Summary
We examined whether men with anabolic steroid induced hypogonadism (ASIH) seeking testosterone supplementation therapy (TST) regretted their decision to use anabolic androgenic steroids (AAS) and what their reasons were for this regret. An anonymous, prospective survey was distributed to 382 men seeking follow-up treatment for hypogonadism. Prior AAS use was confirmed by self-report and men were categorised based upon whether they regretted (R) or did not regret (NR) their use of AAS. The average patient age was 40±0.9 years (n=79) and 15.2% expressed regret over AAS use. No demographic differences were identified between those who regretted AAS use (n=12) and those who did not (n=67). Regret was not related to ASIH diagnosis or to AAS-related side effects like increased aggression, mood disorders, erectile dysfunction, acne, fluid retention or dyslipidemia. Those who regretted AAS use were significantly more likely to have not comprehended the negative impact on future fertility (p<0.030). Actual fertility issues were comparable in men who regretted AAS use (16.7%) and those who did not (13%). A total of 15.2% of men regretted using AAS. A lack of awareness regarding the negative long-term effects on fertility was the primary factor related to regret of AAS use in men with ASIH.
Keywords: Anabolic steroids, hypogonadism, infertility, regret, testosterone
INTRODUCTION
The isolation of the testosterone molecule has resulted in the development of multiple synthetic derivatives. Classified as anabolic-androgenic steroids (AAS), the desirable effects of these products included increased muscle mass and strength along with decreased recovery times and improved healing (Hough, 1990). Amongst the general population, an estimated 3 million people have reported using AAS (Maravelias et al., 2005). Prior exposure to AAS has recently been postulated to contribute to profound hypogonadism (serum testosterone <50 ng/ml) as well as anabolic steroid induced hypogonadism (ASIH) (Coward et al., 2013). Indeed, a preliminary study in 1990 identified inhibited levels of luteinising (LH) and follicle stimulating hormone (FSH) for 1–3 years following last documented use of AAS (Jarow & Lipshultz, 1990).
While the duration of gonadotrophin suppression in response to AAS is unknown, it is likely related to the types of AAS used as well as the duration (Tan & Scally, 2009). While AAS contributes to effective muscle building by diversion of nitrogenic compounds to lean body mass (Sjoqvist et al., 2008), not all AAS compounds function equally with regard to the ratio of anabolic to androgenic effects (Kicman, 2008). In high doses, all AAS have the potential to virilise (Shahidi, 2001) with other, adverse effects being similarly both type- and dose-dependent (Landry & Primos, 1990).
Of the numerous AAS side effects known, fluid retention, decreased testicular size, acne, aggressiveness and negative effects on sperm counts, cholesterol and hematocrit have been noted to be the most common (Coward et al., 2013). Interestingly, the decreased sperm counts, motility and altered morphology (Dohle et al., 2003) associated with AAS are typically transient, with evidence that semen parameters can spontaneously recover within 4–12 months after discontinuation (Knuth et al., 1989; Turek et al., 1995). Recovery may be delayed due to testicular atrophy and dysfunctional spermatogenesis (Schurmeyer et al., 1984; Turek et al., 1995; Gazvani et al., 1997; Menon, 2003) along with germ cell apoptosis (Moretti et al., 2007; Shokri et al., 2010).
In other studies examining illicit substance use, decisions made in youth and adolescence tends to lead to regret later in adult life (Byrnes, 2002). Since usage of these agents (such as AAS) may be ascribed to impulsiveness, and given that a person's values can change over time leading to regret, it is tempting to speculate that prior AAS use could lead to regret as individuals' priorities and values change over time (Igra & Irwin, 1995). Indeed, regret has previously been shown to play a role in the decision to alter substance abuse patterns (Blume & Schmaling, 1998) and has been associated with less alcohol and marijuana use in young adults (Stoddard et al., 2012) suggesting that men with prior AAS use are more likely to seek medical assistance for ASIH given their familiarity with the symptoms and long-term side effects. Furthermore, previous studies (Sanford, 2012; Van den Broek et al., 2013;) have found spousal and partner feelings and anxiety to play a role in regret so we hypothesised that regretting AAS use may be associated with a lack of spousal awareness that would then impact a couples relationship. We therefore sought to determine whether patients presenting with ASIH regretted their decision to use AAS and what factors drove this regret.
MATERIALS AND METHODS
After Institutional Review Board (IRB) approval, a self-administered anonymous survey was distributed to male patients seeking treatment for hypogonadism in a tertiary, academic urology clinic. The survey was self-administered, confidential and anonymous. Hypogonadism was diagnosed with serum total testosterone values combined with patient history of hypogonadal symptoms and treatments included injections, gels or pellets with modality of TST not considered with regards to responses. Patients seeking treatment for infertility were excluded and surveys were also excluded from analysis if they contained incomplete or conflicting responses. Follow-up visits for hypogonadism and TST were used when administering the survey. Patients undergoing initial consultations were excluded since given the anonymous nature of the survey, it was impossible to know whether these men were actually hypogonadal and if they were treated or not.
The survey included basic patient demographics such as age, sexual orientation, marital status, level of education and income. Those men who self-reported AAS use were asked to complete a follow-up second survey. This second portion gathered data that determined characteristics of those patients who engaged in AAS use. Regret was determined using a question in this second part of the survey that stated “Do you regret using anabolic steroids” and could have been answered either Yes or No. In no survey was this question left blank. The effect of AAS use on relationship was addressed by the question “Does your spouse/significant other know about your previous or current steroid use”?. The answer options were: (1) Yes – Has not affected relationship, (2) Yes – Has affected relationship, (3) No – Has not affected relationship, (4) No – Has affected relationship and, (5) Not applicable. No patient selected option 5 as a response. To further assess impact of AAS use the following question was asked: When you first decided to use anabolic steroids, did you understand the potential long-term effect it could have on your (a) natural testosterone production (Yes/No) (b) fertility (Yes/No). A follow up question asked: “Have anabolic steroids affected your: (a) Erections (Yes/No), (b) Fertility (Yes/No)”. The majority of the questions produced single-answer responses via multiple choice or yes/no answers.
Data were analysed using Student's t-test for scalar variables and Fisher's exact test for categorical variables. Correlation analysis between variables was performed using Spearman's rank correlation, and odds ratios were calculated when appropriate. Analysis was performed using Microsoft Excel (Microsoft, Redmond, WA) and GraphPad Prism 6 software (GraphPad Software Inc.; La Jolla, CA) with a p<0.05 considered statistically significant. All values were reported as means±SEM, unless otherwise noted.
RESULTS
Results from an anonymous, prospective survey distributed to hypogonadal men being treated with TST at a high-volume academic Urology clinic were assessed. A total of 79 men stated that they had previously used AAS (Table 1). From these men, 84.8% (n=67) stated that they had no regret (NR) while 15.2% (n=12) expressed regret (R) about their AAS use (Table 1). A total of 382 surveys were distributed with 20.8% of patients reported prior AAS exposure. No statistical differences were identified between mean age (p=0.070), height (p=0.450), weight (p=0.620) and BMI (p=0.300). The majority of the men were heterosexual and married. Men who regretted use were more likely to have no children (58.3%) while men who were not regretful of their prior AAS use were more likely to have 1–2 children (58.2%). Both groups were educated with a sizable percentage having current income levels of >$150,000 (Table 1).
Table 1.
Comparison of the demographics between patients who either regret their decision to use AAS (n=12) or are unaffected (n=68) by prior AAS use. Values within the columns are depicted as mean±SEM with the total number of patients for each section shown in brackets.
| No Regret of AAS use | Regret of AAS use | |
|---|---|---|
| Percentage of survey respondents who noted previous AAS use (n=79) | 84.8% (n=67/79) | 15.2% (n=12/79) |
| Mean Age (Years) | 41.1±1.0 (n=67) | 36.4±1.9 (n=12) |
| Height (inches) | 70.8±0.3 (n=67) | 71.4±0.6 (n=12) |
| Weight (Lbs) | 213.1±3.5 (n=65) | 208.3±10.2 (n=12) |
| Mean BMI (kg/m2) | 29.9±0.5 (n=65) | 28.7±1.3 (n=12) |
| Sexual Orientation: | ||
| Heterosexual (n) | 98.5% (n=66/67) | 100% (n=12) |
| Homosexual (n) | 1.5% (n=1/67) | 0% |
| Marital Status: | ||
| Single (n) | 26.9% (n=18/67) | 41.7% (n=5/12) |
| Married (n) | 55.2% (n=37/67) | 41.7% (n=5/12) |
| Divorced (n) | 10.4% (n=7/67) | 16.7% (n=2/12) |
| Cohabitating (n) | 7.5% (n=5/67) | 0% |
| Number of Children: | ||
| 0 | 35.8% (n=24/67) | 58.3% (n=7/12) |
| 1–2 | 58.2% (n=39/67) | 33.3% (n=4/12) |
| 3–5 | 4.5% (n=3/67) | 0% |
| 6 or more | 1.5% (n=1/67) | 8.3% (n=1/12) |
| Highest Level of Education: | ||
| Grade School | 1.5% (n=1/66) | 0% |
| High School | 13.6% (n=9/66) | 0% |
| Some College/University | 33.3% (n=22/66) | 50% (n=6/12) |
| College/University | 33.3% (n=22/66) | 41.7% (n=5/12) |
| Graduate Level | 18.2% (n=12/66) | 8.3% (n=1/12) |
| Current Income: | ||
| <25,000 | 6.1% (n=4/66) | 9.1% (n=1/11) |
| 25,000–50,000 | 1.5% (n=1/66) | 9.1% (n=1/11) |
| 50,001–75,000 | 24.2% (n=16/66) | 36.4% (n=4/11) |
| 75,001–100,000 | 18.2% (n=12/66) | 9.1% (n=1/11) |
| 100,001–150,000 | 21.2% (n=14/66) | 0% |
| 150,001–200,000 | 13.6% (n=9/66) | 27.3% (n=3/11) |
| >200,000 | 15.2% (n=10/66) | 9.1% (n=1/11) |
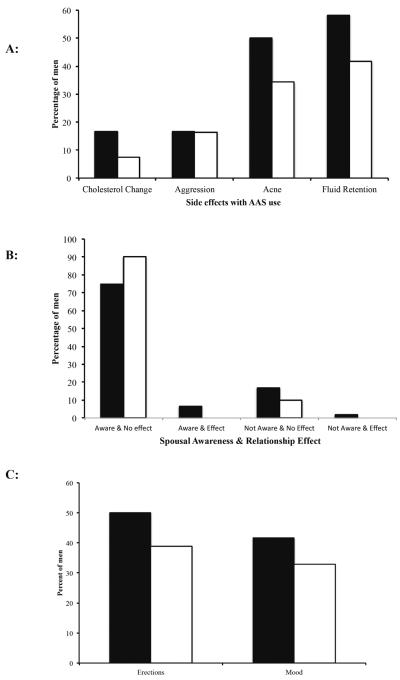
In order to determine whether side effects from AAS use contributed to an individual's regret, we asked men whether they experienced negative impacts on their cholesterol levels or noted changes in their aggression, acne and levels of fluid retention (Figure 1A). In each case, the differences between men who did and did not have regret were not statistically significant, suggesting these side effects did not contribute to their feelings (Figure 1A). Since previous studies (Sanford, 2012; Van den Broek et al., 2013) found partner effects and anxiety to play a role in regret, we hypothesised that regretting AAS use may be associated with a lack of spousal awareness that would then impact a couples relationship (Figure 1B). Surprisingly, most partners were aware of the AAS use, and it had no effect on a couple's relationship (Figure 1B).
Figure 1.
AAS use and regret. (A) Men who reported regret of AAS use experienced similar side-effects for cholesterol (p=0.29), aggression (p=1), acne (p=0.34) and fluid retention (p=0.35) to those who did not regret AAS use. (B) No differences in regret were found in situations where men had spouses who were aware of their use of AAS with no relationship effects (Column 1; p=0.44). No statistical differences were found between men who regretted prior AAS use and those who did not in the following situations: (Column 2) Spouse was aware of AAS use and this had effects on the relationship (Aware & Effect, p=1); (Column 3) Spouse was not aware of AAS use and this had no effects on the relationship (Not Aware & No Effect, p=1) and; (Column 4) Spouse was not aware and this effected the relationship (p=1). (C) Regret had no effect on erections and mood in men who reported AAS use. Decreased frequency and quality of erections (p=0.54) as well as poor mood (p=0.74) affected men who regretted AAS use to a similar degree as those who did not (Black bars=men with regret; white bars=men with NO regret).
Erectile dysfunction has previously been shown to cause a high source of bother and regret for men post-prostatectomy due to shame, embarrassment, and a reduction in general life happiness (Nelson et al., 2010). We thus sought to determine whether men regretting AAS use had erectile dysfunction and whether it affected their mood. Interestingly, a total of 40.5% (n=32/79) of men with prior AAS use indicated having erectile dysfunction. A similar number of people in those men with R (50%, n=6/12) and those with NR (38.8%, n=26/67) experienced erectile dysfunction (p=0.537, Figure 1C). Mood was similarly affected (R=41.7%, n=5/12; NR=32.8%, n=22/67).
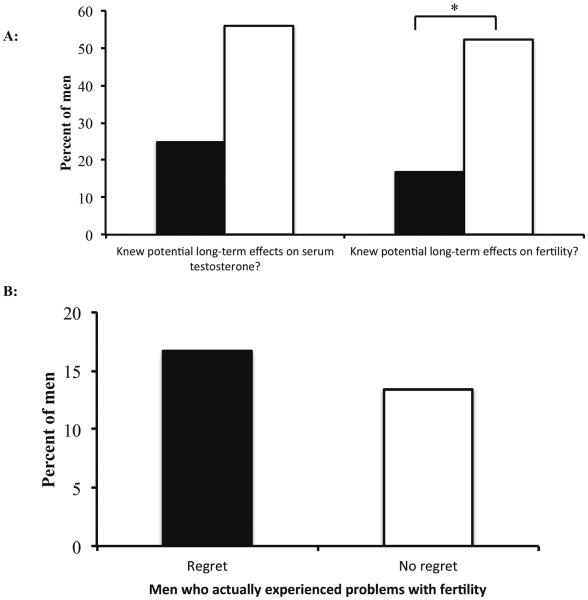
Lastly, we evaluated whether perception of future long-term effects on testosterone and fertility contributed to feelings of regret (Figure 2). Of those men with regret, 25% (n=3/12) stated that they did not understand the potential future impact of AAS use on their serum testosterone levels (Figure 2A). Compared with the 56.7% of (n=38/67) men who did not experience regret, this value approached statistical significance (p=0.06). However, men with regret were significantly less likely to have understood the potential long-term, negative effects on fertility from AAS use (R=16.6% understood, n=2/12 vs. NR=52.2% understood, n=35/67; p=0.029). Interestingly, the amount of men who actually experienced fertility difficulties was similar between the subgroups (R=16.7%, n=2/12; NR=13.4%, n=9/67)(Figure 2B). This suggests that the perception of future long-term fertility problems is more important in regretting AAS use than actual difficulties with conception.
Figure 2.
Regret, AAS and the long-term effects on serum testosterone levels and fertility. (A) Men who regretted their AAS use (black bars) did not understand the potential long-term effects on their serum testosterone levels; however, this relationship did not reach statistical significance (p=0.06). Patients who regretted their prior AAS use were less aware of the potential long-term effects on future fertility (*, p=0.029) compared to those that did not regret AAS use (white bars). (B) Men with regret were equally as likely to experience actual problems with fertility as those without regret. These results suggest that patient perceptions had more impact on regret than actual events (Black bars=men with regret; white bars=men with NO regret).
DISCUSSION
AAS use in hypogonadal men seeking TST is common (Coward et al., 2013). Since AAS use has been suggested to be addictive, and other addictive substances such as marijuana are associated with regret following use, we sought to elucidate whether hypogonadal patients with a history of AAS regretted their decision to use AAS and what factors drove these feelings (Buckley et al., 1988; Kanayama et al., 2001; Evans, 2004; Parkinson & Evans, 2006; Cohen et al., 2007). Most men who understood the effects that AAS would have on their fertility did not experience regret; however, those who regretted AAS use were significantly more likely to not have comprehended the negative impact on fertility. In contrast, the rates of actual fertility issues were equal in men who regretted AAS use and those who did not.
Given the illicit nature of AAS, information regarding their usage patterns is difficult to identify in the context of the medical literature. The only study to date that has examined feelings of regret with regards to AAS use was conducted in 1995; however, it was focused on professional athletes who used AAS for competitive reasons (Silvester, 1995). In that study by Silvester et al. (1995) from the cohort of 22 competitive shot-put/discus athletes studied, only 9.l% (n=2) noted that they regretted their prior AAS use. Furthermore, a total of 86% believed there were no long-term physical health problems, with only one patient (4.5%) stating that AAS caused a permanent reduction in the size of his testicles (Silvester, 1995). Indeed, the majority of the patients in the Silvester study (1995) perceived their health problems to be a nuisance, rather than a severe or debilitating condition. While the population in the current study is different, the frequency at which patients reported regret is similar.
When considering the long-term impact of AAS on health, the current study examined whether the well-known side-effects of altered cholesterol values and increased levels of aggression, acne, and fluid retention were a reason for regret (Figure 1). While no differences were found, a possible limitation to our anonymous survey approach was that it was not possible to ascertain the exact medical history of our study participants. As such, we relied on patient self-report rather than actual medical records. Furthermore, given the anonymous nature of the survey, we were unable to ascertain the reliability of the survey or to correlate serum levels to patient responses.
Given that AAS users have previously been shown to experience poor parental relationships (Skarberg & Engstrom, 2007), and partner/spousal anxiety and depression results in emotional distress (Van den Broek et al., 2013), we sought to analyse whether spousal awareness and the resultant relationship consequences had any influence on AAS-induced regret. In our population, the majority of spouses and partners were aware of their partners AAS use; with most mentioning that it had no effect on their relationship (Figure 1). Similarly, mood affective disorders have been recognised as a complication of AAS use with case reports describing everything from unspecified mood disturbances (Lindstrom et al., 1990) as well as hypomania, mania, irritability, and feelings of power and invincibility (Rashid et al., 2007). In our study population, spousal communication does not appear to be an issue, and while we were unable to capture any psychiatric disorders, mood disturbances were similar between men with and without regret.
Unfortunately, since most men begin AAS at a young age and are presumed to obtain the medication from illicit sources, they are not educated regarding the possible negative outcomes that come with their use. AAS suppress LH and FSH leading to acute hypogonadotropic hypogonadism (Coward et al., 2013). In a subset of men, this anabolicsteroid induced hypogonadism (ASIH) can result in long-term, or permanent inhibition of their hypothalamic-pituitary axis (Jarow & Lipshultz, 1990; Pirola et al., 2010; Boregowda et al., 2011). Furthermore, the exogenously elevated serum testosterone levels obtained from AAS results in oligospermia and azoospermia (Dohle et al., 2003). A primary finding of our study was that regretful prior AAS users did not understand the possibility that ASIH could result and future fertility could be hampered (Figure 4). Indeed those men with regret were less likely to have understood the potential negative impacts on both long-term testosterone levels (p=0.06) and fertility (p=0.029) than men without regret.
The findings in the current study are important when dealing with hypogonadal patients with ASIH seeking TST. Since a proportion of patients may be hypogonadal due to ASIH (Coward et al., 2013), asking about AAS is important. Furthermore, it is valuable for men with ASIH to understand the impact that exogenous AAS may have had on their fertility. In these men with a previous history of AAS who present with infertility and hypogonadism, multiple pharmacological treatments are available, including exogenous testosterone, human chorionic gonadotropin (hCG), human menopausal gonadotropin (hMG), recombinant FSH, and clomiphene citrate (Martikainen et al., 1986; Gill, 1998; Menon, 2003; Ioannidou-Kadis et al., 2006; Hsieh et al., 2013; Rahnema et al., 2014).
ACKNOWLEDGEMENTS
JRK and RR are NIH K12 Scholars supported by a Male Reproductive Health Research Career (MHRH) Development Physician-Scientist Award (HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program.
REFERENCES
- Blume AW, Schmaling KB. Regret, substance abuse, and readiness to change in a dually diagnosed sample. Addict Behav. 1998;23:693–697. doi: 10.1016/s0306-4603(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Boregowda K, Joels L, Stephens JW, Price DE. Persistent primary hypogonadism associated with anabolic steroid abuse. Fertil Steril. 2011;96:e7–8. doi: 10.1016/j.fertnstert.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Buckley WE, Yesalis CE, 3rd, Friedl KE, Anderson WA, Streit AL, Wright JE. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260:3441–3445. [PubMed] [Google Scholar]
- Byrnes JP. The development of decision-making. J Adolescent Health. 2002;31:208–215. doi: 10.1016/s1054-139x(02)00503-7. [DOI] [PubMed] [Google Scholar]
- Cohen J, Collins R, Darkes J, Gwartney D. A league of their own: demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States. J Int Soc Sports Nutr. 2007;4:12. doi: 10.1186/1550-2783-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW, Lipshultz LI. Anabolic steroid induced hypogonadism in young men. J Urol. 2013;190:2200–2205. doi: 10.1016/j.juro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Dohle GR, Smit M, Weber RF. Androgens and male fertility. World J Urol. 2003;21:341–345. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- Evans NA. Current concepts in anabolic-androgenic steroids. Am J SportsMed. 2004;32:534–542. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- Gazvani MR, Buckett W, Luckas MJ, Aird IA, Hipkin LJ, Lewis-Jones DI. Conservative management of azoospermia following steroid abuse. Hum Reprod. 1997;12:1706–1708. doi: 10.1093/humrep/12.8.1706. [DOI] [PubMed] [Google Scholar]
- Gill GV. Anabolic steroid induced hypogonadism treated with human chorionic gonadotropin. Postgrad Med J. 1998;74:45–46. doi: 10.1136/pgmj.74.867.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough DO. Anabolic steroids and ergogenic aids. Am Fam Physician. 1990;41:1157–1164. [PubMed] [Google Scholar]
- Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189:647–650. doi: 10.1016/j.juro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Igra V, Irwin C. Theories of adolescent risk-taking behavior. In: DiClemente R, Hanson W, Ponton L, editors. Handbook of Adolescent Health Risk Behavior. Plenum; New York: 1995. pp. 35–51. [Google Scholar]
- Ioannidou-Kadis S, Wright PJ, Neely RD, Quinton R. Complete reversal of adult-onset isolated hypogonadotropic hypogonadism with clomiphene citrate. Fertil Steril. 2006;86:1513, e1515–1519. doi: 10.1016/j.fertnstert.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Jarow JP, Lipshultz LI. Anabolic steroid-induced hypogonadotropic hypogonadism. Am J Sports Med. 1990;18:429–431. doi: 10.1177/036354659001800417. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Jr., Hudson JI. “Body image” drugs: a growing psychosomatic problem. Psychother Psychosom. 2001;70:61–65. doi: 10.1159/000056228. [DOI] [PubMed] [Google Scholar]
- Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154:502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth UA, Maniera H, Nieschlag E. Anabolic steroids and semen parameters in bodybuilders. Fertil Steril. 1989;52:1041–1047. doi: 10.1016/s0015-0282(16)53172-0. [DOI] [PubMed] [Google Scholar]
- Landry GL, Primos WA., Jr Anabolic steroid abuse. Adv Pediatr. 1990;37:185–205. [PubMed] [Google Scholar]
- Lindstrom M, Nilsson AL, Katzman PL, Janzon L, Dymling JF. Use of anabolic-androgenic steroids among body builders--frequency and attitudes. J Int Med. 1990;227:407–411. doi: 10.1111/j.1365-2796.1990.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Maravelias C, Dona A, Stefanidou M, Spiliopoulou C. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol Lett. 2005;158:167–175. doi: 10.1016/j.toxlet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Martikainen H, Alen M, Rahkila P, Vihko R. Testicular responsiveness to human chorionic gonadotrophin during transient hypogonadotrophic hypogonadism induced by androgenic/anabolic steroids in power athletes. J Steroid Biochem. 1986;25:109–112. doi: 10.1016/0022-4731(86)90288-8. [DOI] [PubMed] [Google Scholar]
- Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril. 2003;79(Suppl 3):1659–1661. doi: 10.1016/s0015-0282(03)00365-0. [DOI] [PubMed] [Google Scholar]
- Moretti E, Collodel G, La Marca A, Piomboni P, Scapigliati G, Baccetti B. Structural sperm and aneuploidies studies in a case of spermatogenesis recovery after the use of androgenic anabolic steroids. J Assist Reprod Genet. 2007;24:195–198. doi: 10.1007/s10815-005-9002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Deveci S, Stasi J, Scardino PT, Mulhall JP. Sexual bother following radical prostatectomyjsm. J Sex Med. 2010;7:129–135. doi: 10.1111/j.1743-6109.2009.01546.x. [DOI] [PubMed] [Google Scholar]
- Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006;38:644–651. doi: 10.1249/01.mss.0000210194.56834.5d. [DOI] [PubMed] [Google Scholar]
- Pirola I, Cappelli C, Delbarba A, Scalvini T, Agosti B, Assanelli D, Bonetti A, Castellano M. Anabolic steroids purchased on the Internet as a cause of prolonged hypogonadotropic hypogonadism. Fertil Steril. 2010;94:2331, e2331–2333. doi: 10.1016/j.fertnstert.2010.03.042. [DOI] [PubMed] [Google Scholar]
- Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic-steroid induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101:1271–279. doi: 10.1016/j.fertnstert.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Rashid H, Ormerod S, Day E. Anabolic androgenic steroids: what the psychiatrist needs to know. Adv Psychiat Treat. 2007;13:203–211. [Google Scholar]
- Sanford K. The communication of emotion during conflict in married couples. J Fam Psychol. 2012;26:297–307. doi: 10.1037/a0028139. [DOI] [PubMed] [Google Scholar]
- Schurmeyer T, Knuth UA, Belkien L, Nieschlag E. Reversible azoospermia induced by the anabolic steroid 19-nortestosterone. Lancet. 1984;1:417–420. doi: 10.1016/s0140-6736(84)91752-5. [DOI] [PubMed] [Google Scholar]
- Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- Shokri S, Aitken RJ, Abdolvahhabi M, Abolhasani F, Ghasemi FM, Kashani I, Ejtemaeimehr S, Ahmadian S, Minaei B, Naraghi MA, Barbarestani M. Exercise and supraphysiological dose of nandrolone decanoate increase apoptosis in spermatogenic cells. Basic Clin Pharmacol Toxicol. 2010;106:324–330. doi: 10.1111/j.1742-7843.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- Silvester LJ. Self-perceptions of the acute and long-range effects of anabolic-androgenic steroids. J Strength Cond Res. 1995;9:95–98. [Google Scholar]
- Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–1882. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- Skarberg K, Engstrom I. Troubled social background of male anabolic-androgenic steroid abusers in treatment. Subst Abuse Treat Prevent Policy. 2007;2:20. doi: 10.1186/1747-597X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard SA, Bauermeister JA, Gordon-Messer D, Johns M, Zimmerman MA. Permissive norms and young adults' alcohol and marijuana use: the role of online communities. J Stud Alcohol Drugs. 2012;73:968–975. doi: 10.15288/jsad.2012.73.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RS, Scally MC. Anabolic steroid-induced hypogonadism--towards a unified hypothesis of anabolic steroid action. Med Hypotheses. 2009;72:723–728. doi: 10.1016/j.mehy.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Turek PJ, Williams RH, Gilbaugh JH, 3rd, Lipshultz LI. The reversibility of anabolic steroid-induced azoospermia. J Urol. 1995;153:1628–1630. [PubMed] [Google Scholar]
- Van den Broek KC, Heijmans N, Van Assen ALM. Anxiety and depression in patients with an implantable cardioverter defibrillator and their partners: a longitudinal study. Pacing Clin Electrophysiol. 2013;36:362–371. doi: 10.1111/pace.12055. [DOI] [PubMed] [Google Scholar]