque A, Zhang Y, Gettys S, Nguyen T, Bui K, Morrison CD, Heike M. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. American Journal of Physiology - Endocrinology and Metabolism. 2013;304:E999–E1011. doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li A, Nattie E. Focal microdialysis of CO(2) in the perifornical – hypothalamic area increases ventilation during wakefulness but not NREM sleep. Respir Physiol Neurobiol. 2013;185(2):349–355. doi: 10.1016/j.resp.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LT, Biancardi V, Vieira EB, Leite-Panissi C, Bícego KC, Gargaglioni LH. Participation of the dorsal periaqueductal grey matter in the hypoxic ventilator response in unanaesthetized rats. Acta Physiol (Oxf) 2014;211(3):528–537. doi: 10.1111/apha.12254. [DOI] [PubMed] [Google Scholar]
- McDowall LM, Horiuchi J, Dampney RAL. Effects of disinhibition of neurons in the dorsomedial hypothalamus on central respiratory drive. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1728–R1735. doi: 10.1152/ajpregu.00503.2007. [DOI] [PubMed] [Google Scholar]
- Malan A. Ventilation measured measured by body plethysmography in hibernating mammals and in poikitotherms. Respir Physiol. 1973;17:32–44. doi: 10.1016/0034-5687(73)90108-4. [DOI] [PubMed] [Google Scholar]
- Mark AL, Agassandian K, Morgan DA, Liu X, Cassel MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Schuwartz MW. Leptin and CNS control of glucose metabolism. Physiol Rev. 2011;91(2):389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky VY, Smaldone MC, Scharf MT, Li J, Tankersley CG, Smith PL, Schwartz AR, O’Donnell CP. Impact of interrupted leptin pathways on ventilatory control. J Appl Physiol. 2004;96:991–998. doi: 10.1152/japplphysiol.00926.2003. [DOI] [PubMed] [Google Scholar]
- Pokorski M, Takeda K, Sato Y, Okada Y. The hypoxic ventilatory response and TRPA1 antagonism in conscious mice. Acta Physiol (oxf) 2014;210:928–938. doi: 10.1111/apha.12202. [DOI] [PubMed] [Google Scholar]
- Song N, Zhang G, Geng W, Liu Z, Jin W, Li L, Cao Y, Zhu D, Yu J, Shen L. Acid Sensing Ion Channel 1 in Lateral Hypothalamus Contributes to Breathing Control. PLoSONE. 2012;7(7):e399, 82. doi: 10.1371/journal.pone.0039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MR, Calaresu FR. Responses of single units in the medial hypothalamus to electrical stimulation of the carotid sinus nerve in the cat. Brain Res. 1972;44:49–62. doi: 10.1016/0006-8993(72)90365-4. [DOI] [PubMed] [Google Scholar]
- Wang TL, Hartzell DL, Rose BS, Flatt WP, Hulsey MG, Menin NK, Makula RA, Baile CA. Metabolic responses to intracerebroventricular leptin and restricted feeding. Physiol Behav. 1999;65:839–848. doi: 10.1016/s0031-9384(98)00243-1. [DOI] [PubMed] [Google Scholar]
- Yeh ER, Erokwu B, LaManna JC, Haxhiu MA. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci Lett. 1997;232:63–66. doi: 10.1016/s0304-3940(97)00579-x. [DOI] [PubMed] [Google Scholar]