Abstract
Neurodegenerative diseases share common features, including catastrophic neuronal loss that leads to cognitive or motor dysfunction. Neuronal injury occurs in an inflammatory milieu that is populated by resident and sometimes, infiltrating, immune cells-all of which participate in a complex interplay between secreted inflammatory modulators and activated immune cell surface receptors. The importance of these immunomodulators is highlighted by the number of immune factors that have been associated with increased risk of neurodegeneration in recent genome-wide association studies. One of the more difficult tasks for designing therapeutic strategies for immune modulation against neurodegenerative diseases is teasing apart beneficial from harmful signals. In this regard, learning more about the immune components of these diseases has yielded common themes. These unifying concepts should eventually enable immune-based therapeutics for treatment of Alzheimer’s and Parkinson’s diseases and amyotrophic lateral sclerosis. Targeted immune modulation should be possible to temper maladaptive factors, enabling beneficial immune responses in the context of neurodegenerative diseases.
Keywords: Central Nervous System, Neuroimmunology, Neuroinflammation, Alzheimer’s Disease, Parkinson’s Disease, Amyotrophic Lateral Sclerosis
1. Introduction
1.1 The immune system in neurodegenerative disorders
Neurodegenerative disorders are associated with age-dependent deposition of aggregated and misfolded proteins, cognitive disturbance, locomotive dysfunction, and neuronal loss (Forman et al., 2004). Importantly, evolution of these diseases occurs against a dysregulated neuroinflammatory backdrop (Glass et al., 2010). It has become clear in genetically modified animal models and from longitudinal patient studies that neuroinflammation and immune activation in the CNS develop early on in the course of disease, likely prior to large-scale neuronal loss. Activated microglia, the CNS-resident macrophage population, are present in nearly all neurodegenerative disorders (Long-Smith et al., 2009; Prokop et al., 2013; Sargsyan et al., 2005). In addition to microglia, activated astrocytes and peripheral monocytes or lymphocytes can be detected in the diseased CNS under certain conditions. Studies linking immune activation to poor prognosis in patients are raising a major question: are all neuroinflammatory pathways detrimental to CNS health?
A broad view would argue that inflammation in the CNS creates a neurotoxic environment, and must be sanctioned in order to prevent disease and to support recovery. In contrast to this ‘inflammation is strictly damaging’ view of neurodegeneration, several studies in animal models have revealed that inhibition of anti-inflammatory factors or expression of pro-inflammatory molecules can improve disease-relevant outcomes. This dichotomy supports the notion that broad-based manipulation of the immune system should likely be switched in favor of targeted immunomodulation on key effectors. This review will focus on current models of immune modulators, cytokines, chemokines, and receptors that are thought to drive immune responses within the CNS. It is becoming clear that all neurodegenerative diseases have a dominant inflammatory phenotype, and we will pay particular attention to advances in understanding immune-based mechanisms of Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), and Parkinson's disease (PD). It is particularly striking that recent mechanistic studies into these debilitating diseases have provided common nodes of innate immune cell dysfunction, yielding important insight into immune modulation therapeutic strategies.
1.2 Immune players and responders within the CNS
Inflammation plays such a central role in health, that even subtle dysregulation of this highly ordered response can lead to myriad pathologies. Restricting expression of secreted inflammatory molecules and receptor/signaling cascades to certain cell types is a key physiological regulatory strategy. Resident immune cells in the CNS include microglia and astrocytes; but microglia are the only CNS cells of hematopoietic origin. Early in embryonic development, myeloid progenitors differentiate into primitive macrophage progenitors in the yolk sac (Ginhoux et al., 2010). Later, these cells engraft into the CNS, where they differentiate into mature microglia that are capable of local expansion and maintenance of a population of phagocytic monocytes for the life of the animal (Ajami et al., 2007; Elmore et al., 2014). Under physiologic conditions, microglia largely exhibit a ramified morphology with highly mobile processes optimized for sampling the local environment (Nimmerjahn et al., 2005). In this surveillance state, microglia monitor neuronal firing and synaptic function (Wake et al., 2009), drive programed cell death of neurons during development (Cunningham et al., 2013), and are responsible for synaptic pruning (Schafer et al., 2012; Stevens et al., 2007). The numerous roles microglia play in development and homeostasis have been thoroughly reviewed elsewhere (Salter and Beggs, 2014; Schwartz et al., 2013). Upon detection of molecular pathogens or danger signals, microglia switch from a ramified to a classically activated state with accompanying changes in morphology, function, and transcriptional repertoire (Hanisch and Kettenmann, 2007). A second glial cell population that is central to neuroprotection and brain homeostasis is the astrocyte. Astrocytes are multifunctional cells, which are derived from the neuroectoderm and play essential roles in neural circuit development, synaptic pruning (Chung et al., 2013), and metabolism (reviewed in; Bouzier-Sore and Pellerin, 2013; Clarke and Barres, 2013; Sofroniew and Vinters, 2010). When activated by stimuli such as innate immune modulators, cytokines, neurotransmitters, hypoxia, and others, astrocytes release cytokines and other immune signaling molecules. In concert with microglia, these immune molecules mediate inflammatory responses that can be beneficial or deleterious in neurodegenerative diseases (Colangelo et al., 2014).
The general dogma for ‘CNS immune privilege’, that peripheral immune cells do not cross the blood brain barrier (BBB) and penetrate the CNS, has been revised in recent years. Under steady-state conditions in the healthy individual, the BBB surrounding the microvasculature and the epithelial blood-cerebrospinal fluid barrier (BCSFB) within the choroid plexus prevents passage of peripheral immune cells into the CNS parenchyma (Engelhardt and Ransohoff, 2012; Ransohoff and Cardona, 2010). However, monocyte-derived macrophages are present in the perivascular space and are able to survey the CNS and respond to stimuli (Hawkes and McLaurin, 2009; Lampron et al., 2013; Michaud et al., 2013a). This complex physiological structure has been thoroughly reviewed elsewhere (Ransohoff and Engelhardt, 2012). In response to acute injury or neurodegenerative diseases, these peripheral macrophages can infiltrate the CNS, pass through the BCSFB, and support healing (Shechter et al., 2009; Simard et al., 2006; Town et al., 2008; reviewed in Ransohoff and Engelhardt, 2012; Schwartz et al., 2013). Furthermore, lymphocytes are a major component of the CSF that can traverse the BCSFB, but do not actively penetrate the CNS parenchyma under steady-state, healthy conditions (Liblau et al., 2013; Ransohoff and Engelhardt, 2012; Schwartz et al., 2013). The majority of these lymphocytes are CD4+ T cells, with characteristics of central and effector memory T cells and very few naïve cells (Ransohoff and Engelhardt, 2012). These cells play roles in homeostasis by regulating neurogenesis (Ziv et al., 2006) and brain plasticity (Kipnis et al., 2004), and can infiltrate the CNS parenchyma during inflammatory processes driven by tissue injury and disease (Ransohoff and Brown, 2012; Schwartz et al., 2013; Town et al., 2005).
1.3 Inflammatory signaling
Much of what is known regarding immune cell signaling has been established through mechanistic studies of innate immune responses to microbial pathogens. Under healthy conditions, inflammatory molecules are transcriptionally inhibited, but stand at the ready to be induced once the inflammatory process is initiated. Typically, the inflammatory response is triggered by detection of highly conserved pathogen associated molecular patterns (PAMPs) by germ-line encoded pattern recognition receptors (PRRs)(Takeuchi and Akira, 2010). One class of PRRs are the Toll-like receptors (TLRs), which has members that specialize in detecting a broad range of PAMPs that are not found in the host organism (thoroughly reviewed in (Kawai and Akira, 2010; Uematsu and Akira, 2008)). These receptors are expressed on cells that play central roles in the inflammatory response, including macrophages and microglia. In addition to non-self epitopes, PRRs can respond to host derived molecules, referred to as danger associated molecular patterns (DAMPs) (Newton and Dixit, 2012). DAMPs can be released by necrotic cells or as a result of a pathogenic condition, and likely play roles in age-related diseases (Shimada et al., 2012). In addition to PRRs, microglia and astrocytes express a number of purinergic receptors that are capable of responding to extracellular nucleotides (i.e., ATP or UTP) and nucleosides (i.e., adenosine) released from cells after injury or death (Di Virgilio et al., 2009).
Activation of PRRs via PAMP recognition initiates several signal transduction pathways that control diverse transcriptional processes resulting in a highly regulated inflammatory response. As one example, TLR4 activation recruits Toll-interleukin 1 receptor (TIR) domain-containing adaptor protein (TIRAP) and myeloid differentiation primary response 88 (MyD88) initiating ‘MyD88-dependent’ early and late-phase activation of nuclear factor-kappaB (NF-κB) and mitogen-activated protein (MAP) kinases. Additionally, TLR4 activates interferon regulatory factor 3 (IRF3) via a TRIF-dependent’ signaling pathway (Kawai and Akira, 2011). These are just a few examples of the complex network of signaling initiated by innate immune cells specialized in surveying the local environment. The inflammatory response is often characterized by activation of a number a transcription factor families including NF-κB, activator protein-1 (AP-1), c-AMP response element-binding protein (CREB), CCAAT/enhancer binding protein (c/EBP), and IRF. Combinatorial activation of transcriptional pathways results in expression of specific gene subsets. However, mechanisms of gene specificity are not well established, and more work is needed to decipher the molecular steps in transcriptional activation of key inflammatory mediators. Expression of specific cytokine and chemokine networks may resolve or drive pathology, and specific effectors for each disease scenario will be discussed below.
Activation of microglial innate immune signaling leads to secretion of inflammatory molecules, and also drives expression of numerous immune receptors that can further modulate their phenotype. This autocrine phenomenon seems to be particularly important for microglial cells, and modulates phagocytosis responses. Phagocytosis by microglia occurs throughout CNS development, and is necessary for brain health by removing dead neurons and protein aggregates (Zabel and Kirsch, 2013). Activation of the complement cascade, purinergic receptors, and the TREM2/TYROBP receptor complex have the ability to regulate microglial phagocytosis of neurons during development, homeostasis, and disease (Schafer et al., 2012; Schwartz et al., 2013; Stevens et al., 2007).
Lastly, but inextricably tied to inflammatory responses within in the CNS, are factors that temper and resolve neuroinflammation. Many negative feedback mechanisms have been identified within the CNS that function to attenuate inflammation. Secreted anti-inflammatory molecules, such as transforming growth factor beta (TGF-β) and interleukin 10 (IL-10), are expressed along with pro-inflammatory cytokines and chemokines. Inhibitors of inflammatory signal transduction pathways, including suppressor of cytokine signaling (SOCS), and transcriptional modulators such as activating transcription factor 3 (ATF3) and nuclear factor erythroid 2 related factor 2 (NRF2), can also be induced during neuroimmune responses (Glass et al., 2010). The complexity of these regulatory cascades can be reduced to several nodes of interaction, many of which are likely deficient as a result of genetics, aging, and disease stage. Herein lies the challenge that understanding neuroinflammation and neurodegenerative diseases poses.
2. Alzheimer’s disease
2.1 Neuroinflammatory genetic risk factors
Alzheimer’s disease (AD) is the most common form of dementia, and is pathologically characterized by extracellular deposition of amyloid-β (Aβ) peptides into β-amyloid plaques, formation of neurofibrillary tangles composed largely of hyperphosphorylated tau protein, neuroinflammation, and large-scale neuronal injury and loss. The amyloid cascade hypothesis holds that the primary etiopathological event in AD is Aβ aggregation and deposition followed by downstream neurotoxicity (Hardy and Allsop, 1991). Aβ found in senile plaques is generated from sequential endoproteolytic cleavage of the type I transmembrane glycoprotein, β-amyloid precursor protein β-APP), by β- and γ-secretases (Hardy and Selkoe, 2002). Familial (also known as early-onset) AD has been linked to rare mutations in APP and the presenilin (PSEN) components of γ-secretase (PSEN1 and PSEN2). However, the vast majority of sporadic AD cases are thought to have a complex etiology impacted by lifestyle and environmental factors. Importantly though, strong genetic susceptibility factors have been reported for sporadic or late-onset AD (LOAD); however, these polymorphisms generally act in non-Mendelian fashion as AD risk factors.
The strongest genetic risk factor for AD is generally considered to be a double polymorphism within the apolipoprotein E (ApoE) gene. In humans, there are three ApoE allelic variants: ε2, ε3, and ε4. While the most common allele is ε3, the ε4 allele is associated with increased AD risk, while the ε2 allele is protective (Kim et al., 2009). Major focus has been directed toward the role of ApoE ε4 in Aβ metabolism and amyloid formation; yet, numerous studies support a complex function of ApoE in the context of AD pathogenesis. As a modulator of Aβ-driven neuroinflammation, ApoE is induced as part of the innate immune response. In this regard, the ApoE ε4 isoform has been reported to be pro-inflammatory, inducing more activated microglia and higher levels of the cytokine interleukin-1beta (IL-1β; Guo et al., 2004). While more work is needed in this important area, it remains possible that ApoE ε4 confers risk for AD at least partially by exacerbating Aβ-driven neuroinflammation.
Just recently, a rare allele has been identified and reported to confer as much AD risk as the more common ApoE ε4 allele. Strikingly, this polymorphism lies within a key innate immune gene known as triggering receptor expressed on myeloid cells 2 (TREM2) (Guerreiro et al., 2013; Jonsson et al., 2013). As a receptor on microglia, TREM2, along with its core molecular complex partner TYRO protein tyrosine kinase-binding protein (TYROBP/DAP12), regulates phagocytosis. Expression of both of these genes is up-regulated in microglia surrounding amyloid plaques in transgenic mouse models of cerebral amyloidosis (Jiang et al., 2013). In humans, the risk-incurring TREM2 allele is thought to result in TREM2 loss-of-function, possibly reducing microglial capacity to phagocytose and clear β-amyloid. Additionally, signaling through the TREM2/TYROBP complex modulates the anti-inflammatory response through inhibition of TLR signaling, which is also likely dysfunctional in individuals bearing the AD risk allele. In a separate study utilizing whole-genome expression and genotype data sets from hundreds of LOAD individuals, network-based gene-module approaches identified TYROBP as the highest-ranking causal gene (Zhang et al., 2013). These genetic findings place innate immune responses at the epicenter of LOAD risk.
2.2 Cytokines and chemokines
If two of the greatest known risk factors for LOAD can act as neuroinflammatory mediators, this would strongly suggest that pro-inflammatory responses endorse AD progression. Indeed, pro-inflammatory cytokines are up-regulated in AD patient brains, and it is widely thought that these inflammatory signals are pathological in nature, resulting in neuronal dysfunction and death. This model is supported by over 25 retrospective epidemiologic studies to date, where broad-spectrum inhibition of pro-inflammatory mediators via on-steroidal anti-inflammatory drugs reduces AD risk by up to 50% (in t’ Veld et al., 2001; Szekely and Zandi, 2010).
Interestingly, polymorphisms within the pro-inflammatory Il1b cytokine gene are associated with greater AD risk (Rothwell, 2000). Induction of IL-1β by microglia can be the result of 1) amyloid recognition through one of several hypothesized Aβ receptors, 2) detection of extracellular ATP as the result of tissue injury and signaling through purinergic receptors, or 3) TLR or inflammasome activation by β-amyloid (Heneka et al., 2012) or DAMPs. IL-1β signaling initiates activation of NF-κB family members and other transcription factors, which drive pro-inflammatory gene expression. One target of IL-1β signaling is inducible nitric oxide synthase (iNOS), which is responsible for production of nitric oxide (NO) and reactive oxygen species (ROS). Under physiological conditions, ROS have a potent anti-microbial innate immune role; however, these factors can also promote bystander neuronal death (Khandelwal et al., 2011). IL-1β signaling in astrocytes results in activation and secretion of a number of pro-inflammatory molecules, including the cytokine-like molecule S100B. In Tg2567 mice harboring the ‘Swedish’ APP mutation, overexpression of S100B accelerates AD-like pathology by increasing amyloidogenic APP cleavage, astrocytosis, microgliosis, and pro-inflammatory cytokine expression (Mori et al., 2010). IL-1β, like IL-18, is produced as a biologically inactive pro-form and matures after inflammasome activation. In mouse models of AD, genetic depletion of the IL-1β promoting NLRP3 inflammasome reduces amyloid burden and rescues special memory defects (Halle et al., 2008; Heneka et al., 2012). Additionally, the endogenous inhibitor of IL-1β, IL-1Rα, is protective and can attenuate neuronal cell death (Rothwell, 2000). For this reason, it has been hypothesized that IL-1Rα therapy (currently being used for rheumatoid arthritis) could benefit AD patients (Lucas et al., 2006; Rothwell, 2000). In sum, expression of IL-1β is thought to perpetuate inflammatory conditions in the CNS, increasing damaging ROS and supporting the chronic inflammatory environment present in the AD brain (Figure 1).
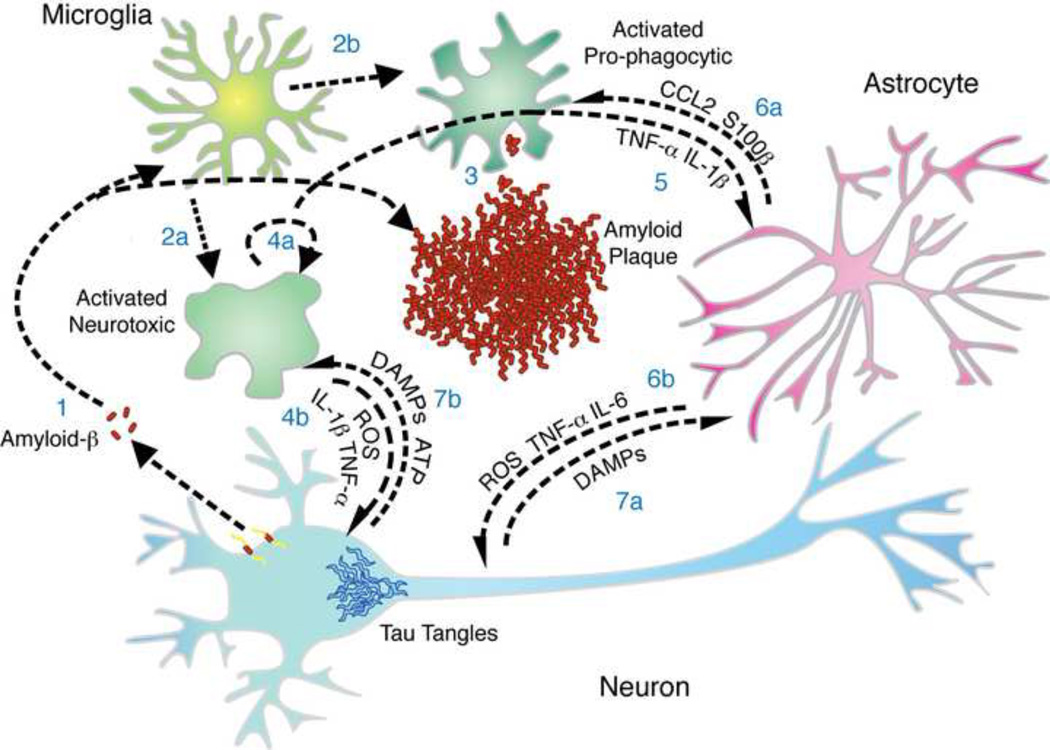
Figure 1. Inflammation in Alzheimer’s Disease.
There is a complex interplay between mediators of inflammation at sites of amyloid deposition in Alzheimer’s disease (AD). (1) Amyloid-β peptide forms aggregates and activates microglia. Microglia transition from ramified to activated, which can be (2a) neurotoxic, producing ROS, IL-1β, and TNF-α, or (2b) pro-phagocytic. (3) Phagocytic microglia will clear amyloid while neurotoxic microglia (4a) will secrete factors that act in an autocrine manner to reinforce/maintain the inflammatory phenotype and (4b) factors that directly damage neurons. (5) Factors secreted by microglia stimulate a response by astrocytes that can (6a) feedback onto microglia, driving recruitment and neuroinflammatory modulation, or (6b) act on neurons, which can be either neurotoxic or neurotrophic. Neurons, as a response to intracellular injury by tau tangles or neurotoxic ROS and cytokines, may die and release DAMPs and ATP that influence the inflammatory phenotype of (7a) astrocytes and (7b) microglia.
In addition to IL-1β, myriad other cytokines and chemokines have been detected in cerebrospinal fluid (CSF), surrounding plaques, or in plaque-associated microglia in AD patients, including: tumor necrosis factor-alpha (TNF-α), IL-6, IL-8, IL-12, IL-23, CCL2, CCL3, CCL4, CXCL2, and cyclo-oxygenase (COX)-1/2, amongst others (Akiyama, 2000; Glass et al., 2010; Vom Berg et al., 2012). The role of many of these inflammatory molecules in AD pathogenesis is not yet clear. TNF-α is a pluripotent cytokine that is an early responder of the innate immune system and stimulates expression of other pro-inflammatory cytokines. In the healthy brain, TNF-α expression is low, but it is elevated in multiple disease states, such as AD. Promoter polymorphisms that influence TNF-α expression are present in some populations and have been reported to be associated with AD (Culpan et al., 2003; Ma et al., 2004; Ramos et al., 2006). In mouse models, TNF-α has been shown to be both protective and pathogenic. Specifically, when TNF-α expression is induced by a viral system in the hippocampus of APP transgenic TgCRND8 animals that overexpress APP KM670/671NL (Swedish) and APP V717F (Indiana) mutations, plaque deposition is reduced, supporting a pro-phagocytic response from TNF-α expression (Chakrabarty et al., 2011). However, in another study, removal of TNF-α receptors also reduces plaque load (He et al., 2007), and supraphysiologic levels of this cytokine can be directly neurotoxic (Tan et al., 1999). Therefore, the actions of TNF-α are complex, and are likely dependent on concentration, target cell(s), TNF-α receptor type, and disease stage.
This degree of complexity has been observed with numerous other pro-inflammatory cytokines and chemokines. For example, a similar dichotomy has been reported for IL-6. IL-6, originally thought to be strictly a pro-inflammatory cytokine, has more recently been shown to have anti-inflammatory and neurotrophic properties as well (Spooren et al., 2011). In the CNS, IL-6 is produced by microglia, astrocytes, and even neurons, and influences microgliosis, astrogliosis, and BBB integrity. By participating in physiological and healthy actions in the brain, IL-6 is a neurotophic factor and is required for neurogenesis, astrogliogenesis, oligodendrogenesis, and proliferation, differentiation, and regeneration of neurons (Spooren et al., 2011). In AD, IL-6 is induced surrounding plaques and in the CSF, and higher concentrations of this cytokine are associated with terminal disease stage. A polymorphism in the Il6 promoter region has been shown to increase gene expression both in plasma and in promoter reporter assays (Fishman et al., 1998). This polymorphism has been investigated in several sporadic LOAD studies, and there has been disagreement as to whether it represents a significant AD risk factor (Flex et al., 2013; Spooren et al., 2011). Although it is possible that the IL-6 polymorphism is AD risk-incurring, this would most likely occur in the context of other risk-associated genetic loci.
Several studies have attempted to establish a mechanism for IL-6 risk for AD. In vitro studies have mainly supported a pathological role, as IL-6 drives gliosis in response to Aβ, increases APP expression in neurons, and promotes tau hyperphosporylation (Erta et al., 2012; Spooren et al., 2011). However, in vivo studies have revealed a role for IL-6 in resolution of disease. Specifically, overexpression of IL-6 in the brains of TgCRND8 and Tg2576 animals with established disease results in increased gliosis, decreased Aβ deposition, and increased phagocytosis (Chakrabarty et al., 2010). This could be due to modification of the inflammatory microenvironment near plaques and/or modulation of microglial phenotype, resulting in acidified lysosomes and, therefore, more efficient Aβ degradation (Majumdar et al., 2007). In the context of human AD, it remains unclear if the pro-inflammatory and pro-phagocytic actions of IL-6 are protective or act to drive the chronic neuroinflammatory environment.
In addition to the cytokines already mentioned, expression of several chemokines is increased in AD patients, such as: CCL2, CCL3, CCL4, IL-8, and CXCL2. Additionally, microglia express chemokine receptors, including CCR3, CCR5, and CX3CR1, and CXCR2 and CXCR3 expression is elevated around β-amyloid plaques (Xia and Hyman, 1999; Guillot-Sestier and Town, 2013). While only a few of these factors have been mechanistically studied in the context of AD, their inducible expression in AD brains likely points to ‘primed’ cerebral innate immunity. One group of early studies reported that CCL2 is highly expressed around amyloid plaques and induces chemotaxis of microglia in a transgenic mouse model of cerebral amyloid deposition (El Khoury et al., 2003). Further, Tg2576 animals deficient in CCR2, the cognate receptor for CCL2, have less recruitment to sites of Aβ plaque deposition, earlier plaque formation, and premature death (El Khoury et al., 2007). However, one report suggested that CCR2 is not expressed by microglia (Mizutani et al., 2012). Therefore, it has been hypothesized that defective amyloid clearance in CCR2 deficient transgenic mice is due to reduced recruitment of bone marrow-derived circulating mononuclear phagocytes (Naert and Rivest, 2011), which can directly clear cerebral amyloid (reviewed in Naert and Rivest, 2013). In one interpretation, these data support a model where recruitment of mononuclear phagocytes is required for beneficial amyloid clearance; however, a corollary of this hypothesis is that blood-borne monocytes must be dysfunctional in the context of cerebral amyloidosis, thereby endorsing cerebral amyloid accumulation and allowing disease progression.
As mentioned at the beginning of this section, NSAID users have up to 50% reduced risk of AD (Akiyama, 2000). NSAIDs are well-established COX-1/2 inhibitors. In peripheral tissues, COX-1 is constitutively expressed and COX-2 is induced as part of the inflammatory response; however, in the brain, there is evidence of the converse (McGeer and McGeer, 2007). COX-1/2 is induced as part of the plaque-associated inflammatory response in AD patients, and one of its major roles is to metabolize prostaglandins, which can be neurotoxic at supraphysiologic levels (Lucas et al., 2006). In mouse models of cerebral amyloidosis, both selective and non-selective COX inhibitors are able to reduce amyloid burden and neuroinflammation (McGeer and McGeer, 2007). However, NSAID/AD clinical trials have not yet produced definitive results (Breitner et al., 2011; ADAPT, 2013; Szekely and Zandi, 2010), and future studies on the roles of the COX enzymes and prostaglandins in AD are warranted. It is clear that a deeper understanding of specific neuroinflammatory factors is vital to developing new therapeutic strategies that target cerebral innate immunity.
2.3 Anti-inflammatory factors
Genetic and pharmacological modulation of key pro-inflammatory molecules in mouse models of AD has illustrated a complex interplay that is both target cell and disease stage dependent. In this regard, immunomodulation of cardinal anti-inflammatory molecules has also been attempted. TGF-β is a central anti-inflammatory regulator that provides control of homeostasis and tissue repair mechanisms. Interestingly, release of this cytokine significantly increases in response to CNS injury, and astrocytes, microglia and oligodendroglia seem to be the major sources of TGF-β1 in the damaged brain (Constam et al., 1992; Finch et al., 1993). In AD patient brains, TGF-β1 is detected in amyloid plaques (van der Wal et al., 1993) and increased levels have been detected in cerebrospinal fluid and serum of AD patients (Chao et al., 1994a, 1994b). In addition, TGF-β1 receptor immunoreactivity is higher in reactive glia in AD cases than in nondemented controls (Lippa et al., 1998) suggesting a pathogenic role for this cytokine (Wyss-Coray et al., 1997). Yet, in vivo studies in transgenic mouse models have yielded conflicting roles of TGF-β1, with over-expression promoting microgliosis and accelerated Aβ deposition (Wyss-Coray et al., 2000, 1997), or resolution of neuroinflammation and increased Aβ phagocytosis in other reports (Frautschy et al., 1992; Wyss-Coray et al., 2001). Another study designed to examine neuron-specific signaling revealed increased Aβ accumulation, dendritic loss, and age-dependent neurodegeneration (Tesseur et al., 2006). In vitro work suggests that TGF-β1 amplifies Aβ-mediated neurodegeneration via a Smad7/β-catenin-dependent mechanism (Salins et al., 2008).
In order to determine the effect of blocking the TGF-β1 signaling pathway in peripheral mononuclear phagocytes, our group developed a transgenic mouse model with blockade of TGF-β receptor and downstream Smad2/3 signaling in peripheral macrophages (designated CD11c–DNR mice). When crossed with Tg2576 mice, bi-transgenic animals had reduced Aβ deposits in brain parenchyma, accompanied by increased infiltration of peripheral macrophages in and around β-amyloid plaques (Town et al., 2008). In vitro, CD11c–DNR-derived peripheral macrophages exhibit blockade of the classical TGF-β-activated Smad2/3 pathway accompanied by hyperactivation of the alternative Smad1/5/8 signaling cascade and markedly increased Aβ phagocytosis. At least in this context then, blockade of a key anti-inflammatory signaling pathway activates innate immunity, promoting clearance and resolution of cerebral amyloidosis.
2.4 The role of innate immune receptors
The signaling cascades responsible for activation of innate immunity and release of inflammatory mediators initiate at receptors. A prototypical example is the TLR family. In this regard, TLR4 and TLR2 have been the major focus in AD. In studies of LOAD in both Italian and Han Chinese populations, two polymorphisms have been identified that occur at higher frequencies in AD verses healthy controls. Interestingly, both polymorphisms reduce the activity of TLR4 (Minoretti et al., 2006; Wang et al., 2011). A beneficial role of TLR4 is further supported by a recent study in which detoxified monophosphoryl lipid A (MPL), a TLR4 ligand, significantly improved AD-like pathology in APP/PS1 mice (Michaud et al., 2013b). These studies compliment other reports that TLR4 and TLR2 participate in Aβ phagocytosis (Reed-Geaghan et al., 2009). In contrast, another study showed that loss of IRAK4, a common TLR downstream mediator, results in increased amyloid clearance, reduced microgliosis, and reduced astrogliosis in aged mice (Cameron et al., 2012). These contradictory studies in mice and observations in patients highlight the challenge of attempting to modulate TLR signaling as an AD therapeutic modality. Further, these and other results underscore the notion that broad-based inhibition of cerebral innate immunity is not likely to be beneficial, because some aspects are adaptive and necessary for normal brain function and homeostasis.
In addition to the TLR family of receptors, there are other major innate immune receptors that have been identified as risk factors in AD. Reduction in TREM2 activity, as highlighted in section 2.1, is likely to attenuate phagocytic capacity of microglia in individuals carrying the mutant allele. In addition to TREM2, genome-wide association studies (GWAS) have identified several other innate immune receptors that confer risk, such as the complement component (3b/4b) receptor 1 (CR1) and CD33. CR1 inhibits complement activation and the risk allele is expressed at lower levels, resulting in increased complement activation and modulation of a possible route for Aβ clearance (Hazrati et al., 2012). CD33 is a sialic acid binding immunoglobulin-like lectin that regulates innate immunity, is increased in AD patients relative to age-matched controls, and inhibits Aβ uptake and clearance (Griciuc et al., 2013). A common observation that links these innate immune gene risk alleles is modulation of microglial phagocytosis. In principle then, rescue of these defective alleles would require compensatory activation of the pro-phagocytic microglial phenotype and therefore, the specific molecular mechanisms of this pathway need to be delineated. The myriad types of cerebral innate immunity in the context of AD are summarized in Figure 1.
3. Parkinson’s Disease
3.1 Multiple immune players
Parkinson’s disease (PD) is the second-most common neurodegenerative disorder after AD. It is one of the three main types of α-synucleopathies, along with dementia with Lewy bodies and multiple system atrophy, characterized by abnormal accumulation of α-synuclein aggregates in neurons, nerve fibers or glial cells (for review see McCann et al., 2014). The histopathological hallmarks of PD are 1) age-related neurodegeneration of extrapyramidal motor neurons: in particular, loss of dopaminergic neurons of the basal ganglia from the substantia nigra pars compacta (SNpc) to the striatum (caudate and putamen), 2) Lewy bodies formed of intracellular proteinaceous inclusions containing α-synuclein (AS), and 3) chronic neuroinflammation. The third feature is characterized by the presence of activated microglia in the putamen, hippocampus, transentorhinal cortex, cingulate cortex, and temporal cortices of patients associated with mild astrogliosis and increased numbers of infiltrating lymphocytes (Brochard et al., 2009; Hirsch and Hunot, 2009; Imamura et al., 2003; McGeer et al., 1988; J Miklossy et al., 2006; Mirza et al., 2000; Orr et al., 2005). The density of microglia in the SNpc is higher than in other brain regions, and microglia are found predominantly in the vicinity of degenerated dopaminergic neurons in PD brains (Banati et al., 1998; Lawson et al., 1990). Furthermore, microglia are activated by substances produced by damaged neurons, such as α-synuclein aggregates (Zhang et al., 2005), ATP (Davalos et al., 2005), MMP-3 (Kim et al., 2007) and neuromelanin (Wilms et al., 2003). In return, activated microglia produce excessive quantities of various pro-inflammatory and neurotoxic factors including cytokines and free radicals (discussed below in sections 3.4 and 3.5); triggering a vicious cycle of inflammation that likely exacerbates neurotoxicity in PD. Importantly, activated microglia may also contribute to the non-motor symptoms of the disease (e.g., dementia) (Emre, 2003). This self-amplifiying cycle of neuronal injury and microglial activation may be one of the key processes driving progressive neurodegeneration. In experimental PD models, anti-inflammatory neuroprotective agents have proved to reduce microglial activation in association with reduced neuronal loss (Liu, 2006). Less is known about the exact role of astrocytes in PD pathogenesis. Animal studies suggest that astrocytes recruit microglia during the early phases of the disease via expression of pro-inflammatory molecules (Halliday and Stevens, 2011; Lee et al., 2010), but a potential neuroprotective role has also been proposed (Chen et al., 2006; Ishida et al., 2006; Lin et al., 1993).
3.2 Genetic risk factors for both familial and sporadic cases
PD is a multifactorial neurodegenerative disease considered to involve genetic defects and environmental factors that act together to promote disease progression. Unlike AD, where less than 1% of cases are due to mutations in APP or PSEN1/2, roughly 10% of PD cases are linked to a purely genetic cause. Interestingly, discrepancies exist between genetic and sporadic forms of the disease; however, similar disease features tend to indicate a common pathological mechanism for both forms of PD. The most common autosomal-dominant causes of PD are mutations in α-synuclein (SNCA) and leucine-rich repeat kinase 2 (LRRK2), whereas parkin (PARK2), PTEN-induced putative kinase 1 (PINK1), and DJ-1 (Parkinson protein 7; PARK7) are known to be responsible for autosomal-recessive cases (Trinh and Farrer, 2013). Remarkably, mutations in PARK2, SNCA, and LRRK2 have also been reported in sporadic PD, suggesting that these genes influence the development of the disease with age (Moore et al., 2005). Importantly, SNCA (Austin et al., 2006; Gu et al., 2010; Klegeris et al., 2008), LRRK2 (Gardet et al., 2010; Gillardon et al., 2012; Judith Miklossy et al., 2006; Moehle et al., 2012), PARK2 (Frank-Cannon et al., 2008; Ledesma et al., 2002; Watson et al., 2012), PINK1 (Akundi et al., 2011), and DJ-1 (Bandopadhyay et al., 2004; Mullett et al., 2009; Waak et al., 2009) have all been shown to regulate adaptive and innate immune responses; notably, activation of T and B cells, microglia and astrocytes, and regulation of cytokine production. Thus, it remains possible that these genetic risk factors contribute to disease by inducing immune cell dysfunction.
More recently, several genetic studies have identified polymorphisms in immune-related genes associated with risk for PD. For example, GWAS has identified genetic variation of the HLA region (Hamza et al., 2010), the T cell receptor signaling pathway (Holmans et al., 2013; Srinivasan et al., 2009), and polymorphisms within inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-18, IL-10, and the TLR4 co-receptor, CD14 (Deleidi and Gasser, 2013; Wahner et al., 2007) as risk factors for late-onset PD. In addition, TLRs have been implicated as genetic factors for sporadic and familial forms of PD, with proposed roles in dopaminergic neuronal degeneration and α-synucleinopathy. Indeed, transcription of PARK2 is regulated by TLR4-MyD88-NF-κB and TNF-β-NF-κB-dependent pathways in microglia and macrophages (Tran et al., 2011), suggesting that activation of TLR-dependent pathways could play a role in dysregulation of neuroinflammation during PD progression (Noelker et al., 2013; Panaro et al., 2008).
3.3 Toll-like receptor dependent neuroinflammation
As mentioned above, activation of TLR-dependent pathways could play a role in Neuroinflammation and endorse PD progression. Studies have found increased abundance of TLRs in α-synucleinopathies, and TLR4 has been shown to be elevated in brains of patients suffering from α-synucleinopathy and in a transgenic mouse model of the disease (Letiembre et al., 2009; Stefanova et al., 2011). Interestingly, DJ-1−/−astrocytes selectively induce TLR4-dependent increased NO production, which likely contributes to neuronal death (Waak et al., 2009). In microglial cultures, blockade or knockdown of TLR4 suppresses phagocytosis of α-synuclein. Furthermore, genetic ablation of TLR4 in mice reduces phagocytosis by microglia, leading to α-synuclein accumulation, loss of dopaminergic neurons, and motor disability (Cookson, 2009; Fellner et al., 2013; Stefanova et al., 2011; Zhang et al., 2005). These results suggest that α-synuclein-dependent activation of microglia, their phagocytic capacity, and liberation of cytokines and ROS are modulated by TLR4. One important implication is that TLR4 may have a neuroprotective role via α-synuclein clearance (Stefanova et al., 2011). Interestingly, misfolded α-synuclein itself seems to be responsible for microglial activation by up-regulation of cytokines, increased expression of antioxidant response enzymes, and altered TLR gene expression (Béraud et al., 2011).
TLR2 may also be involved in the microglial response in PD. Indeed, TLR2 expression is increased in the transgenic mouse model expressing the α-synuclein mutation A30P (Martin et al., 2011), and extracellular α-synuclein released from neuronal cells activates inflammatory responses in microglia via association with TLR2 (Kim et al., 2013).
3.4 Molecular mediators of neuroinflammation
Although the pathomechanisms of PD are still unclear, neuroinflammation and microglial activation are now thought to play central roles in the disease. Numerous markers of microglial activation in PD-relevant brain regions have been reported in patients, including: human leukocyte antigen-DR (HLA-DR; McGeer et al., 1988), complement receptor (CR)3/43, the activated macrophage/microglial marker CD68 (Banati et al., 1998), intracellular adhesion molecule-1 (ICAM-1), lymphocyte function-associated antigen-1 (LFA-1), and major histocompatibility (MHC) class II (Imamura et al., 2003). On one hand, a plethora of studies indicate that pro-inflammatory cytokine and chemokine abundance is altered in PD compared to control brains. Elevated levels of IL-1β, IL-2, IL-4, CCL5, and TGF-α have all been reported in the CSF of PD patients (Blum-Degen et al., 1995; Mogi et al., 1996; Tufekci et al., 2011). Furthermore, IFN-γ, NF-κB, IL-1β, TGF-α (Mogi et al., 2007, 1994a), COX-1and COX-2 (Knott et al., 2000; Teismann et al., 2003) are up-regulated in the SN and in the striatum, and TNF-α and IL-6 are increased in CSF as well as in PD-relevant brain regions (Blum-Degen et al., 1995; Boka et al., 1994; Dobbs et al., 1999; Imamura et al., 2003; Mogi et al., 1996, 1994b). In addition, the hippocampus, cerebellum, and mesencephalon of PD patients present with increased abundance of IL-2, IFN-γ, and NF-κB (Hunot et al., 1997; Mogi et al., 2007, 1996). On the other hand, anti-inflammatory cytokines (such as TGF-β1) have also been detected in the CSF and in brain parenchyma of patients suffering from PD (Mogi et al., 1995; Nagatsu et al., 2000). These data reflect the complexity of the neuroinflammatory milieu present in PD patients’ brains.
3.5 The role of oxidative stress
Oxidative stress (OS) is also thought to contribute to neuronal degeneration and microglial activation in PD. The dopaminergic neurons present in the SNpc exhibit high oxygen consumption and low levels of the antioxidant enzymes superoxide dismutase (SOD), glutathione (GSH), and catalase, making them particularly susceptible to oxidative damage (Floyd, 1999). Several lines of evidence strongly suggest that oxidative stress is involved in microglial activation during PD evolution. Markers of oxidative stress, such as NOS and inducible NOS (iNOS), are increased in PD patients’ brains (Hirsch et al., 2012; Hunot et al., 1996; Knott et al., 2000; Varçin et al., 2012). Reactive oxygen species (ROS) are downstream products of the free radical superoxide, and are generated by NADPH oxidase (NOX)-dependent transfer of electrons from NADPH to molecular oxygen. Interestingly, NOX isoforms are widely expressed in the CNS; in particular, by neurons, astrocytes and microglia. NOX-dependent generation of ROS relies on the highly expressed voltage–gated proton channel HV1 that regulates intracellular pH (Wu, 2014). The NOX family and its regulator HV1 are an important source of CNS oxidative stress, and NOX-derived ROS participate in various microglia-mediated events such as microglial proliferation, neuronal apoptosis, glutamate neurotransmitter release and astrocyte signaling (for a review see Gao et al., 2012). Further, dopamine can undergo auto-oxidation to produce cytotoxic ROS, and in vitro studies suggest that dopaminergic neuron cell death could be induced by L-DOPA-dependent generation of ROS (Coyle and Puttfarcken, 1993; Lipski et al., 2011). ROS can also perpetrate excitotoxicity via over-activation of N-methyl-D-aspartate (NMDA) receptors (implicated in control of synaptic plasticity and memory function), leading to a hyper-production of NO (Barnham et al., 2004). Additionally, increased levels of neuromelanin (that pigments neurons of the substantia nigra and acts as a chelating agent and neuroprotector) released from dying cells can activate microglia, increasing the sensitivity of dopaminergic neurons to oxidative stress-induced cell death (Halliday et al., 2005; Kastner et al., 1992; Zhang et al., 2011). Finally, dopaminergic neurons are more vulnerable to pro-inflammatory cytokines such as TNF-α, and this phenomenon has been linked to elevated oxidative stress (McGuire et al., 2001).
Astrocytes may play a protective role vis-à-vis oxidative stress in PD. The dopaminergic neurons in the SNpc of PD patients exhibit high levels of nuclear factor erythroid 2-related factor 2 (NFR2), which has the ability to activate endogenous antioxidant responses to oxidative stress (Kumar et al., 2012). In fact, NRF2 is a transcription factor present in astrocytes that plays a major role in protection against ROS injury by triggering cytoprotective products (Tanji et al., 2013). Interestingly, NRF2 deficiency diminishes striatal dopamine and increases neurotoxicity in the MPTP mouse model of PD (Chen et al., 2009; Innamorato et al., 2010). In dopaminergic neurons in vitro, stimulation of NRF2-induced anti-oxidant and neuroprotective effects oppose neurotoxicity induced by MPTP or 6-OHDA (a dopamine analog) treatment (Jakel et al., 2007; Yamamoto et al., 2007). In vivo, mice receiving transplantation of NRF2-overexpressing astrocytes in the striatum are more resistant to 6-OHDA, and dopaminergic neuronal loss in the SNpc is rescued (Chen et al., 2009; Jakel et al., 2007). Additionally, greater loss of dopamine transporter levels in striatum of NRF2 knockout mice post-MPTP has also been reported (Burton et al., 2006). Therefore, NRF2 represents an important anti-oxidant factor that is neuroprotective in the context of PD.
4. Amyotrophic lateral sclerosis
4.1 Brain inflammation
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that is characterized by selective loss of motor neurons in the brainstem, spinal cord, and motor cortex. Individuals suffering from ALS experience fasciculation, muscle wasting, and hyper-reflexia, making this neurodegenerative disease both debilitating and fatal. The vast majority of ALS cases are sporadic in nature, likely resulting from a complex interplay between polygenetic variants and environment, and it is currently estimated that only ~10% of ALS is familial (Renton et al., 2014). The first mutation reported for familial ALS was in the Cu/Zn-superoxide dismutase gene (SOD1; Rosen et al., 1993). Since the introduction of next-generation high-throughput sequencing, the pace of identifying causal genes has greatly increased. In European populations, it is estimated that the genetic etiology of about two-thirds of familial ALS and 11% of sporadic ALS has been accounted for (Renton et al., 2014). In addition to SOD1, the most common mutations in ALS are found in C9orf72 (DeJesus-Hernandez et al., 2011; Renton et al., 2011), RNA-binding gene fused in sarcoma (FUS) (Kwiatkowski et al., 2009; Vance et al., 2009), TDP-43 (TARDBP) (Kabashi et al., 2008; Sreedharan et al., 2008; Van Deerlin et al., 2008), and sequestosome-1 (SQSTM1) (Shimizu et al., 2013; Teyssou et al., 2013). A common pathological hallmark in ALS is the presence of ubiquitin-positive cytoplasmic inclusions containing TDP-43 in pathologic neurons (McGeer and McGeer, 2002). The genetic and mechanistic linkage of the RNA-binding proteins TDP-43 and FUS, as well as the intronic expansion of C9orf72, suggests that defective RNA metablolism, ribonucleoprotein binding, and splicing support ALS proteinopathy (Renton et al., 2014). In general, genetic abnormalities associated with ALS support the hypothesis of neurotoxicity due to abnormal protein aggregation, ineffective ubiquitin-mediated protein clearance, and faulty RNA processing.
Similar to AD and PD, neuroinflammation in ALS is routinely documented in pathologically affected brain areas, and is characterized by gliosis, activated microglia and astrocytes, and elevated production of pro-inflammatory cytokines (McGeer and McGeer, 2002). Additionally, infiltrating lymphocytes and macrophages are also observed in ALS-associated brain areas. Two general approaches to modeling ALS in rodents have been taken. The first has been to express mutant human SOD1 and, more recently, to express or deplete TDP-43. The most well-established mouse model of ALS expresses human SOD1G93A and recapitulates both motor neuron loss and neuroinflammation observed in human ALS (Gurney et al., 1994). More recently, SOD1G93A, SOD1G37R, or SOD1G37R expression in neurons vs. glia was explored and it was shown that onset and progression represent distinct disease phases. Specifically, mSOD1 expression in motor neurons could establish an early disease phase; however, microglial expression is required to perpetrate late stage neuronal pathology and loss (Boillée et al., 2006; Clement et al., 2003). Rodent models of ALS that express wild-type TDP-43 (Wils et al., 2010; Xu et al., 2010) or ALS-linked mutant TDP-43A315T (Wegorzewska et al., 2009) or TDP-43M337V (Huang et al., 2012; Xu et al., 2011) have a neurodegeneration phenotype (reviewed in Tsao et al., 2012). Interestingly, a recent study utilizing an RNAi transgene targeting TARDBP reported neurodegeneration with only partial TDP-43 depletion (Yang et al., 2014). Similar to the human mSOD1 overexpression model, this phenotype is dependent on another glial cell population, astrocytes, in addition to neurons, and highlights the key role of glial cells in ALS pathogenesis.
4.2 Neuroinflammation is linked to motor neuron loss
Similar to AD, activated microglia and reactive astrocytes can be found in spinal cords of ALS patients and in transgenic mouse models of the disease (McGeer and McGeer, 2002). Reactive glial cells have been reported to mediate both neuroprotective and neurotoxic signals; therefore, the relative contribution of each to disease pathogenesis remains unclear (Bowerman et al., 2013). Cytokines and chemokines detected in ALS spinal cords are similar to AD and PD, and include: IL-1β, TNF-α, CCL2, and IL-6, amongst others. For IL-1β, much of the evidence supports increased abundance in conjunction with the pro-inflammatory cascade of factors that drive chronic inflammation in ALS. Mutant human SOD1 can form aggregates that activate the inflammasome, (including caspase-1 and IL-1β release; Ilieva et al., 2009; Meissner et al., 2010). When this signaling cascade is interrupted in SOD1G93A mice by removing caspase-1, IL-1β, or overexpressing the IL-1β inhibitory receptor (IL-1Rα), SOD1G93A transgenic mice live longer and have less neuroinflammation (Meissner et al., 2010). Interestingly, while TNF-α is up-regulated in this ALS mouse model, genetic ablation of TNF-α does not impact lifespan or motor neuron loss in these animals (Gowing et al., 2006). This somewhat puzzling result may be specific to this model, or it could represent a compensatory mechanism due to elevated IL-1β and TLR2 expression (Gowing et al., 2006).
Increased abundance of chemokines, including CCL2, has been reported in patients and in animal models of ALS in early stages of disease, and these changes track well with development of pathology (Henkel et al., 2006; Kuhle et al., 2009). It is not clear if this expression pattern is injurious or protective, although recruitment of monocytes could play an important role in disease progression. Much akin to other neurodegenerative disorders, it has been demonstrated that microglia can have neuroprotective or neurotoxic phenotypes in ALS (reviewed in Appel et al., 2011). Specifically, reconstitution of PU.1 null mice that are unable to develop myeloid and lymphoid cells with wild-type or mSOD1 bone marrow revealed that wild-type microglia are protective while mSOD1 microglia are toxic (Beers et al., 2006). Additionally, ex vivo microglia from early-stage mSOD1 mice were shown to be alternatively activated (M2 phenotype) and neuroprotective, whereas late-stage microglia were more classically activated (M1) and neurotoxic to motor neurons in co-culture (Liao et al., 2012). Nonetheless, the role of most cytokines and chemokines in ALS pathogenesis remains enigmatic. Establishing which secreted factors are neuroprotective, neurotoxic, serve to recruit immune cells, or to activate these cells toward a particular phenotype, is needed to determine the best candidates for therapeutic intervention.
As previously addressed, cytokine and chemokine expression are often induced by activation of innate immune receptors, such as TLRs. Elevated expression of TLR2, TLR4, and CD14 have been observed in animal models of ALS; however, something similar was not observed in patients (Casula et al., 2011; Letiembre et al., 2009). In vitro studies have demonstrated that extracellular mSOD1 can induce microglial secretion of pro-inflammatory IL-1β and TNF-α, and this effect is TLR2/4 and CD14 dependent (Liu et al., 2009; Zhao et al., 2010). While increased abundance of TLRs could potentiate microglial activation, ALS patient data suggests that this phenomenon is associated with general neuroinflammatory events as opposed to disease-specific pathogenesis. For example, large-scale motor neuron degeneration releases ATP that signals through P2×7 receptors on microglia and astrocytes resulting in the expression of inflammatory molecules, including IL-1β, TNF-α, COX-1/2, and ROS (Apolloni et al., 2013; Bowerman et al., 2013; Gandelman et al., 2010). This neurotoxic response can be attenuated by inhibition of P2×7 signaling (Apolloni et al., 2013; Gandelman et al., 2010). The intracellular nature of ALS inclusions suggests that closer investigation into potential immune responses and associated transcriptional changes is required.
4.3 Messenger RNA splicing may impact inflammatory gene expression
The frequent presence of TDP-43/FUS in ALS intracellular inclusions, regardless of pathogenic allelic variant, is likely to have broad implications for transcriptional regulation (Ling et al., 2013). Aggregation of splicing factors in the cytoplasm leads to depletion in the nucleus and dysregulation of thousands of target mRNAs (Ling et al., 2013). It is unclear if this phenomenon plays a dominant role in production of neurotoxic vs. neuroprotective mediators. Increased NF-κB has been reported in TDP-43 aggregates, and work in the mSOD1 model suggests that activation of the NF-κB and AP-1 families are key transcriptional inducers in ALS (Glass et al., 2010; Swarup et al., 2011). One factor that is down-regulated by NF-κB activation, but is thought to be neuroprotective, is NRF2. Insufficient activation of NRF2, as observed in aging and in neurodegenerative disease, has been linked to reduced ability to activate cytoprotective genes, such as those required for protection from oxidative stress (Sandberg et al., 2014). Glial transcriptional dysregulation, disrupting the balance of pro-inflammatory and cytoprotective transcription factors as illustrated by NRF2, has been reported in both PD and in ALS, and may be linked to neuronal survival.
4.4 CNS infiltrating immune cells
In contrast to AD, copious blood-derived immune cells are associated with pathological ALS tissue. In patients suffering from ALS, the BBB and the blood-spinal cord barrier are damaged (Winkler et al., 2013). These pathologies are associated with reduced numbers of pericytes, vascular dysfunction, and infiltration of immune cells. Peripheral macrophages, mast cells, and lymphocytes are all found in close association with brain and spinal cord lesions in ALS patients (Graves et al., 2004; Lewis et al., 2012). Yet, lymphocyte populations have been hypothesized to have diametric roles in disease progression. For example, when mSOD1 transgenic mice were bred with mice lacking functional T cells, motor neuron disease was accelerated (Beers et al., 2008).
Additionally, T regulatory cell numbers were found to inversely correlate with disease, and this population of CD4+ T cells is believed to be responsible for neuroprotection (Beers et al., 2011; Bowerman et al., 2013; Henkel et al., 2013). Alternatively, CD8 cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are increased in number surrounding ALS lesions (Rentzos et al., 2012). CTLs express Fas ligand and could induce Fas-mediated cell death in motor neurons, which are more susceptible to injury when expressing human mSOD1 (Raoul et al., 2006, 2002). The role of NK cells in ALS progression is unknown; yet, increased expression of IFN-γ or other NK-associated cytokines could potentiate CTL/T helper type I-mediated motor neuron death (Bowerman et al., 2013).
5. Therapeutic implications and conclusions
Progress toward understanding of the role of inflammation in neurodegenerative diseases is driving innovative work that will be central to the development of immune-based therapies for these disorders. While there are certainly maladaptive components of neuroinflammatory events that serve to perpetuate a neurotoxic environment and to drive neuropathogenic lesions, there is also the opportunity to use newly-deciphered immune pathways to steer beneficial neuroimmune responses (Figure 2). One therapeutic target, common amongst these disorders, includes altering capacity of phagocytic monocytes to clear protein aggregates (i.e., Aβ, α-synuclein). Secondly, proper expression and regulation of ROS by astrocytes through the cytoprotective role of NRF2 target genes would be predicted to ameliorate neurotoxicty. Finally, proper recruitment of beneficial immune cells from within the CNS and from the periphery needs to be explored as a potential strategy to promote healthy CNS immune responses.
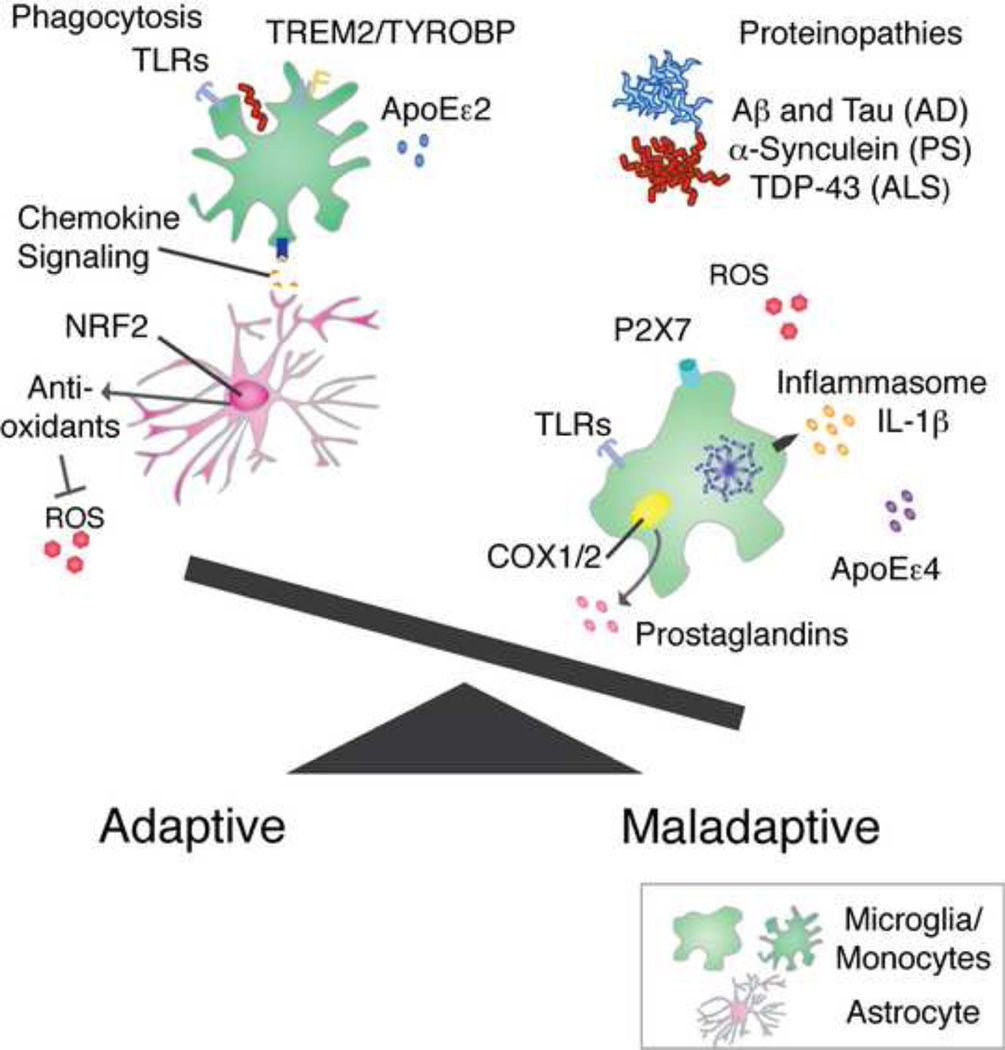
Figure 2. Microglial phenotype is a major determinant of adaptive vs. maladaptive inflammation in neurodegenerative disorders.
Once neurodegenerative disease has been diagnosed, the scale has already been tipped towards maladaptive immunity for a significant amount of time. Specifically, protein aggregates have become established and a pro-inflammatory/pro-neurotoxic microglial phenotype predominates. This phenotype is characterized by ROS production, inflammasome activation and secretion of IL-1β, COX1/2 mediated prostaglandins, activation of P2×7 and TLRs by DAMPs, and immune modulation by ApoEε4 in carriers. Adaptive and beneficial microglial/monocyte phenotypes are pro-phagocytic and are accompanied by signaling by astrocytes (i.e. CXCR2/IL-8 or other chemokines in microglia or CCR2/CCL2 in peripheral monocytes), activation of TREM2/TYROBP and TLRs in a pro-phagocytic manner, reduction of ROS by NRF2 target genes, and immune modulation by the protective ApoEε2 allele in carriers.
Whereas the death and dysfunction of neurons is the main phenomenon leading to neurological syndromes, it is becoming increasingly evident that neuroinflammation and oxidative stress are substantial players in the pathoethiology of neurodegenerative disorders. Genetic variants may modulate immune and inflammatory pathways both in familial and in sporadic forms of these diseases, and modify disease onset and progression. Neuroinflammatory mediators (i.e., cytokines, receptors and transcription factors) are driving forces in neurodegenerative diseases that have a profound impact on progression of inflammation and neuronal injury. Thus, regulating the production or action of these mediators may prove to be an effective approach to mitigate CNS inflammatory processes and neurodegeneration.
Highlights.
Teasing apart immune modulation signals is key for neurodegenerative diseases.
Determining specific phenotypes of microglia and astrocytes is critical.
Engaging beneficial immune responses is important to promote CNS health.
One therapeutic target to clear misfolded proteins is phagocytic monocytes.
These unifying themes should enable immunotherapeutics against neurodegeneration.
Acknowledgements
The authors are supported by the National Institute on Aging (5R00AG029726-04 and 3R00AG029726-04S1, to T.T.), the National Institute on Neurologic Disorders and Stroke (1R01NS076794-01, to T.T.), an Alzheimer’s Association Zenith Fellows Award (ZEN-10-174633, to T.T.), an American Federation of Aging Research/Ellison Medical Foundation Julie Martin Mid-Career Award in Aging Research (M11472, to T.T.), and generous faculty start-up funds from the Zilkha Neurogenetic Institute (to T.T.).
Abbreviations
- AD
Alzheimer’s Disease
- ALS
Amyotrophic Lateral Sclerosis
- ApoE
Apolipoprotein E
- AP-1
Activator Protein-1
- β-APP
β-amyloid precursor protein
- ATF
Activating Transcription Factor
- ATP
Adenosine Triphosphate
- BBB
Blood Brain Barrier
- BCSFB
Blood-cerebrospinal fluid barrier
- BSCB
Blood-spinal cord barrier
- c/EBP
CCAAT/enhancer binding protein
- CCL
Chemokine ligand
- CCR
C-C Chemokine Receptor
- CNS
Central Nervous System
- COX
Cyclo-OXygenase
- CR
Complement Receptor
- CREB
C-AMP Response Element-Binding protein
- CSF
Cerebrospinal Fluid
- CTLs
Cytotoxic T Lymphocytes
- CX3CR
CX3C Chemokine Receptor
- CXCL
Chemokine (C-X-C motif) ligand
- DAMPs
Danger Associated Molecular Patterns
- GSH
glutathione
- HLA-DR
human leukocyte antigen-DR
- ICAM-1
intracellular adhesion molecule-1
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IRAK
Interleukin-1 Receptor-Associated Kinase
- IRF
interferon regulatory factor
- LFA-1
lymphocyte function-associated antigen-1
- LOAD
Late-Onset AD
- LRRK
Leucine-Rich Repeat Kinase
- MAPK
mitogen-activated protein kinases
- MHC
major histocompatibility
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MyD88
myeloid differentiation primary response 88
- NF-κB
nuclear factor-kappaB
- NFR
nuclear factor erythroid 2-related factor
- NK
Natural Killer
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NRF
Nuclear factor erythroid 2 Related Factor
- NSAID
Non-steroidal anti-inflammatory drugs
- OS
Oxidative Stress
- PAMPs
Pathogen Associated Molecular Patterns
- PD
Parkinson’s Disease
- PRRs
Pattern Recognition Receptors
- PS
presenilin
- ROS
Reactive Oxygen Species
- SNpc
Substantia Nigra Pars Compacta
- SOCS
Suppressor Of Cytokine Signaling
- SOD
superoxide dismutase
- TIR
Toll-Interleukin Receptor
- TIRAP
Toll-Interleukin Receptor domain-containing Adaptor Protein
- TGF-β
Transforming Growth Factor beta
- TLRs
Toll-Like Receptors
- TNF-α
tumor necrosis factor-alpha
- TREM
Triggering Receptor Expressed on Myeloid cells
- TRIF
TIR-domain-containing adapter-inducing interferon- β
- TYROBP
TYRO protein tyrosine kinase-binding protein
- UTP
Uridine Triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akiyama H. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akundi RS, Huang Z, Eason J, Pandya JD, Zhi L, Cass WA, Sullivan PG, Büeler H. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS One. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carrì MT, Cozzolino M, Volonté C, D’Ambrosi N. The NADPH oxidase pathway is dysregulated by the P2×7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J. Immunol. 2013;190:5187–5195. doi: 10.4049/jimmunol.1203262. [DOI] [PubMed] [Google Scholar]
- Appel SH, Zhao W, Beers DR, Henkel JS. The microglial-motoneuron dialogue in ALS. Acta Myol. 2011;30:4–8. [PMC free article] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J. Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov. Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Huang A, Wen S, Liao B, Appel SH. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, Chasovskikh S, Mhyre TR, Maguire-Zeiss KA. α-Synuclein Alters Toll-Like Receptor Expression. Front. Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson's disease patients. Neurosci. Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore A-K, Pellerin L. Unraveling the complex metabolic nature of astrocytes. Front. Cell. Neurosci. 2013;7:179. doi: 10.3389/fncel.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman M, Vincent T, Scamps F, Perrin FE, Camu W, Raoul C. Neuroimmunity dynamics and the development of therapeutic strategies for amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2013;7:214. doi: 10.3389/fncel.2013.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers. Dement. 2011;7:402–411. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay J-M, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cameron B, Tse W, Lamb R, Li X, Lamb BT, Landreth GE. Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer’s disease. J. Neurosci. 2012;32:15112–15123. doi: 10.1523/JNEUROSCI.1729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula M, Iyer AM, Spliet WGM, Anink JJ, Steentjes K, Sta M, Troost D, Aronica E. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience. 2011;179:233–243. doi: 10.1016/j.neuroscience.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine TNFα results in attenuation of amyloid deposition in vivo. Mol. Neurodegener. 2011;6:16. doi: 10.1186/1750-1326-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Ala TA, Hu S, Crossley KB, Sherman RE, Peterson PK, Frey WH. Serum cytokine levels in patients with Alzheimer’s disease. Clin. Diagn. Lab. Immunol. 1994a;1:433–436. doi: 10.1128/cdli.1.4.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Frey WH, Ala TA, Tourtellotte WW, Peterson PK. Transforming growth factor beta in Alzheimer’s disease. Clin. Diagn. Lab. Immunol. 1994b;1:109–110. doi: 10.1128/cdli.1.1.109-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-C, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-S, Peng G-S, Li G, Yang S, Wu X, Wang C-C, Wilson B, Lu R-B, Gean P-W, Chuang D-M, Hong J-S. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Julien J-P, Goldstein LSB, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci. Lett. 2014 doi: 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J. Immunol. 1992;148:1404–1410. [PubMed] [Google Scholar]
- Cookson MR. alpha-Synuclein and neuronal cell death. Mol. Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Culpan D, MacGowan SH, Ford JM, Nicoll JAR, Griffin WS, Dewar D, Cairns NJ, Hughes A, Kehoe PG, Wilcock GK. Tumour necrosis factor-alpha gene polymorphisms and Alzheimer’s disease. Neurosci. Lett. 2003;350:61–65. doi: 10.1016/s0304-3940(03)00854-1. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung G-YR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell. Mol. Life Sci. 2013;70:4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol. Scand. 1999;100:34–41. doi: 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-Stimulating Factor 1 Receptor Signaling Is Necessary for Microglia Viability, Unmasking a Microglia Progenitor Cell in the Adult Brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2:229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM. TGF-beta 1 is an organizer of responses to neurodegeneration. J. Cell. Biochem. 1993;53:314–322. doi: 10.1002/jcb.240530408. [DOI] [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flex A, Giovannini S, Biscetti F, Liperoti R, Spalletta G, Straface G, Landi F, Angelini F, Caltagirone C, Ghirlanda G, Bernabei R. Effect of Proinflammatory Gene Polymorphisms on the Risk of Alzheimer’s Disease. Neurodegener. Dis. 2013 doi: 10.1159/000353395. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Forman MS, Trojanowski JQ, Lee VM-Y. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat. Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Treviño I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM, Baird A. Phagocytosis and deposition of vascular beta-amyloid in rat brains injected with Alzheimer beta-amyloid. Am. J. Pathol. 1992;140:1389–1399. [PMC free article] [PubMed] [Google Scholar]
- Gandelman M, Peluffo H, Beckman JS, Cassina P, Barbeito L. Extracellular ATP and the P2×7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J. Neuroinflammation. 2010;7:33. doi: 10.1186/1742-2094-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H-M, Zhou H, Hong J-S. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012;33:295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardet A, Benita Y, Li C, Sands BE, Ballester I, Stevens C, Korzenik JR, Rioux JD, Daly MJ, Xavier RJ, Podolsky DK. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 2010;185:5577–5585. doi: 10.4049/jimmunol.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G, Dequen F, Soucy G, Julien J-P. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J. Neurosci. 2006;26:11397–11402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves MC, Fiala M, Dinglasan LAV, Liu NQ, Sayre J, Chiappelli F, van Kooten C, Vinters HV. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004;5:213–219. doi: 10.1080/14660820410020286. [DOI] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X-L, Long C-X, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert J-C, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier M-V, Town T. Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol. Disord. Drug Targets. 2013;12:593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, LaDu MJ, Van Eldik LJ. A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J. Mol. Neurosci. 2004;23:205–212. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, Fedorow H, Cartwright MI, Griffiths FM, Shepherd CE, Double KL. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain. 2005;128:2654–2664. doi: 10.1093/brain/awh584. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov. Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati L-N, Van Cauwenberghe C, Brooks PL, Brouwers N, Ghani M, Sato C, Cruts M, Sleegers K, St George-Hyslop P, Van Broeckhoven C, Rogaeva E. Genetic association of CR1 with Alzheimer’s disease: a tentative disease mechanism. Neurobiol. Aging. 2012;33 doi: 10.1016/j.neurobiolaging.2012.07.001. 2949.e5-2949.e12. [DOI] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J. Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng T-C, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2012;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol. Cell. Neurosci. 2006;31:427–437. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, Zhao W, Moore DH, Powell SZ, Appel SH. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol. Med. 2013;5:64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18(Suppl 1):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F, Saad M, Sadd M, Bras JM, Bettella F, Nicolaou N, Simón-Sánchez J, Mittag F, Gibbs JR, Schulte C, Durr A, Guerreiro R, Hernandez D, Brice A, Stefánsson H, Majamaa K, Gasser T, Heutink P, Wood NW, Martinez M, Singleton AB, Nalls MA, Hardy J, Morris HR, Williams NM. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum. Mol. Genet. 2013;22:1039–1049. doi: 10.1093/hmg/dds492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tong J, Bi F, Zhou H, Xia X-G. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J. Clin. Invest. 2012;122:107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Boissière F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Innamorato NG, Jazwa A, Rojo AI, García C, Fernández-Ruiz J, Grochot-Przeczek A, Stachurska A, Jozkowicz A, Dulak J, Cuadrado A. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS One. 2010;5:e11838. doi: 10.1371/journal.pone.0011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Nagai A, Kobayashi S, Kim SU. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J. Neuropathol. Exp. Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu J-T, Zhu X-C, Tan L. TREM2 in Alzheimer’s disease. Mol. Neurobiol. 2013;48:180–185. doi: 10.1007/s12035-013-8424-8. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard J-P, Lacomblez L, Pochigaeva K, Salachas F, Pradat P-F, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kastner A, Hirsch EC, Lejeune O, Javoy-Agid F, Rascol O, Agid Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson’s disease related to their neuromelanin content? J. Neurochem. 1992;59:1080–1089. doi: 10.1111/j.1471-4159.1992.tb08350.x. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Khandelwal PJ, Herman AM, Moussa CE-H. Inflammation in the early stages of neurodegenerative pathology. J. Neuroimmunol. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho D-H, Suk J-E, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee H-J, Lee S-J. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Choi DH, Block ML, Lorenzl S, Yang L, Kim YJ, Sugama S, Cho BP, Hwang O, Browne SE, Kim SY, Hong J-S, Beal MF, Joh TH. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- Kuhle J, Lindberg RLP, Regeniter A, Mehling M, Steck AJ, Kappos L, Czaplinski A. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur. J. Neurol. 2009;16:771–774. doi: 10.1111/j.1468-1331.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- Kumar H, Koppula S, Kim I-S, More SV, Kim B-W, Choi D-K. Nuclear factor erythroid 2-related factor 2 signaling in Parkinson disease: a promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS Neurol. Disord. Drug Targets. 2012;11:1015–1029. doi: 10.2174/1871527311211080012. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lampron A, Pimentel-Coelho PM, Rivest S. Migration of bone marrow-derived cells into the central nervous system in models of neurodegeneration. The Journal of. J. Comp. Neurol. 2013;521:3863–3876. doi: 10.1002/cne.23363. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Galvan C, Hellias B, Dotti C, Jensen PH. Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J. Neurochem. 2002;83:1431–1440. doi: 10.1046/j.1471-4159.2002.01253.x. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Suk J-E, Patrick C, Bae E-J, Cho J-H, Rho S, Hwang D, Masliah E, Lee S-J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol. Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Lewis C-A, Manning J, Rossi F, Krieger C. The Neuroinflammatory Response in ALS: The Roles of Microglia and T Cells. Neurol. Res. Int. 2012;2012:803701. doi: 10.1155/2012/803701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao B, Zhao W, Beers DR, Henkel JS, Appel SH. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 2012;237:147–152. doi: 10.1016/j.expneurol.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau RS, Gonzalez-Dunia D, Wiendl H, Zipp F. Neurons as targets for T cells in the nervous system. Trends Neurosci. 2013;36:315–324. doi: 10.1016/j.tins.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Ling S-C, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM, Iwatsubo T, Trojanowski JQ. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am. J. Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Nistico R, Berretta N, Guatteo E, Bernardi G, Mercuri NB. L-DOPA: a scapegoat for accelerated neurodegeneration in Parkinson’s disease? Prog. Neurobiol. 2011;94:389–407. doi: 10.1016/j.pneurobio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Liu B. Modulation of microglial pro-inflammatory and neurotoxic activity for the treatment of Parkinson’s disease. AAPS J. 2006;8:E606–E621. doi: 10.1208/aapsj080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hao W, Dawson A, Liu S, Fassbender K. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J. Biol. Chem. 2009;284:3691–3699. doi: 10.1074/jbc.M804446200. [DOI] [PubMed] [Google Scholar]
- Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Lucas S-M, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147(Suppl):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SL, Tang NLS, Lam LCW, Chiu HFK. Association between tumor necrosis factor-alpha promoter polymorphism and Alzheimer’s disease. Neurology. 2004;62:307–309. doi: 10.1212/01.wnl.0000103288.49441.e4. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Genomics Hum. Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014;20(Suppl 1):S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol. Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM. Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp. Neurol. 2001;169:219–230. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13046–13050. doi: 10.1073/pnas.1002396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J-P, Bellavance M-A, Préfontaine P, Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013a;5:646–653. doi: 10.1016/j.celrep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Michaud J-P, Hallé M, Lampron A, Thériault P, Préfontaine P, Filali M, Tribout-Jover P, Lanteigne A-M, Jodoin R, Cluff C, Brichard V, Palmantier R, Pilorget A, Larocque D, Rivest S. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc. Natl. Acad. Sci. U. S. A. 2013b;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Arai T, Guo J-P, Klegeris A, Yu S, McGeer EG, McGeer PL. LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 2006;65:953–963. doi: 10.1097/01.jnen.0000235121.98052.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Minoretti P, Gazzaruso C, Vito C, Di Emanuele E, Bianchi M, Coen E, Reino M, Geroldi D. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer’s disease. Neurosci. Lett. 2006;391:147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience. 2000;95:425–432. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994a;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994b;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P, Nagatsu T. Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci. Lett. 1995;193:129–132. doi: 10.1016/0304-3940(95)11686-q. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Kondo T, Mizuno Y, Nagatsu T. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci. Lett. 2007;414:94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer’s disease. Glia. 2010;58:300–314. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullett SJ, Hamilton RL, Hinkle DA. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology. 2009;29:125–131. doi: 10.1111/j.1440-1789.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Rivest S. A deficiency in CCR2+ monocytes: the hidden side of Alzheimer’s disease. J. Mol. Cell Biol. 2013;5:284–293. doi: 10.1093/jmcb/mjt028. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J. Neural Transm. 2000;(Suppl):143–151. [PubMed] [Google Scholar]
- Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012;4:a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci. Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Lofrumento DD, Saponaro C, De Nuccio F, Cianciulli A, Mitolo V, Nicolardi G. Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson’s-like disease. Immunopharmacol. Immunotoxicol. 2008;30:729–7240. doi: 10.1080/08923970802278557. [DOI] [PubMed] [Google Scholar]
- Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1182-x. [DOI] [PubMed] [Google Scholar]
- Ramos EM, Lin M-T, Larson EB, Maezawa I, Tseng L-H, Edwards KL, Schellenberg GD, Hansen JA, Kukull WA, Jin L-W. Tumor necrosis factor alpha and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Arch. Neurol. 2006;63:1165–1169. doi: 10.1001/archneur.63.8.1165. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J. Clin. Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Estévez AG, Nishimune H, Cleveland DW, deLapeyrière O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita V-M, Kaivorinne A-L, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzos M, Evangelopoulos E, Sereti E, Zouvelou V, Marmara S, Alexakis T, Evdokimidis I. Alterations of T cell subsets in ALS: a systemic immune activation? Acta Neurol. Scand. 2012;125:260–264. doi: 10.1111/j.1600-0404.2011.01528.x. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- ADAPT. Results of a follow-up study to the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) Alzheimers. Dement. 2013;9:714–723. doi: 10.1016/j.jalz.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Salins P, He Y, Olson K, Glazner G, Kashour T, Amara F. TGF-beta1 is increased in a transgenic mouse model of familial Alzheimer’s disease and causes neuronal apoptosis. Neurosci. Lett. 2008;430:81–86. doi: 10.1016/j.neulet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Salter MW, Beggs S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology. 2014;79C:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J. Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Toyoshima Y, Shiga A, Yokoseki A, Arakawa K, Sekine Y, Shimohata T, Ikeuchi T, Nishizawa M, Kakita A, Onodera O, Takahashi H. Sporadic ALS with compound heterozygous mutations in the SQSTM1 gene. Acta Neuropathol. 2013;126:453–459. doi: 10.1007/s00401-013-1150-5. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien J-P, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan BS, Doostzadeh J, Absalan F, Mohandessi S, Jalili R, Bigdeli S, Wang J, Mahadevan J, Lee CLG, Davis RW, William Langston J, Ronaghi M. Whole genome survey of coding SNPs reveals a reproducible pathway determinant of Parkinson disease. Hum. Mutat. 2009;30:228–238. doi: 10.1002/humu.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SWM, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Dupré N, Petri S, Strong M, Kriz J, Julien J-P. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways. J. Exp. Med. 2011;208:2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS Neurol. Disord. Drug Targets. 2010;9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Saxe M, Paris D, Wu Y, Mullan M. Ligation of microglial CD40 results in p44/42 mitogen-activated protein kinase-dependent TNF-alpha production that is opposed by TGF-beta 1 and IL-10. J. Immunol. 1999;163:6614–6621. [PubMed] [Google Scholar]
- Tanji K, Maruyama A, Odagiri S, Mori F, Itoh K, Kakita A, Takahashi H, Wakabayashi K. Keap1 is localized in neuronal and glial cytoplasmic inclusions in various neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2013;72:18–28. doi: 10.1097/NEN.0b013e31827b5713. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi D-K, Wu D-C, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can J, Van, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J. Clin. Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssou E, Takeda T, Lebon V, Boillée S, Doukouré B, Bataillon G, Sazdovitch V, Cazeneuve C, Meininger V, LeGuern E, Salachas F, Seilhean D, Millecamps S. Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: genetics and neuropathology. Acta Neuropathol. 2013;125:511–522. doi: 10.1007/s00401-013-1090-0. [DOI] [PubMed] [Google Scholar]
- Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer’s disease. Neuromolecular Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee J-K, Tansey MG. Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B. PLoS One. 2011;6:e23660. doi: 10.1371/journal.pone.0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- Tsao W, Jeong YH, Lin S, Ling J, Price DL, Chiang P-M, Wong PC. Rodent models of TDP-43: recent advances. Brain Res. 2012;1462:26–39. doi: 10.1016/j.brainres.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufekci KU, Genc S, Genc K. The endotoxin-induced neuroinflammation model of Parkinson’s disease. Parkinsons. Dis. 2011;2011:487450. doi: 10.4061/2011/487450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-Like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 2008:1–20. doi: 10.1007/978-3-540-72167-3_1. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu I-L, Yang W-S, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM-Y, Schellenberg GD, Yu C-E. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wal EA, Gómez-Pinilla F, Cotman CW. Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport. 1993;4:69–72. doi: 10.1097/00001756-199301000-00018. [DOI] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo J-M, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varçin M, Bentea E, Michotte Y, Sarre S. Oxidative stress in genetic mouse models of Parkinson’s disease. Oxid. Med. Cell. Longev. 2012;2012:624925. doi: 10.1155/2012/624925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Berg J, Prokop S, Miller KR, Obst J, Kälin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O, Winter Y, Becher B, Heppner FL. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012;18:1812–1819. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- Waak J, Weber SS, Waldenmaier A, Görner K, Alunni-Fabbroni M, Schell H, Vogt-Weisenhorn D, Pham T-T, Reumers V, Baekelandt V, Wurst W, Kahle PJ. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch. Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-Z, Yu J-T, Miao D, Wu Z-C, Zong Y, Wen C-Q, Tan L. Genetic association of TLR4/11367 polymorphism with late-onset Alzheimer’s disease in a Han Chinese population. Brain Res. 2011;1381:202–207. doi: 10.1016/j.brainres.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet M-F. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp. Neurol. 2012;237:318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J. 2003;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-J. Voltage-Gated Proton Channel HV1 in Microglia. Neuroscientist. 2014 doi: 10.1177/1073858413519864. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am. J. Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature. 1997;389:603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Hyman BT. Chemokines/chemokine receptors in the central nervous system and Alzheimer’s disease. J. Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- Xu Y-F, Gendron TF, Zhang Y-J, Lin W-L, D’Alton S, Sheng H, Casey MC, Tong J, Knight J, Yu X, Rademakers R, Boylan K, Hutton M, McGowan E, Dickson DW, Lewis J, Petrucelli L. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-F, Zhang Y-J, Lin W-L, Cao X, Stetler C, Dickson DW, Lewis J, Petrucelli L. Expression of mutant TDP-43 induces neuronal dysfunction in transgenic mice. Mol. Neurodegener. 2011;6:73. doi: 10.1186/1750-1326-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Sawada H, Izumi Y, Kume T, Katsuki H, Shimohama S, Akaike A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J. Biol. Chem. 2007;282:4364–4372. doi: 10.1074/jbc.M603712200. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang H, Qiao T, Yang B, Aliaga L, Qiu L, Tan W, Salameh J, McKenna-Yasek DM, Smith T, Peng L, Moore MJ, Brown RH, Cai H, Xu Z. Partial loss of TDP-43 function causes phenotypes of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1322641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel MK, Kirsch WM. From development to dysfunction: microglia and the complement cascade in CNS homeostasis. Ageing Res. Rev. 2013;12:749–756. doi: 10.1016/j.arr.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea L-G, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, Clurman B, Melquist S, Narayanan M, Suver C, Shah H, Mahajan M, Gillis T, Mysore J, MacDonald ME, Lamb JR, Bennett DA, Molony C, Stone DJ, Gudnason V, Myers AJ, Schadt EE, Neumann H, Zhu J, Emilsson V. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong J-S, Sulzer D, Zecca L. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotox. Res. 2011;19:63–72. doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong J-S, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien J-P, Appel SH. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58:231–243. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]