Abstract
Background
Neuropsychiatric systemic lupus erythematosus (NPSLE) is a common and potentially fatal manifestation of SLE. Antiphospholipid antibodies (aPL) such as lupus anticoagulant (LA), anticardiolipin (aCL) and antibodies to β2glycoprotein I (anti-β2GPI), the most important aPL antigen, are thought to play a role in some forms of NPSLE. As of yet, their specific roles in NPSLE manifestations remain to be elucidated.
Methodology/Principal Findings
57 SLE patients (53 women) were assessed for LA, aCL and anti-β2GPI twice, to determine persistent positivity. All patients were examined by neurology and psychiatry specialists. 69 healthy subjects were assessed as controls. NPSLE was diagnosed in 74% of patients. Headaches were the most prevalent manifestation of NPSLE (39%), followed by cerebrovascular disease (CVD) (23%), depressive disorders (19.0%), and seizures (14%). NPSLE and non-NPSLE patients showed comparable SLE activity and corticosteroid use. In 65% of patients neuropsychiatric manifestations preceded SLE diagnosis. aPL profiles of NPSLE patients and non-NPSLE patients were similar. Headaches and ischemic stroke were independently associated with anti-β2GPI-IgM (OR=5.6; p<0.05), and seizures were linked to anti-β2GPI-IgG (OR=11.3; p=0.01).
Conclusions
In SLE patients, neuropsychiatric manifestations occur frequently and early, often before the disease is diagnosed. Autoantibodies to β2GPI are linked to non-specific headaches, ischemic stroke and seizures, and show a better predictive value than aCL and LA. These findings may help to improve the diagnosis of NPSLE and should prompt further studies to characterize the role of anti-β2GPI in the pathogenesis of this condition.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystem, autoimmune disease with autoantibody-mediated tissue damage. Clinically, SLE is characterized by heterogeneous symptoms and may involve almost all tissues and organs, including the nervous system. Neuropsychiatric lupus encompasses a wide spectrum of neurologic and psychiatric disorders resulting from the involvement of the central, peripheral and autonomic nervous system due to SLE-related pathology. The attribution of different neurologic and psychiatric disorders to neuropsychiatric SLE (NPSLE) is still a matter of debate. NPSLE is a relatively frequent and potentially fatal presentation of SLE, and the involvement of CNS especially is associated with a more serious course and increased mortality [1–3].
The pathogenesis of NPSLE remains largely unclear, but the occlusion of vessels supplying the nervous tissue and direct interaction of antibodies with phospholipids of neural cells appear to be important. Antiphospholipid antibodies (aPL) may contribute to both of these pathogenic mechanisms [4–6]. aPL are a heterogeneous group of autoantibodies, such as anticardiolipin antibodies (aCL), lupus anticoagulant (LA) and anti-β2-glycoprotein-I (anti-β2GPI), that are frequently observed in autoimmune disorders, especially in SLE. aPL share the ability to bind to phospholipid binding proteins or to complexes of these proteins with phospholipids. β2GPI is the most important aPL antigen [7]. In recent years, the role and relevance of anti-β2GPI in autoimmune conditions have been better characterized. The presence of anti-β2GPI was included in the list of diagnostic criteria for antiphospholipid syndrome (APS) and, recently, in the Systemic Lupus International Collaborating Clinics classification criteria for SLE [8, 9]. Nonetheless, little is known about the frequency of expression of anti-β2GPI in NPSLE, and their role in its pathology.
The aim of the study was to evaluate NPSLE and non-NPSLE patients for the presence of anti-β2GPI and other aPL such as aCL and LA and to assess the association between these antibodies and the presence of NPSLE disorders.
Materials and Methods
Subjects and diagnostic measures
This study was approved by the local Ethics Committee and conducted in accordance with the Declaration of Helsinki. The study was performed at a university hospital dermatology department, which is a regional reference center for patients with cutaneous and systemic lupus erythematosus. Informed consent was obtained from each participant prior to inclusion into the study. Every patient recruited was interviewed, examined physically by a dermatologist (TH) and blood was sampled. Subsequently, every patient was referred to and examined by a neurologist (AB) and a psychiatrist (MKK).
The examined group comprised 57 consecutive Caucasian SLE in- and out-patients (53 women, 4 men). Each patient met 4 or more ACR classification criteria for SLE [10]. The disease duration was calculated as the time from the first time when at least 4 SLE criteria were fulfilled until the inclusion in the study. Disease activity was assessed once, with the Systemic Lupus Activity Measure (SLAM) score, which ranges from 0 (no disease activity) to 86 (maximum disease activity) [11]. The assessment was performed and material for evaluation of disease activity (blood and urine) sampled during the visit in the department of dermatology. Patients’ corticosteroid doses were calculated for prednisone equivalency. The average corticosteroid dose administered during the last 14 days before examination was defined as the “current daily corticosteroid dose”. Patients’ life cumulative corticosteroid doses were calculated based on their history and medical documentation. A control group of 69 healthy Caucasian volunteers were age and gender matched with the SLE group (62 women, 7 men). The control group had the same blood tests performed as the SLE patients.
Neurologic and psychiatric assessment
Neurological and psychiatric features of NPSLE were classified according to the current ACR nomenclature, after exclusion of other causes of neuropsychiatric signs and symptoms, defined by ACR as “exclusions” and “associations” [12]. Headaches were classified into one of the three categories: migraine, tension-type headache and cluster headache, which are included in the ACR nomenclature for NPSLE and in the classification of the international headache society (IHS) in the category of primary headaches [12, 13]. Therapy resistant, severe, disabling persistent headache that did not fit any of these categories, after exclusion of secondary causes, was categorized as “intractable headache, nonspecific”. This headache category is included in the ACR classification, but not the IHS classification [12]. Cognitive function was tested in all patients with a Mini Mental State Examination (MMSE) employed as a screening tool [14]. Patients who scored lower than 27 or reported a history of cognitive impairment were further examined neuropsychologically.
Laboratory measures
All blood samples were collected in a fasting state. Peripheral blood cell counts, erythrocyte sedimentation rate, serum creatinine, and urine were analysed. The presence (dilution > 1:160) and titres of antinuclear antibodies (ANA) were assessed with an indirect immunofluorescence method, by use of a standard diagnostic test, Europlus ANA Mosaic 20 (Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany) [15]. ANA specificity was determined by the line-blot method with the ANA Euroline Profile 3 kit (Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany).
The lupus anticoagulant assay was performed according to the recommendations of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis [16]. The assessment was performed on plasma, obtained after centrifugation of the blood sample, collected in a tube with sodium citrate. In the first stage lupus anticoagulant was screened by activated Partial Thromboplastin Time (aPTT) and diluted Russel Viper Venom Time (dRVVT). None of the subjects were being treated with anticoagulants 14 days preceding this testing.
aCL IgG, IgM, IgA and anti-β2GPI IgG, IgM, and IgA were assessed with an immunoassay method by a commercial microplate ELISA kit (Euroimmun Medizinische Labordiagnostika AG). Blood samples were obtained by venipuncture (5 ml) from each subject and collected into glass tubes without anticoagulant. The blood was left to clot and then centrifuged. Serum was frozen and stored in aliquots at -80°C degrees. The cut-off value was calculated for each type and class of antibodies at the 98 percentile level of results obtained for the control group, according to previous recommendations [17]. LA, aCL and anti-β2GPI assays in SLE patients were performed twice at an interval of at least 12 weeks, according to the current Sydney classification criteria for antiphospholipid antibody syndrome [9]. Only patients who showed aPL (> 98 percentile level of the control group) in both tests were considered positive. In the control group, the antiphospholipid assays were performed only once.
Statistical analysis
Descriptive statistics including mean, standard deviation, and range were used to present outcomes, and the two tailed Student t-test was used to compare variables with normal distribution. Homogeneity of variance was tested with the Levene’s test. If variances were not homogenous, the Cochran-Cox test was employed. For non-normally distributed variables, medians, upper and lower quartiles and the Mann-Whitney test were used. To compare dichotomic variables, Chi square and whenever appropriate, the Fisher exact test were used. To quantify the strength of the probability of occurrence of neuropsychiatric manifestations in patients with anti-β2GPI and other aPL, odds ratio (OR) was used. Statistical significance was set at a level of 0.05.
Results
Age of study subjects and SLE duration
The mean age of patients was 42.5 ± 12.2 years (range: 20–69 years), and the mean age of control group subjects was 42.7 ± 10.9 years. The mean SLE duration was 8.3 ± 8.9 years (range: 6 months to 47 years).
Neuropsychiatric manifestations are frequent and often precede SLE diagnosis
NPSLE was diagnosed in 42 (74%) of 57 consecutive SLE patients. The prevalence of NPSLE manifestations ranged from 39% for headaches to one patient each with acute confusional state and myasthenia gravis (Table 1). In 18 patients only one NPSLE manifestation was diagnosed; most frequently it was headache, which was found, as an isolated manifestation, in 8 patients (14% of SLE patients), 6 (11%) of whom had migraine headaches. Two further patients were not included in the NPSLE-headache group as their headaches were associated with seizures and diagnosed as ictal headaches.
Table 1. Prevalence of neuropsychiatric systemic lupus erythematosus (NPSLE) in patients with systemic lupus erythematosus (SLE).
| Number of subjects (percentage of all SLE patients, N = 57) | |
|---|---|
| non-NPSLE | 15 (26.3%) |
| NPSLE | 42 (73.7%) |
| Headache | 22 (38.6%) |
| Migraine | 10 (17.5%) |
| Tension headache | 2 (3.5%) |
| Nonspecific, intractable headache | 10 (17.5%) |
| Cerebrovascular disease (CVD) | 13 (22.8%) |
| Ischaemic stroke | 10 (17.5%) |
| Transient ischemic attack (TIA) | 3 (5.3%) |
| Intracranial hemorrhage | 1 (1.8%) |
| Seizures | 8 (14.0%) |
| Grand mal seizures | 6 (10.5%) |
| Partial simple seizures | 2 (3.5%) |
| Partial complex seizures | 1 (1.8%) |
| Multiple mononeuropathy | 6 (10.5%) |
| Single mononeuropathy | 1 (1.8%) |
| Sensory polyneuropathy | 3 (5.3%) |
| Cranial neuropathy (of the 5th and the 8th nerve) | 2 (3.5%) |
| Myasthenia gravis | 1 (1.8%) |
| Acute confusional state | 1 (1.8%) |
| Cognitive dysfunction | 5 (8.8%) |
| Depressive disorders | 11 (19.3%) |
| Anxiety disorders | 6 (10.5%) |
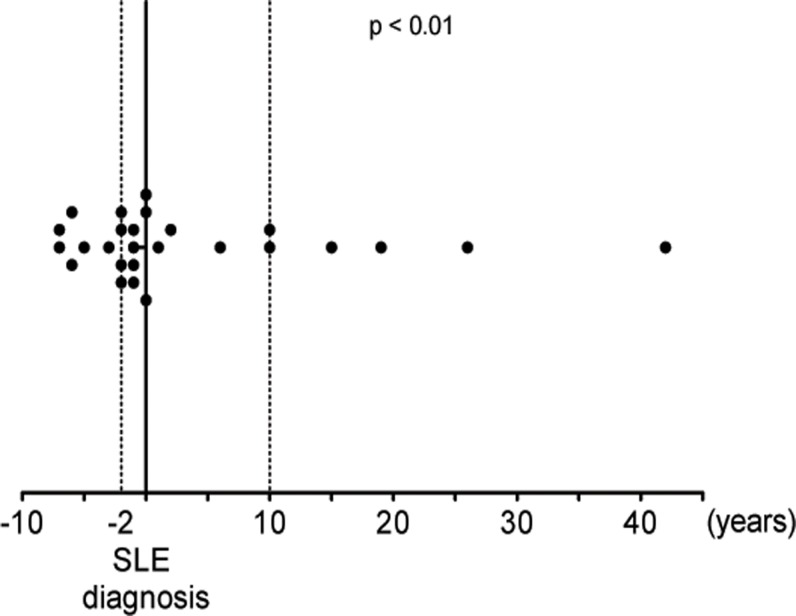
In 26 patients it was possible to assess the temporal relationship of the first neuropsychiatric symptoms and SLE diagnosis. In 17 (65.4%) of these NPSLE patients, neuropsychiatric symptoms preceded SLE diagnosis, and in 9 (34.6% of NPSLE patients) the onset of symptoms followed the diagnosis of SLE. In the 17 patients who showed neuropsychiatric symptoms before they were diagnosed with SLE, the median interval was 2 years (lower quartile: 1; upper quartile: 5.5). This was significantly (p<0.01) shorter than the median interval of 10 years (lower quartile: 4; upper quartile: 22.5) in patients who developed neuropsychiatric manifestations after they had been diagnosed with SLE (Fig. 1).
Fig 1. Relation of diagnosis of SLE and the onset of neuropsychiatric symptoms.
Values are negative in those patients, who developed neuropsychiatric symptoms before they were diagnosed with SLE. Dotted lines show the median intervals between the onset of neuropsychiatric symptoms and diagnosis of SLE, i.e. 2 years in patients who developed neuropsychiatric symptoms before they were diagnosed with SLE and 10 years in patients who developed neuropsychiatric symptoms after they were diagnosed with SLE.
NPSLE and non-NPSLE patients show comparable SLE activity, corticosteroid use, antiphospholipid antibody profiles, and frequency of APS
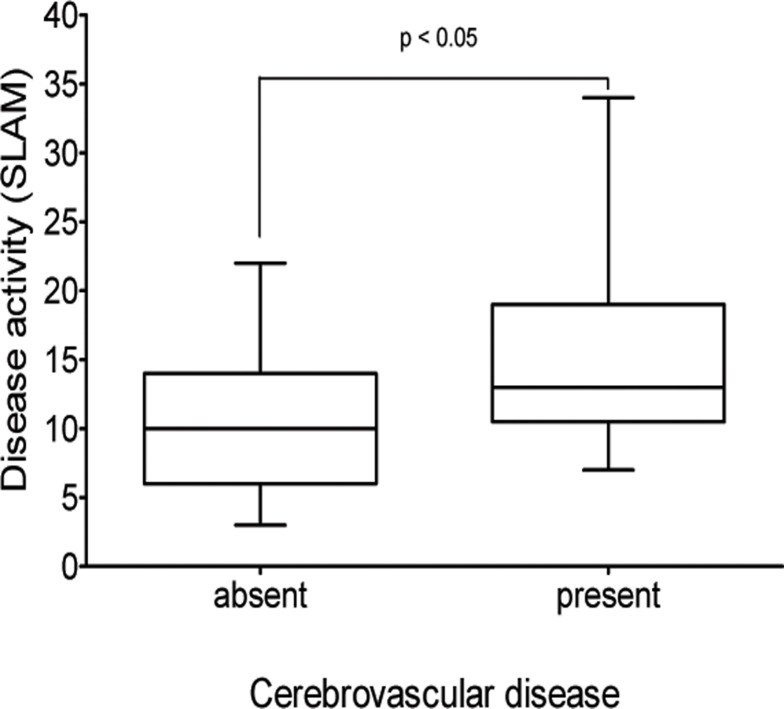
The mean disease activity in our SLE patients was 11.3 ± 6.0, range 3–34, as assessed by SLAM. We did not find a significant difference in the mean SLE activity between NPSLE and non-NPSLE patients. The only NPSLE subgroup that exhibited increased disease activity was patients with cerebrovascular diseases (CVD): median disease activity in these patients was significantly higher (13.0; lower quartile 10.5, upper quartile 19.0) than in patients without cerebrovascular diseases (10.0; lower quartile 6.0, upper quartile 14.0; p<0.05) (Fig. 2). We did not observe any statistically significant differences in the frequency of ACR criteria for SLE between NPSLE and non-NPSLE patients (Table 2). The mean current daily corticosteroid dose of all patients was 11.2 ± 13.8 mg (range 0–60 mg), and the mean life cumulative corticosteroid dose was 25.4 ± 32.3 g (range 0–123 g). There was no difference between NPSLE and non-NPSLE groups in corticosteroid doses, neither current nor cumulative.
Fig 2. Comparison of systemic lupus erythematosus (SLE) activity, as measured with the Systemic Lupus Activity Measure (SLAM), in patients with and without cerebrovascular diseases.
Median values expressed as horizontal lines, interquartile ranges as boxes, and ranges as whiskers.
Table 2. Clinical and laboratory characteristics of NPSLE and non-NPSLE patients according to the American College of Rheumatology (ACR) classification criteria.
| ACR criteria | NPSLE N = 42 (%) | non-NPSLE, N = 15 (%) |
|---|---|---|
| Malar rash | 34 (81.0) | 10 (66.7) |
| Discoid rash | 10 (23.8) | 4 (26.7) |
| Photosensitivity | 38 (90.5) | 11 (73.3) |
| Oral ulcers | 22 (52.4) | 4 (26.7) |
| Arthritis | 38 (90.5) | 14 (93.3) |
| Serositis | 3 (7.1) | 3 (20.0) |
| Renal disorder | 13 (31.0) | 7 (46.7) |
| Hematologic disorder | 34 (81.0) | 13 (86.7) |
| hemolytic anemia | 19 (45.2) | 8 (53.3) |
| leukopenia | 27 (64.3) | 10 (66.7) |
| lymphopenia | 14 (33.3) | 8 (53.3) |
| thrombocytopenia | 17 (40.5) | 4 (26.7) |
| Immunologic disorder | 23 (54.8) | 8 (53.3) |
| anti-dsDNA antibodies | 8 (19.0) | 3 (20.0) |
| anti-Sm antibodies | 8 (19.0) | 2 (13.3) |
| VDRL—false positive | 1 (2.3) | 1 (7.1) |
| anticardiolipin antibodies IgG or IgM | 11 (26.2) | 3 (20.0) |
| lupus anticoagulant | 5 (11.9) | 2 (13.3) |
| ANA positive (titer > 1/160) | 42 (100) | 15 (100) |
NPSLE—neuropsychiatric systemic lupus erythematosus; ANA—antinuclear antibodies
Differences between NPSLE and non-NPSLE groups are statistically insignificant.
Antiphospholipid antibodies, i.e. IgG, IgM, or IgA antibodies against β2GPI or CL as well as LA, were found in 11% to 26% of patients (Table 3). There were no significant differences of the rates of any of these aPL between NPSLE patients and non-NPSLE patients.
Table 3. Prevalence of antiphospholipid antibodies in patients with systemic lupus erythematosus (SLE) and in the subgroups with and without neuropsychiatric (NP) manifestations.
| Antiphospholipid antibodies | SLE, N = 57 | NPSLE, N = 42 | non-NPSLE, N = 15 | |
|---|---|---|---|---|
| Anti-β2glycoprotein antibodies | IgG | 8 (14.0%) | 5 (11.9%) | 3 (20%) |
| IgM | 9 (15.8%) | 8 (19.0%) | 1 (6.7%) | |
| IgA | 6 (10.5%) | 4 (9.5%) | 2 (13.3%) | |
| Anticardiolipin antibodies | IgG | 6 (10.5%) | 4 (9.5%) | 2 (13.3%) |
| IgM | 9 (15.8%) | 8 (19.0%) | 1 (6.7%) | |
| IgA | 15 (26.3%) | 11 (26.2%) | 4 (26.7%) | |
| Lupus anticoagulant | 7 (12.3%) | 5 (11.9%) | 2 (13.3%) | |
Differences between NPSLE and non-NPSLE groups are statistically insignificant.
13 of all SLE patients (23%) were previously diagnosed with APS. 12 of these 13 patients were confirmed to be positive for aCL in the current examination. Diagnosis of APS was not related to any of the NPSLE disorders, with the exception of CVD, and ischemic stroke specifically, which itself is a clinical criterion of APS.
Patients with anti-β2GPI present more frequently with headaches, ischemic stroke or seizures, and seizures are also linked to aCL and LA
When we compared patients with certain aPL for differences in their NPSLE manifestations we found that patients with anti-β2GPI IgM have an almost six times higher likelihood of having nonspecific, intractable headaches (Table 4) or ischemic stroke (Table 5) than patients negative for these antibodies. Patients with anti-β2GPI IgG were found to be 11 times more likely to exhibit seizures (Table 6) and 9 times more likely to have grand mal seizures specifically (Table 7) than patients negative for these antibodies. We found seizures to also be linked to LA and aCL IgA (Table 6), and grand mal seizures to aCL IgA only (Table 7). The remaining NPSLE subgroups showed no apparent association with the investigated antibodies.
Table 4. Association of antiphospholipid antibodies with nonspecific headaches.
| Headaches nonspecific | Fisher exact test, p value | OR | 95% CI | |
|---|---|---|---|---|
| anti-β2GPI IgM (+) n = 9 | 4 (44.4%) | < 0.05 | 5.60 | 1.17–26.88 |
| anti-β2GPI IgM (-) n = 48 | 6 (12.5%) |
anti-β2GPI—anti-β2-glycoprotein-I; IgM—immunoglobulin M; OR—odds ratio; CI—confidence interval
Table 5. Association of antiphospholipid antibodies with ischemic stroke.
| Ischemic stroke | Fisher exact test, p value | OR | 95% CI | |
|---|---|---|---|---|
| anti-β2GPI IgM (+) n = 9 | 4 (44.4%) | < 0.05 | 5.60 | 1.17–26.88 |
| anti-β2GPI IgM (-) n = 48 | 6 (12.5%) |
anti-β2GPI—anti-β2-glycoprotein-I; IgM—immunoglobulin M; OR—odds ratio; CI—confidence interval
Table 6. Association of antiphospholipid antibodies with seizures.
| Seizures | Fisher exact test, p value | OR | 95% CI | |
|---|---|---|---|---|
| anti-β2GPI IgG (+) n = 8 | 4 (50.0%) | 0.010 | 11.25 | 2.01–62.97 |
| anti-β2GPI IgG (-) n = 49 | 4 (8.2%) | |||
| LA (+) n = 7 | 3 (42.9%) | 0.05 | 6.75 | 1.16–39.20 |
| LA (-) n = 50 | 5 (10.0%) | |||
| aCL IgA (+) n = 15 | 5 (33.3%) | < 0.05 | 6.50 | 1.32–31.91 |
| aCL IgA (-) n = 42 | 3 (7.1%) |
anti-β2GPI—anti-β2-glycoprotein-I; aCL—anticardiolipin; IgG—immunoglobulin G; IgA—immunoglobulin A; LA—lupus anticoagulant; OR—odds ratio; CI—confidence interval
Table 7. Association of antiphospholipid antibodies with grand mal seizures.
| Grand mal seizures | Fisher exact test, p value | OR | 95% CI | |
|---|---|---|---|---|
| anti-β2GPI IgG (+) n = 8 | 3 (37.5%) | < 0.05 | 9.20 | 1.45–58.36 |
| anti-β2GPI IgG (-) n = 49 | 3 (6.1%) | |||
| aCL IgA (+) n = 15 | 4 (26.7%) | < 0.05 | 7.27 | 1.17–45.06 |
| aCL IgA (-) n = 42 | 2 (4.8%) |
anti-β2GPI—anti-β2-glycoprotein-I; aCL—anticardiolipin; IgG—immunoglobulin G; IgA—immunoglobulin A; OR—odds ratio; CI—confidence interval
Discussion
Here, we characterized the prevalence, disease activity, onset and aPL profiles of patients with NPSLE. Most importantly, we demonstrate, to our knowledge for the first time, that specific neuropsychiatric manifestations in SLE, namely intractable headaches, ischemic stroke and seizures are linked to the presence of anti-β2GPI. Notably, anti-β2GPI were the only or the most predictive antibodies for all of these manifestations.
The overall prevalence of NPSLE in our patients was high and consistent with data from studies using the same ACR nomenclature [18–23]. Ainiala as well as Hanly and co-workers proposed stricter criteria excluding manifestations which relationship with SLE pathology is controversial [24–26]. Here, we decided to include all ACR neuropsychiatric manifestations, to further test their relationship with objective clinical and laboratory findings including aPL.
We observed, consistent with previous findings, that the onset of neuropsychiatric manifestations preceded SLE diagnosis in the majority of cases, and at the same time it took many years for the other patients to develop NPSLE [27, 28]. This indicates the need to educate clinicians to consider SLE in patients who show neurologic manifestations, but also to continuously monitor SLE patients for symptoms of NPSLE. It may be speculated that immunosuppressive therapy, which is often started once the diagnosis of SLE is established, might delay progression to NPSLE, resulting in its delayed onset.
We did not find any differences in disease activity and corticosteroid use comparing NPSLE and non-NPSLE patients. Analyzing individual NPSLE manifestations separately, only CVD was linked to a higher SLE activity. We also did not find any differences in the frequency of ACR criteria for SLE between NPSLE and non-NPSLE patients, including hematologic criteria, which were previously reported to be related to NPSLE [27, 29].
Positivity to aCL appeared to be stable in patients with APS, and almost all patients with pre-existing APS appeared to be positive to aCL. Frequency of APS did not differ between NPSLE and non-NPSLE patients.
In our patients, NPSLE was not related to differences in aPL expression, and this is in line with previous studies that used the same diagnostic criteria we did [20, 30].
Anti-β2GPI were demonstrated in the 1990s to play a crucial role in APS [7, 31]. In contrast, the role of anti-β2GPI in SLE including NPSLE is less well characterized and understood [32]. As of now, there are only a few studies that have investigated anti-β2GPI in NPSLE, and all of them failed to show that anti-β2GPI are associated with any of the individual NPSLE manifestations [19, 25, 33–37]. Importantly, we found specific NP manifestations of SLE, namely intractable headaches, ischemic stroke and seizures to be linked to the presence of anti-β2GPI and other aPL.
The attribution of headaches to NPSLE was previously questioned and remains controversial [26, 38–40]. In accordance with most but not all previous reports [36, 40–42] we did not find a link between the occurrence of headaches and SLE activity. Interestingly, we found that headaches classified according to the ACR nomenclature as intractable, nonspecific were present in 18% of our patients, and they were associated with anti-β2GPI IgM. This suggests that intractable headaches can be a manifestation of NPSLE and that anti-β2GPI IgM may be useful in their diagnostic workup. In contrast to a previous report [43], we did not observe an association of aPL with migraine, which confirms other recent studies, including prospective ones [44, 45]. The most likely reason for the fact that the majority of previous studies did not find headaches to be linked to aPL is that these studies only looked at all headaches as a group or at migraine but not other subtypes of headache [36, 40, 41, 46, 47]. Interestingly, five APS patients with intractable headaches were previously reported to benefit from anticoagulation therapy and to relapse after its cessation [48].
In SLE, a link between CVD and aPL was previously confirmed for LA [19, 25, 49, 50] and IgG [22, 49–51] as well as IgM class aCL [22]. We found ischemic stroke to be associated with anti-β2GPI, which is in line with a previous study in children with SLE and APS [52]. Stroke is the second most frequent vascular thrombosis, after deep vein thrombosis, and it is the most frequent presentation of arterial thrombosis in APS [53, 54]. Seizures are a well known manifestation of NPSLE, included also in the list of ACR criteria for SLE [10]. Epilepsy is also associated with APS, and as many as 10% of patients with primary APS develop seizures [55]. Patients with epilepsy are more frequently positive for aCL and anti-β2GPI [56–58].
In SLE, an association between seizures and IgG [22, 50, 59, 60] and IgM class aCL [22] was reported in some studies but not others [19, 33, 49, 51]. For LA, Herranz and co-workers found 44% of SLE patients with epilepsy to be positive, as compared to 43% in our study [60]. We failed to find any study that previously investigated IgA aCL in NPSLE. Interestingly, there is one study that tested and confirmed an association between seizures and IgA aCL in APS, and its results are consistent with our results in NPSLE, indicating a possible role of IgA aCL in the pathogenesis of seizures and confirming the importance of IgA isotype testing [58].
Strengths and limitations
Our study has several strengths and limitations. Most previous studies on aPL in NPSLE relied on single aPL measurements whereas we performed two independent measurements at least 12 weeks apart, in accordance with current diagnostic laboratory criteria for APS [9]. Single antibody measurements may result in decreased specificity, leading to classify as positive also transiently positive results [61]. ELISA assays for aPL and anti-β2GPI in particular are still inadequately standardized [62, 63], and the use of local cut-off values is recommended [17, 64]. These cut-off values should be based on control values from age matched subjects, as children and elderly may express higher serum levels of aPL [65, 66]. We employed this approach to minimize this known problem.
The limitations of our study include the limited number of SLE patients. Further studies on larger samples of SLE patients are required to draw firm conclusions on the associations of autoantibodies with specific NPSLE manifestations, especially to investigate rare NPSLE disorders. Secondly, we did not reassess the expression of aPL and neuropsychiatric signs and symptoms after many years, which is needed to learn about the long term influence of persistent aPL. Finally, cognitive impairment was, differently than recommended by ACR criteria for NPSLE, screened with MMSE and clinical history only, instead of a battery of neuropsychological tests, which may have resulted in lower sensitivity.
Conclusions
In summary, our results confirm a high prevalence of NPSLE in SLE patients and show that neuropsychiatric manifestations often precede SLE diagnosis. Most importantly, we found a link between anti-β2GPI and non-specific, intractable headaches, ischemic stroke and seizures, and these autoantibodies show a better predictive value than other aPL such as aCL and LA. Further studies on the role and relevance of persistent anti-β2GPI in headaches, ischemic stroke, and seizures are needed and may elucidate pathogenic effects of anti-β2GPI as possible, novel targets for more effective therapies of NPSLE.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by the grant from the Medical University of Lodz nr. 503/1-152-01/503-01. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sibley JT, Olszynski WP, Decoteau WE, Sundaram MB. The incidence and prognosis of central nervous system disease in systemic lupus erythematosus. J Rheumatol 1992;19(1):47–52. [PubMed] [Google Scholar]
- 2. Mevorach D, Elkon KB. Neuropsychiatric lupus: getting beyond the barrier of the brain. Lupus 1996;5(3):173–174. [DOI] [PubMed] [Google Scholar]
- 3. Ward MM, Pyun E, Studenski S. Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus. Arch Intern Med. 1996;156:1337–1344. [PubMed] [Google Scholar]
- 4. Harris EN, Gharavi AE, Asherson RA, Boey ML, Hughes GR. Cerebral infarction in systemic lupus: association with anticardiolipin antibodies. Clin Exp Rheumatol. 1984;2:47–51. [PubMed] [Google Scholar]
- 5. Asherson RA, Khamashta MA, Gil A, Vazquez JJ, Chan O, Baguley E, et al. Cerebrovascular disease and antiphospholipid antibodies in systemic lupus erythematosus, lupus-like disease, and the primary antiphospholipid syndrome. Am J Med. 1989;86:391–399. [DOI] [PubMed] [Google Scholar]
- 6. Chapman J, Cohen-Armon M, Shoenfeld Y, Korczyn AD. Antiphospholipid antibodies permeabilize and depolarize brain synaptoneurosomes. Lupus. 1999;8:127–133. [DOI] [PubMed] [Google Scholar]
- 7. Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJ, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–1547. [DOI] [PubMed] [Google Scholar]
- 8. Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 10. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725 [DOI] [PubMed] [Google Scholar]
- 11. Liang MH, Socher SA, Roberts WN, Esdaile JM. Measurement of systemic lupus erythematosus activity in clinical research. Arthritis Rheum. 1988;31:817–825. [DOI] [PubMed] [Google Scholar]
- 12. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 13. Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160. [DOI] [PubMed] [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15. Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum. 1997;40:1601–1611. [DOI] [PubMed] [Google Scholar]
- 16. Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost. 1995;74:1185–1190. [PubMed] [Google Scholar]
- 17. Tincani A, Allegri F, Balestrieri G, Reber G, Sanmarco M, Meroni P, et al. Minimal requirements for antiphospholipid antibodies ELISAs proposed by the European Forum on antiphospholipid antibodies. Thromb Res. 2004;114:553–558. [DOI] [PubMed] [Google Scholar]
- 18. Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–500. [DOI] [PubMed] [Google Scholar]
- 19. Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58:1214–1220. [DOI] [PubMed] [Google Scholar]
- 20. Afeltra A, Garzia P, Mitterhofer AP, Vadacca M, Galluzzo S, Del Porto F, et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology. 2003;61:108–110. [DOI] [PubMed] [Google Scholar]
- 21. Robert M, Sunitha R, Thulaseedharan NK. Neuropsychiatric manifestations systemic lupus erythematosus: a study from South India. Neurol India. 2006;54:75–77. [DOI] [PubMed] [Google Scholar]
- 22. Sanna G, Bertolaccini ML, Cuadrado MJ, Laing H, Khamashta MA, Mathieu A, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30:985–992. [PubMed] [Google Scholar]
- 23. Unterman A, Nolte JE, Boaz M, Abady M, Shoenfeld Y, Zandman-Goddard G. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum. 2011;41:1–11. 10.1016/j.semarthrit.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 24. Hanly JG, Urowitz MB, Sanchez-Guerrero J, Bae SC, Gordon C, Wallace DJ, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56:265–273. [DOI] [PubMed] [Google Scholar]
- 25. Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Clarke A, et al. Autoantibodies as biomarkers for the prediction of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1726–1732. 10.1136/ard.2010.148502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45:419–423. [DOI] [PubMed] [Google Scholar]
- 27. Feinglass EJ, Arnett FC, Dorsch CA, Zizic TM, Stevens MB. Neuropsychiatric manifestations of systemic lupus erythematosus: diagnosis, clinical spectrum, and relationship to other features of the disease. Medicine (Baltimore). 1976;55:323–339. [DOI] [PubMed] [Google Scholar]
- 28. Hawro T, Bogucki A, Sysa-Jedrzejowska A, Bogaczewicz J, Wozniacka A. [Neurological disorders in systemic lupus erythematosus patients]. Pol Merkur Lekarski. 2009;26:43–48. [PubMed] [Google Scholar]
- 29. Chiewthanakul P, Sawanyawisuth K, Foocharoen C, Tiamkao S. Clinical features and predictive factors in neuropsychiatric lupus. Asian Pac J Allergy Immunol. 2012;30:55–60. [PubMed] [Google Scholar]
- 30. Singh S, Gupta MK, Ahluwalia J, Singh P, Malhi P. Neuropsychiatric manifestations and antiphospholipid antibodies in pediatric onset lupus: 14 years of experience from a tertiary center of North India. Rheumatol Int. 2009;29:1455–1461. 10.1007/s00296-009-0887-6 [DOI] [PubMed] [Google Scholar]
- 31. Willis R, Pierangeli SS. Anti-beta2-glycoprotein I antibodies. Ann N Y Acad Sci. 2013;1285:44–58. 10.1111/nyas.12080 [DOI] [PubMed] [Google Scholar]
- 32. Sciascia S, Bertolaccini ML, Roccatello D, Khamashta MA, Sanna G. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol. 2014;261:1706–1714. 10.1007/s00415-014-7406-8 [DOI] [PubMed] [Google Scholar]
- 33. Hanly JG, Urowitz MB, Su L, Gordon C, Bae SC, Sanchez-Guerrero J, et al. Seizure disorders in systemic lupus erythematosus results from an international, prospective, inception cohort study. Ann Rheum Dis. 2012;71:1502–1509. 10.1136/annrheumdis-2011-201089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanly JG, Urowitz MB, Siannis F, Farewell V, Gordon C, Bae SC, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58:843–853. 10.1002/art.23218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kozora E, Filley CM, Zhang L, Brown MS, Miller DE, Arciniegas DB, et al. Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus. 2012;21:402–411. 10.1177/0961203311429116 [DOI] [PubMed] [Google Scholar]
- 36. Hanly JG, Urowitz MB, O'Keeffe AG, Gordon C, Bae SC, Sanchez-Guerrero J, et al. Headache in systemic lupus erythematosus: results from a prospective, international inception cohort study. Arthritis Rheum. 2013;65:2887–2897. 10.1002/art.38106 [DOI] [PubMed] [Google Scholar]
- 37. Avcin T, Benseler SM, Tyrrell PN, Cucnik S, Silverman ED. A followup study of antiphospholipid antibodies and associated neuropsychiatric manifestations in 137 children with systemic lupus erythematosus. Arthritis Rheum. 2008;59:206–213. 10.1002/art.23334 [DOI] [PubMed] [Google Scholar]
- 38. Mitsikostas DD, Sfikakis PP, Goadsby PJ. A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth. Brain 2004;127:1200–1209. [DOI] [PubMed] [Google Scholar]
- 39. Rasmussen BK. Epidemiology of headache. Cephalalgia. 2001;21:774–777. [DOI] [PubMed] [Google Scholar]
- 40. Sfikakis PP, Mitsikostas DD, Manoussakis MN, Foukaneli D, Moutsopoulos HM. Headache in systemic lupus erythematosus: a controlled study. Br J Rheumatol. 1998;37:300–303. [DOI] [PubMed] [Google Scholar]
- 41. Fernandez-Nebro A, Palacios-Munoz R, Gordillo J, Abarca-Costalago M, De Haro-Liger M, Rodriguez-Andreu J, et al. Chronic or recurrent headache in patients with systemic lupus erythematosus: a case control study. Lupus. 1999;8):151–156. [DOI] [PubMed] [Google Scholar]
- 42. Amit M, Molad Y, Levy O, Wysenbeek AJ. Headache in systemic lupus erythematosus and its relation to other disease manifestations. Clin Exp Rheumatol. 1999;17:467–470. [PubMed] [Google Scholar]
- 43. Cavestro C, Micca G, Molinari F, Bazzan M, C DIP, Aloi R, et al. Migraineurs show a high prevalence of antiphospholipid antibodies. J Thromb Haemost. 2011;9:1350–1354. 10.1111/j.1538-7836.2011.04348.x [DOI] [PubMed] [Google Scholar]
- 44. Sanna G, Bertolaccini ML, Cuadrado MJ, Khamashta MA, Hughes GR. Central nervous system involvement in the antiphospholipid (Hughes) syndrome. Rheumatology (Oxford). 2003;42:200–213. [DOI] [PubMed] [Google Scholar]
- 45. Tietjen GE, Day M, Norris L, Aurora S, Halvorsen A, Schultz LR, et al. Role of anticardiolipin antibodies in young persons with migraine and transient focal neurologic events: a prospective study. Neurology. 1998;50:1433–1440. [DOI] [PubMed] [Google Scholar]
- 46. Markus HS, Hopkinson N. Migraine and headache in systemic lupus erythematosus and their relationship with antibodies against phospholipids. J Neurol. 1992;239:39–42. [DOI] [PubMed] [Google Scholar]
- 47. Montalban J, Cervera R, Font J, Ordi J, Vianna J, Haga HJ, et al. Lack of association between anticardiolipin antibodies and migraine in systemic lupus erythematosus. Neurology. 1992;42:681–682. [DOI] [PubMed] [Google Scholar]
- 48. Cuadrado MJ, Khamashta MA, D'Cruz D, Hughes GR. Migraine in Hughes syndrome—heparin as a therapeutic trial? QJM. 2001;94:114–115. [DOI] [PubMed] [Google Scholar]
- 49. Mok CC, Lau CS, Wong RW. Neuropsychiatric manifestations and their clinical associations in southern Chinese patients with systemic lupus erythematosus. J Rheumatol. 2001;28:766–771. [PubMed] [Google Scholar]
- 50. Mikdashi J, Handwerger B. Predictors of neuropsychiatric damage in systemic lupus erythematosus: data from the Maryland lupus cohort. Rheumatology (Oxford). 2004;43:1555–1560. [DOI] [PubMed] [Google Scholar]
- 51. Sabbadini MG, Manfredi AA, Bozzolo E, Ferrario L, Rugarli C, Scorza R, et al. Central nervous system involvement in systemic lupus erythematosus patients without overt neuropsychiatric manifestations. Lupus. 1999;8:11–19. [DOI] [PubMed] [Google Scholar]
- 52. von Scheven E, Glidden DV, Elder ME. Anti-beta2-glycoprotein I antibodies in pediatric systemic lupus erythematosus and antiphospholipid syndrome. Arthritis Rheum. 2002;47:414–420. [DOI] [PubMed] [Google Scholar]
- 53. Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. [DOI] [PubMed] [Google Scholar]
- 54. Sastre-Garriga J, Montalban X. APS and the brain. Lupus. 2003;12:877–882. [DOI] [PubMed] [Google Scholar]
- 55. de Carvalho JF, Pasoto SG, Appenzeller S. Seizures in primary antiphospholipid syndrome: the relevance of smoking to stroke. Clin Dev Immunol. 2012;2012:981519 10.1155/2012/981519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peltola JT, Haapala A, Isojarvi JI, Auvinen A, Palmio J, Latvala K, et al. Antiphospholipid and antinuclear antibodies in patients with epilepsy or new-onset seizure disorders. Am J Med. 2000;109:712–717. [DOI] [PubMed] [Google Scholar]
- 57. Verrot D, San-Marco M, Dravet C, Genton P, Disdier P, Bolla G, et al. Prevalence and signification of antinuclear and anticardiolipin antibodies in patients with epilepsy. Am J Med. 1997;103:33–37. [DOI] [PubMed] [Google Scholar]
- 58. Lakos G, Kiss E, Regeczy N, Tarjan P, Soltesz P, Zeher M, et al. Isotype distribution and clinical relevance of anti-beta2-glycoprotein I (beta2-GPI) antibodies: importance of IgA isotype. Clin Exp Immunol. 1999;117:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mikdashi J, Krumholz A, Handwerger B. Factors at diagnosis predict subsequent occurrence of seizures in systemic lupus erythematosus. Neurology. 2005;64:2102–2107. [DOI] [PubMed] [Google Scholar]
- 60. Herranz MT, Rivier G, Khamashta MA, Blaser KU, Hughes GR. Association between antiphospholipid antibodies and epilepsy in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:568–571. [DOI] [PubMed] [Google Scholar]
- 61. Martinez-Berriotxoa A, Ruiz-Irastorza G, Egurbide MV, Garmendia M, Gabriel Erdozain J, Villar I, et al. Transiently positive anticardiolipin antibodies and risk of thrombosis in patients with systemic lupus erythematosus. Lupus. 2007;16:810–816. [DOI] [PubMed] [Google Scholar]
- 62. Lakos G, Favaloro EJ, Harris EN, Meroni PL, Tincani A, Wong RC, et al. International consensus guidelines on anticardiolipin and anti-beta2-glycoprotein I testing: report from the 13th International Congress on Antiphospholipid Antibodies. Arthritis Rheum. 2012;64:1–10. 10.1002/art.33349 [DOI] [PubMed] [Google Scholar]
- 63. Pierangeli SS, Favaloro EJ, Lakos G, Meroni PL, Tincani A, Wong RC, et al. Standards and reference materials for the anticardiolipin and anti-beta2glycoprotein I assays: a report of recommendations from the APL Task Force at the 13th International Congress on Antiphospholipid Antibodies. Clin Chim Acta. 2012;413:358–360. 10.1016/j.cca.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 64. Tincani A, Filippini M, Scarsi M, Galli M, Meroni PL. European attempts for the standardisation of the antiphospholipid antibodies. Lupus. 2009;18:913–919. 10.1177/0961203309106919 [DOI] [PubMed] [Google Scholar]
- 65. Avcin T, Cimaz R, Meroni PL. Recent advances in antiphospholipid antibodies and antiphospholipid syndromes in pediatric populations. Lupus. 2002;11:4–10. [DOI] [PubMed] [Google Scholar]
- 66. Piette JC, Cacoub P. Antiphospholipid syndrome in the elderly: caution. Circulation. 1998;97:2195–2196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.