Abstract
Background
Functional magnetic resonance imaging (fMRI) research suggests that both adult and adolescent major depressive disorder (MDD) is marked by aberrant connectivity of the default mode network (DMN) during resting-state. However, emotional dysresgulation is also a key feature of MDD. No studies to date have examined emotion-related DMN pathology in adolescent depression. Comprehensively understanding the dynamics of DMN connectivity across brain states in depressed individuals with short disease histories could provide insight into the etiology of MDD.
Methods
We collected fMRI data during an emotion identification task and also during resting-state from 26 medication-free adolescents (13-17 years) with MDD and 37 wellmatched healthy controls (HCL). We examined between-group differences in blood oxygenation level-dependent task responses, emotion-dependent, and resting-state functional connectivity of the two primary nodes of the DMN: medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC). Additionally, we examined between-group differences in DMN functional connectivity and its relationship to depression severity.
Results
Relative to HCL, unmedicated MDD adolescents demonstrated reduced mPFC and PCC emotion-related deactivation and greater mPFC and PCC emotion-dependent functional connectivity with precuneus, cingulate gyrus, and striatum/subcallosal cingulate gyrus. Importantly, PCC-subcallosal cingulate connectivity remained inflexibly elevated in MDD versus HCL during resting-state. Lastly, stronger PCC emotion-dependent functional connectivity was associated with greater depression severity and an earlier age of depression onset.
Conclusions
Adolescent depression is associated with inflexibly elevated DMN connections. Given recent evidence of DMN maturation throughout adolescence, our findings suggest that early-onset depression adversely impacts normal development of functional brain networks.
Keywords: functional magnetic resonance imaging, major depressive disorder, default mode network, functional connectivity, psychophysiological interaction, adolescence
Introduction
The incidence of depression rises alarmingly during adolescence (1, 2), affecting 4-17% of youth worldwide (3-5). Adolescent-onset depression is over four times more likely to lead to a recurrent episode than adult-onset depression (6), and is associated with greater symptom severity, likelihood of relapse, and suicidality across the lifespan (7-9). Despite its clinical significance, relatively few studies have examined the neurobiological underpinnings of depression during this sensitive period of development. The adolescent brain, and especially the circuits supporting emotional processing, continues to undergo neural pruning and maturation (10-12). Thus, investigating the neurobiological mechanisms of emotional processing early in the disease course is critical for elucidating the etiology of depression.
Functional connectivity methods in neuroimaging have led to examinations of intrinsic brain networks in depression and other disorders, in particular the default mode network (DMN) (13-18). The DMN is a network of functionally connected brain regions whose activity becomes synchronously elevated during undirected or internally-directed mental states (e.g., passively resting, recalling the past, imagining the future) but dampens during goal-directed cognition (19). Most studies suggest that the DMN is further comprised of interconnected subsystems that converge on key nodes, with the two most notable being the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC) (19-22). Activity in the mPFC is believed to support self-referential mentation (21, 23-25), while the PCC is a highly connective area that is heavily implicated in memory-related processes, consciousness and awareness (26, 27).
Both the anterior and posterior hubs of the DMN have been linked to social and affective cognition and are anatomically connected to regions involved in emotion generation, salience monitoring, and self-inspection (16, 21, 23, 28). Aberrant DMN function could therefore lead to dysfunctional self-referential and affective processing in the form of excessively negative self-focus (16, 17, 24). Indeed, altered resting-state connectivity has been demonstrated in depressed adults (29-44), adolescents (40, 45-51), and even children (52, 53). These studies tend to report altered, albeit directionally variable, functional coupling between nodes in the salience network and regions in the DMN in depressed individuals, with one study even reporting no significant differences in resting-state DMN functional connectivity between depressed and healthy adolescents (50). These inconsistencies, particularly in the adolescent literature, are compelling and highlight the need for more thorough examinations of the DMN in this population (51). Moreover, emotional dysregulation is also a core feature of MDD (54, 55). Thus, functional connectivity analyses of the DMN during a task that appropriately probes emotional processing are needed to target brain areas relevant to this feature of depression.
Presently, there are few studies examining the DMN during emotional processing in either depressed adults or adolescents. Ineffective suppression of the DMN during affective processing has been reported in depressed adults (31, 56), with greater mPFC and PCC activation correlating positively with feelings of depression and hopelessness (56). However, these studies did not examine functional connectivity. Prior studies examining emotion-dependent functional connectivity in depression have typically focused their analyses on nodes in the salience network, reporting diminished functional connectivity of the amygdala, anterior cingulate cortex, and mPFC in depressed versus healthy individuals (57-63). While this research suggests that MDD may be characterized by altered connectivity between the anterior hub of the DMN and structures involved in affective and salience processing (13, 16, 17), no work to date has examined both mPFC and PCC connectivity during emotional processing in depressed adolescents. To our knowledge, there are only two studies examining functional connectivity of emotional processing in depressed adolescents and they are limited in their sample sizes and focus only on regions involved in affective processing (57, 58). Furthermore, recent evidence shows that the DMN undergoes developmental changes (64-67). It is therefore imperative that DMN dynamics are investigated in early-onset depression free from chronic illness and pharmacological treatment so that the pathophysiology of this disease at the level of brain networks may be elucidated.
To this end, we recruited a relatively large sample of medication-free adolescents with major depressive disorder (MDD) and a healthy control (HCL) group that was well-matched on several demographic variables. Subjects underwent fMRI scanning during an emotion identification task and also during resting-state. Clusters in the mPFC and PCC exhibited significantly less emotion-related deactivation in MDD compared to HCL. Subsequently, context-dependent and resting-state functional connectivity analyses were conducted using these mPFC and PCC regions as seeds. Based on previous studies implicating aberrant functional connectivity in MDD, we predicted that depressed adolescents would exhibit altered emotion-dependent connectivity of the DMN and that the strength of these connections would correlate positively with depression severity (57-63). Given the role of the DMN in self-referential processing and its association with depression in adults, we hypothesized that emotion-dependent functional connectivity of the DMN would correlate with clinical measures of rumination (29, 34, 36, 39). Finally, in light of growing evidence showing developmental changes of the DMN (64, 65), we also hypothesized that emotion dependent functional connectivity of the DMN may be associated with age of depression onset.
Methods and Materials
Subject recruitment
A total of 83 right-handed adolescents (13-17 years) were recruited for this study. Six subjects (3 MDD) were excluded due to a failure to record behavioral responses in the scanner. We applied rigorous motion outlier thresholds to the remaining subjects (see Inclusion/exclusion criteria in the supplement for more details), resulting in whole brain and emotion-dependent functional connectivity results for 63 subjects (26 MDD) and resting-state results for 57 subjects (23 MDD). Depressed adolescents were recruited from psychiatric and primary care clinics in San Diego; HCL were recruited from the same geographic area via e-mail, internet, or flyers. Adolescents from both genders and all ethnicities were allowed to participate and all subjects received financial compensation for their participation. All participating adolescents provided written informed assent and their parent(s)/legal guardian(s) provided written informed consent in accordance with the Declaration of Helsinki and the Institutional Review Boards at the University of California, San Diego (UCSD), UC San Francisco, Rady Children's Hospital in San Diego, and the County of San Diego.
Diagnostic assessment of subjects
The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (68) was administered to all potentially depressed subjects. The computerized Diagnostic Interview Schedule for Children 4.0 (69) and the Diagnostic Predictive Scale (69) were used to screen for the presence of any Axis I diagnoses in HCL.
Depression symptoms were measured with the Children's Depression Rating Scale-Revised (70) and the Beck Depression Inventory-II (BDI-II) (71), anxiety with the Multidimensional Anxiety Scale for Children (MASC) (72), and rumination with the Ruminative Responses Styles (RRS) inventory (73).
The groups were well-matched on age, gender distribution, ethnicity, pubertal status, socioeconomic status, and general intelligence. Details regarding diagnostic assessment, demographic measures, and inclusion/exclusion criteria are presented in the supplement. See Table 1 for a summary of the characteristics of the depressed subjects and Table S1 for a summary of their psychosocial treatment history.
Table 1. Summary of subject groups’ demographic, clinical characteristics, and behavioral performance of task.
Entries are of the form: mean ± SEM. Statistical analyses of between-group differences were conducted with chi-squared tests (χ2), Student t-tests (t), and Wilcoxon Rank Sum test (U). All p-values indicate two-tailed statistical significance levels. Details on the scales used can be found in the supplement. A summary of the psychosocial treatment history of our depressed subjects can be found in Table S1. All depressed subjects were naïve to antidepressants at the time of scanning except for two: one had last been exposed 4 months prior to their scan and one had been last exposed 4 years prior to their scan.
| Characteristic | Depressed | Control | Statistic | P-value |
|---|---|---|---|---|
| Number of participants in final analysis | 26 | 37 | ||
| Gender (M / F) | 7 / 19 | 14 / 23 | χ2 =0.819 | 0.366 |
| Age at time of scan (years) | 16.1 ± 0.3 | 16.0 ± 0.2 | t61=0.29 | 0.77 |
| Ethnicity (Asian / Black / Caucasian / Hispanic / Mixed) | 3 / 3 / 8 / 10 / 2 | 4 / 2 / 13 / 14 / 4 | U=9 | 0.458 |
| Hollingshead Socioeconomic Score | 40 ± 25.2 | 29 ± 16.3 | U=432 | 0.36 |
| Tanner Score | 4.2 ± 0.4 | 4.0 ± 0.7 | U=509 | 0.90 |
| Wechsler Abbreviated Scale of Intelligence (Standardized) | 104.2 ± 4.6 | 107.3 ± 3.3 | t61=0.56 | 0.58 |
| Beck's Depression Inventory II | 28.4 ± 2.0 | 3.4 ± 0.7 | t60=13.10 | <0.0001 |
| Children's Depression Rating Scale-Revised (Standardized) | 73.1 ± 1.8 | 34.3 ± 1.2 | t61=18.64 | <0.0001 |
| Multidimensional Anxiety Scale for Children (Standardized) | 59.8 ± 1.8 | 42.1 ± 1.4 | t60=7.88 | <0.0001 |
| Ruminative Responses Scale | 67.6 ± 3.3 | 33.7 ± 1.7 | t51=9.81 | <0.0001 |
| Age of depression onset (years) | 12.28 ± 0.56 | |||
| Comorbidities | n (%) | |||
| No comorbidities | 9 (34.62%) | |||
| Generalized Anxiety Disorder (GAD) | 7 (26.92%) | |||
| Post Traumatic Stress Disorder (PTSD) | 3 (11.5%) | |||
| Specific Phobia | 2 (7.7%) | |||
| GAD + PTSD | 1 (3.8%) | |||
| Anxiety Disorder Not Otherwise Specified (NOS) | 1 (3.8%) | |||
| Attention Deficit Hyperactivity Disorder NOS | 1 (3.8%) | |||
| Social Phobia | 2 (7.7%) | |||
| Behavioral performance on emotion identification task | ||||
| Accuracy (%) | 80.73 ± 6.44 | 80.06 ± 9.73 | t61=0.053 | 0.958 |
| Response Time (s) | 2.25 ± 0.52 | 2.16 ± 0.59 | t61=0.110 | 0.913 |
Experimental stimuli and paradigm
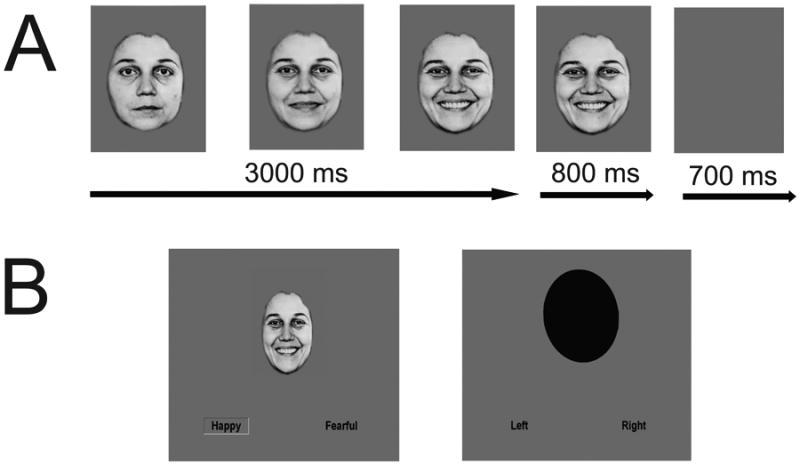
All subjects completed one run each of the emotion identification task and resting-state in the same scan session. Emotional face stimuli have been shown to deactivate regions of the DMN in healthy individuals (74). However, the only other functional connectivity study using emotional faces to examine adolescent depression not only did not examine the DMN, but also tested only static fearful faces (58). Because dynamic face stimuli are considered more ecologically valid and more robustly activate affective processing regions compared to static face stimuli (75, 76), our emotion identification task employed dynamically morphing faces expressing fear, happiness, and sadness. We used ten standardized faces (5 female) expressing fear, happiness, and sadness (77) morphed with computer graphical manipulation (78, 79). On FACE trials, a screen displaying text of the possible emotions to discern (FEAR, HAPPY, SAD) was presented for 1500ms. Next, a neutral face morphed smoothly to an emotion of prototypical intensity over the span of 3000ms and remained onscreen for an additional 800 ms before the screen turned blank for 700ms, signifying the trial's end (Figure 1A). At stimulus onset, two possible emotion choices were displayed in the bottom left and right corners; subjects were instructed to press one of two buttons corresponding to their choice as soon as they recognized the facial emotion. OVAL trials (6 s per trial), where subjects had to determine if a morphing oval was tilting left or right (maximal tilt angle=10°), were used as a sensorimotor control (Figure 1B). At the end of the scan, a blank screen was presented for 10s. On run contained 80 trials (60 facial trials and 20 oval trials; total run=490s). Response time and accuracy on every trial during scanning was recorded. See also Figure 1 and the supplement.
Figure 1. Emotion identification task with dynamically morphing face stimuli.
On FACE trials, a screen displaying text of the three possible emotions to discern (FEAR, HAPPY, SAD) was presented for 1500 ms. Next, a neutral face morphed smoothly and dynamically to an emotion of prototypical intensity over the span of 3000 ms. At maximal emotion intensity, the face then remained on the screen for an additional 800 ms of the trial before the screen turned blank for 700 ms (Figure 1A depicts an example face and is enlarged for purposes of clarity). At stimulus onset, two possible emotion choices were displayed in text on the bottom left and right corners; subjects were instructed to press one of two buttons corresponding to the displayed emotion as soon as they recognized the emotion of the face. As a sensorimotor control, we also had OVAL trials (6 s per trial), where subjects had to determine if the top of an oval was tilting to the left or right and make a button response accordingly as soon as they recognized the tilt direction (Figure 1B depicts a sample FACE and OVAL trial). See the supplement for more details.
Image acquisition
All scanning was conducted on a General Electric 3T MR750 System (General Electric Healthcare, Milwaukee, WI) with Twin Speed gradients and a GE 8-channel head coil at the Center of Functional MRI at UCSD. See the supplement for more details.
Image analysis
All image processing and analyses were conducted with AFNI (80) and FSL (81). The T1-weighted images were skull-stripped and transformed to MNI152 (Montreal Neurological Institute, Montreal, Quebec, Canada) with an affine transform (82, 83) followed by nonlinear refinement (84). EPI data were slice-time and motion corrected and then aligned to the T1-weighted images using a Localized Pearson Correlation function (85). Next, the EPI data were convolved with a 4.2 mm full-width half-maximum (FWHM) isotropic Gaussian filter and grand mean scaled before being transformed to MNI152 space at 3x3x3 mm resolution. A generalized least squares regression model that estimates the serial correlation of noise with an autoregressive moving average method was used to fit each voxel's time-series. Each stimulus type was included as a regressor-of-interest (FEAR, HAPPY, SAD, OVAL). Each time-series of interest spanned stimulus onset until the first valid (≥ 150 ms) response, thus providing a naturally jittered reference function, before being convolved with a gamma-variate function (86). Demeaned motion parameters (3 rotational and 3 translational) and a second order Legrendre polynomial were included as nuisance regressors (i.e., baseline). Volumes where the Euclidean norm of the motion derivatives exceeded 0.2 or where more than 10% of voxels exceeded the median absolute deviation of the detrended time-series were censored. Subjects where more than 20% of their volumes were censored were removed from the final analysis (see Inclusion/exclusion criteria in the supplement for more details). A general linear test for FACE-OVAL was computed for each participant. Brain activation was operationally defined as percentage signal change relative to baseline.
Functional connectivity analyses
Context-dependent functional connectivity analyses were conducted according to prior studies utilizing facial stimuli from our laboratory (58) and others (87-89). Specifically, we employed a seed-based approach using the psychophysiological interaction (PPI) (90) method developed for AFNI (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html), with clusters in the left mPFC and left PCC exhibiting significant between-group differences on the emotion identification task defined as distinct seeds (Figure 2). Details on our functional connectivity analyses can be found in the supplement under Emotion-dependent functional connectivity (PPI) image analysis and Resting-state image analysis. Additional exploratory PPI analyses were also performed to examine DMN functional connectivity to each emotion (see Exploratory emotion-dependent functional connectivity analysis and Tables S4-S6 in supplement).
Figure 2. Mean activation to each emotion type in the mPFC and PCC regions (i.e., seeds for the PPI analysis) showing significant between-group differences on the task (FACE-OVAL).
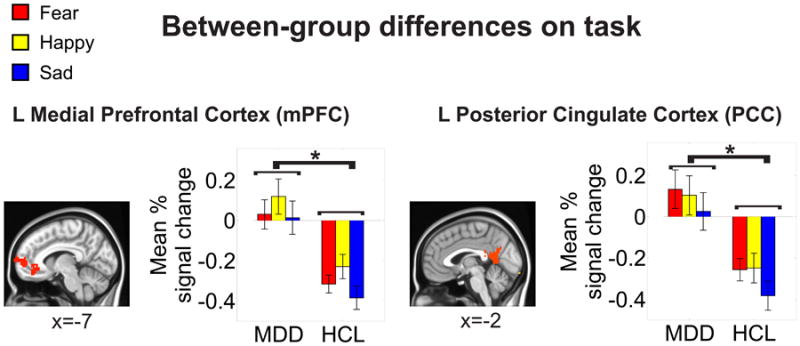
Mean percentage signal change to each condition (FEAR-OVAL, HAPPY-OVAL, SAD-OVAL) was extracted and subjected to post-hoc t-tests (Fisher's LSD). These post-hoc t-tests indicated a main effect of group at p<0.05 (denoted by *). These areas survived correction for multiple comparisons at a cluster-wise threshold of p<0.05. Locations are reported in MNI coordinates (radiological convention). See Figure S1 and Table S2 for a full summary of the between-group differences on the task.
Between-group whole brain task analysis
Group differences on the emotion identification task were assessed using a linear mixed effects (LME) model on the estimates from the regression model, with group (MDD, HCL) modeled as a fixed factor and participant modeled as a random factor.
Between-group emotion-dependent functional connectivity analysis
Group differences in the PPI analysis were assessed using a LME on the Fisher's z-scores of the interaction effect, with group modeled as a fixed factor and participant modeled as a random factor. A LME was run for each seed separately.
Relating emotion-related activation of mPFC and PCC with elevated emotion-dependent functional connectivity
We correlated mean percentage signal change of emotion-related activation in the mPFC and PCC (i.e., the seeds in the PPI analysis) with the mean beta-values from each of the regions exhibiting significant between-group differences in the PPI analysis with that respective seed using two-tailed tests of the non-parametric Spearman's correlation coefficient (rs). For details, see the supplement.
Controlling for multiple comparisons
We computed the minimum number of contiguous voxels passing the voxel-wise threshold for a significant group difference that would result in a cluster-wise p<0.05 corrected threshold at the whole brain level using Monte Carlo simulations: 51 voxels (1377 μL). For details on our simulation parameters, see the supplement.
Comparison between emotion-dependent and resting-state functional connectivity
Resting-state functional connectivity values (i.e., Fisher's z-scores) in the regions showing significant between-group differences with either the mPFC or PCC seeds on the PPI analyses were extracted and subjected to a LME with group and brain-state (task-activated, resting-state) modeled as fixed factors, participant modeled as a random factor, and with the Satterthwaite approximation for degrees of freedom.
Correlations between clinical variables and emotion-dependent functional connectivity
Statistical analyses of all demographic and clinical scales were computed with R (91) and Matlab (Natwick, MA). Within the MDD group only, the relationships between CDRS-R, BDI-II, RRS, MASC, and the mean Fisher's z-score within each of the significant regions identified by the between-group PPI analysis were examined with two-tailed tests of Pearson's correlation coefficient (r).
Results
Sociodemographic, clinical, and behavioral data
The groups did not differ on age, gender distribution, ethnicity, pubertal status, socioeconomic status, general intelligence, or behavioral performance on the emotion identification task (Table 1, all p's>0.30), but differed significantly as expected on depression, anxiety, and rumination measures (Table 1, all p's<0.001).
Between-group whole brain task results
The MDD group showed less deactivation in the left mPFC and left PCC (Figure 2) relative to HCL during emotional processing (see also supplement, Figure S1, and Table S2). Emotion-related activation in the mPFC and PCC showed no significant differences between depressed subjects who were undergoing psychosocial therapy at the time of scanning versus those who were not (all p's>0.59; see the supplement and Table S7 for more details).
Between-group emotion-dependent functional connectivity results
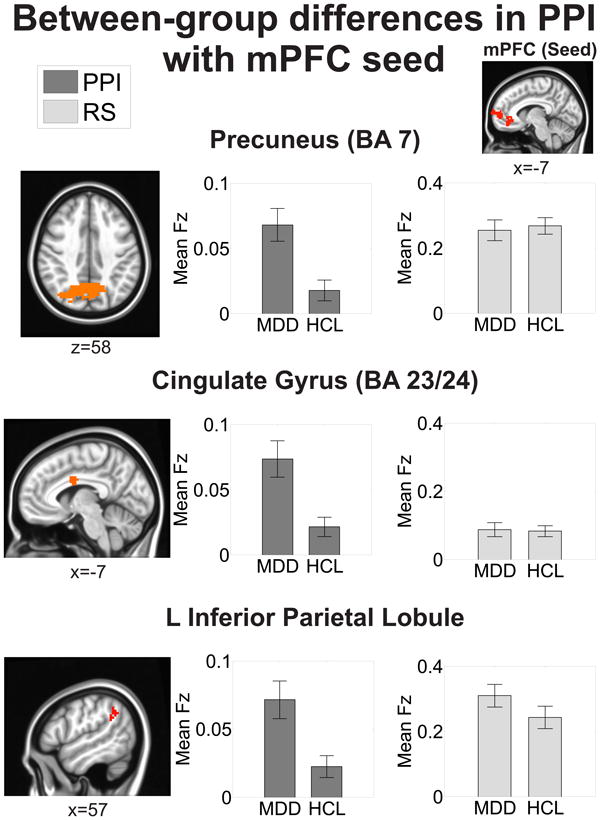
We seeded the left mPFC and PCC clusters that exhibited significant between-group differences on the emotion identification task in a seed-based PPI analysis (Figure 2). These same seeds were also used in the resting-state functional connectivity analyses. MDD exhibited greater mPFC emotion-dependent functional connectivity with bilateral precuneus (BA 7), cingulate gyrus (BA 23/24), and left inferior parietal lobule/supramarginal gyrus compared to HCL (Figure 3, Table 2). MDD also exhibited greater PCC emotion-dependent functional connectivity with bilateral precuneus (BA 7), right cingulate gyrus (BA 23/24), and left lentiform nucleus/subcallosal cingulate gyrus compared to HCL (Figure 4, Table 3). These areas showed no differences in emotion-dependent functional connectivity between depressed subjects who were undergoing psychosocial therapy at the time of scanning versus those who were not (all p's>0.27; see the supplement and Table S6 for more details).
Figure 3. Regions showing significant between-group differences in emotion-dependent functional connectivity of the mPFC seed.
All areas survived correction for multiple comparisons at a cluster-wise threshold of p<0.05. Mean functional connectivity values are reported as Fisher's z-scores (Fz). The mean resting-state (RS) Fz were extracted from the regions exhibiting significant between-group differences on the psychophysiological interaction (PPI) analysis (see Methods for more details). Locations are reported in MNI coordinates (radiological convention). See also Table 2.
Table 2. Summary of significant clusters from the between-group emotion-dependent functional connectivity analysis of mPFC.
Regions are the result of a psychophysiological interaction (PPI) method of functional connectivity and corrected for multiple comparisons at a cluster-wise threshold of p<0.05. Locations are reported according to the center of mass of cluster in MNI coordinates (radiological convention). Functional connectivity values are reported as mean ± SEM. Resting-state (RS) functional connectivity values were extracted from the regions exhibiting significant between-group differences on the PPI analysis (see Methods for more details). See also Figure 3.
| Region (Brodmann's Area) | Location (x,y,z) | # of voxels | Statistic(F1,61) | MDD (PPI) | HCL (PPI) | MDD(RS) | HCL (RS) |
|---|---|---|---|---|---|---|---|
| Directionality: MDD > HCL | |||||||
| Precuneus (BA 7) | 8, -67, 38 | 321 | 6.39 | 0.068 ± 0.01 | 0.018 ± 0.008 | 0.255 ± 0.03 | 0.268 ± 0.03 |
| Cingulate gyrus (BA23/24) | -6, -1, 30 | 176 | 6.30 | 0.073 ± 0.01 | 0.021 ± 0.007 | 0.087 ± 0.02 | 0.083 ± 0.02 |
| L inferior parietal lobule, supramarginal gyrus | -50, -52, 34 | 72 | 5.43 | 0.071 ± 0.01 | 0.022 ± 0.008 | 0.310 ± 0.03 | 0.242 ± 0.03 |
Figure 4. Regions showing significant between-group differences in emotion-dependent functional connectivity with the PCC seed.
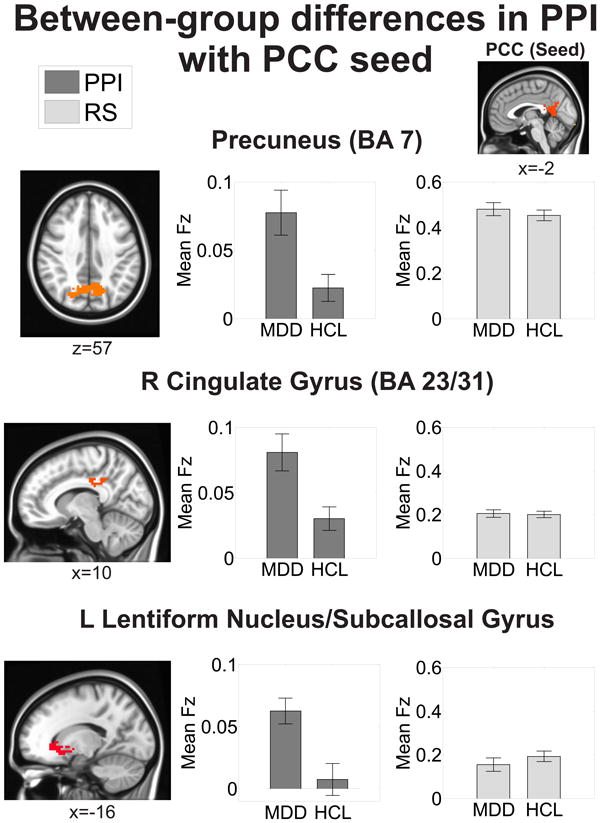
All areas survived correction for multiple comparisons at a cluster-wise threshold of p<0.05. Mean functional connectivity values are reported as Fisher's z-scores (Fz). The mean resting-state (RS) Fz were extracted from the regions exhibiting significant between-group differences on the psychophysiological interaction (PPI) analysis (see Methods for more details). Locations are reported in MNI coordinates (radiological convention). See also Table 3.
Table 3. Summary of significant clusters from the between-group emotion-dependent functional connectivity analysis of PCC.
Regions are the result of a psychophysiological (PPI) method of functional connectivity and corrected for multiple comparisons at a cluster-wise threshold of p<0.05. Locations are reported according to the center of mass of cluster in MNI coordinates (radiological convention). Functional connectivity values are reported as mean ± SEM. Resting-state (RS) functional connectivity values were extracted from the regions exhibiting significant between-group differences on the PPI analysis (see Methods for more details). See also Figure 4.
| Region(Brodmann's Area) | Location (x,y,z) | # of voxels | Statistic(F1,61) | MDD (PPI) | HCL (PPI) | MDD (RS) | HCL (RS) |
|---|---|---|---|---|---|---|---|
| Directionality: MDD > HCL | |||||||
| Precuneus (BA 7) | 5, -65, 37 | 195 | 6.25 | 0.078 ± 0.02 | 0.023± 0.009 | 0.481 ± 0.03 | 0.454 ± 0.02 |
| R cingulate gyrus (BA 31/23) | 14, -26, 32 | 179 | 5.42 | 0.081 ± 0.01 | 0.030± 0.008 | 0.205 ± 0.02 | 0.200 ± 0.01 |
| L dorsal striatum, subcallosal cingulate gyrus | -17, 17, -4 | 63 | 5.49 | 0.063 ± 0.01 | 0.008 ± 0.01 | 0.155 ± 0.03 | 0.193 ± 0.02 |
Relating emotion-related activation of mPFC and PCC with elevated emotion-dependent functional connectivity
Greater emotion-related activation in the PCC correlated significantly with stronger PCC emotion-dependent functional connectivity with dorsal striatum/subcallosal cingulate gyrus (rs=0.271, p=0.032). Detailed methods and results can be found in the supplement.
Comparison between emotion-dependent and resting-state functional connectivity
We compared mPFC and PCC-based functional connectivity in areas showing between-group differences from our PPI analysis across brain-states (task-activated, resting-state). We found that PCC functional connectivity with the dorsal striatum/subcallosal cingulate gyrus exhibited a significant interaction between group and brain-state (F1,54=5.410, p=0.023; Figure 3; Table 3). Post-hoc t -tests (Fisher's LSD) further revealed that this difference was driven by reduced functional connectivity during the emotion identification task in HCL. While all other areas displayed a significant effect of brain-state (resting-state > task activated, all p's<0.01), no other areas showed a significant main effect of group nor a significant interaction between group and brain-state in resting-state functional connectivity (all p's>0.05).
Correlations between clinical variables and emotion-dependent functional connectivity
All correlations were performed between the mean Fisher's z-scores of every significant cluster arising from the between-group PPI analysis for each DMN seed with levels of depression, rumination, anxiety, and age of depression onset. CDRS-R scores correlated positively with mPFC-cingulate functional connectivity (r =0.483, p=0.014; Figure S2A) and PCC-cingulate functional connectivity (r=0.436, p=0.029; Figure S2B). Functional connectivity between the PCC seed and dorsal striatum/subcallosal cingulate gyrus correlated negatively with age of onset (r=-0.527, p=0.014; Figure S2C). No functional connections showed significant associations with RRS or MASC scores (all p's>0.05), suggesting our results are selective to depression.
Discussion
Examining the dynamics of intrinsic functional networks associated with emotional dysregulation in early-onset depression is critical for elucidating the neurobiological mechanisms of MDD. Here, we examined functional connectivity of one of the major intrinsic brain networks, the default mode network (DMN), during active emotional processing and also during resting-state in a relatively large sample of unmedicated adolescents with MDD and well-matched healthy controls. We hypothesized that depressed adolescents would exhibit altered emotion-dependent connectivity of the DMN compared to healthy controls and that the strength of these aberrant connections would correlate with clinical measures of depression. Our investigation yielded three primary results. First, depressed adolescents exhibited relatively greater mPFC and PCC functional connectivity with areas involved in default mode (precuneus, posterior cingulate gyrus), cognitive executive (middle cingulate gyrus, inferior parietal lobule), and salience and affective processing (dorsal striatum/subcallosal cingulate gyrus). Second, functional connectivity between PCC and dorsal striatum/subcallosal cingulate gyrus remained inflexibly elevated in the depressed group during resting-state. Lastly, stronger PCC emotion-dependent functional connectivity was associated with greater depression severity and an earlier age of depression onset. Taken together, these findings advance a dynamic network model of depression, whereby depressed adolescents exhibit altered intrinsic functional connections that remain inflexibly elevated.
The finding of reduced deactivation in the DMN during emotional processing in depressed adults (24, 56, 92) was replicated in our adolescent sample (Figure 2), providing additional validation of DMN dysfunction in depressive illness and extending the relevance of DMN pathology to adolescent depression. Importantly, the same mPFC and PCC areas exhibiting reduced emotion-related deactivation in MDD coactivated with several regions during affective challenge (Figures 3-4, Tables 3-4). Specifically, depressed adolescents showed comparatively elevated mPFC and PCC functional coupling with default mode regions involved in self-referential processing (precuneus, posterior cingulate gyrus) (19, 25, 93), areas in the central executive network governing attentional selection of external stimuli (middle cingulate gyrus, inferior parietal lobule) (94-103), and affective processing structures best associated with the salience network (subcallosal cingulate gyrus/dorsal striatum) (31, 35, 40, 104-118). In healthy individuals, the DMN is anticorrelated with the central executive and salience networks (119, 120). However, our results demonstrate that these functional connections coactivate strongly together during emotional processing in adolescent depression.
Our emotion-dependent functional connectivity results may appear upon initial examination to differ from other recent studies of depression also employing seed-based psychophysiological interaction (PPI) analyses. Specifically, studies in medication-naïve adults (63) and adolescents (57) with depression reported decreased connectivity between mPFC and amygdala during emotional processing in depressed versus healthy individuals. Similarly, in a PPI study of unmedicated adolescents (58), depressed individuals showed reduced functional connectivity between the subcallosal cingulate area and PCC when evaluating fearful faces (58). However, all of these studies used static images and faces; dynamic face stimuli have been shown to be more ecologically valid than static faces and more robustly activate affective processing regions (75). Our present study uses dynamically morphing faces, thereby representing a more optimal functional assay to probe the neurocircuitry of emotional dysregulation. Additionally, none of the aforementioned studies explicitly examined both the mPFC and PCC. Critically, none of these studies defined their seeds functionally (i.e., they were derived from atlases or based on previously published coordinates). While hypothesis-driven, such a method may be suboptimal when trying to specifically target areas underlying behavioral and cognitive symptoms related to a disease state—namely, emotional dysregulation in depression.
Prior resting-state (37, 40, 46, 49, 50, 121, 122) and PPI studies (58, 59, 62, 63) have suggested that MDD is characterized by alterations within and between intrinsic functional brain networks. Our previously described functional connectivity results support this idea, but our finding that depressed adolescents failed to show brain-state (task-activated versus resting-state) dependent changes in PCC connectivity with the subcallosal cingulate gyrus (Figure 4) also suggests that depression may be characterized by inflexible functional connections. These findings corroborate a recent study in adolescents and young adults (15-24 years) that compared functional connectivity of the subcallosal cingulate area during a cognitive control task and resting-state, and reported inflexible functional connectivity between the subcallosal cingulate area and ventral striatum in the depressed group (107). By targeting emotionally-selective areas of the DMN and comparing DMN patterns across brain-states, our results build from the extant literature to advance a network model of depression characterized by both altered and inflexible connections of intrinsic brain networks.
Consistent with prior reports of significant associations between clinical measures of depression and resting-state DMN connectivity (40, 48, 49) we found that greater DMN functional connectivity during emotional processing correlated positively with depression severity (Figure S2A and S2B). Contrary to our hypothesis and findings in resting-state adult studies (29, 33, 36, 39), we did not find significant associations with rumination. Rumination as measured by the RRS may not capture negative self-focus as it pertains to adolescent depression (123), or it may reflect more general negative affect (124, 125). Given that emotion-dependent functional connectivity also did not correlate significantly with anxiety suggests that our results are specific to adolescent depression. Supporting this notion is our finding that stronger PCC connectivity during emotional processing was significantly associated with an earlier age of depression onset (Figure S2C). Connectivity between the PCC and parahippocampal cortex during recollection of negative life events has also been shown to increase with the number of depressive episodes, as well as the frequency and subsequent prediction of sad mood in daily life (128). Individuals with an increased genetic risk for developing depression also exhibit altered PCC connectivity with the amygdala during emotional regulation—but not during resting-state (129). These findings in concert with our results suggest that stronger emotion-dependent functional connections between the PCC and limbic regions may be a contributing mechanism to the development of depression. Another interpretation is that depression may impact the normal development of intrinsic brain networks through the manifestation of inflexible connections. The DMN follows a developmental trajectory in humans wherein the coherence between the mPFC and PCC subsystems is initially weak but solidifies by adolescence (64-66). Recent work has even suggested developmental changes selective to anticorrelated networks based in the mPFC during both resting-state (66) and a social emotion task (130). These results indicate that DMN maturation is vulnerable to experiences such as early life stressors and other risk factors associated with the development of depression (131). Under this framework, our findings suggest that early-onset depression adversely impacts normal maturation of intrinsic brain networks.
Although this study is the first to compare emotion-dependent and resting-state functional connectivity in adolescent depression, our results must be interpreted in light of their limitations. Future studies with larger samples are needed to replicate our results and different probes of affective processing are needed to assess the generalizability of our findings. The impact of depression on brain maturation remains a critical research question; future work examining dynamic brain-state patterns in participants spanning a wider age range may yield clearer answers. Finally, longitudinal research is needed to address the limitations of our cross-sectional design and to determine whether the aberrant dynamic DMN patterns we report are an enduring trait of MDD or whether they resolve with successful treatment.
In summary, the findings reported here are notable in several respects. First, no other study has investigated functional connectivity dynamics of the DMN in adolescent depression. Second, our finding of inflexibly elevated DMN functional connectivity in our depressed sample may reflect core features of depressive illness (e.g., emotional dysregulation) and thus, represent a candidate biomarker for evaluating treatment outcomes. Lastly, our unique study population of medication-free depressed adolescents offers potential insight regarding the influence of depression on normal brain development. Nevertheless, the neurobiological mechanisms by which these alterations occur and the optimal interventions needed requires additional study.
Supplementary Material
Acknowledgments
We would like to thank Gang Chen for helpful discussions on the neuroimaging analyses; Robert Hendren and Larissa Duncan for helpful comments on the manuscript; Napoleon Hoang, Guang Yang, Dipavo Banerjee, Audrey Fortezzo, Catherine Rios, Mary Luna, and Kevin Hahn for assistance with subject recruitment and data collection.
Financial Disclosures: This work was supported by grants from NIMH (R01MH085734, R01MH085734-02S1, and R01MH085734-05S1 to TTY and K01MH097978 to KZL); from the NARSAD Foundation to TTY; from the Center of Excellence in Stress and Mental Health and a Veteran's Affairs Merit Grant to ANS; from the Swedish Research Council (350-2012-303), Swedish Society of Medicine (SLS-244671), and the Sweden American Association to EHB; from the Veteran's Affairs CSR&D Merit Review to IAS.
Dr. Martin Paulus receives grant or research support from the National Institutes of Health. Dr. Guido Frank receives grant or research support from the National Institutes of Health. Dr. Jeffrey Max receives grant or research support from the National Institutes of Health. He also provides expert testimony in cases of traumatic brain injury on an ad hoc basis for plaintiffs and defendants on a more or less equal ratio. This activity constitutes approximately 5% of his professional activities. Dr. Susan Tapert receives grant or research support from the National Institutes of Health.
The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures All authors declare no potential biomedical or financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Walters EE. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depression and anxiety. 1998;7:3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child and adolescent psychiatric clinics of North America. 2002;11:619–637. doi: 10.1016/s1056-4993(02)00011-1. x. [DOI] [PubMed] [Google Scholar]
- 3.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello EJ, Mustillo S, Keller G, Angold A. Prevalence of psychiatric disorders in childhood and adolescents. In: Levin BL, Petrila J, Hennessy KD, editors. Mental Health Services: A Public Health Perspective. 2nd. Oxford, U.K: Oxford University Press; 2004. pp. 111–128. [Google Scholar]
- 5.Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biological psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 6.Naicker K, Galambos NL, Zeng Y, Senthilselvan A, Colman I. Social, demographic, and health outcomes in the 10 years following adolescent depression. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;52:533–538. doi: 10.1016/j.jadohealth.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, et al. Lost human capital from early-onset chronic depression. The American journal of psychiatry. 2000;157:940–947. doi: 10.1176/appi.ajp.157.6.940. [DOI] [PubMed] [Google Scholar]
- 8.Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, et al. Effect of age at onset on the course of major depressive disorder. The American journal of psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
- 9.Hollon SD, Shelton RC, Wisniewski S, Warden D, Biggs MM, Friedman ES, et al. Presenting characteristics of depressed outpatients as a function of recurrence: preliminary findings from the STAR*D clinical trial. Journal of psychiatric research. 2006;40:59–69. doi: 10.1016/j.jpsychires.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Molecular and cellular endocrinology. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Romeo RD, McEwen BS. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 12.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Cerebral cortex. Vol. 19. New York, NY: 2009. Development of anterior cingulate functional connectivity from late childhood to early adulthood; pp. 640–657. 1991. [DOI] [PubMed] [Google Scholar]
- 13.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 14.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Frontiers in systems neuroscience. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Raichle ME. Disease and the brain's dark energy. Nature reviews Neurology. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 16.Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological medicine. 2013:1–11. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- 17.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual review of clinical psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of disease. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 20.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain research bulletin. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 21.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 23.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta analysis. Journal of cognitive neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 26.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. Journal of neurophysiology. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, et al. Resting state default-mode network connectivity in early depression using a seed region-of -interest analysis: decreased connectivity with caudate nucleus. Psychiatry and clinical neurosciences. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 31.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, et al. Increased neural resources recruitment in the intrinsic organization in major depression. Journal of affective disorders. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biology of mood & anxiety disorders. 2011;1:11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social cognitive and affective neuroscience. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biological psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, et al. Resting-state functional connectivity in treatment-resistant depression. The American journal of psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological medicine. 2012;42:2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y, et al. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry research. 2010;182:211–215. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain : a journal of neurology. 2012;135:1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Kong L, Wu F, Womer F, Jiang W, Cao Y, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychological medicine. 2012:1–7. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Zhu X, Wang X, Zhu X, Zhong M, Yi J, et al. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PloS one. 2014:9–e85241. doi: 10.1371/journal.pone.0085241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain imaging and behavior. 2011;5:307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao Q, Ding J, Lu G, Su L, Zhang Z, Wang Z, et al. Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PloS one. 2011:6–e25159. doi: 10.1371/journal.pone.0025159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin C, Gao C, Chen C, Ma S, Netra R, Wang Y, et al. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neuroscience letters. 2011;503:105–109. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pannekoek JN, van der Werff SJ, Meens PH, van den Bulk BG, Jolles DD, Veer IM, et al. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naive clinically depressed adolescents. Journal of child psychology and psychiatry, and allied disciplines. 2014 doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- 51.Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: A systematic review. NeuroImage Clinical. 2013;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, et al. Functional connectivity of the amygdala in early-childhood-onset depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:1027–1041. e1023. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. Journal of child psychology and psychiatry, and allied disciplines. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual review of psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 55.Kovacs M, Joormann J, Gotlib IH. Emotion (Dys)regulation and Links to Depressive Disorders. Child development perspectives. 2008;2:149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 57.Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, et al. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. Journal of affective disorders. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, et al. Functional connectivity of negative emotional processing in adolescent depression. Journal of affective disorders. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Friedel E, Schlagenhauf F, Sterzer P, Park SQ, Bermpohl F, Strohle A, et al. 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology. 2009;205:261–271. doi: 10.1007/s00213-009-1536-1. [DOI] [PubMed] [Google Scholar]
- 61.Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Moller HJ, et al. Functional connectivity of emotional processing in depression. Journal of affective disorders. 2011;134:272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 62.Park CH, Wang SM, Lee HK, Kweon YS, Lee CT, Kim KT, et al. Affective state-dependent changes in the brain functional network in major depressive disorder. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong L, Chen K, Tang Y, Wu F, Driesen N, Womer F, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. Journal of psychiatry & neuroscience : JPN. 2013;38:120117. doi: 10.1503/jpn.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS computational biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of cognitive neuroscience. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saverino C, Grigg O, Churchill NW, Grady CL. Age differences in the default network at rest and the relation to self-referential processing. Social cognitive and affective neuroscience. 2014 doi: 10.1093/scan/nsu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 69.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Poznanski EO. Children's Depressing Rating Scale-Revised (CDRS-R) Western Psychological Services; 1996. [Google Scholar]
- 71.Beck A, Steer R, Brown G. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 72.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 73.Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- 74.Sreenivas S, Boehm SG, Linden DE. Emotional faces and the default mode network. Neuroscience letters. 2012;506:229–234. doi: 10.1016/j.neulet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 75.LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Cerebral cortex. Vol. 13. New York, NY: 2003. Dynamic perception of facial affect and identity in the human brain; pp. 1023–1033. 1991. [DOI] [PubMed] [Google Scholar]
- 76.Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Brain research Cognitive brain research. 2004;20:81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Ekman P, Friesen WV. In: Pictures of facial affect. Press CP, editor. Palo Alto, CA: 1975. [Google Scholar]
- 78.Morris JS, Friston KJ, Dolan RJ. Neural responses to salient visual stimuli. Proceedings Biological sciences / The Royal Society. 1997;264:769–775. doi: 10.1098/rspb.1997.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perrett DI, May KA, Yoshikawa S. Facial shape and judgements of female attractiveness. Nature. 1994;368:239–242. doi: 10.1038/368239a0. [DOI] [PubMed] [Google Scholar]
- 80.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 81.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 82.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 83.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 84.Andersson JLR, Jenkinson M, Smith S. Non-Linear Optimisation 2007 [Google Scholar]
- 85.Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. NeuroImage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, et al. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. The American journal of psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, et al. Altered functional and structural brain network organization in autism. NeuroImage Clinical. 2012;2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 91.R Development Core Team. A Language and Environment for Statistical Computing. 2nd. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 92.Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2011;12:627–637. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- 93.Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social cognitive and affective neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, et al. Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event-related fMRI study. Human brain mapping. 2007;28:263–274. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 96.Marois R, Leung HC, Gore JC. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25:717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 97.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Science. Vol. 280. New York, NY: 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance; pp. 747–749. [DOI] [PubMed] [Google Scholar]
- 98.Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vogt BA, Finch DM, Olson CR. Cerebral cortex. Vol. 2. New York, NY: 1992. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions; pp. 435–443. 1991. [DOI] [PubMed] [Google Scholar]
- 101.Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. The Journal of neuropsychiatry and clinical neurosciences. 2011;23:121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- 102.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diekhof EK, Falkai P, Gruber O. Functional interactions guiding adaptive processing of behavioral significance. Human brain mapping. 2009;30:3325–3331. doi: 10.1002/hbm.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Davey CG, Yucel M, Allen NB, Harrison BJ. Task-related deactivation and functional connectivity of the subgenual cingulate cortex in major depressive disorder. Frontiers in psychiatry. 2012;3:14. doi: 10.3389/fpsyt.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 109.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 110.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biological psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 111.Holtzheimer PE, 3rd, Mayberg HS. Deep brain stimulation for treatment-resistant depression. The American journal of psychiatry. 2010;167:1437–1444. doi: 10.1176/appi.ajp.2010.10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rogers MA, Bradshaw JL, Pantelis C, Phillips JG. Frontostriatal deficits in unipolar major depression. Brain research bulletin. 1998;47:297–310. doi: 10.1016/s0361-9230(98)00126-9. [DOI] [PubMed] [Google Scholar]
- 113.Drevets WC. Neuroimaging studies of mood disorders. Biological psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 114.Drevets WC. Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 115.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American journal of psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of affective disorders. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, et al. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, affective & behavioral neuroscience. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, et al. Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:628–641. e613. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang Y, Kong L, Wu F, Womer F, Jiang W, Cao Y, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychological medicine. 2013;43:1921–1927. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- 122.Ho TC, Wu J, Shin DD, Liu TT, Tapert SF, Yang G, et al. Altered cerebral perfusion in executive, affective, and motor networks during adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:1076–1091. e1072. doi: 10.1016/j.jaac.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Michl LC, McLaughlin KA, Shepherd K, Nolen-Hoeksema S. Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: longitudinal evidence in early adolescents and adults. Journal of abnormal psychology. 2013;122:339–352. doi: 10.1037/a0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nolen-Hoeksema S, Stice E, Wade E, Bohon C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. Journal of abnormal psychology. 2007;116:198–207. doi: 10.1037/0021-843X.116.1.198. [DOI] [PubMed] [Google Scholar]
- 125.Thomsen DK. The association between rumination and negative affect: a review. Cognition and Emotion. 2006;20:1216–1235. [Google Scholar]
- 126.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of abnormal psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- 127.Muris P, Roelofs J, Meesters C, Boomsma P. Rumination and worry in nonclinical adolescents. Cognitive Therapy and Research. 2004;28:539–554. [Google Scholar]
- 128.Zamoscik V, Huffziger S, Ebner-Priemer U, Kuehner C, Kirsch P. Increased involvement of the parahippocampal gyri in a sad mood predicts future depressive symptoms. Social cognitive and affective neuroscience. 2014 doi: 10.1093/scan/nsu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fang Z, Zhu S, Gillihan SJ, Korczykowski M, Detre JA, Rao H. Serotonin transporter genotype modulates functional connectivity between amygdala and PCC/PCu during mood recovery. Frontiers in human neuroscience. 2013;7:704. doi: 10.3389/fnhum.2013.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burnett S, Blakemore SJ. Functional connectivity during a social emotion task in adolescents and in adults. The European journal of neuroscience. 2009;29:1294–1301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HP A axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


