Abstract
Objective:
To characterize autonomic impairment in motor neuron disease.
Methods:
Neurological evaluations and autonomic testing were analyzed retrospectively in 132 patients: 86 classic amyotrophic lateral sclerosis (ALS), 36 lower motor neuron disease (LMN), and 10 upper motor neuron predominant (UMN).
Results:
One-third of patients were symptomatic; urinary urgency and constipation were the most frequent symptoms. Increased Composite Autonomic Severity Score (CASS) was present in 75% with mild impairment (CASS 1-3) in 85% and moderate (CASS 4-7) in 15%. The frequencies of testing abnormalities were: sudomotor 46%, cardiovagal 50%, and adrenergic 14%. The UMN group had significantly higher median CASS scores than the classic ALS (P=0.021) and LMN group (P=0.018).
Discussion:
We found predominantly mild autonomic impairment in ALS patients, with mostly cardiovagal and sudomotor involvement. Moderate autonomic failure occurred in 1 of 7 patients, especially those with an UMN presentation. Patients with selective corticospinal tract involvement may have more impairment of autonomic pathways.
Keywords: Motor neuron disease, Amyotrophic lateral sclerosis, Autonomic impairment, Composite Autonomic Severity Score, Sudomotor
Introduction
There is increasing evidence that amyotrophic lateral sclerosis (ALS) is a multisystem disease that extends beyond the anterior horn cells and corticospinal tracts. Transactive response DNA binding protein 43 Kda (TDP-43) links ALS to frontotemporal dementia, and disease progression fits a model of prion-like spread of TDP-43 protein in a corticofugal direction.1 Impairment of the sensory system has also been reported.2-4 The degree and type of autonomic impairment in this disease continues to be poorly understood. Autonomic involvement has been investigated, but studies to date have included small numbers of subjects or were focused on a very limited part of the autonomic nervous system. We performed a retrospective review of symptoms and deficits in ALS patients who underwent standardized autonomic evaluation. This approach ensures a comprehensive and uniform evaluation from which one could define the severity and distribution of autonomic involvement.
Materials and Methods
After institutional review board approval, a search was performed on the autonomic database of Mayo Clinic-Rochester by laboratory codes for ALS and motor neuron disease. The records of 203 patients seen between 1990 and 2013 were retrieved. Patients with diabetes mellitus, incomplete autonomic testing, on confounding medications (opioids, anticholinergics), or with a final diagnosis different than motor neuron disease were excluded.
Based on these criteria, 132 patients were included. All had undergone neurological evaluation (by peripheral nerve or neuromuscular specialists) and NCS/EMG at Mayo Clinic. Autonomic screen was done in standardized fashion with normative data for age and gender. The autonomic screen consisted of sympathetic sudomotor (quantitative sudomotor axon reflex test-QSART or QSWEAT), cardiovagal [heart rate variability to deep breathing and Valsalva maneuver (VM)], and cardiovascular adrenergic testing (blood pressure profile during VM and tilt table testing).5 The neurological evaluations and nerve conduction studies (NCS)/electromyography (EMG) were reviewed to extract information about type of motor neuron disease. The presence and degree of autonomic symptoms was extracted from both standard review of systems questionnaires and neurologist evaluations. Results of autonomic testing were reviewed, and the composite autonomic severity score (CASS) based on the above tests of sudomotor, cardiovagal, and adrenergic functions, corrected for the confounding effects of age and gender,6 were calculated. The CASS has been validated to provide a measure of severity and distribution of autonomic failure and is derived from the autonomic reflex screen as described previously.6 The CASS score ranges from 0 to 10 points and is subdivided into 3 subscores: CASS sudomotor (range 0-3), CASS cardiovagal (range 0-3), and CASS adrenergic (range 0-4). Each score has been normalized for the confounding variables of age and gender. Laboratory evidence of autonomic failure is graded as follows: 0, absent; 1-3, mild; 4-6, moderate; and 7-10, severe failure.7
Prior to analysis of autonomic testing, the patient cohort was divided into 3 groups based on neurological evaluation and NCS/EMG. The lower motor neuron predominant group included progressive muscular atrophy (PMA) and patients who lacked significant upper motor neuron disease (“suspected” and “probable” ALS in El Escorial criteria). The classic ALS group included patients with evidence of significant upper and lower motor neuron disease. Patients with 1, 2, or 3 neuraxis segments affected were included (“possible”, “probable”, and “definite” ALS, respectively, in El Escorial criteria). Finally, the upper motor neuron predominant group included patients with primary lateral sclerosis (PLS) and those without significant lower motor neuron disease (“possible” ALS in El Escorial criteria).8,9 Most patients were not followed longitudinally, so it is unknown whether the lower or upper motor neuron predominant groups remained as such or developed classic ALS.
Continuous variables are presented as mean ± standard deviation. Due to sample size differences and data skewness, nonparametric statistical comparisons were performed. The Kruskal-Wallis Test was used for multiple group comparisons, and the Mann-Whitney Test was used to examine comparisons between 2 groups. All statistical tests were 2-tailed, and P<0.05 was considered significant for all comparisons. SPSS statistical software, version 21 for Windows (SPSS Inc, Chicago, IL), was used for all statistical analyses.
Results
A total of 132 patients (73 men and 59 women, mean age 57 +/− 15, range 24-84) were included (Table 1). There were 86 patients with classic ALS, 36 with lower motor neuron predominant disease (including 8 cases of PMA), and 10 with upper motor neuron predominant disease (including 2 PLS). Mean duration of symptoms before evaluation at our institution was 21.8 months (range 3-240).
Table 1.
General features of the study population
| Sex | Mean Age +/−SD (range) |
Mean duration (months) of symptoms |
|||
|---|---|---|---|---|---|
| Patients | N | M | W | 57 +/−15 (24-84) |
22 +/− 29 (3-240) |
| Total | 132 | 73 | 59 | ||
| UMN group |
10 | 2 | 8 | 63 +/−11 (43-78) |
41 +/−51 (10-180) |
| LMN group |
36 | 27 | 9 | 55 +/−14 (24-80) |
28 +/−43 (4-240) |
| Classic group |
86 | 44 | 42 | 57 +/−12 (25-84) |
16 +/−13 (1.3-72) |
More than one-quarter (29%, 38/132) reported autonomic symptoms; urinary and gastro-intestinal symptoms (20% and 11%) were the most frequent. Of those, urinary urgency and constipation were the most common manifestations. Male erectile dysfunction (10%) and orthostatic intolerance (7%) were also reported.
The CASS was increased in 75% of patients (99/132), with a mean score of 1.6 +/− 1.4 (+/− SD). Autonomic impairment was mild (CASS 1-3) in 85% and moderate (CASS 4-7) in the remainder (15%), and no patients had severe generalized autonomic failure (Table 2). We analyzed the degree of impairment in the different components of autonomic testing. Sudomotor abnormalities were present in 46% (61/132). Based on the QSART abnormalities, distal or length-dependent (54%) was the most common sweat loss pattern. Patchy and global or diffuse patterns were less frequent (32% and 14%, respectively).
Table 2.
CASS Abnormalities
| Increased CASS | 75% (99/132) |
CASS 1-3: 85% (85/99) |
| CASS 4-7: 15% (14/99) | ||
| CASS-Sudomotor abnormalities |
46% (61/132) |
Length- dependent: 54% (33/61) |
| Patchy: 32% (20/61) and global: 14% (8/61) | ||
| CASS-Cardiovagal abnormalities |
50% (66/132) |
Decreased HRDBa: 65% (43/66) |
| Decreased VRb: 14% (9/66) | ||
| Decreased VR and HRDB: 21% (14/66) | ||
| CASS- Adrenergic abnormalities |
14% (18/132) |
Abnormal VMc: 55% (10/18) |
| OHd: 28% (5/18) | ||
| OH and abnormal VM: 17% (3/18) |
Heart rate to deep breathing
Valsalva ratio
Valsalva maneuver
Orthostatic hypotension
Cardiovagal abnormalities were present in 50% (66/132) of patients, with heart rate to deep breathing being the most common abnormality (86%). Finally, adrenergic vasomotor impairment was present in 14% (18/132) of the patients. An abnormal blood pressure response to the VM was more frequent than orthostatic hypotension.
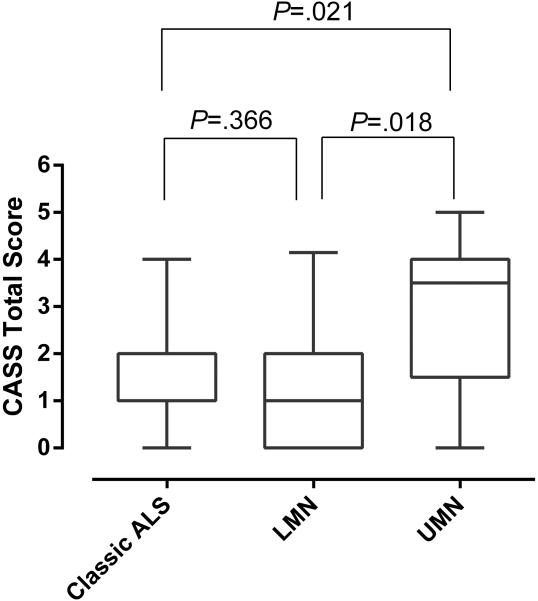
A comparison of median CASS scores among the 3 groups is shown in Figure 1. The upper motor neuron predominant group showed significantly higher CASS scores when compared to both the classic ALS and lower motor neuron predominant groups. Although CASS scores were higher in the classic ALS group compared to lower motor neuron predominant, this difference was not significant statistically.
Figure 1.
Comparison of median total CASS scores among the 3 groups of motor neuron disease.
The bars represent the 5/95th percentiles. Note significantly higher scores in the upper motor neuron group (Median: 3.5, SD: 1.75, SEM: 0.55) when compared to classic ALS (Median:1.0, SD:1.27, SEM: 0.13) and lower motor neuron (Median: 1.0, SD: 1.33, SEM: 0.22) predominant groups. There was no significant difference between the LMN and classic ALS groups. SD: standard deviation. SEM: standard error of the mean.
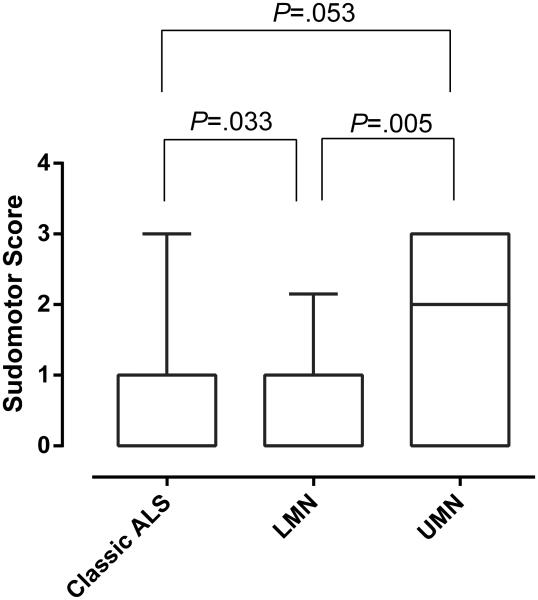
Comparison of the median CASS score components among the 3 groups showed that only the sudomotor component was significantly different between groups (Kruskal-Wallis Test: P=0.008). Further analysis showed that both the UMN and Classic ALS groups had higher sudomotor deficits than the LMN group (Fig 2).
Figure 2.
Comparison of the CASS sudomotor component among the 3 motor neuron disease groups.
The bars represent the 5/95th percentiles. Note significantly greater sudomotor impairment in the UMN group compared to the LMN group and classic ALS vs LMN group. The comparison of classic VS UMN was not significant.
Since the UMN group seemed to segregate from the other groups, we reviewed records for further details and follow-up. Among the upper motor neuron group, 2 patients had evidence of superimposed mild parkinsonism and 1 had mild frontotemporal lobe dysfunction at presentation. Follow-up was available for 6/10 patients; no alternative diagnosis had been made. Two patients died within the first year after the evaluation. One patient was alive 5 years after evaluation, and 2 others were alive 13 and 21 years after evaluation using walking aids for ambulation.
Discussion
There are 2 key findings in this study. The first and main finding is that autonomic dysfunction and deficits are frequent but modest in ALS. The second finding is that autonomic deficits are greater when upper motor neurons are affected predominantly.
Autonomic symptoms are relatively common, occurring in 29% of patients. Symptoms of urinary or gastrointestinal dysfunction or of orthostatic intolerance were the most common. Prior studies have reported similar findings.10,11 We also found frequent male erectile dysfunction, which has not been reported extensively. Although dietary changes, decrease in ambulation, and psychological stress may contribute to these symptoms, prior studies have shown changes in the intermediolateral columns and the Onuf nucleus which could provide an anatomical explanation for these clinical manifestations.12,13
Autonomic deficits were common (75% of patients) but mild in most patients (85%), and they were moderate involvement in a minority (15%). The most common abnormality was that of cardiovagal impairment, occurring in half of the patients. These findings are consonant with prior reports14,15 and provide further evidence of cardiovagal parasympathetic involvement in ALS.
Sudomotor abnormalities are common, occurring in 46% of our cohort. Although prior studies have reported sudomotor impairment in ALS,16 we also recorded the pattern of sweat loss on QSART. The most common distribution was distal or length-dependent. However, a smaller number of patients had patterns that were non-length-dependent, including patchy or diffuse sweating loss. Although immobilization and muscular atrophy alone can affect sweat production,17 the length-dependent pattern could suggest a peripheral or postganglionic origin. For instance, prior studies have found dermal ultrastructural changes and decreased density of epidermal nerve fibers in ALS when compared to controls, which could account for our findings.18,19 It is also possible that degeneration of intermediolateral columns or central pathways could contribute to sudomotor changes.
We found that neurogenic orthostatic hypotension was rare. Minor changes in beat-to-beat blood pressure responses to the VM occurred, suggestive of only modest adrenergic involvement. A small number of patients showed orthostatic hypotension without abnormalities in VM (Table 2). Although this finding may be explained by non-neurogenic causes, such as hypovolemia or venous pooling secondary to muscle atrophy, the fact that these patients had positive CASS scores (due to cardiovagal and sweat abnormalities), suggests an alternative explanation. They could have subtle autonomic failure, which, coupled with deconditioning, results in mild orthostatic hypotension. The presence of orthostatic hypotension in a small percentage of patients could not be explained solely by age; 4 patients were older than 70 years, and 4 were younger than that age. Overall, the observation that generalized autonomic failure was not a feature of ALS is in keeping with prior reports.14,20
The pathogenesis of ALS is largely unknown. There is increasing evidence that ALS is a proteinopathy with varying degrees of central involvement.1 There is also a suggestion that autonomic failure is greater in cases where there are clinical manifestations beyond the anterior horn cells. In our cohort, 2 of 10 in the upper motor neuron predominant group demonstrated mild parkinsonism, and 1 of 10 had frontotemporal impairment at the time of evaluation. The more severe autonomic failure in this group is consonant with our prior study of Guamanian ALS,21 where we reported that patients with parkinsonism and dementia had much greater autonomic failure than did our present cohort. This observation leads us to hypothesize that the upper motor neuron predominant group has more widespread central neurologic disease, including autonomic involvement.
The main limitation of the study is the retrospective design and possible selection bias, since patients were selected from the Autonomic Laboratory database. Therefore, results of testing in a “general” ALS population could be less pronounced. However, we chose this approach so that subjects were studied uniformly in an environment where confounding medications were largely withheld and interpretation was robust in comparison with a large normative database. Another limitation is that the study is cross-sectional with limited longitudinal follow-up.
Acknowledgments
This work was supported in part by National Institutes of Health (NS 32352 Autonomic Disorders Program Project (PAL); NS 44233 Pathogenesis and Diagnosis of Multiple System Atrophy (PAL), U54 NS065736 Autonomic Rare Disease Clinical Consortium (PAL) and CA 169443 (NPS)), Mayo CTSA (UL1 TR000135), and Mayo Funds.
The Autonomic Diseases Consortium is a part of the NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by U54 NS065736 from the National Institute of Neurological Diseases and Stroke (NINDS) and the NIH Office of Rare Diseases Research (ORDR).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- CASS
Composite Autonomic Severity Score
- HRDB
Heart rate to deep breathing
- NCS
Nerve conduction studies
- OH
Orthostatic hypotension
- PLS
Primary lateral sclerosis
- PMA
Progressive muscular atrophy
- QSART
Quantitative sudomotor axon reflex test
- TDP-43
Transactive response DNA binding protein 43 Kda
- VR
Valsalva ratio
- VM
Valsalva maneuver
References
- 1.Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Del Tredici K. Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nature Reviews Neurology. 2013;9(12):708–714. doi: 10.1038/nrneurol.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pugdahl K, Fuglsang-Frederiksen A, de Carvalho M, Johnsen B, Fawcett PR, Labarre-Vila A, Liguori R, Nix WA, Schofield IS. Generalised sensory system abnormalities in amyotrophic lateral sclerosis: a European multicentre study. J Neurol Neurosurg Psychiatry. 2007;78(7):746–749. doi: 10.1136/jnnp.2006.098533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammad M, Silva A, Glass J, Sladky JT, Benatar M. Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology. 2007;69(24):2236–2242. doi: 10.1212/01.wnl.0000286948.99150.16. [DOI] [PubMed] [Google Scholar]
- 4.Dyck PJ, Stevens JC, Mulder DW, Espinosa RE. Frequency of nerve fiber degeneration of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. Morphometry of deep and superficial peroneal nerves. Neurology. 1975;25(8):781–785. doi: 10.1212/wnl.25.8.781. [DOI] [PubMed] [Google Scholar]
- 5.Low PA. Autonomic nervous system function. J Clin Neurophysiol. 1993;10(1):14–27. doi: 10.1097/00004691-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68(8):748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa JJ, Dyck PJ, Laughlin RS, Mercado JA, Massie R, Sandroni P, Dyck PJ, Low PA. Autonomic dysfunction in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2012;78(10):702–708. doi: 10.1212/WNL.0b013e3182494d66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 9.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. Diseases. WFoNRGoMN. [DOI] [PubMed] [Google Scholar]
- 10.Toepfer M, Folwaczny C, Klauser A, Riepl RL, Muller-Felber W, Pongratz D. Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1(1):15–19. doi: 10.1080/146608299300079484. [DOI] [PubMed] [Google Scholar]
- 11.Nübling GS, Mie E, Bauer RM, Hensler M, Lorenzl S, Hapfelmeier A, Irwin DE, Borasio GD, Winkler AS. Increased prevalence of bladder and intestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2014 doi: 10.3109/21678421.2013.868001. in press. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy PG, Duchen LW. A quantitative study of intermediolateral column cells in motor neuron disease and the Shy-Drager syndrome. J Neurol Neurosurg Psychiatry. 1985;48:1103–1106. doi: 10.1136/jnnp.48.11.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kihira T, Yoshida S, Yoshimasu F, Wakayama I, Yase Y. Involvement of Onuf's nucleus in amyotrophic lateral sclerosis. J Neurol Sci. 1997;147(1):81–88. doi: 10.1016/s0022-510x(96)05313-0. [DOI] [PubMed] [Google Scholar]
- 14.Chida K, Sakamaki S, Takasu T. Alteration in autonomic function and cardiovascular regulation in amyotrophic lateral sclerosis. J Neurol. 1989;236:127–130. doi: 10.1007/BF00314326. [DOI] [PubMed] [Google Scholar]
- 15.Pisano F, Miscio G, Mazzuero G, Lanfranchi P, Colombo R, Pinelli P. Decreased heart rate variability in amyotrophic lateral sclerosis. Muscle Nerve. 1995;18(11):1225–1231. doi: 10.1002/mus.880181103. [DOI] [PubMed] [Google Scholar]
- 16.Beck M, Giess R, Magnus T, Puls I, Reiners K, Toyka KV, Naumann M. Progressive sudomotor dysfunction in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2002;73(1):68–70. doi: 10.1136/jnnp.73.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos-Bento M, de Carvalho M, Evangelista T, Sales Luis ML. Sympathetic sudomotor function and amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2(2):105–108. doi: 10.1080/146608201316949550. [DOI] [PubMed] [Google Scholar]
- 18.Weis J, Katona I, Muller-Newen G, Sommer C, Necula G, Hendrich C, Ludolph AC, Sperfeld AD. Small-fiber neuropathy in patients with ALS. Neurology. 2011;76(23):2024–2029. doi: 10.1212/WNL.0b013e31821e553a. [DOI] [PubMed] [Google Scholar]
- 19.Provinciali L, Cangiotti A, Tulli D, Carboni V, Cinti S. Skin abnormalities and autonomic involvement in the early stage of amyotrophic lateral sclerosis. J Neurol Sci. 1994;126(1):54–61. doi: 10.1016/0022-510x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 20.Sachs C, Conradi S, Kaijser L. Autonomic function in amyotrophic lateral sclerosis: a study of cardiovascular responses. Acta Neurol Scand. 1985;71(5):373–378. doi: 10.1111/j.1600-0404.1985.tb03215.x. [DOI] [PubMed] [Google Scholar]
- 21.Low PA, Ahlskog JE, Petersen RC, Waring SC, Esteban-Santillan C, Kurland LT. Autonomic failure in Guamanian neurodegenerative disease. Neurology. 1997;49:1031–1034. doi: 10.1212/wnl.49.4.1031. [DOI] [PubMed] [Google Scholar]