Abstract
Research has suggested that chronic low-level lead exposure diminishes neurocognitive function in children. Tests that are sensitive to behavioral effects at lowest levels of lead exposure are needed for the development of animal models. In this study we investigated the effects of chronic low-level lead exposure on exploratory activity (unbaited nose poke task), exploratory ambulation (open field task) and motor coordination (Rotarod task) in pre-adolescent mice. C57BL/6J pups were exposed to 0 ppm (controls), 30 ppm (low-dose) or 230 ppm (high-dose) lead acetate via dams’ drinking water administered from birth to postnatal day 28, to achieve a range of blood lead levels (BLLs) from not detectable to 14.84 μg dl−1). At postnatal day 28, mice completed behavioral testing and were killed (n = 61). BLLs were determined by inductively coupled plasma mass spectrometry. The effects of lead exposure on behavior were tested using generalized linear mixed model analyses with BLL, sex and the interaction as fixed effects, and litter as the random effect. BLL predicted decreased exploratory activity and no threshold of effect was apparent. As BLL increased, nose pokes decreased. The C57BL/6J mouse is a useful model for examining effects of early chronic low-level lead exposure on behavior. In the C57BL/6J mouse, the unbaited nose poke task is sensitive to the effects of early chronic low-level lead exposure. This is the first animal study to show behavioral effects in pre-adolescent lead-exposed mice with BLL below 5 μg dl−1.
Keywords: developmental lead exposure, mouse model, exploratory activity, neurobehavioral toxicity, cognition
Introduction
Substantial progress has been made in reducing the numbers of children exposed to higher levels of environmental lead; however, early chronic low-level lead exposure remains an unresolved child public health problem and child health disparity. Over the past 30 years more than 50 longitudinal and cross-sectional studies have shown that blood lead levels (BLLs) as low as 2 μg dl−1 are associated with lower measured intelligence, reduced neurocognitive function and/or impaired motor functions (Bellinger and Needleman, 2003; Canfield et al., 2003; CDC, 2005; Gilbert and Weiss, 2006; Jusko et al., 2008; Landrigan et al., 2006; Lanphear et al., 2005). In response to these findings, in January 2012, the Centers for Disease Control and Prevention (CDC) recommended 5 μg dl−1 as a reference value for identifying children with elevated blood lead (CDC, 2012). How and why early chronic low-level lead exposure alters behavior and brain is not yet understood however.
Very few animal studies have examined the effects on behavior at the lowest levels of lead exposure. In a recent comprehensive review of the literature, five animal studies were identified and included two mouse studies, two rat studies and one monkey study. In mouse studies, as compared with controls, developmental exposure to 27 ppm lead acetate delivered in dams’ drinking water, yielding mean BLL ≤ 10 μg dl−1 (specific BLL values not reported), was associated with decreased exploratory ambulation (open field task) and decreased motor coordination (Rotarod task) in adult male but not female C57BL/6J mice (Leasure et al., 2008). In a second mouse study, as compared with controls, adult BALB/c mice with developmental exposure to 20 ppm lead acetate delivered in dams’ drinking water (specific BLL values were not reported) had decreased exploratory ambulation (open field task) and decreased memory (water maze task) (Kasten-Jolly, Pabello, Bolivar, and Lawrence, 2012).
In a study of adult Wistar rats with and without exposure to 20 ppm lead acetate delivered in dams’ drinking water during development, low-level lead-exposed animals had decreased recognition memory (novel object recognition task), and increased exploratory ambulation (open field task) (Azzaoui, Ahami, and Khadmaoui, 2009). In another study of adult Sprague–Dawley rats exposed to 5 and 50 ppm lead acetate delivered in dams’ drinking water, the lowest exposure group had decreased exploratory ambulation on an open field task (Reiter, Anderson, Laskey, and Cahill, 1975) (specific BLL values not reported). In a study of adult Rhesus monkeys with developmental exposure to lead acetate (0.7 mg kg−1) and with mean BLL < 5 μg dl−1, exploration of a novel space was increased following habituation (Ferguson and Bowman, 1990).
In these five studies, the effects of chronic low-level lead exposure on behavior in adult animals were assessed. Consistent effects on memory and inconsistent effects on exploratory ambulation were observed. To increase relevance to the findings observed in the child clinical literature, and to promote the development of animal models, studies are needed examining effects of early chronic low-level lead exposure in young animals. The mouse as a model organism has several advantages. Mouse physiology, anatomy and genetics closely approximate human systems; the relatively short gestation and accelerated lifespan of mice reduce expense and improve feasibility. Importantly, studies of proteins that influence lead absorption, including δ-aminolevulinic acid dehydratase (ALAD) and metallothioneins, have been modeled in mice (Takahashi, 2012).
The goal of this study was to contribute to the small but growing animal literature of studies examining effects on behavior of early chronic low-level lead exposure yielding BLLs similar to those observed in the child clinical literature. We attempted to predict behavioral performance from BLL at death in pre-adolescent C57BL/6J mice, with and without early chronic low-level lead exposure. Behavioral tasks used in this study included exploratory ambulation (open field task), exploratory activity (unbaited nose poke task) and motor coordination (Rotarod task). We hypothesized linear inverse relationships between BLL and exploratory ambulation (number of quadrants crossed in open field), exploratory activity (number of nose pokes) and motor coordination (number of seconds on Rotarod).
Materials and Methods
Animals
This study was done in accordance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Research Council, 2011) and with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Texas at El Paso (UTEP). C57BL/6J mice were purchased at the Jackson Laboratory, and then bred and housed in the animal vivarium of the Biosciences Research Building at UTEP. Animals were housed in a room that was kept at a temperature that ranged from 20 to 26 °C and at a relative humidity that ranged from 30 to 70% with a 12 h light–dark cycle. Animals were housed in individual ventilated cages (22.22 cm × 36.83 cm × 13.97 cm) with an air handling that provided the air supply to the cages and exhausted the ammonia gases and CO2 buildup within the cage. All animals were fed ad libitum with Harlan irradiated global 18% protein rodent diet.
Dams were mated at postnatal day (PND) 30. Each dam was housed with a male mouse in individual cages until a vaginal plug was detected, at which time the male mouse was removed from the cage. At birth, eight unculled litters (36 males and 25 females) were assigned to one of three treatment groups, including 0 ppm lead acetate (controls), 30 ppm lead acetate (low dose) and 230 ppm lead acetate (high dose).
Lead Exposure
Water bottles of experimental groups were filled with lead acetate treated distilled water (30 ppm or 230 ppm) at PND 0. Water bottles of control pups were treated with 30 ppm sodium acetate. Pups were weaned at PND 21, and males and females were separated and group housed. Exposure to lead continued until PND 28 when mice were placed in individual cages, tested behaviorally and killed.
Behavioral Tests
Mice completed three behavioral tests, including the open field task (5 min), the unbaited nose poke task (3 min) and the Rotarod task (four trials of maximum 300 s each). All animals completed the three tests in the same order (open field, nose poke and Rotarod) with a 5 min break between tasks. Animals completed behavioral testing in a room with standard-level fluorescent overhead lighting. All testing occurred between 10.00 and 13.00 h.
Nose Poke Task
The test was conducted in a 16 × 16 × 16 inch square Plexiglas arena with a fitted raised platform with 16 evenly spaced unbaited holes (1 inch in diameter). At the start of testing, each mouse was released into the lower right corner of the arena and left to explore the platform freely for 3 min. A video camera mounted at the level of the platform recorded each testing session. Following the completion of all behavioral testing, raters trained to reliability and blind to experimental condition rated the number of nose pokes (head dips) per minute. Video recordings for each mouse were projected on to a 45 × 60 in high-resolution and high-reflectivity projection screen by a Toshiba DLP high-resolution projector.
Open Field Task
The test was conducted in a 16 × 16 × 16 inch square Plexiglas arena with four quadrant markings. At the start of testing, each mouse was released into the lower right corner of the field and allowed to explore it freely for 5 min.
A video camera mounted at the level of the open field recorded each testing. Following the completion of all behavioral testing, raters trained to reliability and blind to experimental condition rated the number of crosses from one quadrant into another with all four paws. Video recordings for each mouse were projected on to a 45 × 60 inch high-resolution and high-reflectivity projection screen by a Toshiba DLP high-resolution projector.
Rotarod Task
The Rotarod device used in this study was stand-alone unit designed for mouse testing (Med Associates, Inc., St. Albans, VT, USA), including five testing stations each with its own beam-activated timer. The rotating barrel was 3.2 cm in diameter and the lane widths were 5.7 cm; the fall height was 16.5 cm. Mice were tested on an accelerating rod that increased from 3.5 to 35 rpm over the 300 s trial. Three researchers observed the mice throughout the procedure and noted session details. At the start of each trial, researchers placed one mouse per station facing towards the wall and the motor was started. Mice were given a 10 s stabilization period on the rod before trial timing began. If a mouse fell during the 10 s stabilization period, the mouse was replaced on the rotating rod. After the 10 s stabilization, a mouse drop from the rod triggered the electric beam and stopped the timer at the bottom of each station. The trial time for each mouse was recorded. Each mouse completed four trials for a maximum of 300 s per trial, with a 3 min inter-trial interval.
Blood Collection
After behavioral testing was completed, animals were anesthetized with Avertin ranging from 5 to 10 ml depending on body weight. When mice were unresponsive to corneal touch and paw pinch tests, mice were sexed and weighed. The chest was opened and heart blood was extracted via syringe puncture at the heart apex, yielding approximately 50 μl of whole blood per animal. Blood samples were refrigerated until processing for inductively coupled plasma mass spectrometry (ICP-MS) analysis, which occurred with 72 h of sample collection.
Inductively Coupled Plasma Mass Spectrometry Analysis of Blood Lead
A complete description of ICP-MS apparatus and procedures were previously provided (Sobin, Parisi, Schaub, and de la Riva E, 2011a). Briefly, an Agilent 7500ce ICP-MS with an octopole reaction system and a CETAC ASX-520 autosampler was used (Agilent Technologies, Inc., Santa Clara, CA). A Micro Mist U-series nebulizer and a double-pass quartz spray chamber were used to introduce the samples into plasma. The instrument parameters were a carrier gas of 0.78 l min−1, makeup gas of 0.15 l min−1, RF power of 1420 W and a spray chamber that was set at a temperature of 2 °C. For sample processing, a propylene tube was filled with 5.58 ml of water, 300 μl of blood, 60 μl of aqueous internal standard solution and 60 μl of aqueous 10 ppm gold in 3% hydrochloric acid solution. The samples were vortexed and centrifuged for 1 min at 2000 g and the supernatant was analyzed by ICP-MS. BLLs were determined in μg dl−1.
Data Analyses
Data were analyzed using SAS Version 9.3 and R (R Development Core Team, 2009) with package lme4 (Bates, Maechler, and Bolker, 2011). Distributions of variables were examined and residual plots were visually inspected. No obvious deviations from homoscedasticity or normality were observed. BLLs and body weights were compared for males and females by exposure group. Generalized linear mixed model regression analyses were used with BLL, sex and the interaction as fixed effects and litter as the random effect (to account for possible non-independence of behavior among litter mates). Random intercepts and random slopes were included. For each of the three behavioral tests, one dependent variable (task parameter score) was initially tested. When a significant effect was observed, additional models were conducted to test for effects during each minute of task performance. The purpose of this approach was to determine whether behavioral differences occurred consistently throughout the task, or were evident only in selected task segments. Discrete data (number of nose pokes and number of quadrants crossed) were modeled with a Poisson distribution; Rotarod time was modeled with a Gaussian distribution. Residual pseudo-likelihood estimation and dual quasi-Newton optimization were used. Fit was evaluated by examining the ratio of the generalized chi-squared test to its degrees of freedom; a value close to 1.0 indicated that variability in the data had been adequately modeled with no residual overdispersion (Schabenberger, 2005). Covariance parameter estimates were evaluated. Finally, the significance of added variance explained in models with vs. without BLL was determined in two ways, by evaluation of the Akaike Information Criterion (AIC) value decrease (>2 indicating significant model improvement) (Burnham and Anderson, 2002), and the reduction in model deviance tested with the likelihood ratio test (P < 0.05) (Bolker et al., 2009; Pinheiro and Bates, 2000).
Results
Animals
Eight unculled litters (36 males and 25 females) were randomly assigned to one of three treatment conditions resulting in 19 control animals (eight males, 11 females), 26 low-dose animals (16 males, 10 females) and 16 high-dose animals (12 males, four females). Three treatment conditions were used to produce a distribution of BLLs for regression analyses. Lead at the levels administered in this study had no observable effects on mortality or morbidity of the exposed mice, and had no observable adverse physical effects, such as abnormal motor function or ataxia. Table 1 shows BLLs after 28 days of lead exposure treatment and body weights at death in males and females. As shown, BLLs and body weights differed between exposure groups, and body weight was dependent on BLL (males, r = −0.77; females r = −0.78). The ranges of BLL for males and females did not differ significantly (means ± SD for males = 4.9 ± 3.7 and females = 3.3 ± 4.3, t59 = 1.59, P = NS). All animals completed the open field and nose poke tasks. One control animal did not stabilize on the rotating rod (repeatedly fell off during the 10 s stabilization period) and did not complete the task. Overall, the results showed that BLL affected nose poke behavior; no effects of BLL on open field behavior or Rotarod behavior were observed. Table 2 shows the means ± SD from each of the behavioral tests for males and females, illustrated in Figs. 1–3.
Table 1.
Blood lead levels and body weight of males and females by exposure group after 28 days of lead exposure
| Lead exposure group | Blood lead levels (μg dl−1)
|
Body weight (g)
|
||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| 230 ppm | 9.39 ± 1.90 | 12.14 ± 2.90 a** b** | 8.65 ± 1.94 | 8.83 ± 1.11d** |
| 30 ppm | 3.93 ± 0.91 | 3.19 ± 0.75c** | 14.97 ± 1.59 | 12.50 ± 1.82 |
| 0 ppm | 0.20 ± 0.12 | 0.19 ± 0.09 | 15.75 ± 1.57 | 13.93 ± 1.09 |
Each value represents the least square mean ± SD of the blood lead levels (μg dl−1) and body weight (g) for males and females by lead exposure group.
Compared with 30 ppm group and 0 ppm group.
Compared with 230 ppm lead-exposed males.
Compared with 0 ppm group.
Compared with 30 ppm group and 0 ppm group.
P < 0.001.
Table 2.
Performance for males and females on the three behavioral tests with and without detectable blood lead measured after 28 days of exposure to lead acetate or sodium
| Behavioral test | Mean ± SD
|
||
|---|---|---|---|
| Total | Males | Females | |
| Nose poke (number of head-dips) | 12.01 ± 4.38 | 11.67 ± 3.99 | 12.51 ± 4.93 |
| Open field (quadrants crossed) | 13.89 ± 4.05 | 13.70 ± 3.97 | 14.18 ± 4.24 |
| Rotarod (seconds spent on the rod) | 90.42 ± 55.68 | 78.37 ± 40.77 | 107.28 ± 68.98 |
Each value represents the mean ± SD for the performance of males and females on each of the behavioral tests.
Figure 1.
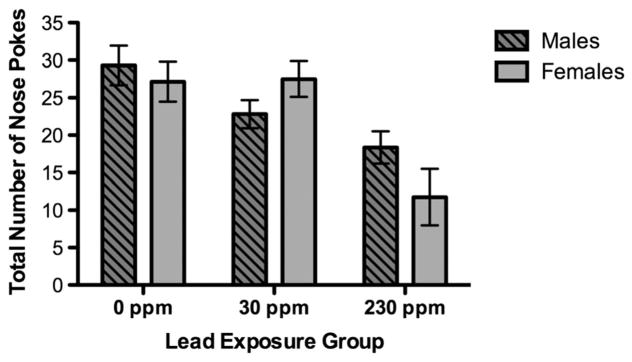
Total number of nose pokes of males and females by lead exposure group during 3 min. Data are expressed as least square means ± SEM 230 ppm (12 males, four females), 30 ppm (16 males, 10 females) and 0 ppm (eight males, 11 females).
Figure 3.
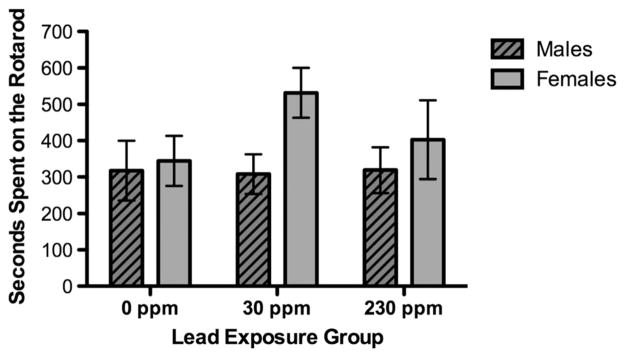
Seconds spent on the Rotarod of males and females by lead exposure group. Data are expressed as least square means ± SEM 230 ppm (12 males, four females), 30 ppm (16 males, 10 females) and 0 ppm (eight males, 11 females).
Nose Poke Task
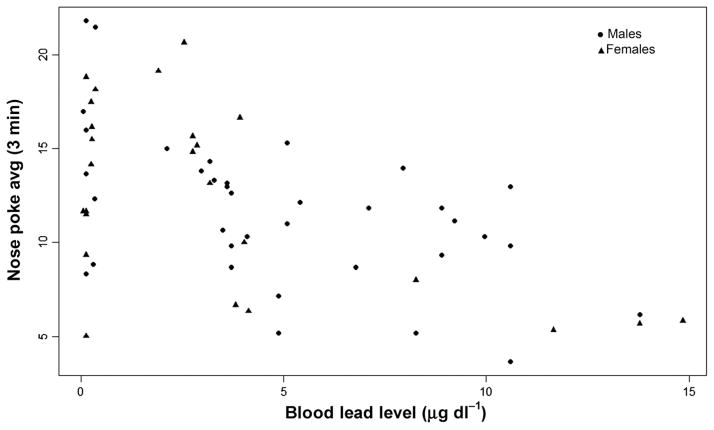
Animals were placed in the lower right corner of the arena. All animals moved either horizontally or vertically from the initial placement corner moving at a relatively steady pace, moving from hole to hole. There were no notable differences in how animals completed the task and no animals fell through the unbaited holes to the arena floor below. The model solution for nose poke average required five iterations with no restarts, and convergence criterion met. The fit statistics suggested that the variability was adequately modeled (chi-squared/df. = 1.4). The litter random effect covariance parameter estimate was small (est = 0.06, SE = 0.04). Controlling for litter, the main effect for BLL was significant (type III SS F = 4.60, P = 0.037); no effect was observed for sex (F = 0.49), or the interaction of BLL by sex (F = 0.73). Comparison of models without (AIC = 334.4) and with (AIC = 331.6) BLL showed that BLL affected the nose pokes (chi-squared/1 = 4.79, P = 0.03), lowering the average by approximately 0.55 ± 0.26. Figure 4 shows that as BLL increased, the number of nose pokes decreased for males and females. Given the significant findings for the model testing average nose pokes, each minute of nose poke performance was also modeled to determine whether effects were present consistently throughout the task. For the model examining effects of BLL on nose pokes during minute 1 (five iterations, no restarts, convergence criterion met), fit statistics suggested that the variability was adequately modeled (chi-squared/df. = 0.70) and the litter random effect covariance parameter estimate was small (est = 0.03, SE = 0.03). Controlling for litter, a main effect for BLL was found (type III SS F = 10.6, P < 0.01), with no effect for sex (F = 0.33) and no effect for the interaction (F = 0.31). Comparison of models without (AIC = 348.4) and with (AIC = 341.4) BLL showed that BLL affected nose pokes during minute 1 (chi-squared/1 = 9.01, P = 0.003), lowering the average by approximately 0.71 ± 0.17. During minute 1 of the nose poke task, as BLL increased, the number of nose pokes decreased for males and for females. For the nose poke task minute 2 model (six iterations no restarts, convergence criterion met), fit statistics suggested the data variability was adequately modeled (chi-squared/df. = 1.10) and the litter random effect covariance parameter estimate was small (est = 0.04, SE = 0.04). In contrast to minute 1, no effects for BLL (type III SS F = 0.58), sex (type III SS F = 0.02) or the interaction (type III SS F = 0.01) were observed for minute 2 nose pokes. Comparison of models without (AIC = 372.8) and with (AIC = 373.9) BLL confirmed that BLL did not affect nose pokes during minute 2 (chi-squared/1 = 0.90, P = NS). Thus, there was no measureable association between BLL and nose poke behavior during minute 2. For the nose poke task minute 3 model (six iterations, no restarts, convergence criterion met) fit statistics again suggested that the data variability was adequately modeled (chi-squared/df. = 0.89) and the litter random effect covariance parameter estimate was small (est = 0.06, SE = 0.05). Similar to minute 1, in minute 3, a main effect for BLL was observed (type III SS F = 12.7, P < 0.01), with no effect for sex (F = 0.09) or the interaction (F = 1.42). Comparison of models without (AIC = 364.5) and with (AIC = 360.9) BLL showed that BLL affected nose pokes during minute 3 of the task (chi-squared/1 = 5.63, P = 0.02), lowering the average by approximately 0.78 ± 0.19. As for behavior in minute 1 of the task, as BLL increased, the number of nose pokes during minute 3 decreased for males and for females.
Figure 4.
Association between blood lead levels and number of nose pokes during 3 min (total exploration). As blood lead levels increased, the number of nose pokes decreased for males and females (chi-squared/1 = 4.79, P = 0.03). Males (n = 36) and females (n = 25).
Open Field Task
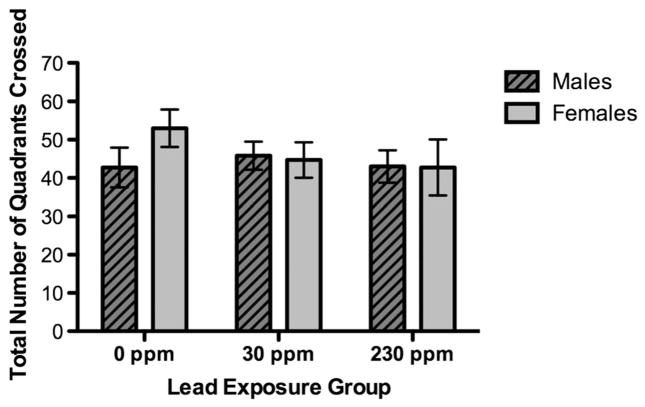
Animals were placed in the lower right corner of the arena and allowed to explore freely. The immediacy with which animals explored the open field varied considerably across animals; however, all animals moved in the space to some extent during each minute of the task. For the model testing average number of quadrants crossed (seven iterations, no restarts, convergence criterion met) fit statistics suggested that the data variability was adequately modeled (chi-squared/df. = 3.13) and the litter random effect covariance parameter estimate was small (est = 0.04, SE = 0.03). No effects were observed for BLL (type III SS F = 0.37), sex (F = 1.71) or the interaction (F = 1.73). Comparison of models with and without BLL confirmed that BLL did not affect open field behavior (chi-squared/1 = 0.38, P = NS).
Rotarod Task
Animals were placed on the rod in one of five bin sections by three researchers and positioned to be facing away from the experimenters. Placing the mice took less than 20 s; when all mice were in place, the rod was started. Mice were allowed a 10 s stabilization period and after this, task timing began. Twelve animals (20%) fell and had to be replaced on the rod during the stabilization period; one animal fell twice and then completed the task. One control animal fell repeatedly and did not complete the task. Timing began after the 10 s stabilization. The ranges of time on the accelerating rod were large for all groups across all trials. Among the control animals, time on the rod ranged from 2 to 300 s; among low-dose animals from 3 to 300 s; and among high-dose animals from 10 to 300 s. For trial 1, no animal stayed on the rod for the full trial period (300 s). For trials 2 and 3, five animals stayed on the rod for each full trial period; for trial 4, seven animals stayed on the rod for the full trial period. No animal stayed on the rod for the full period more than once. For the model of average time on rod (in seconds) for four trials (four iterations, no restarts, convergence criterion met) fit statistics suggested that the data variability was not adequately modeled (chi-squared/df. = 42764). The litter random effect covariance parameter estimate was large and the standard error exceeded the parameter (est = 8145, SE = 8437). The model was not evaluated further (and did not suggest significant main effects).
Discussion
The findings from this study contribute to the small but growing number of animal studies investigating the effects on behavior of early chronic low-level lead exposure yielding low BLLs. Our studies of minority children living in lower socio-economic conditions showed that 60% had BLLs ≥ 2.5 μg dl−1 as determined by ICP-MS (Sobin, Parisi, Schaub, Gutierrez, and Ortega, 2011b) and suggested that large numbers of lower-income minority children are likely to be exposed to low-level environmental lead. Animal models are needed to understand how early chronic low-level lead exposure alters development, and behavioral tests sensitive to very low-level lead exposure are needed. The two previous mouse studies examining effects of early chronic low-level lead exposure suggested that early chronic low-level lead exposure reduced exploratory ambulation (open field task) in adult animals (Kasten-Jolly et al., 2012; Leasure et al., 2008). The lack of significant effects for exploratory ambulation in the present study of pre-adolescent animals suggested that some effects of early chronic low-level lead exposure might not emerge until adulthood. Additional studies are needed to determine whether behavioral differences observed at pre-adolescence are sustained into adulthood, whether early chronic low-level lead exposure disrupts pathways associated with exploratory activity at pre-adolescence and whether early exposure predisposes animals to poorer behavioral and brain resilience in late life. For these studies, we administered two standard measures of exploration, the open field task and the unbaited nose poke task (Wahlsten, 2011). These tasks are used together to differentiate exploratory ambulation (horizontal locomotion) from exploratory activity (head-dips in unbaited holes) (Hoffman, Hornig, Yaddanapudi, Jabado, and Lipkin, 2004). Exploratory animal behaviors are critical for survival, reflect adaptation, learning and memory, and provide the means by which animals acclimate to a new environment and monitor a known environment. Horizontal movement in an open arena provides a measure of the simplest type of exploration via ambulation. Complex exploratory activity such as head-dipping during ambulation represents the animal’s behavioral resolution of an approach-avoidance conflict in a novel environment. Studies have shown that in a novel environment, animals simultaneously experience curiosity and fear (Hughes, 2007). The completion of a nose poke is interpreted as curiosity over-riding fear, and thus the animal’s capacity for this to occur. In this study, early chronic exposure to low-level lead decreased this exploratory activity in pre-adolescent lead-exposed animals. To explain the observed association, we speculated that in pre-adolescent mice, lead exposure somehow disrupted the capacity for curiosity to override fear, at the level observed in the control animals. To explore this possibility, additional studies are needed to distinguish effects of low-level lead exposure on fear vs. curiosity. Interestingly, the significant inverse associations between BLL and exploratory activity were observed during minute 1 and minute 3, and not during minute 2 of the nose poke task. The amount of activity for control mice was consistent across all minutes of the task, and for all mice, consistent within minute 2. For this reason, we speculated that the results observed in minute 1 vs. minute 3 might reflect disruption of two distinct processes. As discussed above, during initial exploration of the novel space (task minute 1), less exploratory activity in lead exposed as compared with control animals could have resulted from fear predominating over curiosity, which resolved by task minute 2, as suggested by similar exploratory activity among all animals in this epoch. If fear in fact had resolved by task minute 2, then fewer nose pokes in lead-exposed animals observed during task minute 3, rather than indicating the predominance of fear over curiosity, may have indicated loss of curiosity relative to the control animals. This is of course speculative. As suggested above, studies are needed to tease apart the possible effects of low-level lead exposure on the fear vs. curiosity components of exploration. The C57BL/6J mouse was selected for these studies because this outbred strain is a well-characterized animal model for neuroscience and toxicology research. Studies of metal-binding proteins such as ALAD and metallothioneins that influence lead absorption, and thus its neurotoxicity, have been modeled in mice (Gonick, 2011). Furthermore, studies have suggested that metallothioneins and ALAD dynamics in mice are highly comparable to those in humans (Gonick, 2011; Kenaga, Cherian, Cox, and Oberdorster, 1996; Takahashi, 2012) suggesting the value of a mouse model for understanding the effects of chronic low-level lead exposure on behavior. For future studies, it may be useful to consider briefly a few possible mechanistic sources of the behavioral differences observed. It has been suggested that activation of amygdalar GABA neurons, which underlie the fear response, also influence head-dipping (“nose poke”) behavior in novel environments (Takeda, Tsuji, and Matsumilla, 1998). Thus, it could be useful to investigate whether early chronic low-level lead exposure specifically targets amygdalar GABA pathways. In addition, acetylcholine pathways linking the hippocampus and entorhinal cortex support and influence differences in exploratory activity in a novel environment, and specifically, curiosity for a novel environment in the nose poke task (Brodkin, 1999; Crusio, 1995). Studies of these specific pathways could build on the current findings and contribute to the development of an animal model of early chronic low-level lead exposure. Perhaps consistent with these findings, memory impairments have been found in children with BLLs similar to those of the low-dose mice in this study (<5 μg dl−1) (e.g., Min et al., 2007; Surkan et al., 2007). In addition, in brain studies of pre-adolescent C57BL/6J mice exposed to chronic low-level lead, abnormalities in hippocampus/dentate gyrus were identified (Sobin et al., 2013). The results of these studies should be interpreted cautiously. These studies used unculled litters and the number of females across the exposure groups was unbalanced. Sex was included as a control factor in all models tested and the lack of effects observed for sex may be attributable to lower numbers of females with higher-level exposure. Additional studies with balanced numbers of males and females at higher levels of exposure are needed. In addition, the statistical model tested for Rotarod performance did not return an adequate fit suggesting that different tests of motor coordination may be needed. Whether chronic low-level lead exposure disrupts motor coordination cannot be determined from these studies. Finally, this study examined behavior at one time point (pre-adolescence). Additional studies are needed to replicate these findings, and to examine effects of early chronic low-level lead exposure on exploratory activity at different developmental stages, perhaps using observational measures of home-cage behavior for younger mice.
Conclusion
Early chronic low-level lead exposure yielding BLLs ranging from 1.98 to 14.84 μg dl−1 disrupt exploratory activity in pre-adolescent mice. The effects of early chronic low-level lead exposure on pre-adolescent behavior can be meaningfully modeled in the C57BL/6J mouse.
Figure 2.
Total number of quadrants crossed of males and females by lead exposure group during 5 min. Data are expressed as least square means ± SEM 230 ppm (12 males, four females), 30 ppm (16 males, 10 females) and 0 ppm (eight males, 11 females).
Acknowledgments
The authors would like to acknowledge Mari Golub, Environmental Toxicology, UC Davis, for her assistance in the preparation of the final manuscript. This research was made possible by grants from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, (R21HD060120, CS, PI); the National Center for Research Resources, a component of the National Institutes of Health (5G12RR008124); the Center for Clinical and Translational Science, The Rockefeller University, New York, New York; the Paso del Norte Health Foundation, El Paso, Texas; and from funding sources at the University of Texas, El Paso, including the Border Biomedical Research Center (BBRC); the University Research Institute (URI); and by funds provided from the J. Edward and Helen M.C. Stern Endowed Professorship in Neuroscience (CS). The funders had no role in the design, collection, data analyses and interpretation, implementation and manuscript preparation for this study, or the decision to submit the article for publication.
Footnotes
Conflict of Interest
The Authors did not report any conflict of interest.
References
- Azzaoui FZ, Ahami AO, Khadmaoui A. Impact of lead sub-chronic toxicity on recognition memory and motor activity of Wistar rat. Pak J Biol Sci. 2009;12:173–177. doi: 10.3923/pjbs.2009.173.177. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. R package version 0.999375-42. 2011. lme4: Linear mixed-effects models using S4 classes. [Google Scholar]
- Bellinger DC, Needleman HL. Intellectual impairment and blood lead levels. N Engl J Med. 2003;349:500–502. doi: 10.1056/NEJM200307313490515. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Brodkin J. Assessing memory in mice using habituation of nose-poke responding. Behav Pharmacol. 1999;10:445–451. doi: 10.1097/00008877-199909000-00002. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multi-model Inference: A practical information-theoretic approach. Springer-Verlag; New York: 2002. [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 micrograms per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. A review of evidence of health effects of blood lead levels < 10 micrograms per deciliter in children. 2005 Retrieved from http://www.cdc.gov/nceh/lead/ACCLPP/meetingMinutes/lessThan10MtgMAR04.pdf.
- Centers for Disease Control and Prevention. CDC response to advisory committee on childhood lead poisoning prevention recommendations in “low level lead exposure harms children: A renewed call of primary prevention”. 2012 Retrieved from http://www.cdc.gov/nceh/lead/ACCLPP/Final_Document_030712.pdf.
- Crusio WE. Behavioural Brain Research in Naturalistic and Seminaturalistic Settings. Kluwer Academic Press; The Netherlands: 1995. Natural selection on hippocampal circuitry underlying exploratory behaviour in mice: Quantitative-genetic analysis; pp. 323–342. [Google Scholar]
- Ferguson SA, Bowman RE. Effects of postnatal lead exposure on open field behavior in monkeys. Neurotoxicol Teratol. 1990;12:91–97. doi: 10.1016/0892-0362(90)90118-v. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 μg/dL. Neurotoxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonick HC. Lead-binding proteins: A review. J Toxicology. 2011;2011:686050. doi: 10.1155/2011/686050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), & National Academies Press (U.S.) Guide for the care and use of laboratory animals. National Academies Press; Wasingthon DC: 2011. [Google Scholar]
- Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. 2004;24:1780–1791. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Neotic preferences in laboratory rodents: Issues, assessment and substrates. Neurosci Biobehav Rev. 2007;31:441–464. doi: 10.1016/j.neubiorev.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Jr, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 μg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten-Jolly J, Pabello N, Bolivar VJ, Lawrence DA. Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. Neurotoxicology. 2012;33:1005–1020. doi: 10.1016/j.neuro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenaga C, Cherian MG, Cox C, Oberdorster G. Metallothionein induction and pulmonary responses to inhaled cadmium chloride in rats and mice. Fundam Appl Toxicol. 1996;30:204–212. doi: 10.1006/faat.1996.0057. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Trasande L, Thorpe LE, Gwynn C, Lioy PG, Lipkind HS, Susser E. The national children’s study: A 21-year prospective study of 100 000 American children. Pediatrics. 2006;118:2173–2186. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Jr, Pothakos K, Lau YS, Fox DA. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J-Y, Min K-B, Cho S-I, Kim R, Sakong J, Paek D. Neurobehavioral function in children with low blood lead concentrations. Neurotoxicology. 2007;28:421–425. doi: 10.1016/j.neuro.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed Effects Models in S and S-Plus. Springer-Verlag; New York: 2000. [Google Scholar]
- Reiter LW, Anderson GE, Laskey JW, Cahill DF. Developmental and behavioral changes in the rat during chronic exposure to lead. Environ Health Perspect. 1975;12:119–123. doi: 10.1289/ehp.7512119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabenberger O. Introducing the GLIMMIX procedure for generalized linear mixed models. Proceedings of the Thirtieth Annual SAS User’s Group International Conference; 2005. pp. 196–305. [Google Scholar]
- Sobin C, Parisi N, Schaub T, de la Riva E. A Bland-Altman comparison of the Lead Care(R) System and inductively coupled plasma mass spectrometry for detecting low-level lead in child whole blood samples. J Med Toxicol. 2011a;7:24–32. doi: 10.1007/s13181-010-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Parisi N, Schaub T, Gutierrez M, Ortega AX. delta-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 and peptide transporter 2*2 haplotype may differentially mediate lead exposure in male children. Arch Environ Contam Toxicol. 2011b;61:521–529. doi: 10.1007/s00244-011-9645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Montoya MG, Parisi N, Schaub T, Cervantes M, Armijos RX. Microglial disruption in young mice with early chronic lead exposure. Toxicol Lett. 2013;15:00151–00153. doi: 10.1016/j.toxlet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels < 10 μg/dL. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S. Molecular functions of metallothionein and its role in hematological malignancies. J Hematol Oncol. 2012;5:41. doi: 10.1186/1756-8722-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol. 1998;350:21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Mouse Behavioral Testing: How to use mice in behavioral neuroscience. Academic Press; New York: 2011. [Google Scholar]