Abstract
In the adult, immune and neural processes jointly modulate pain. During development, both are in transition and little is known about the role that the immune system plays in pain processing in infants and children. The objective of this study was to determine if inhibition or augmentation of the immune system would alter pain processing in the infant rat, as it does in the adult. In Experiment 1, rat pups aged 3, 10 or 21 (PN3, PN10, PN21) days of age were pre-treated with NS398 (selective COX-2 inhibitor) or SC560 (selective COX-1 inhibitor) and tested in the intraplantar formalin test to assess effects of COX inhibition on nociception. Neither drug had an effect on the behavioral response at PN3 or PN10 pups but both drugs attenuated nociceptive scores in PN21 pups. cFos expression in the spinal cord likewise was reduced only at PN21. In Experiment 2, pups were injected with LPS prior to the formalin test at PN3 and PN21. LPS increased the nociceptive response more robustly at PN21 than at PN3, while increasing cytokine mRNA equally at both ages. The augmentation of pain responding at PN21 was largely during the late stages of the formalin test, as reported in the adult. These data support previous findings demonstrating late maturing immune modulation of nociceptive behaviors.
INTRODUCTION
The immune system is intimately involved in pain. But during development there are a number of transitions, some gradual, some abrupt, in both neural processes that regulate pain (Fitzgerald, 2005) and in the immune system (Bilbo and Schwarz, 2012). How these processes come together during development to process and modulate pain following tissue damage is not known in any detail (Titinchi and Clark, 1984; Bilbo and Schwarz, 2012). Because infection in human neonates is more common than at any other age (Fanos et al., 2007; Polin et al., 2012) and because large numbers of infant and child patients undergo multiple and long-term medical procedures that are necessary but painful, it is important clinically to understand how immune processes and neural systems engage each other during development.
In the study presented here, we examined the age at which specific cyclooxygenase (COX) inhibition, the mechanism by which NSAID’s act, transitions from inactive to analgesic in the preweaning rat pup. The extent data show that COX inhibitors do not alleviate pain following peripheral tissue injury in infant rats. For example, general COX inhibitor (ketorolac) was not analgesic in the formalin model of nociception in 3 day old rat pups (Gupta et al., 2001), and systemic or intrathecal administration of SC560 (a selective COX-1 inhibitor) did not alter paw withdrawal thresholds in two-week old animals (Ririe et al., 2004; Ririe et al., 2006). Thus it appears that at least some pain inhibitory actions of COX inhibitors are not functional in young rat pups. The first goal of these studies was to study more closely the developmental course of action of specific COX-1 and COX-2 inhibitors on the pain response in infant rats and to define the age at which both inhibitors become effective.
Upregulation of the background immune system augments pain (Grace et al., 2014). In the adult, lipopolysaccharide (LPS), stimulates the immune system (Voituron et al., 2012) and enhances pain in human and non-human models through cytokine activation (Watkins et al., 1994; Yirmiya et al., 1994; Cunha et al., 2000; Reeve et al., 2000; Cahill et al., 2003; Bressan et al., 2006; Cao et al., 2009; Benson et al., 2012; Yoon et al., 2012). In the few studies that have investigated LPS and nociception in infants, a single intrathecal injection of LPS had a small but statistically significant hyperalgesic action at 10 days of age, which was less than that of the adult (Moss et al., 2007; Vega-Avelaira et al., 2007). LPS injected systemically at PN2 and PN4 (50 μg/kg; PN0 is birth) induced hyperalgesia in the formalin test at PN 12 and PN21, but not PN6, demonstrating an age dependent effect. The interpretation of this age dependent effect is complicated because the age of LPS injection and the time between LPS and nociceptive testing were not independently varied. Therefore the LPS effect may be developmental but also might be due to the delay between LPS treatment and testing (Zouikr et al., 2014). We hypothesized that LPS would increase nociception and enhance nociception-induced pro-inflammatory processes only in older rat pups, and further predicted that in the older pups, the effect would be mainly in the later phases of the formalin test, as it is in the adult (Padi and Kulkarni, 2005).
MATERIALS AND METHODS
Experimental Animals
All experiments were approved by IACUC’s at New York State Psychiatric Institute and the Children’s Hospital of Philadelphia, where the experiments were conducted, and followed Ethical Guidelines of the Society for Neuroscience and the International Society Developmental Psychobiology.
Subjects were Long-Evans hooded rats (3, 10 or 21 days old) bred and born in our animal facility. Within a given litter, animals were randomly assigned to experimental groups. The pups were housed with their mothers in plastic cages measuring 40 × 20 × 24 cm with bedding, food and water available ad libitum. The colony room was maintained at 24°C with a 12 h light-dark cycle (lights on at 08:00 hours). Cages were checked twice a day at approximately 9:00 and 18:00 hours and pups found at either time were designated 0 days of age. Pups were separated from their mothers immediately prior to the experiment and were kept warm using incubators and heating pads.
Formalin test
To assess nociception, formalin (2%) was injected into the plantar surface of the left hind paw. Female and male pups, 3 days of age (PN) received 10μl, PN10 pups received 20 μl and PN21 pups 50μl of formalin (all ~1 μl/g body weight). The formalin test was chosen because it produces a robust response across ages and for which there are substantial prior data (Guy and Abbott, 1992; Barr, 1998; Teng and Abbott, 1998). We did not include saline injections because intraplantar saline does not elicit nociceptive responding. Nociceptive behaviors were scored and recorded for 45 minutes, starting immediately after the formalin injection. These behaviors were recorded at 1-minute intervals using a 5 point score scale. Score 0= paw resting on surface (similar to the un-injected paw), score 1= body weight favoring injected paw, score 2= injected paw lifted up above the surface, score 3=shaking injected paw, score 4= licking and/or biting injected paw. The scale includes a range of behaviors allows for the changing motor abilities of the pups with age. The scores are lower in the younger animals because of limited motor abilities (e.g. licking and biting are rare at PN3), and the interphase of the test, seen in adults and older pups, is diminished at PN3, likely due to limited descending inhibition (Guy and Abbott, 1992; Teng and Abbott, 1998). It should be noted, that despite their youth, and fetuses and pups increase their behavioral responses to increased nociceptive input, and that measures such as lifting or shaking the paw correlate highly with formalin concentrations(Teng and Abbott, 1998).
Paw Edema
To assess whether COX inhibition altered paw edema, the dorsal-ventral and lateral diameter of the right and left hindpaw of each pup were measured two hours after formalin injection using calipers accurate to 0.001 mm. These measurements were multiplied to obtain a “volume” score for the 1 mm segment that was measured. The right hindpaw served as a control for the degree of inflammation in the formalin-injected left hindpaw.
Drug preparation and administration
The drugs used were NS398 and SC560 [Cayman Chemical (Ann Arbor, MI)]. 70% DMSO (Sigma Aldrich) served as the vehicle. Rats were injected with DMSO (all injections, 1ml/100g body weight, i.p.), or NS398 (COX-2 Inhibitor) or SC560 (COX-1 inhibitor). Three drug doses were used for each drug (3, 10 and 30mg/kg i.p.); these doses were taken from the literature in adult and infant animals (Tegeder et al., 2001; Ririe et al., 2004; Ndengele et al., 2008). Although the experimenters knew the drug that was tested, they were blind to the dose, including vehicle. After the drug injection, rats were then placed into the temperature controlled testing chamber for a one-hour habituation period and then injected with formalin.
LPS (Sigma, Cat. # L6511), an endotoxin from gram-negative bacteria, acts through TLR4 receptors, and was initially tested at several concentrations taken from the literature (0.001%–0.04%; 10 μl/g body weight). We tested only 3- and 21-day old pups, and not PN10 pups. We settled on a dose that did not produce obvious illness or weight loss (0.001%; 100 μg LPS/kg body weight; ~1μg or ~4 μg/animal in 100 or 400 μl i.p. for 3 or 21 day old pups respectively). This dose is consistent with those reported in the literature for adult rats [e.g. (Yirmiya et al., 1994)]. We injected LPS or saline 4, 8, 16 or 72 hours before the formalin test and assessed the nociceptive responses as described. In studies in adult animals, LPS enhanced formalin induced nociception at 12 and 16 hours after LPS, but at not at 0 (immediate) or 4 hours after LPS (Padi and Kulkarni, 2005). Immune activation occurs early after LPS (Philbin et al., 2010; Voituron et al., 2012) and in our model, mRNA cytokines and immune related cells were stimulated at 4 hours and remained so for up to 16 hours.
Quantitative PCR
Spinal cord lumbar dorsal horn was excised at different times after the formalin injection for the 3- and 21-day-old pups. c-Fos mRNA was analyzed by quantitative PCR (qPCR) using Taqman methods for both PN03 and PN21 pups. We also assayed c-fos at the 2 hour post-formalin time point in pups that had been treated with SC560 and NS498 to provide a separate confirmation of the behavioral data (Barr et al., 2005; Barr et al., 2009). For the LPS experiments gene expression of a number of immune markers and cytokines was assessed at 4, 16 and 72 hours after LPS injection to parallel the time course for the behavioral experiment. All primers are shown in Table 1. We did not have complete data at the 8 hour time point for the PCR analysis and do not include it here.
Table 1.
Taqman sequences for the quantitative PCR
| Name | Gene ID | Amplicon Length | Location | Sequence |
|---|---|---|---|---|
|
| ||||
| c-fos | X06769.1 | 58 | 274 | aggacttttgcgcagatctgtccgtctctagtgccaactttatccccacggtgacagc |
| IBA-1 | NM_017196.3 | 86 | 289 | atatcgatattatgtccttgaagcgaatgctggagaaacttggggttcccaagacccatctagagctgaagaaattaattagagag |
| GFAP | NM_017009.2 | 76 | 1185 | tccgagaaaccagcctggacaccaaatctgtgtcagaaggccacctcaagaggaacatcgtggtaaagacggtgga |
| COX-2 | NM_017232.3 | 112 | 312 | gtccagatcacatttgattgacagcccaccaacttacaatgtgcactacggttacaaaagttgggaagctttctccaacctctcctactacaccagggcccttcctcctgtg |
| IL-1α | NM_017019.1 | 73 | 682 | gaaggagattccggaaacaccaaaactcatcacaggtagtgagaccgacctcattttcttctgggaaaaaatc |
| IL-β | NM_031512.2 | 74 | 525 | gtattctccatgagctttgtacaaggagagacaagcaacgacaaaatccctgtggccttgggcctcaaggggaa |
| IL-6 | NM_012589.1 | 121 | 383 | gaaatttgcctattgaaaatctgctctggtcttctggagttccgtttctacctggagtttgtgaagaacaacttacaagataacaagaaagacaaagccagagtcattcagagcaatactg |
| CCL2 | NM_031530.1 | 95 | 155 | cagttaatgccccactcacctgctgctactcattcactggcaagatgatcccaatgagtcggctggagaactacaagagaatcaccagcagcagg |
| CCL3 | NM_013025.2 | 63 | 253 | gtcattttcctgaccaagagaaaccggcagatctgcgctgaccccaaagagacctgggtccaa |
| CCL5 | NM_031116.3 | 80 | 228 | agtcgtctttgtcactcgaaggaaccgccaagtgtgtgccaacccagagaagaagtgggttcaagaatacatcaactatt |
| TNF-α | NM_012675.3 | 92 | 329 | agaagttcccaaatgggctccctctcatcagttccatggcccagaccctcacactcagatcatcttctcaaaactcgagtgacaagcccgta |
Note: All primers were purchased from Applied Biosciences.
Statistics
All data were analyzed by factorial analysis of variance (ANOVA) followed by posthoc analysis using Sidak’s test to correct for multiple comparisons. For the formalin test, the behaviors were combined into 3-minute bins to reduce minute-by-minute variability and were a within-subjects variable. The dose response studies were conducted within a single litter, with different pups within a litter receiving all doses and the vehicle. Thus these doses were within litter variables and were analyzed by a repeated measures design to account for the genetic and environmental commonalities within litters (Wainwright, 1998; Festing, 2006). Although the rating scale is ordinal, parametric statistics were used because the distribution of the scores was reasonably normal. A discussion of the type of analysis appropriate to types of statistical variables is found in Marascuilo and McSweeney [(Marascuilo and McSweeney, 1977), see also (Ponten et al., 2012)].
The overall edema “volume” score was analyzed by an ANOVA with the drug dose a repeated measures (e.g. litter) effect. For the PCR data, relative expression (2−ΔΔCt) was compared using GAPDH as the loading control. Separate analysis of GAPDH cycles showed no effects of experimental treatment. Different ages were analyzed separately by a one-way repeated measures ANOVA on the 2−ΔCt PCR cycles for each primer. Posthoc comparisons between the saline and LPS injections were at each time point (4, 16 or 72 hours post-injection), also by Sidak’s test.
RESULTS
Behavior
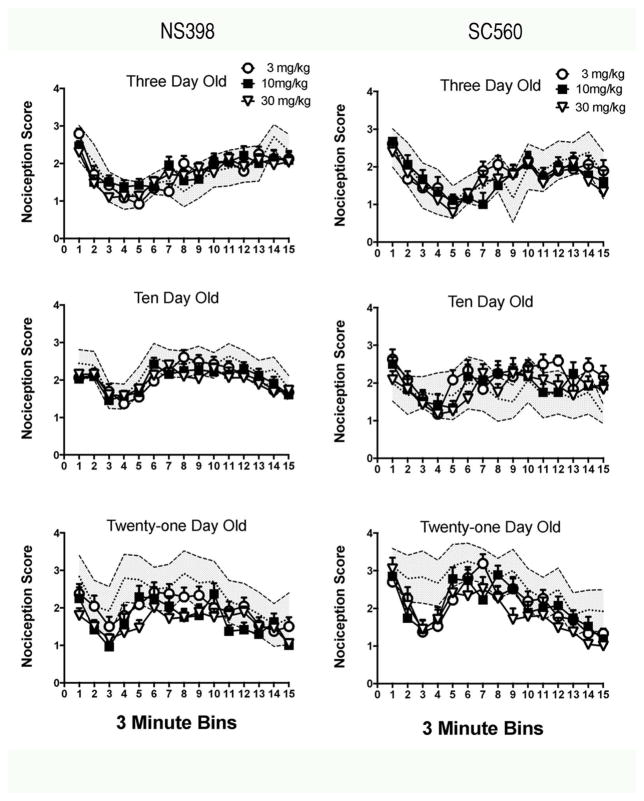
The results for both drugs were similar. SC560 and NS398 attenuated nociceptive scores in PN21 animals but had no effect in either PN3 or PN10 pups (PN3 dose effects: p=0.638 and 0.939 for SC560 and NS398 respectively; PN10 dose effects: p=0.065 and 0.277 for SC560 and NS398). Note that the trend in main effects and the trend for a bin X drug dose interaction (see below) for SC560 are towards increased nociception towards the end of the test session. Each drug was quite effective at PN21 [F(3,30)=11.89, p<0.0001 for SC560; F(3,21)=7.35, p=0.0015 for NS398; Figure 1]. Post hoc comparisons of the doses with the control at PN21 showed significant effects of all three doses of SC560 (p=0.0057, p=0.0007, p<.0001 for the low, medium and high dose) and for the medium and high dose for NS398 (p= 0.052, 0.001, 0.004). For each drug at all ages there was a bins effect (p<0.0001) but no bin X drug dose interaction (all p’s>0.10 except SC560 at PN10, p=.093). Thus there is a transition in the age of analgesic onset of COX-1 and COX-2 inhibitors that is late in the preweaning period, confirming and expanding on prior studies (Gupta et al., 2001; Ririe et al., 2004).
Figure 1. Behavioral effects of SC560 and NS398.
All pups were injected with formalin since saline injections to the paw do not produce nociceptive responses. The nociceptive response is on the Y-axis. The gray shaded area is the 95% confidence interval for the vehicle + formalin treated pups. The confidence interval shows both the effect size and significance (points outside the 95% confidence interval are significant p<.05) for individual bins. There was no drug effect or drug X bin interaction at 3 or 10 days of age for either drug, although there was a trend for SC560 to increase responding at 10 days of age. In contrast, at 21 days of age both drugs were equally effective at reducing the nociceptive response. The effect is consistent at all phases of the response. N=8, 9, and 8 for PN3, PN10 and PN21 for SC560; N=8, 12, and 8 for PN3, PN10 and PN21 for NS398.
c-Fos expression
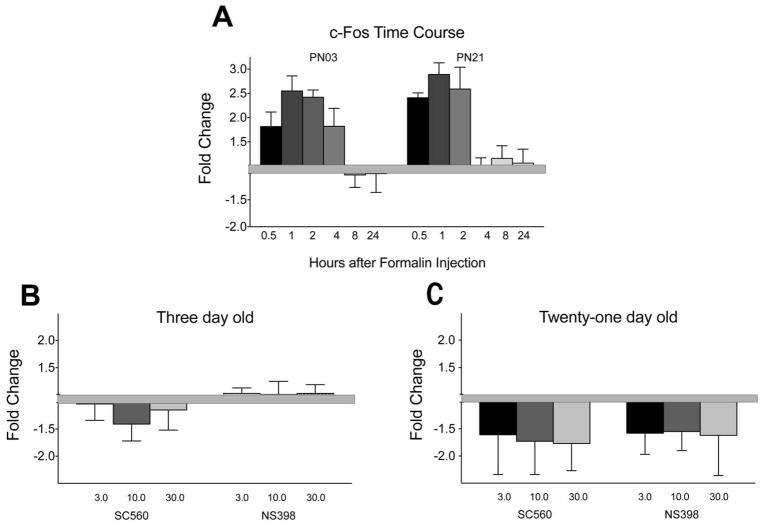
c-Fos mRNA in un-drugged animals was increased at both ages for up to 4 hours at PN3 and up to 2 hours for PN21 after the formalin injection (Figure 2A). At three days of age, neither SC560 nor NS398 altered c-fos expression at two hours (Figure 2B; all <1.5 fold compared to the vehicle control) whereas at PN21, both drugs reduced expression over 1.5 fold at each dose for both drugs, confirming the behavioral results (Figure 2C).
Figure 2. Effects of formalin and SC560 and NS398 on c-fos mRNA.
Panel A. This panel shows the effects of the formalin injection on c-Fos mRNA. c-Fos mRNA was increased for 2–4 hours after the injection in the ipsilateral spinal cord dorsal, consistent with the literature. Panel B. In the 3 day old pups, neither drug reduced c-fos mRNA. All fold changes were <1.5. Panel C. Both NS398 and SC560 reduced c-fos mRNA between 1.5–1.8 fold in the 21-day-old pups compared to the drug vehicle. N=7–9 per time point at each age. N for the drug effect on c-fos was 4 for PN3 and PN21.
Edema
Paw volumes were calculated for the injected paw (dorsal-ventral X lateral dimensions). These were 35 to 70% larger on the injected side compared to those of the contralateral paw [F(1,32)=366.4 for NS398; F(1,23)=213.4 for SC560; p<0.001 for both; Table 2). There were no drug effects that were specific to the injected paw.
TABLE 2.
Edema
| SC560 Ipsilateral |
|||||
|---|---|---|---|---|---|
| Age | N | Vehicle | 3 mg/kg | 10 mg/kg | 30 mg/kg |
| 3 | 5 | 13.7±1.1 | 13.2±0.7 | 13.3±1.0 | 14.3±0.8 |
| 10 | 10 | 25.6±0.8 | 24.1±1.0 | 25.1±1.1 | 24.5±0.8 |
| 21 | 11 | 36.7±1.7 | 37.1±2.5 | 35.7±1.4 | 32.5±1.2 |
| Contralateral | |||||
|---|---|---|---|---|---|
| Age | N | Vehicle | 3 mg/kg | 10 mg/kg | 30 mg/kg |
| 3 | 5 | 8.9±0.7 | 8.4±0.8 | 8.6±1. 2 | 8.8±0.5 |
| 10 | 10 | 15.4±1.0 | 15.3±1.1 | 14.9±0.6 | 14.2±0.5 |
| 21 | 11 | 22.1±1.6 | 26.5±1.8 | 24.6±0.8 | 23.0±0.7 |
| NS398 Ipsilateral |
|||||
|---|---|---|---|---|---|
| Age | N | Vehicle | 3 mg/kg | 10 mg/kg | 30 mg/kg |
| 3 | 4 | 11.2±1.6 | 10.1±1.8 | 10.8±1.9 | 11.5±1.1 |
| 10 | 12 | 25.8±0.9 | 26.1±1.0 | 24.0±0.9 | 24.4±0.9 |
| 21 | 8 | 34.2±2.7 | 35.5±2.7 | 38.0±3.2 | 36.4±3.4 |
| Contralateral | |||||
|---|---|---|---|---|---|
| Age | N | Vehicle | 3 mg/kg | 10 mg/kg | 30 mg/kg |
| 3 | 4 | 8.3±0.5 | 8.1±0.9 | 7.7±0.6 | 8.5±0.6 |
| 10 | 12 | 15.2±0.6 | 14.9±0.7 | 15.3±0.6 | 14.9±0.7 |
| 21 | 8 | 23.2±2.0 | 26.3±2.1 | 25.7±2.7 | 24.0±1.8 |
Note: Entries are means (± one SEM). The values are calculated as depth X width of paw in mm. There were no effects of either drug specific to the inflamed paw.
LPS Effects on Behavior
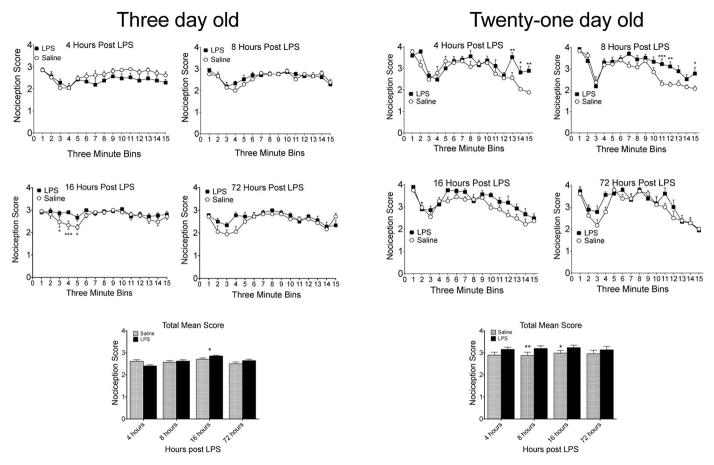
We compared LPS to saline across the 15 three-minute bins at each age and time point with an overall ANOVA. For the older 21 day old pups, there were main effects of LPS treatment at 8 and 16 hours points after the LPS injection and trends at 4 and 72 hours [main effects of treatment; 4 hours: F(1,8)=4.64, p=.063; 8 hours: F(1,8)=14.59, p=.005; 16 hours: F(1,8)=9.38, p=.016; 72 hours: F(1,5)=6.13, p=.076]. At PN21, interactions of the effects of LPS at different bins in the test were significant at 4 and 8 hours [Interaction effects: F(14,112)=2.87, p=.001; 8 hours: F(14,112)=2.58, p=.003]. LPS enhanced nociception only in the later time points, consistent with the adult literature (Padi and Kulkarni, 2005). At three days of age, there was a significant treatment effect and interaction of bins and LPS only at 16 hours [Interaction effects: F(14,84)=6.93, p=0.040; main effects of treatment F(1,6)=6.93, p=.039; (Figure 3)].
Figure 3. Behavioral effects of LPS effects formalin test.
LPS treated animals were compared to their within-litter vehicle controls at each of 4 times post-LPS injection. The top panels show the pretreatment effects of LPS compared to saline in the formalin test. The bar graphs show the data collapsed over all bins. For the 3-day-old pups, there were no significant interactions between the LPS treatment and the formalin time scores at 4, 8 or 72 hours post-LPS but a significant interaction at 16 hours. The major effect of LPS at this time was in the interphase. When the overall treatment effect was analyzed, LPS enhanced responding at 16 and 72 hours. For the PN21 pups, there were significant interactions at 4 and 8 hours, with the major effects seen in phase 2. There were overall treatment effects, with LPS enhancing nociception. N=7 and 9 for the PN3 and PN21 respectively. *=p<.05; **=p<.01; ***=p<.001.
LPS effects on immune related mRNA
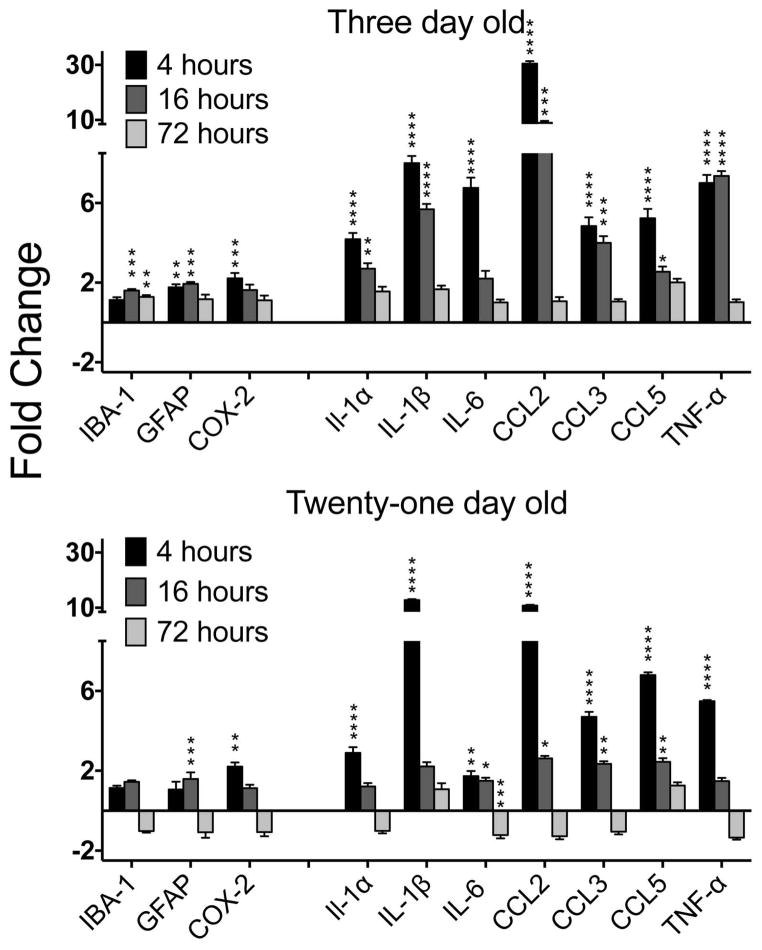
LPS in the formalin test had significant and broad effects on inflammatory cytokines at both ages. There were smaller but significant effects on GFAP, IBA-1 and COX-2 at PN3 and GFAP and COX-2 at PN21, At 3 days of age, the effects lasted through 16 hours, returning to controls levels by 72 hours (Figure 4A; PN03, N=6, all F’s (4, 25) F= 5.35 to 50.27; p=.005 to .0001). Results were similar for PN21 except the effects were shorter-lived (Figure 4B; PN21, N=5, all F’s (4, 20) F= 5.35 to 48.72; p=.005 to .0001).
Figure 4. LPS effects on immune markers.
At 4 and 16 hours after LPS + formalin injection, all proinflammatory cytokines were elevated at both ages, compared to saline + formalin controls. Microglial and astrocyte markers were also significantly elevated but to a lesser degree. All genes declined to baseline by 72 hours. N=6 for each condition. Posthoc significance levels: *=p<.05; **=p<.01; ***=p<.001; ****=p<.0001.
DISCUSSION
Major findings
The major behavioral findings are two-fold and are consistent with the literature in infants. First, SC560 and NS398, which are consistently analgesic in acute inflammatory pain in adults, were not analgesic at PN3 or PN10 pups but were strongly so at PN21. Both COX inhibitors also reduced c-fos mRNA in the spinal cord at PN21 but not PN3.
LPS enhanced nociception to a greater extent at PN21 than at PN3. The effects were modest, largely in the later phases of the formalin test, as has been reported for both adult and infant animals (Padi and Kulkarni, 2005; Zouikr et al., 2014). The second, prolonged phase in the formalin test is likely mediated by NMDA glutamate receptors (Smythe and Pappas, 1985; Ignar and Kuhn, 1988). The late onset of the pro-nociceptive effect of LPS In the infant rat is consistent with results of others using different methods. Intrathecal injection of LPS had a small effect on tactile withdrawal thresholds at PN10 compared to PN21 (Moss et al., 2007). ATP-activated microglia injected intrathecally had no effect on tactile allodynia at 10 days of age, a small effect at PN16, and an adult-like action at PN21. In the present studies, the effects of LPS on behavior did not parallel either the time course its effects on the immune system, nor the age dependent differences. In particular, at 3 days of age the proinflammatory effects of LPS lasted 4 to 16 hours after LPS treatment, whereas at PN21, these effects were largely at baseline levels by 16 hours. Intrathecal LPS also increased numbers of microglia (Iba-1 staining) at ages at which it had no effect on nociception (Moss et al., 2007). Thus although LPS is less pronociceptive in the younger pups, it is effective in stimulating cytokine and microglia production at these ages. Because we did not assay protein levels, we do not know time course of changes, if any, in cytokine protein levels.
Effects in the human infant
The strong NSAIDs ketorolac and ibuprofen, or the weak NSAID paracetamol (acetaminophen) are routinely given to infants and neonates. Open trials and clinical experience suggest that they are effective. There are, however, few double-blinded studies to corroborate these clinical observations. Ketorolac or ibuprofen can be effective in children (Bertin et al., 1996; Tuomilehto and Kokki, 2002; Hong et al., 2010), even as efficacious as opiates (Keidan et al., 2004; Jo et al., 2011), although the effect may be short lasting [(Bean-Lijewski and Stinson, 1997; Pickering et al., 2002); see (Michelet et al., 2012; Wong et al., 2013) for reviews]. Others however have found no pain relief greater than placebo (Schoeler et al., 2012). When combined with other analgesics, ketorolac is reported by some to be as effective in reducing opiate use (Laudenbach et al., 2002; Yan et al., 2011) whereas other found no advantage adding NSAIDs to other analgesics (Enkvist et al., 1996; Brum et al., 2006; Lynn et al., 2011).
Two recent meta-analyses found that both ketorolac and paracetamol reliably reduces pain and opiate requirements in children older than three years of age (Michelet et al., 2012; Wong et al., 2013). Their effectiveness is less clear in infants. In three studies with infants 17–28 months of age on average, there was significant sparing of opiate use with paracetamol (Hong et al., 2010; Hong et al., 2010; Mireskandari and Makarem, 2011). However, in younger infants aged 0–9 (median age 0) or 12 months, closer to the PN3 and PN10 rats used here, paracetamol was not effective as an adjunctive treatment nor did it reduce pain scores (Bremerich et al., 2001; van der Marel et al., 2007). Thus the question of efficacy of cyclooxygenase inhibitors in neonates is still open.
Possible Mechanisms
In the infant, activation of the immune system has consequences. For example, LPS worsens outcomes following brain injury as early as 1 day of age in rats and mice (Lee et al., 2000; Xue and Del Bigio, 2005; Brochu et al., 2011) and human infants can mount an inflammatory immune response within a few days after birth (Fotopoulos et al., 2005),
Since the immune system can be stimulated early in life, why peripheral tissue injury does not engage in immune system in the infant puzzling. Many pain processes that activate the immune system in the adult are immature in the infant. For example substance P and GABA are immune-modulators [see for review: (Bost, 2004; Tuluc et al., 2009; Douglas and Leeman, 2011; Jin et al., 2013) but have delayed developmental trajectories in modulating pain (Fitzgerald and Gibson, 1984; King et al., 2000; King et al., 2000; King and Barr, 2003; Baccei and Fitzgerald, 2004; Hathway et al., 2006; Zouikr et al., 2014). Thus injury that involves immune processes through either of these two neurotransmitters would have no consequence early in life. In contrast, glutamate immune interactions (Pascual et al., 2012; Takaki et al., 2012; McKenna, 2013) would be expected to alter pain processes in the neonate since glutamate has an early role in pain modulation (Bardoni et al., 1998; Bardoni et al., 2000; King and Barr, 2000; Baccei et al., 2003; King and Barr, 2007). It also follows that the timing of the maturation of immune function relative to that of pain processes will have long-term consequences for later nociceptive processes (Boisse et al., 2005; Hodyl et al., 2010; Wang et al., 2011).
COX inhibitors may act through different, yet unspecified, mechanisms that are immature at the ages tested here. One possibility is that the products of the cyclooxygenases, PGH2, one of the downstream synthases, or the prostaglandins themselves, are not pro-nociceptive in the infant as they are in the adult (Hu et al., 2008). If prostaglandins have no role in pain induction in the infant, their inhibition would have no effect. We know of no data that addresses this question.
LPS stimulated the production of multiple cytokines at both ages, yet was more pronociceptive at PN21. LPS has complex effects on multiple physiological systems that may be age specific. To provide but two examples, LPS in the adult rat is pyrogenic (Romanovsky et al., 1996; Fraifeld and Kaplanski, 1998) but produces hypothermia prior to 21 days of age (Kaplanski et al., 1997). It is possible that the mild hyperthermia reported at PN21 (~0.6° C) contributed to the effect at that age (Kaplanski et al., 1997). Likewise, LPS stimulates an inflammatory response that disrupts the blood brain barrier triggering microglia to release a number of neurotoxic substances [reviewed in (Hagberg and Mallard, 2005)]. The blood brain barrier is developing during early postnatal life and its immaturity may attenuate the effects of LPS by some as of yet unknown mechanism.
Conclusions
Not all drugs that target the immune system and that are effective in adults may be equally effective for infants. Due to the limited availability of effective pain relieving drugs for this population of infants, opiates remain the most used analgesic. Ketorolac and other NSAIDs are routinely given to infants and neonates and open trials and clinical experience suggest that they are effective after 2 years of age, but good controlled studies in neonates and premature infants are few.
Given the major side effects that accompany opiate use, a better understanding of specific pain pathways and the role that the immune system plays in mediating and modulating pain in infants and children can provide new targets that can guide the development of more effective therapeutic approaches to treating infants and children in pain.
Acknowledgments
We thank, J. Cheng, J. Verlus, P. Singh, N. Tanizaki, S. Wang, and B. Woods for their assistance in collecting these data and Kevin Bass for reviewing drafts of the manuscript. This work was supported in part a by Ford Foundation Postdoctoral fellowship to D.A. Hunter, RIMI support to C. Chai through Mercy College (P20MD002717), NIH grants NS047159 and DA000325 to G.A. Barr, and funds from the James Battaglia Endowment.
References
- Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J Physiol. 2003;549:231–242. doi: 10.1113/jphysiol.2003.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Magherini PC, MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. Journal of Neuroscience. 1998;18:6558–6567. doi: 10.1523/JNEUROSCI.18-16-06558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Magherini PC, MacDermott AB. Activation of NMDA receptors drives action potentials in superficial dorsal horn from neonatal rats. Neuroreport. 2000;11:1721–1727. doi: 10.1097/00001756-200006050-00025. [DOI] [PubMed] [Google Scholar]
- Barr GA. Maturation of the biphasic behavioral and heart rate response in the formalin test. Pharmacol Biochem Behav. 1998;60:329–335. doi: 10.1016/s0091-3057(97)00602-3. [DOI] [PubMed] [Google Scholar]
- Barr GA, Gao P, Wang S, Cheng J, Qin J, Sibille EL, Pavlidis P. Microarray analysis of gene expression following the formalin test in the infant rat. Pain. 2005;117:6–18. doi: 10.1016/j.pain.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, Sullivan RM. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean-Lijewski JD, Stinson JC. Acetaminophen or ketorolac for post myringotomy pain in children? A prospective, double-blinded comparison. Paediatr Anaesth. 1997;7:131–137. doi: 10.1046/j.1460-9592.1997.d01-47.x. [DOI] [PubMed] [Google Scholar]
- Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit JS, Engler H, Oberbeck R, Schedlowski M, Elsenbruch S. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012;153:794–799. doi: 10.1016/j.pain.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Bertin L, Pons G, d’Athis P, Duhamel JF, Maudelonde C, Lasfargues G, Guillot M, Marsac A, Debregeas B, Olive G. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387–392. doi: 10.1111/j.1472-8206.1996.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–141. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Bost KL. Tachykinin-mediated modulation of the immune response. Front Biosci. 2004;9:3331–3332. doi: 10.2741/1484. [DOI] [PubMed] [Google Scholar]
- Bremerich DH, Neidhart G, Heimann K, Kessler P, Behne M. Prophylactically-administered rectal acetaminophen does not reduce postoperative opioid requirements in infants and small children undergoing elective cleft palate repair. Anesth Analg. 2001;92:907–912. doi: 10.1097/00000539-200104000-00020. [DOI] [PubMed] [Google Scholar]
- Bressan E, Cunha Fde Q, Tonussi CR. Contribution of TNFalpha, IL-1beta and CINC-1 for articular incapacitation, edema and cell migration in a model of LPS-induced reactive arthritis. Cytokine. 2006;36:83–89. doi: 10.1016/j.cyto.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J Neuroinflammation. 2011;8:55. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum PC, Hurt CM, Shcherbakova OG, Kobilka B, Angelotti T. Differential targeting and function of alpha2A and alpha2C adrenergic receptor subtypes in cultured sympathetic neurons. Neuropharmacology. 2006;51:397–413. doi: 10.1016/j.neuropharm.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Dray A, Coderre TJ. Enhanced thermal antinociceptive potency and anti-allodynic effects of morphine following spinal administration of endotoxin. Brain Res. 2003;960:209–218. doi: 10.1016/s0006-8993(02)03885-4. [DOI] [PubMed] [Google Scholar]
- Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130:1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist MO, Hamalainen H, Jansson CC, Kukkonen JP, Hautala R, Courtney MJ, Akerman KE. Coupling of astroglial alpha 2-adrenoreceptors to second messenger pathways. J Neurochem. 1996;66:2394–2401. doi: 10.1046/j.1471-4159.1996.66062394.x. [DOI] [PubMed] [Google Scholar]
- Fanos V, Cuzzolin L, Atzei A, Testa M. Antibiotics and antifungals in neonatal intensive care units: a review. J Chemother. 2007;19:5–20. doi: 10.1179/joc.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Gibson S. The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience. 1984;13:933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Fotopoulos S, Mouchtouri A, Xanthou G, Lipsou N, Petrakou E, Xanthou M. Inflammatory chemokine expression in the peripheral blood of neonates with perinatal asphyxia and perinatal or nosocomial infections. Acta Paediatr. 2005;94:800–806. doi: 10.1111/j.1651-2227.2005.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Fraifeld V, Kaplanski J. Brain eicosanoids and LPS fever: species and age differences. Prog Brain Res. 1998;115:141–157. doi: 10.1016/s0079-6123(08)62034-8. [DOI] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014 doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Cheng J, Wang S, Barr GA. Analgesic efficacy of ketorolac and morphine in neonatal rats. Pharmacol Biochem Behav. 2001;68:635–640. doi: 10.1016/s0091-3057(00)00475-5. [DOI] [PubMed] [Google Scholar]
- Guy ER, Abbott FV. The behavioral response to formalin in preweanling rats. Pain. 1992;51:81–90. doi: 10.1016/0304-3959(92)90012-Z. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- Hathway G, Harrop E, Baccei M, Walker S, Moss A, Fitzgerald M. A postnatal switch in GABAergic control of spinal cutaneous reflexes. Eur J Neurosci. 2006;23:112–118. doi: 10.1111/j.1460-9568.2005.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodyl NA, Walker FR, Krivanek KM, Clifton VL, Hodgson DM. Prenatal endotoxin exposure alters behavioural pain responses to lipopolysaccharide in adult offspring. Physiol Behav. 2010;100:143–147. doi: 10.1016/j.physbeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Hong JY, Kim WO, Koo BN, Cho JS, Suk EH, Kil HK. Fentanyl-sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent-/nurse-controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology. 2010;113:672–677. doi: 10.1097/ALN.0b013e3181e2c34b. [DOI] [PubMed] [Google Scholar]
- Hong JY, Won Han S, Kim WO, Kil HK. Fentanyl sparing effects of combined ketorolac and acetaminophen for outpatient inguinal hernia repair in children. J Urol. 2010;183:1551–1555. doi: 10.1016/j.juro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol. 2008;153:1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignar DM, Kuhn CM. Relative ontogeny of opioid and catecholaminergic regulation of thyrotropin secretion in the rat. Endocrinology. 1988;123:567–571. doi: 10.1210/endo-123-1-567. [DOI] [PubMed] [Google Scholar]
- Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids. 2013;45:87–94. doi: 10.1007/s00726-011-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YY, Hong JY, Choi EK, Kil HK. Ketorolac or fentanyl continuous infusion for post-operative analgesia in children undergoing ureteroneocystostomy. Acta Anaesthesiol Scand. 2011;55:54–59. doi: 10.1111/j.1399-6576.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- Kaplanski J, Fraifeld V, Rubin M. Body temperature and hypothalamic PGE2 response to LPS in developing rats. Ann N Y Acad Sci. 1997;813:474–479. doi: 10.1111/j.1749-6632.1997.tb51735.x. [DOI] [PubMed] [Google Scholar]
- Keidan I, Zaslansky R, Eviatar E, Segal S, Sarfaty SM. Intraoperative ketorolac is an effective substitute for fentanyl in children undergoing outpatient adenotonsillectomy. Paediatr Anaesth. 2004;14:318–323. doi: 10.1046/j.1460-9592.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- King TE, Barr GA. NMDA and AMPA receptor antagonists attenuate nociceptive responses across early development. The Journal of Pain. 2000;2:33. [Google Scholar]
- King TE, Barr GA. Functional development of neurokinin peptides substance P and neurokinin A in nociception. Neuroreport. 2003;14:1603–1607. doi: 10.1097/00001756-200308260-00012. [DOI] [PubMed] [Google Scholar]
- King TE, Barr GA. Spinal cord ionotropic glutamate receptors function in formalin-induced nociception in preweaning rats. Psychopharmacology (Berl) 2007;192:489–498. doi: 10.1007/s00213-007-0735-x. [DOI] [PubMed] [Google Scholar]
- King TE, Cheng J, Wang S, Barr GA. Maturation of NK1 receptor involvement in the nociceptive response to formalin. Synapse. 2000;36:254–266. doi: 10.1002/(SICI)1098-2396(20000615)36:4<254::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- King TE, Heath MJ, Debs P, Davis MB, Hen R, Barr GA. The development of the nociceptive responses in neurokinin-1 receptor knockout mice. Neuroreport. 2000;11:587–591. doi: 10.1097/00001756-200002280-00031. [DOI] [PubMed] [Google Scholar]
- Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology. 2002;96:134–141. doi: 10.1097/00000542-200201000-00026. [DOI] [PubMed] [Google Scholar]
- Lee SH, Han SH, Lee KW. Kainic acid-induced seizures cause neuronal death in infant rats pretreated with lipopolysaccharide. Neuroreport. 2000;11:507–510. doi: 10.1097/00001756-200002280-00016. [DOI] [PubMed] [Google Scholar]
- Lynn AM, Bradford H, Kantor ED, Andrew M, Vicini P, Anderson GD. Ketorolac tromethamine: stereo-specific pharmacokinetics and single-dose use in postoperative infants aged 2–6 months. Paediatr Anaesth. 2011;21:325–334. doi: 10.1111/j.1460-9592.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–146. [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993;79:270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- Marascuilo LA, McSweeney M. Nonparametric and distribution-free methods for the social sciences. Monterey, CA: Brooks/Cole Publishing Co; 1977. [Google Scholar]
- McKenna MC. Glutamate Pays Its Own Way in Astrocytes. Front Endocrinol (Lausanne) 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet D, Andreu-Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, Mantz J, Dahmani S. A meta-analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesth Analg. 2012;114:393–406. doi: 10.1213/ANE.0b013e31823d0b45. [DOI] [PubMed] [Google Scholar]
- Mireskandari SM, Makarem J. Effect of rectal diclofenac and acetaminophen alone and in combination on postoperative pain after cleft palate repair in children. J Craniofac Surg. 2011;22:1955–1959. doi: 10.1097/SCS.0b013e31822ea7fd. [DOI] [PubMed] [Google Scholar]
- Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway GJ, Salter MW, Fitzgerald M. Spinal microglia and neuropathic pain in young rats. Pain. 2007;128:215–224. doi: 10.1016/j.pain.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. Faseb J. 2008;22:3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- Padi SS, Kulkarni SK. Role of cyclooxygenase-2 in lipopolysaccharide-induced hyperalgesia in formalin test. Indian J Exp Biol. 2005;43:53–60. [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbin KE, Bateman RJ, Mendelowitz D. Clonidine, an alpha2-receptor agonist, diminishes GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2010;1347:65–70. doi: 10.1016/j.brainres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AE, Bridge HS, Nolan J, Stoddart PA. Double-blind, placebo-controlled analgesic study of ibuprofen or rofecoxib in combination with paracetamol for tonsillectomy in children. Br J Anaesth. 2002;88:72–77. doi: 10.1093/bja/88.1.72. [DOI] [PubMed] [Google Scholar]
- Polin RA, Denson S, Brady MT. Epidemiology and diagnosis of health care-associated infections in the NICU. Pediatrics. 2012;129:e1104–1109. doi: 10.1542/peds.2012-0147. [DOI] [PubMed] [Google Scholar]
- Ponten E, Viberg H, Gordh T, Eriksson P, Fredriksson A. Clonidine abolishes the adverse effects on apoptosis and behaviour after neonatal ketamine exposure in mice. Acta Anaesthesiol Scand. 2012;56:1058–1065. doi: 10.1111/j.1399-6576.2012.02722.x. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Ririe DG, Prout HD, Barclay D, Tong C, Lin M, Eisenach JC. Developmental differences in spinal cyclooxygenase 1 expression after surgical incision. Anesthesiology. 2006;104:426–431. doi: 10.1097/00000542-200603000-00008. [DOI] [PubMed] [Google Scholar]
- Ririe DG, Prout HM, Eisenach JC. Effect of cyclooxygenase-1 inhibition in postoperative pain is developmentally regulated. Anesthesiology. 2004;101:1031–1035. doi: 10.1097/00000542-200410000-00034. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. First and second phases of biphasic fever: two sequential stages of the sickness syndrome? Am J Physiol. 1996;271:R244–253. doi: 10.1152/ajpregu.1996.271.1.R244. [DOI] [PubMed] [Google Scholar]
- Schoeler M, Loetscher PD, Rossaint R, Fahlenkamp AV, Eberhardt G, Rex S, Weis J, Coburn M. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol. 2012;12:20. doi: 10.1186/1471-2377-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe JW, Pappas BA. Neonatal 6-hydroxydopamine potentiates clonidine’s locomotor effects throughout maturation in the rat. Pharmacol Biochem Behav. 1985;22:1075–1078. doi: 10.1016/0091-3057(85)90319-3. [DOI] [PubMed] [Google Scholar]
- Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflammation. 2012;9:275. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Niederberger E, Vetter G, Brautigam L, Geisslinger G. Effects of selective COX-1 and -2 inhibition on formalin-evoked nociceptive behaviour and prostaglandin E(2) release in the spinal cord. J Neurochem. 2001;79:777–786. doi: 10.1046/j.1471-4159.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- Teng CJ, Abbott FV. The formalin test: a dose-response analysis at three developmental stages. Pain. 1998;76:337–347. doi: 10.1016/S0304-3959(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Titinchi S, Clark B. Alpha 2-adrenoceptors in human lymphocytes: direct characterisation by [3H]yohimbine binding. Biochem Biophys Res Commun. 1984;121:1–7. doi: 10.1016/0006-291x(84)90679-x. [DOI] [PubMed] [Google Scholar]
- Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Tuomilehto H, Kokki H. Parenteral ketoprofen for pain management after adenoidectomy: comparison of intravenous and intramuscular routes of administration. Acta Anaesthesiol Scand. 2002;46:184–189. doi: 10.1034/j.1399-6576.2002.460211.x. [DOI] [PubMed] [Google Scholar]
- van der Marel CD, Peters JW, Bouwmeester NJ, Jacqz-Aigrain E, van den Anker JN, Tibboel D. Rectal acetaminophen does not reduce morphine consumption after major surgery in young infants. Br J Anaesth. 2007;98:372–379. doi: 10.1093/bja/ael371. [DOI] [PubMed] [Google Scholar]
- Vega-Avelaira D, Moss A, Fitzgerald M. Age-related changes in the spinal cord microglial and astrocytic response profile to nerve injury. Brain Behav Immun. 2007;21:617–623. doi: 10.1016/j.bbi.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Voituron N, Hilaire G, Quintin L. Dexmedetomidine and clonidine induce long-lasting activation of the respiratory rhythm generator of neonatal mice: possible implication for critical care. Respir Physiol Neurobiol. 2012;180:132–140. doi: 10.1016/j.resp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Wainwright PE. Issues of design and analysis relating to the use of multiparous species in developmental nutritional studies. J Nutr. 1998;128:661–663. doi: 10.1093/jn/128.3.661. [DOI] [PubMed] [Google Scholar]
- Wang KC, Wang SJ, Fan LW, Cai Z, Rhodes PG, Tien LT. Interleukin-1 receptor antagonist ameliorates neonatal lipopolysaccharide-induced long-lasting hyperalgesia in the adult rats. Toxicology. 2011;279:123–129. doi: 10.1016/j.tox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013;23:475–495. doi: 10.1111/pan.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Immune pre-activation exacerbates hemorrhagic brain injury in immature mouse brain. J Neuroimmunol. 2005;165:75–82. doi: 10.1016/j.jneuroim.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. The role of cyclooxygenase-1 and -2 in the rat formalin test. Anesth Analg. 2002;94:962–967. doi: 10.1097/00000539-200204000-00035. table of contents. [DOI] [PubMed] [Google Scholar]
- Yan M, Dai H, Ding T, Dai A, Zhang F, Yu L, Chen G, Chen Z. Effects of dexmedetomidine on the release of glial cell line-derived neurotrophic factor from rat astrocyte cells. Neurochem Int. 2011;58:549–557. doi: 10.1016/j.neuint.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rosen H, Donchin O, Ovadia H. Behavioral effects of lipopolysaccharide in rats: involvement of endogenous opioids. Brain Res. 1994;648:80–86. doi: 10.1016/0006-8993(94)91908-9. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Patel D, Dougherty PM. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience. 2012;221:214–224. doi: 10.1016/j.neuroscience.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouikr I, Tadros MA, Barouei J, Beagley KW, Clifton VL, Callister RJ, Hodgson DM. Altered nociceptive, endocrine, and dorsal horn neuron responses in rats following a neonatal immune challenge. Psychoneuroendocrinology. 2014;41:1–12. doi: 10.1016/j.psyneuen.2013.11.016. [DOI] [PubMed] [Google Scholar]