Abstract
Background
Oral fluid (OF) offers a simple, non-invasive and directly observable sample collection for clinical and forensic drug testing. Given that chronic cannabis smokers often engage in drug administration multiple times daily, evaluating OF cannabinoid pharmacokinetics during ad libitum smoking is important for practical development of analytical methods and informed interpretation of test results.
Methods
Eleven cannabis smokers resided on a closed research unit for 51 days, and underwent four 5-day oral delta-9-tetrahydrocannabinol (THC) treatments. Each medication period was separated by 9 days of ad libitum cannabis smoking from 12:00 to 23:00h daily. Ten OF samples were collected from 9:00–22:00h on each of the last ad libitum smoking days (Study Days 4, 18, 32, and 46).
Results
As the number of cannabis cigarettes smoked increased over study days, OF THC, cannabinol (CBN), and 11-nor-9-carboxy-THC (THCCOOH) also increased with a significant effect of time since last smoking (∆time; range, 0.0–17.4h) and ≥88% detection rates; concentrations on Day 4 were significantly lower than those on Days 32 and 46 but not Day 18. Within 30 min post smoking, median THC, CBN, and THCCOOH concentrations were 689µg/L, 116µg/L, and 147ng/L, respectively, decreasing to 19.4µg/L, 2.4µg/L, and 87.6ng/L after 10h. Cannabidiol and 11-hydroxy-THC showed overall lower detection rates of 29 and 8.6%, respectively.
Conclusions
Cannabinoid disposition in OF was highly influenced by ∆time and composition of smoked cannabis. Furthermore, cannabinoid OF concentrations increased over ad libitum smoking days, in parallel with increased cannabis self-administration, possibly reflecting development of increased cannabis tolerance.
Introduction
Research on cannabinoid disposition in oral fluid (OF) proliferated in response to increasing interest in this alternative matrix for drug testing. Controlled drug administration studies allow detailed evaluation of cannabinoid OF pharmacokinetics. Within 12–15 min after a single smoked cannabis cigarette, delta-9-tetrahydrocannabinol (THC) OF concentrations are highly elevated (mean 59.4–4577µg/L[1–5]), followed by a biphasic elimination pattern; after a rapid decline in the first few hours with a mean half-life range of 0.8–2.0h,[2, 3, 6] OF THC more slowly decreases and may persist for several days in low concentrations in chronic frequent smokers.
Niedbala et al. documented mean (SD) last THC detection time in OF of 34 (11)h with 40% participants positive (0.5–1.3µg/L THC) 72h post-smoking; however, participants cannabis abstinence was not observed after 4h.[1] In 28 chronic frequent cannabis smokers during 4–33 days of abstinence, median OF THC detection window of 24 (95% CI 4.8–43.2)h was reported with occasional positive concentrations ≤3.0µg/L beyond 24h, up to 28 days.[7] Minor cannabinoids such as cannabinol (CBN), cannabidiol (CBD), and delta-9-tetrahydrocannabinolic acid (THC-A) followed similar elimination profiles in OF but with much lower concentrations and subsequently shorter detection windows.[5, 8] Detecting 11-nor-9-carboxy THC (THCCOOH), an inactive THC metabolite, can minimize the potential for OF positives due to passive environmental exposure. Peak concentrations of free THCCOOH ranged from 20.6–561ng/L and were detected 0.25–48h after a single smoked cannabis cigarette.[4, 5, 9] 11-hydroxy-THC (11-OH-THC) is an active THC metabolite that also presents in OF at low µg/L concentrations. Following a single smoked cannabis cigarette, 11-OH-THC was detected only in 2 expectorated OF samples (0.3 and 1.2µg/L) after 0.25–1h and none in concurrently collected Quantisal samples with 0.25 and 0.5µg/L limits of quantification (LOQ), respectively.[4, 5]
The results should be interpreted in context of study design not just by time since smoking, because OF cannabinoid concentrations and their quantification are influenced by potency of cannabis cigarettes,[2, 3] participants’ smoking history,[10] and collection methods.[4, 5] Most clinical studies evaluating OF cannabinoids employed single-dose administration. On the other hand, it is well-documented that chronic, frequent cannabis smokers may consume cannabis multiple times a day.[11–13] Cannabinoid OF concentrations reported in field application studies could represent the samples collected after multiple smoking episodes; high concentrations up to 6484µg/L THC, 400ng/L THCCOOH, 12.3µg/L 11-OH-THC, 115µg/L CBD, and 124µg/L CBN were detected in clinical,[14, 15] workplace,[14, 16] and roadside settings.[17, 18] However, time since last smoking is unknown in such studies, preventing assessment of concentration time-courses.
In the present report, we evaluate cannabinoid disposition in OF during multiple, ad libitum smoking periods. OF collection reflected authentic, frequent smoking scenarios of chronic cannabis smokers. THC, THCCOOH, 11-OH-THC, CBD, and CBN OF concentration time-courses were characterized with respect to time since last smoking. These data provide important information for establishing OF cannabinoid cutoff criteria and determining concentration ranges for analytical method development.
Materials and Methods
Participants
Inclusion criteria were age ≥18 years, self-reported cannabis smoking ≥25 days per month during the past 3 months, negative urine immunoassay test for drugs other than cannabinoids, negative breath alcohol test, and negative urine pregnancy test on admission, reported experience of ≥2 cannabis withdrawal symptoms of at least moderate severity in prior periods of abstinence, and ≥8th grade level of education and demonstrated literacy. Participants were excluded if they were taking psychoactive medication; met clinical criteria for Axis I psychiatric disorders (DSM-IV-TR) other than cannabis or nicotine dependence; were seeking treatment for cannabis-related problems or using cannabis for medical purposes; or donated blood within 6 weeks of admission. Participants also were required to have no history of seizure, severe head trauma, dementia, or other condition associated with significant cognitive impairment, heart attack or major cardiac event in the prior 6 months, abnormal electrocardiogram or allergy to sesame oil (dronabinol capsule ingredient). The study was approved by the Johns Hopkins Medicine Institutional Review Board, and participants provided written informed consent.
Study design
Participants resided on the closed Johns Hopkins Bayview Behavioral Pharmacology Research Unit for 51days, during which oral synthetic THC (dronabinol) effects on cannabis withdrawal, side effects, cognitive performance, and subjective and physiological responses to smoked cannabis challenge were evaluated.[19] The first 4 days were the baseline ad libitum smoking period (Days 1–4); cannabis smoking was allowed from 12:00 to 23:00h each day. Four 5-day oral THC sessions (Days 5–9, 19–23, 33–37, and 47–51) followed, during which oral THC was administered at 9:00, 14:00, and 19:00h each day. Participants received in a counterbalanced order, oral THC doses of 0, 30, 60, or 120mg/day on 5 consecutive days during the study. Each oral THC maintenance period was separated by 9-days of ad libitum cannabis smoking between 12:00 and 23:00h (Days 10–18, 24–32, and 38–46). On the last day of each study period (Days 4, 9, 18, 23, 32, 37, 46, and 51) participants were administered 5 controlled puffs of smoked cannabis at approximately 11:30h. This was the only smoked cannabis exposure during the oral THC maintenance periods, and provided a standardized initial exposure for all participants on the last day of each ad libitum cannabis smoking period.
During ad libitum cannabis smoking, participants were able to request cannabis cigarettes from research nurses. Upon request, participants were escorted to a specially ventilated room where cannabis smoking occurred. Each cannabis cigarette was accounted for in an inventory log and consumed under direct observation of study staff.
Cannabis cigarettes for the 4 ad libitum cannabis smoking periods and the controlled smoked cannabis exposures were obtained from the National Institute on Drug Abuse; mean (SD) cannabis cigarette weight was 0.9(0.07) g and contained 5.9 (0.3)% THC, 0.36 (0.04)% CBN, and 0.01 (0.00)% CBD, yielding approximately 53.1, 3.2, and 0.1 mg per cigarette, respectively. In the current report, OF cannabinoid disposition during the last days of ad libitum cannabis smoking sessions (Days 4, 18, 32, and 46) were characterized. Additionally, two other measures were obtained to evaluate association between oral fluid cannabinoid concentrations and tolerance: 1) plasma cannabinoid concentrations at 09:00h the days after ad libitum smoking sessions (Days 5, 19, 33, and 47); plasma samples during ad libitum smoking sessions were incomplete due to limited blood collection volume and 2) subjective drug effect ratings in 100-point visual analogue scale (VAS) at 14:00 and 19:00h on the last days of ad libitum smoking sessions; participants were asked “do you feel a drug effect?” and rated from 0 (“not at all”) to 100 (“extremely”).
Sample collection and analysis
Ten OF samples were collected on each of the last ad libitum smoking days (Days 4, 18, 32, and 46) at 9:00, 11:00, 11:45, 12:30, 14:00, 15:30, 17:00, 19:00, 20:30, and 22:00h. OF (1±0.1mL) was collected with the Quantisal™ device (Immunalysis). OF samples were refrigerated for 24h and then stored at −20°C until analysis.
THC, CBD, CBN, 11-hydroxy-THC (11-OH-THC), and THCCOOH in OF were quantified according to a modified version of our previously published method.[20] Minor changes were made to improve method productivity. Briefly, 1mL cold acetonitrile was added to 1mL Quantisal OF-buffer mixture and centrifuged. Supernatants were decanted onto CEREX® Polycrom™ THC solid-phase extraction columns (SPEware) conditioned with 1mL methanol. Columns were washed with 3mL deionized water/acetonitrile/ammonium hydroxide (85:15:1), dried under positive pressure, and primed with 0.4mL hexane. THC, CBD, CBN, and 11-OH-THC were eluted with 3mL hexane/acetone/ethyl acetate (60:30:20), followed by THCCOOH elution into separate tubes with 3mL hexane/ethyl acetate/glacial acetic acid (75:25:2.5). Eluates were dried under nitrogen and derivatized with 20µL trifluoroacetic anhydride and 40 µL hexafluoroisopropanol for THCCOOH and 20µL trifluoroacetamide with 1 % trimethylchlorosilane for other analytes at 65°C for 35 min. THCCOOH derivatives were evaporated and reconstituted in 20µL toluene. Two-dimensional gas chromatography-mass spectrometry (2D-GC-MS) was utilized for separation and quantification. The GC column configuration for neutral cannabinoid analysis was changed to that of our plasma method,[21] allowing multiple GC instruments for either analysis; instruments were equipped with DB-1MS (Agilent Technologies) as the primary and ZB-50 (Phenomenex) as the secondary column. LOQs were 0.5µg/L for THC and CBD, 1µg/L for CBN and 11-OH-THC, and 15ng/L for THCCOOH. The modified method’s performance was comparable to that of the original with 0.8–6.6%RSD (n=5 per each QC concentration) intra-assay imprecision, 1.8–11.8%RSD (n=10 per QC; 6 assays) inter-assay imprecision, 47.0–101.6% extraction efficiency of d0- and d3-analytes, and 92.2–107.2% (n=15 per QC concentration) analyte recovery.
Blood samples were collected in heparinized tubes and placed on ice until centrifugation within 2 hours to separate plasma. The samples were stored at −20°C until analysis. Plasma THC, 11-OH-THC, and THCCOOH concentrations were analyzed by a previously published 2D-GC-MS method with minor changes.[21, 22] LOQs were 0.5µg/L for THC and THCCOOH and 1.0µg/L for 11-OH-THC.
Data analysis
IBM SPSS Statistics version 20 and Microsoft Excel 2007 were utilized for statistical evaluation. Cannabinoid concentrations were non-normally distributed, as determined by the Kolmogorov-Smirnov test and Normal Q-Q plot. Accordingly, effects of ∆time (calculated by subtraction of last smoking times from sample collection times), study days, and cannabis use history on cannabinoid OF concentrations during ad libitum cannabis smoking sessions were evaluated with Generalized Linear Mixed Models after log transformation of the data and multiple comparisons adjusted with sequential Bonferroni correction. Correlations between cannabinoids were evaluated with nonparametric Spearman’s rho (ρ); only positive pairs were included. Medians were compared for 1) OF cannabinoid concentrations in CBD-/11-OH-THC-positive or negative samples; 2) plasma cannabinoid concentrations on Days 5, 19, 33, and 47; and 3) subjective drug effects ratings on Days 4, 18, 32, and 46 with nonparametric Related-Sample Wilcoxon Signed Rank Test. Values below LOQ were considered as one tenth LOQ for regression analysis to avoid overestimation of the mean but this approach may underestimate variances.[23] Results with 2-tailed P<0.05 were considered significant.
Results
Eleven chronic frequent cannabis smokers (10 males, 1 female; aged 25–52 years) provided 440 OF samples on the last days of ad libitum cannabis smoking sessions. Participants’ demographics, cannabis smoking history, and the number of cannabis cigarettes smoked on the last days of four ad libitum smoking sessions are provided in Table 1. The samples were collected from 0.02–17.1h after last cannabis smoking (Table 2). Before admission, participants reported median (range) average cannabis smoking of 3.4 (3.0–5.9) times per day for 16 (11–38) years. On Days 4, 18, 32, and 46 of ad libitum smoking from 12:00–23:00h, participants smoked medians (ranges) of 16 (2–25), 17 (1–30), 20 (3–40), and 25 (2–40) cannabis cigarettes, respectively; number of joints smoked on Day 4 was significantly fewer than those smoked on Days 32 and 46 (P<0.01) but not on Day 18 (P=0.260).
Table 1.
Eleven chronic, frequent cannabis smokers’ demographics, self-reported cannabis use history, and the number of cannabis cigarettes smoked on the last day of each ad libitum smoking session.
| Cannabis use historya | # cannabis cigarettes smoked | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Age, year |
Sex | Race | BMIb | Days used in past 30 |
Mean times smoked/dayc |
Age at first use, years |
Years of regular use |
Study Day 4 |
Study Day 18 |
Study Day 32 |
Study 46 |
| A | 47 | M | AAd | 19.4 | 30 | 2.1 | 13 | 27 | 2 | 1 | 3 | 2 |
| B | 36 | M | AA | 28.5 | 30 | 5.6 | 17 | 16 | 16 | 8 | 20 | 20 |
| C | 29 | M | AA | 30.2 | 30 | 4.7 | 9 | 17 | 20 | 20 | 25 | 27 |
| E | 31 | F | AA | 36.0 | 30 | 5.0 | 18 | 13 | 8 | 14 | 20 | 25 |
| H | 30 | M | AA | 21.0 | 28 | 5.9 | 14 | 14 | 15 | 24 | 25 | 30 |
| I | 52 | M | AA | 26.1 | 29 | 2.3 | 14 | 38 | 13 | 17 | 20 | 20 |
| J | 39 | M | AA | 48.2 | 28 | 3.4 | 13 | 26 | 25 | 25 | 40 | 25 |
| K | 37 | M | AA | 27.7 | 29 | 2.0 | 16 | 21 | 7 | 6 | 11 | 20 |
| M | 30 | M | AA | 32.4 | 29 | 2.9 | 14 | 16 | 17 | 14 | 13 | 20 |
| N | 25 | M | AA | 22.0 | 30 | 3.0 | 14 | 11 | 18 | 25 | 30 | 40 |
| P | 30 | M | AA | 23.1 | 29 | 3.5 | 16 | 14 | 20 | 30 | 40 | 40 |
| Median | 31.0 | 27.7 | 29.0 | 3.4 | 14.0 | 16.0 | 16.0 | 17.0 | 20.0 | 25.0 | ||
| Mean | 35.1 | 28.6 | 29.3 | 3.7 | 14.4 | 19.4 | 14.6 | 16.7 | 22.5 | 24.5 | ||
| SD | 8.3 | 8.2 | 0.8 | 1.4 | 2.4 | 8.1 | 6.7 | 9.1 | 11.4 | 10.5 | ||
information from the screening assessments collected at a mean of 23 (range 4–49) days prior to study admission
body mass index, calculated as mass (kg) divided by the square of height (m)
calculated from the total number of times used in past 30 days
African American
Table 2.
Detection rates and cannabinoid concentrations in oral fluid collected 0.0–17.1h after last cannabis smoking from 11 chronic frequent cannabis smokers during ad libitum smoking sessions
| ∆time, h (N)a | THC | THCCOOH | CBD | CBN | 11-OH-THC | |
|---|---|---|---|---|---|---|
| 0.0–0.4 (88) | Detection rate, % | 100 | 98.9 | 65.9 | 100 | 33.0 |
| Median (range)b | 689 (6.7–21,180) | 146.9 (<LOQc-488.7) | 1.6 (<LOQ-74.4) | 116 (1.5–2190) | <LOQ (<LOQ-13.9) | |
| 0.5–0.9 (86) | Detection rate, % | 100 | 98.8 | 39.5 | 100 | 7.0 |
| Median (range) | 185 (2.3–10,543) | 117.4 (<LOQ-727.0) | <LOQ (<LOQ-33.5) | 21.9 (1.1–2155) | <LOQ (<LOQ-5.5) | |
| 1.0–1.9 (94) | Detection rate, % | 98.9 | 100 | 27.7 | 94.7 | 3.2 |
| Median (range) | 87.9 (<LOQ-7429) | 105.2 (16.4–506.1) | <LOQ (<LOQ-27.8) | 11.2 (<LOQ-854) | <LOQ (<LOQ-3.8) | |
| 2.0–8.9 (73) | Detection rate, % | 97.3 | 95.9 | 8.2 | 76.7 | 0.0 |
| Median (range) | 18.7 (<LOQ-691) | 78.5 (<LOQ-518.8) | <LOQ (<LOQ-2.5) | 3.5 (<LOQ-136) | <LOQ (<LOQ) | |
| 10.0–17.1 (95) | Detection rate, % | 96.8 | 95.8 | 2.1 | 69.5 | 0.0 |
| Median (range) | 19.4 (<LOQ-1162) | 84.6 (<LOQ-350.1) | <LOQ (<LOQ-3.1) | 2.4 (<LOQ-90.0) | <LOQ (<LOQ) | |
∆time determined as time of last cannabis smoking subtracted from sample collection time; N = number of samples collected during the ∆time period
µg/L except for THCCOOH (ng/L)
concentrations< limit of quantification (0.5µg/L for THC and CBD; 1µg/L for CBN and 11-OH-THC; 15ng/L for THCCOOH)
THC
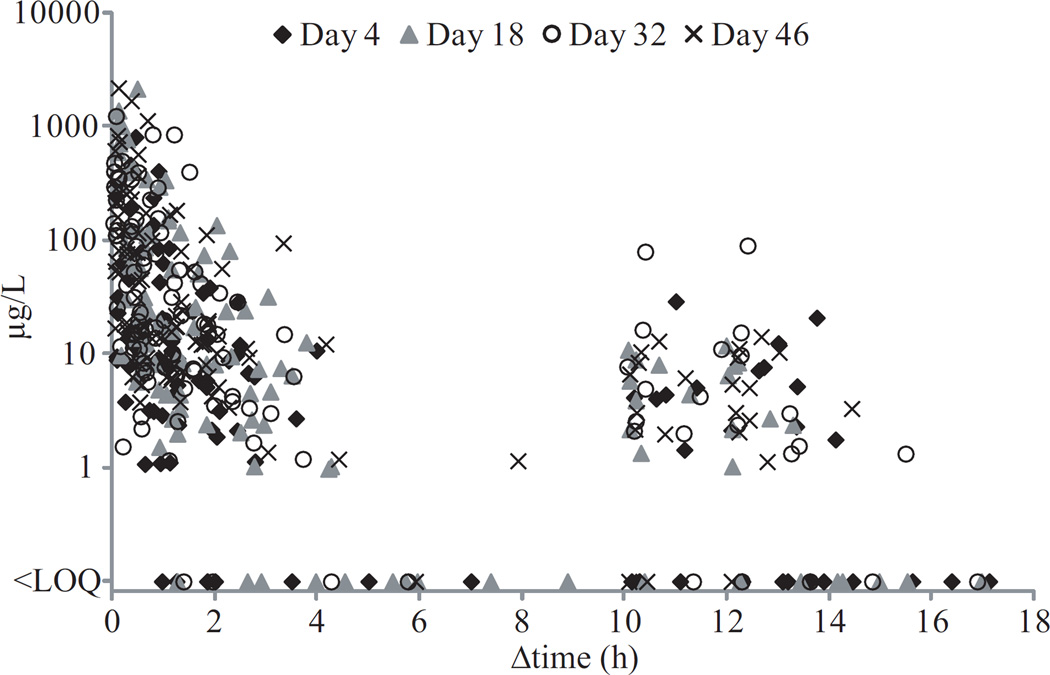
Ninety eight percent of OF samples were positive for THC throughout the collection period. THC OF concentrations were significantly (F1,435= 173.03,P<0.001) dependent on time since last smoking (Figure 1) with large inter-subject variability (Figure 2). THC concentrations also showed a significant difference across study days (F3,435= 12.07,P<0.001); concentrations on Day 4 were lower than those on Days 32 and 46 (P’s <0.001) but not significantly different from those on Day 18 (P=0.062).
Figure 1.
Median oral fluid cannabinoid concentrations in 11 chronic frequent cannabis smokers on the last days (Days 4, 18, 32, and 46) of ad libitum smoking sessions. (A, B, and C) Δ9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH), and cannabinol (CBN) median concentrations over time intervals in logarithmic scales. (D) THCCOOH median concentrations in arithmetic scale for more detailed illustration of the time course. Participants administered controlled (5 puffs) smoked cannabis dose at 11:30h and then allowed ad libitum cannabis smoking from 12:00 to 23:00h while oral fluid collection occurred from 9:00–22:00h. A single participant may contribute multiple data points to a single interval based on their smoking frequency. Concentrations were plotted with respect to time since last smoking. Data table indicates the number of samples included in each time interval.
Figure 2.
Δ9-tetrahydrocannabinol (THC) concentrations in 440 oral fluid samples from 11 chronic frequent cannabis smokers on the last days (Days 4, 18, 32, and 46) of ad libitum smoking sessions. Participants administered controlled (5 puffs) smoked cannabis dose at 11:30h and then allowed ad libitum cannabis smoking from 12:00 to 23:00h while oral fluid collection occurred within 9:00–22:00h. Limit of quantification (LOQ) for THC was 0.5μg/L. Δtime indicates time since last cannabis smoking. Each marker represents an oral fluid sample.
THCCOOH
Similar to THC, high detection rates (98%) were observed for OF THCCOOH, which also exhibited significant effect of ∆time (F1,435=21.03,P<0.001; Figures 1, 3) and study day (F3,435=5.52, P=0.001); THCCOOH concentrations on Day 4 were significantly lower than those on Days 32 and 46 (P<0.01) but not on Day 18 (P=0.634). There was a significant correlation between OF THCCOOH and THC positive pairs (ρ=0.538,P<0.001, n=427).
Figure 3.
11-nor-9-carboxy-tetrahydrocannabinol (THCCOOH) concentrations in 440 oral fluid samples from 11 chronic frequent cannabis smokers on the last days (Days 4, 18, 32, and 46) of ad libitum smoking sessions. Participants administered controlled (5 puffs) smoked cannabis dose at 11:30h and then allowed ad libitum cannabis smoking from 12:00 to 23:00h while oral fluid collection occurred within 9:00–22:00h. Limit of quantification (LOQ) for THCCOOH was 15ng/L. Δtime indicates time since last cannabis smoking. Each marker represents an oral fluid sample.
CBN
CBN was detected in 88% of OF samples with concentrations approximately 10-times lower than THC’s (Figures 1, 4). ∆time significantly affected CBN OF concentrations (F1,435=231.21,P<0.001) that also were significantly different across study days (F3,435=7.77,P<0.001); concentrations on Day 4 were lower than those on Days 32 and 46 (P<0.01) but not significantly different from Day 18 (P=0.084). OF CBN was significantly correlated with OF THC (ρ=0.881,P<0.001,n=389) and THCCOOH (ρ=0.413,P<0.001,n=385).
Figure 4.
Cannabinol (CBN) concentrations in 440 oral fluid samples from 11 chronic frequent cannabis smokers on the last days (Days 4, 18, 32, and 46) of ad libitum smoking sessions. Participants administered controlled (5 puffs) smoked cannabis dose at 11:30h and then allowed ad libitum cannabis smoking from 12:00 to 23:00h while oral fluid collection occurred within 9:00–22:00h. Limit of quantification (LOQ) for CBN was 1μg/L. Δtime indicates time since last cannabis smoking. Each marker represents an oral fluid sample.
CBD and 11-OH-THC
CBD was detected in 29% of OF samples with concentrations ≤74.4µg/L (Table 2). CBD was no longer measurable 12.4h after last smoking (median=0.5h). All samples positive for CBD also were positive for THC. Median (range) THC and THCCOOH concentrations in CBD-positive samples were 860 (121-21180)µg/L and 157.2 (32.4–727.0)ng/L, respectively, significantly higher than THC [42.4 (<LOQ-973) µg/L] and THCCOOH [89.8 (<LOQ-518.8)ng/L] concentrations in CBD-negative samples collected within the similar ∆time interval, 0.07–12.3h post smoking (P’s <0.001). OF CBD was significantly correlated with THC (ρ=0.935,P<0.001, n=127) and to a lesser degree, with THCCOOH (ρ=0.335,P<0.001, n=127).
Only 8.6% of OF samples were positive for 11-OH-THC with a LOQ of 1µg/L. 11-OH-THC concentrations never exceeded 13.9µg/L and were not detected beyond 1.6h after last smoking. As with CBD, 11-OH-THC-positive samples also contained high concentrations of THC and THCCOOH; median (range) THC and THCCOOH concentrations in 11-OH-THC positive samples were 3385 (379-21180) µg/L and 256.2 (71.7–727.0) ng/L, respectively, significantly higher than THC [124 (<LOQ-6845) µg/L] and THCCOOH [117.4 (<LOQ-460.6) ng/L] concentrations in 11-OH-THC-negative samples collected within the similar ∆time interval, 0.02–1.6h post smoking (P’s<0.001). OF 11-OH-THC showed significant correlation with THC (ρ=0.788,P<0.001, n=38) and THCCOOH (ρ=0.489,P<0.001, n=38).
Correlations with cannabis smoking history
Controlling for ∆time and study days, total number of cannabis cigarettes smoked during the day of OF sample collection significantly and positively correlated with THC, THCCOOH, and CBN OF concentrations (F1,432=115.02, 18.15 and 133.42, respectively; P’s<0.001). OF THC and THCCOOH significantly correlated with years of regular cannabis use (F1,432=9.78, P=0.002; F1,432=17.588, P<0.001, respectively), but not with average self-reported number of times cannabis was smoked per day or age 1st used cannabis. OF CBN showed significant correlations with all 3 variables (P<0.05).
OF cannabinoid cutoff criteria
Detection windows could not be determined for THC≥1µg/L, THC≥2µg/L, or THCCOOH≥20ng/L cutoff, because times of last positive OF sample and last OF collection times both occurred 12.3–17.1h after last smoking; 98, 96, and 95% of OF samples, respectively, were positive. Similarly, detection windows could not be fully evaluated with a cutoff of THC≥1 or 2µg/L + CBN≥0.5µg/L; 54% of participants had last positive results in OF samples collected at the latest Δtimes. In the other 5 participants, last positives occurred at 12.2–15.5h after last smoking, followed by 1–4 negative results. A cutoff of THC≥1 or 2µg/L + CBD≥0.5µg/L led to detection windows of 0.5–3.3h after last smoking except for 1 participant whose last positive occurred at 12.4h.
Plasma cannabinoids and subjective drug effect
Δtimes since the last smoking were comparable for the plasma samples collected on the day after each ad libitum smoking session; Δtimes were not significantly different among Days 5, 19, 33, and 47 (P=0.662; One-Way ANOVA) with the medians of 10.4–10.5h. In these samples, median (range) THC concentrations were 7.3 (1.0–11.9), 9.2 (2.0–14.0), 11.3 (2.4–14.2), and 12.0 (1.8–17.0)µg/L on Days 5, 19, 33, and 47, respectively; the median on Day 5 was significantly lower than those on Day 33 (P=0.008) and Day 47 (P=0.006) but not on Day 19 (P=0.139). Plasma 11-OH-THC and THCCOOH concentrations were not significantly different among the study days (P’s ≥0.286) with the medians of 2.2–4.1 and 89.2–158.4µg/L, respectively.
Subjective drug effect ratings were obtained on the last days of ad libitum smoking sessions from 7 instead of 11 participants as acquisition of this measure started in the middle of the study. The values collected at 14:00 and 19:00h were averaged to reduce the Δtime variability; averaged Δtimes since last smoking were not significantly different (P=0.313; One-Way ANOVA) with the medians of 1.3, 1.7, 1.0, and 1.0h on Days 4, 18, 32, and 46, respectively. The medians of averaged drug effect ratings were 31.5, 21.5, 26.5, and 24.0 on Days 4, 18, 32, and 46, respectively, and were not significantly different (P’s≥0.310).
Discussion
The present study examined OF cannabinoid elimination time courses during multiple, ad libitum cannabis smoking episodes in chronic frequent cannabis smokers. OF THC and CBN concentration ranges were slightly higher than what we and others[2–5, 8, 10] showed after smoking a single cannabis cigarette; however, median concentrations throughout the collection period were similar to those after a single cigarette. With use of the same Quantisal collection device and cannabis cigarettes with higher THC content (6.8% vs. 5.9%), median THC concentrations 0.25 and 0.5h after single smoking were 644 and 212 µg/L,[5] respectively. In the current research, median THC concentration ranges within 0.0–0.4h and 0.5–0.9h within the four ad libitum smoking sessions were 110-2257 and 64.9–222 µg/L, respectively (Figure 1). THC and CBN OF concentrations demonstrated overall comparable ranges after single and multiple smoking episodes, large inter-subject variability, and a highly significant effect of ∆time. OF CBD, on the other hand, had detection rates and concentrations much lower than our previous results after single smoked cannabis,[5] likely because of lower CBD content in the present study’s cannabis cigarettes (2.0 vs. 0.1 mg CBD). Moore et al. similarly found that no participants (n=3) were positive for CBD after smoking their cannabis cigarettes (cannabinoid contents not reported).[8] These findings indicate that parent cannabinoid OF concentrations are affected primarily by time since last smoking and composition of cannabis smoke. ∆time also influenced OF THCCOOH although to a lesser extent compared to OF THC and CBN (Table 2), suggesting a longer elimination rate. THCCOOH demonstrated high prevalence in OF samples of these chronic frequent cannabis smokers, but concentrations never exceeded 727ng/L. We detected 11-OH-THC above 1µg/L (≤13.9µg/L) only within 1.6h post smoking when THC was ≥379µg/L. Similarly, after a single smoked cigarette, ≤6% expectorated, Saliva-Sampler™, and Oral-Eze® OF samples were positive for 11-OH-THC at LOQs of 0.25–1.0µg/L; concentrations were 0.3–2.6µg/L, occurring within 2h post smoking.[24–26] Of cannabinoid-positive OF samples from treatment/workplace drug testing programs, 5.7% were positive for 11-OH-THC with a concentration range of 0.01–12.3µg/L.[14] To fully elucidate OF 11-OH-THC elimination pattern, a more sensitive quantification method is needed. Nonetheless, the data suggest the potential for 11-OH-THC to monitor recent cannabis smoking and also, as a metabolite, may minimize the possibility of false positive results from passive environmental contamination.
Study limitations should be considered when interpreting our results. Frequency of cannabis smoking among our participants varied substantially, which contributed to large inter-subject variability in cannabinoid OF concentrations. Participant A, who reported smoking at lower than mean frequency prior to the study, smoked only 1–3 joints on the last days of 4 ad libitum smoking sessions. His THC and THCCOOH concentrations were overall lower than median concentrations (Figure 5). Participant E’s (our only female) number of cannabis cigarettes smoked over time increased from 8 to 14, 20, and 25, while Participant N smoked 18, 25, 30, and 40 cannabis cigarettes on Days 4, 18, 32, and 46, respectively; the number of cannabis cigarettes that Participant E smoked was similar to median values and Participant N smoked more than the median in all four sessions. Predictably, THC and THCCOOH concentrations were slightly higher in Participant N compared to Participant E (Figure 5). OF THC, CBN, and THCCOOH concentrations had significantly positive correlations with total number of cannabis cigarettes smoked during the day of OF sample collection. Because the current study allowed ad libitum smoking 12:00–23:00h with scheduled OF collection times, participants who smoked frequently contributed more data to the initial ∆time intervals than those who smoked less frequently (greater contribution in later time intervals). Moreover, OF samples collected within 10.0–17.1h after last smoking were those collected before ad libitum smoking started for the day (09:00–12:00). This led to spikes in concentration ∆time-course plots (Figure 5) and lower cannabinoid concentrations within the 2.0–8.9h interval than the 10.0–17.1h interval (e.g., median THC concentrations were 18.7µg/L in the 2.0–8.9h ∆time interval and 19.4µg/L in the 10.0–17.1h interval; Table 2). Additionally, shortly before ad libitum smoking started at 12:00h, participants smoked 5 puffs of cannabis following a paced puff procedure (5-sec inhalation, 10-sec breath holding, and 40-sec inter-puff interval). Smoking of 5 puffs on the cannabis cigarette was included to obtain reference values for physiological and subjective measures in assessing dose effect of oral THC on smoked cannabis effects; this was another aim of the current study and the results were described in a previously published report.[19] Since this controlled smoking session contributed to OF cannabinoid concentrations, the time point was considered as a smoking episode in calculating Δtime. However, its weight in OF cannabinoid concentrations would likely have been lower than that of ad libitum smoking.
Figure 5.
Δ9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-THC (THCCOOH) oral fluid concentration time plots in selected 3 participants (A, E, and N) on the last days (Days 4, 18, 32, and 46) of ad libitum smoking sessions. Participants administered controlled (5 puffs) smoked cannabis dose at 11:30h and then allowed ad libitum cannabis smoking from 12:00 to 23:00h, and oral fluid collection occurred between 9:00 and 22:00h. Concentrations were plotted with respect to time since last smoking. Limits of quantification (LOQ) were 0.5μg/L for THC and 15ng/L for THCCOOH.
Cannabinoid OF concentrations indicated tolerance development in chronic, frequent cannabis smokers. Tolerance to THC’s pharmacological effects after prolonged exposure was documented in preclinical and clinical research.[12, 27–29] We and others suggested that cannabinoid tolerance could be due to CB1 receptor down-regulation and desensitization; the extent of the neuronal alteration differed by brain region and down-regulation negatively correlated with years of cannabis smoking.[28, 30] In the present report, controlling for participants and ∆time, THC, CBN, and THCCOOH concentrations were significantly higher on the last days of the third and fourth ad libitum smoking sessions compared to those on the last days of the first smoking session. A possible reason is cannabinoid accumulation in the body over time. However, each ad libitum smoking session was separated by 5 days of oral THC maintenance, during which cannabis smoking was prohibited. As transfer from blood to oral fluid is minimal for parent cannabinoids, median THC, CBN, and CBD concentrations on the last day of each oral THC maintenance phase (i.e., the day before the initiation of ad libitum smoking session) were ≤0.8µg/L. Hence, it does not adequately explain higher cannabinoid OF concentrations on Days 32 and 46 compared to those on Day 4. Conversely, THCCOOH accumulation is possible as this analyte is transferred from blood to OF.[31] Median THCCOOH concentrations increased from 33.7 to 214.7ng/L on the last days of 0 to 120 mg oral THC dosing. However, because participants received oral THC doses in a counterbalanced order, the increase in OF THCCOOH cannot be explained simply by its accumulation over time. Alternatively, increased cannabinoid OF concentrations more likely reflect the increased number of cannabis cigarettes smoked over study days for some study participants. Other studies have similarly documented a rise in the number of cannabis cigarettes smoked during ad libitum smoking access in residential research settings. In a case report, an individual smoked on average 9.5 cannabis cigarettes daily during 21 days and on the 19th, 20th, and 21st days, the person smoked 18, 19, and 25 cannabis cigarettes, respectively.[32] Another study reported an average (range) daily number of cannabis cigarettes of 5.2 (1.7–10.0) during 47–59 days of ad libitum smoking that exceeded each participant’s customary quantity.[33] Georgotas and Zeidenberg also documented that on the 14th day of 4-week ad libitum cannabis smoking, participants smoked an average (range) of 11.6 (9–14) cannabis cigarettes and on the 28th day, increasing to 19.0 (10–22).[34] While participants may have been motivated to smoke more than usual due to availability of free cannabis cigarettes, this alone does not explain the time-dependent increase in smoking frequency.
Furthermore, McClure et al. observed increases in number of cannabis cigarettes smoked, total volume per cigarette, average volume per puff, and puff velocity on the second day of ad libitum smoking compared to the first day.[35] Positive correlations were found between multiple smoking topography measures (number of cigarettes, puff volume, duration, and/or velocity) and cannabis use history, craving, and withdrawal ratings.[35] The researchers noted that the number of years a participant reported having been a frequent cannabis user was significantly associated with larger, longer and more forceful puffs, which, they suggested, was due to compensation for drug tolerance.[35] Interestingly, our previous study showed a significantly positive correlation between years of cannabis smoking and peak THC OF concentrations.[5] The current report also documented significantly positive correlations between years of cannabis smoking and OF THC, THCCOOH and CBN. Possibility of tolerance affecting OF cannabinoid concentrations was further supported by increase in plasma THC concentrations and yet no significant difference in subjective drug effect ratings over study days. Together, the findings suggest association among cannabis tolerance, smoking experience/topography, and cannabinoid OF concentrations.
In conclusion, cannabinoid concentrations increased over ad libitum smoking study days, possibly due to tolerance developed in this chronic, frequent smoker cohort. Further study including pharmacodynamic measures is needed to corroborate this potential relationship. The present report extended previous research that cannabinoid OF disposition was primarily influenced by ∆time and composition of smoked cannabis. Collectively, there was evidence of a possible relationship among cannabis tolerance, smoking topography, duration of use, and cannabinoid OF concentrations. Multiple cannabis smoking episodes also increased the upper limit of the OF concentration range, highlighting the importance of dilution integrity experiments in analytical method validation for cannabinoid OF testing in chronic frequent cannabis smokers. These findings provide important data for interpreting OF cannabinoid test results and demonstrate the ability to obtain repeated, valid oral fluid specimens at defined times relative to drug exposure, which support the value of cannabinoid OF testing as a monitoring tool in clinical and forensic drug testing programs.
Acknowledgements
Research Funding
This research was funded by grant R01 DA025044 from the National Institute on Drug Abuse and by the Intramural Research Program, National Institute on Drug Abuse, NIH. The funding sources had no role in study design, data collection and analysis, or presentation of results. This study was registered at clinicaltrials.gov (NCT00893074).
We acknowledge Sebastien Anizan, Garry Milman, Mateus Bergamaschi, and Marisol Castaneto for analytical assistance in data collection and contributions from the clinical staff at the NIDA Intramural Research Program and Johns Hopkins Behavioral Pharmacology Research Unit.
References
- 1.Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25:289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 2.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288–293. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 3.Huestis MA, Cone EJ. Relationship of ∆9-tetrahydrocannabinold concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J Anal Toxicol. 2004;28:394–399. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- 4.Milman G, Schwope DM, Gorelick DA, Huestis MA. Cannabinoids and metabolites in expectorated oral fluid following controlled smoked cannabis. Clin Chim Acta. 2012;413:765–770. doi: 10.1016/j.cca.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem. 2012;58:748–756. doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niedbala S, Kardos K, Salamone S, Fritch D, Bronsgeest M, Cone EJ. Passive cannabis smoke exposure and oral fluid testing. J Anal Toxicol. 2004;28:546–552. doi: 10.1093/jat/28.7.546. [DOI] [PubMed] [Google Scholar]
- 7.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily Cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–1136. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 8.Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:459–464. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. Detection of conjugated 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. J Anal Toxicol. 2007;31:187–194. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 10.Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Pharmacokinetic properties of delta9-tetrahydrocannabinol in oral fluid of occasional and chronic users. J Anal Toxicol. 2010;34:216–221. doi: 10.1093/jat/34.4.216. [DOI] [PubMed] [Google Scholar]
- 11.Copeland J, Swift W, Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. J Subst Abuse Treat. 2001;20:45–52. doi: 10.1016/s0740-5472(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 12.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 13.Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 2008;69:1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cone EJ, Clarke J, Tsanaclis L. Prevalence and disposition of drugs of abuse and opioid treatment drugs in oral fluid. J Anal Toxicol. 2007;31:424–433. doi: 10.1093/jat/31.8.424. [DOI] [PubMed] [Google Scholar]
- 15.Heltsley R, DePriest A, Black DL, Robert T, Marshall L, Meadors VM, et al. Oral fluid drug testing of chronic pain patients. I. Positive prevalence rates of licit and illicit drugs. J Anal Toxicol. 2011;35:529–540. doi: 10.1093/anatox/35.8.529. [DOI] [PubMed] [Google Scholar]
- 16.Gjerde H, Christophersen AS, Moan IS, Yttredal B, Walsh JM, Normann PT, et al. Use of alcohol and drugs by Norwegian employees: a pilot study using questionnaires and analysis of oral fluid. J Occup Med Toxicol. 2010;5:1–8. doi: 10.1186/1745-6673-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummer OH, Gerostamoulos D, Chu M, Swann P, Boorman M, Cairns I. Drugs in oral fluid in randomly selected drivers. Forensic Sci Int. 2007;170:105–110. doi: 10.1016/j.forsciint.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Simonsen KW, Steentoft A, Hels T, Bernhoft IM, Rasmussen BS, Linnet K. Presence of psychoactive substances in oral fluid from randomly selected drivers in Denmark. Forensic Sci Int. 2012;221:33–38. doi: 10.1016/j.forsciint.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D. The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 2012;128:64–70. doi: 10.1016/j.drugalcdep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217:1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. Simultaneous quantification of Delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J Chromatogr A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milman G, Bergamaschi MM, Lee D, Mendu DR, Barnes AJ, Vandrey R, et al. Plasma cannabinoid concentrations during dronabinol pharmacotherapy for cannabis dependence. Ther Drug Monit. 2014;36:218–224. doi: 10.1097/FTD.0b013e3182a5c446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uh HW, Hartgers FC, Yazdanbakhsh M, Houwing-Duistermaat JJ, et al. Evaluation of regression methods when immunological measurements are constrained by detection limits. BMC Immunol. 2008;9:1–10. doi: 10.1186/1471-2172-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milman G, Schwope DM, Gorelick DA, Huestis MA. Cannabinoids and metabolites in expectorated oral fluid following controlled smoked cannabis. Clin Chim Acta. 2012;413:765–770. doi: 10.1016/j.cca.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anizan S, Milman G, Desrosiers N, Barnes AJ, Gorelick DA, Huestis MA. Oral fluid cannabinoid concentrations following controlled smoked cannabis in chronic frequent and occasional smokers. Anal Bioanal Chem. 2013;405:8451–8461. doi: 10.1007/s00216-013-7291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newmeyer MN, Desrosiers NA, Lee D, Mendu DR, Barnes AJ, Gorelick DA, et al. Cannabinoid disposition in oral fluid after controlled cannabis smoking in frequent and occasional smokers. Drug Test Anal. 2014 doi: 10.1002/dta.1632. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González S, Cebeira M, Fernández-Ruiz J, et al. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Ramaekers JG, Theunissen EL, de Brouwer M, Toennes SW, Moeller MR, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011;214:391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milman G, Schwope DM, Schwilke EW, Darwin WD, Kelly DL, Goodwin RS, et al. Oral fluid and plasma cannabinoid ratios after around-the-clock controlled oral delta9-tetrahydrocannabinol administration. Clin Chem. 2011;57:1597–1606. doi: 10.1373/clinchem.2011.169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendelson JH, Mello NK, Lex BW, Bavli S. Marijuana withdrawal syndrome in a woman. Am J Psychiatry. 1984;141:1289–1290. doi: 10.1176/ajp.141.10.1289. [DOI] [PubMed] [Google Scholar]
- 33.Tashkin DP, Shapiro BJ, Lee YE, Harper CE. Subacute effects of heavy marihuana smoking on pulmonary function in healthy men. N Engl J Med. 1976;294:125–129. doi: 10.1056/NEJM197601152940302. [DOI] [PubMed] [Google Scholar]
- 34.Georgotas A, Zeidenberg P. Observations on the effects of four weeks of heavy marihuana smoking on group interaction and individual behavior. Compr Psychiatry. 1979;20:427–432. doi: 10.1016/0010-440x(79)90027-0. [DOI] [PubMed] [Google Scholar]
- 35.McClure EA, Stitzer ML, Vandrey R. Characterizing smoking topography of cannabis in heavy users. Psychopharmacology (Berl) 2012;220:309–318. doi: 10.1007/s00213-011-2480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]