Abstract
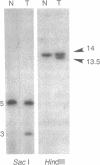
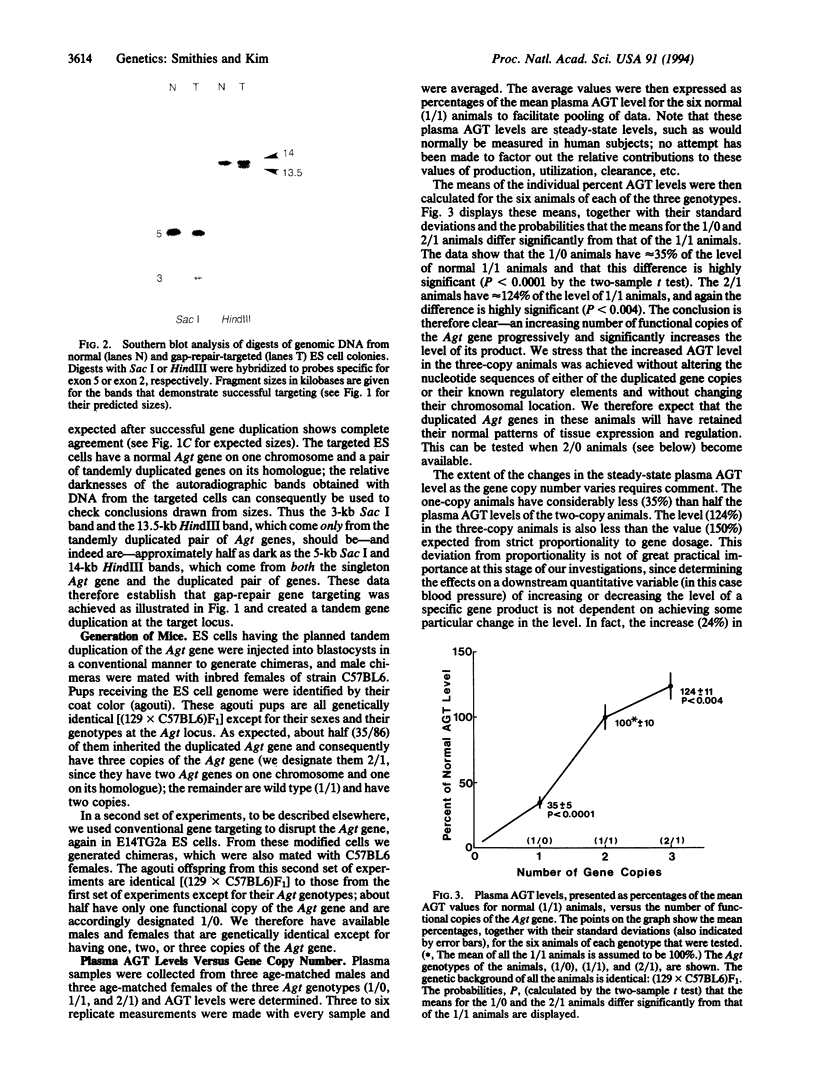
Experimental analysis of complex quantitative genetic traits, such as essential hypertension, should be greatly facilitated by being able to manipulate the expression of a gene in living animals without altering the nucleotide sequence, chromosomal location, or regulatory elements of the gene. To explore this possibility, we have used targeted gene disruption and duplication to generate mice that are genetically identical [(129 x C57BL6)F1] except for having one, two, or three functional copies of the gene coding for angiotensinogen. The two-copy animals have two normal copies of the angiotensinogen gene; the one-copy and three-copy animals have one normal copy with the other either disrupted or duplicated by gene targeting. The duplicated pair of genes was generated by a special form of gap-repair gene targeting that tandemly duplicates the whole of a gene together with 5' and 3' flanking regions. We find progressively and significantly higher levels of the gene product in the animals having increasing numbers of gene copies: the one-copy animals have steady-state plasma angiotensinogen levels approximately 35% of normal (P < 0.0001), and the three-copy animals have levels approximately 124% of normal (P < 0.004). Detailed information about regulatory sequences is not required for this type of experiment; nor is it necessary to have DNA clones or targeting constructs that cover the whole of the target gene. Varying gene copy numbers by targeting consequently offers a promising approach to quantitative genetics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs S. S., Gregg R. G., Borenstein N., Smithies O. Efficient transformation and frequent single-site, single-copy insertion of DNA can be obtained in mouse erythroleukemia cells transformed by electroporation. Exp Hematol. 1986 Nov;14(10):988–994. [PubMed] [Google Scholar]
- Clouston W. M., Evans B. A., Haralambidis J., Richards R. I. Molecular cloning of the mouse angiotensinogen gene. Genomics. 1988 Apr;2(3):240–248. doi: 10.1016/0888-7543(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Iwao H., Nakamura N., Kim S., Ikemoto F., Yamamoto K., Schlager G. Renin-angiotensin system in genetically hypertensive mice. Jpn Circ J. 1984 Nov;48(11):1270–1279. doi: 10.1253/jcj.48.1270. [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X., Soubrier F., Kotelevtsev Y. V., Lifton R. P., Williams C. S., Charru A., Hunt S. C., Hopkins P. N., Williams R. R., Lalouel J. M. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992 Oct 2;71(1):169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Kim H. S., Latour A. M., Brigman K., Boucher R. C., Jr, Scambler P., Wainwright B., Smithies O. Toward an animal model of cystic fibrosis: targeted interruption of exon 10 of the cystic fibrosis transmembrane regulator gene in embryonic stem cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10730–10734. doi: 10.1073/pnas.88.23.10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valancius V., Smithies O. Double-strand gap repair in a mammalian gene targeting reaction. Mol Cell Biol. 1991 Sep;11(9):4389–4397. doi: 10.1128/mcb.11.9.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]