Abstract
BACKGROUND
Previous research has suggested possible sex-related differences in executive functioning among cocaine users; however, no studies specifically explain sex-related differences in neurocognitive impairment (NCI) among cocaine users receiving clinical care. Knowledge about this association can aid in the development of targeted prevention strategies to reduce adverse health outcomes. This study was designed to examine the sex-related differences in NCI among high-risk cocaine users receiving substance-abuse treatment.
METHODS
The Neuropsychological Impairment Scale (NIS) was administered to 199 cocaine users (98 men; 101 women), receiving methadone maintainance treatment, to assess self-reported NCI by identifying the patients’ awareness of neuropsychological symptoms. We used T-test comparison to find differences in NCI between men and women and multiple regression analysis to explore the relative contribution of sex to NCI.
RESULTS
Consistent with prior work, high NCI was evident within this sample, as indicated by high scores on most of the NIS subscales. Women reported greater impairment than men, as evidenced by significantly higher scores on several NIS subscales, after controlling for demographic and other confounding variables. Interestingly, cocaine craving significantly predicted NCI among men but not among women, as suggested by the significant association between cocaine craving and all except one of the NIS subscales.
CONCLUSIONS
These findings suggest that cocaine users enter into treatment with a range of NCI – with women having significantly more neurocognitive deficits than men – that may contribute to differential treatment outcomes. This highlights the need to include additional services such as neuropsychological screening and sex-specific treatment programs to optimally reduce adverse health outcomes in these high-risk, cognitively impaired patients.
Keywords: cocaine, neurocognitive impairment, sex-related differences, substance abuse, methadone maintainance patients, behavioral intervention
Introduction
Cocaine is one of the most commonly abused psychoactive substances in the United States. The National Survey on Drug Use and Health (NSDUH) estimated that in 2012 there were over 1.8 million current cocaine users, of which 639,000 persons had used cocaine for the first time within the past 12 months; this averages to approximately 1,800 initiates per day.1 Studies on the neurocognitive effects of cocaine abuse have shown a significant association between neuropsychological impairment and affective dysfunction.2–5 Paralleling the effects on cognitive performance, evidence from magnetic resonance imaging (MRI) studies has shown that repeated exposure to cocaine produces significant structural and metabolic abnormalities in the brain, predominantly in the areas considered crucial for executive control.2,3,6–12 Given its addictive potential and negative health consequences, cocaine abuse has been regarded as a major public health issue.13
Accumulating research suggests that there are important differences in the patterns of cocaine abuse and addiction between the sexes. For example, women are more likely to have an earlier cocaine use debut than men,14,15 and women are just as likely as men to advance from first cocaine use to dependence16 but tend to experience a more rapid progression to the negative consequences associated with cocaine dependence (ie, “telescoping effect”).17,18 Additionally, some studies report that women seeking treatment tend to have more severe cocaine use problems than men, and have a higher occurrence of comorbid psychiatric conditions such as anxiety disorder, depression, and posttraumatic stress disorder.19–21 This vulnerability of women might be influenced by sex-related differences in cognitive impairment among cocaine users. Consistent with this assumption, a study by van der Plas et al found that decision making was significantly more impaired in women addicted to cocaine as compared to men.22
These findings point to possible sex-related interactions between cocaine use and cognitive deficits; however, they do not specifically explain sex-related differences in neurocognitive impairment (NCI) among chronic cocaine users. Moreover, cocaine use is highly prevalent among patients receiving substance-abuse treatment and therefore is a clinical concern.23 Nevertheless, the interplay of this relationship remains untested among cocaine-using patients receiving clinical care. Therefore, studying sex-related differences in NCI among high-risk cocaine users receiving substance-abuse treatment can aid in the development of targeted prevention strategies.
Accordingly, the objectives of the current study were to examine the sex-related differences in neurocognitive impairment among a sample of cocaine users receiving substance-abuse treatment (ie, methadone maintenance treatment; MMT) and to explore the relative contributions of cocaine-use variables and sex on self-reported NCI. We formulated two main hypotheses: 1) cocaine users participating in methadone maintainance treatment program will have high levels of neurocognitive impairment, and 2) compared to males, female cocaine users participating in methadone maintainance treatment program will have a higher degree of neurocognitive impairment.
Methods
Design
This was a retrospective secondary analysis of a subset of data collected from the parent study, which was a randomized clinical trial of a behavioral HIV-risk reduction intervention designed to reduce HIV risk among high-risk, drug-dependent individuals participating in drug treatment.24 The study protocol was approved by the Investigational Review Board (IRB) at the University of Connecticut, the Human Investigation Committee at Yale University, and received board approval from the APT Foundation MMP, Inc. and the study complied with the principles of the Declaration of Helsinki. Variables for cocaine use had been created for the primary study, and self-reported NCI was assessed using the Neuropsy-chological Impairment Scale (NIS) described below.25
Sample
The parent study included 304 HIV-negative individuals, without any psychiatric comorbidities, enrolled in a community-based methadone maintenance treatment (MMT) program who reported sex- or drug-related HIV transmission risk behavior.24 For the purpose of this study, we included subjects who reported using cocaine in the past week. Among the 199 individuals who met the inclusion criteria for this study, 98 (49.2%) were male. One-hundred and forty-six subjects (73.4%) were white, 18 (9%) were African-American, 30 (15.1%) were Hispanic, and 5 (2.5%) described themselves as “other” minority. The subjects’ average age was 33.9 (±9.5), years and the majority of the participants (67.8%) were never married. One-hundred and forty-nine subjects (74.9%) were high school graduates, and 156 (78.4%) were unemployed.
All subjects were enrolled in an inner-city MMT program and were maintained on a stable dose of methadone. The mean (±SD) daily methadone dose was 57.2 (±24.9) mg. The subjects had been using cocaine regularly for about 11.9 (±8.8) years. Forty-three (21.6%) subjects used cocaine by intravenous route of administration, 112 (56.3%) by smoking, and 44 (22.1%) intranasally. During the week prior to entering the study, subjects used 4.2 (±12.4) g of cocaine over a period of 0.9 (±1.7) days per week. Cocaine craving, which was assessed by asking the subjects to rate their desire for cocaine in the scale of 1 to 5 (1 = “not at all” and 5 = “extreme”) during the previous week, was found to be “extreme” in 7 (3.5%) subjects and “not at all” among 117 (58.8%) subjects.
Assessment instrument
Neurocognitive measures
The NIS was administered to patients individually upon meeting the criteria for the study. It is a brief, self-report screening measure used to assess NCI by identifying patients’ awareness of neu-ropsychological symptoms25 and is generally used as a screening instrument in clinical settings. Although clinical diagnosis of NCI requires the use of a more comprehensive assessment battery of instruments, recent studies have stressed the need for rapid screening tools.26 Validity studies that have compared the NIS with tests known to assess various cognitive impairments have found the NIS to be both a valid and reliable predictor of NCIs in diverse group of patient population.25,27–30
The NIS is composed of 95 items rated on 5-point scales, ranging from 0 (not at all) to 4 (extremely). These responses are used to compute three summary scores and seven clinical subscale scores. There are also validity checks, which provide a background for the interpretation of the other scores (Table 1).28 For each subscale, sums of the point scale scores were totaled and then transformed to T-scores, which were used in the current analysis as a continuous variable. Generally, high levels on any of these scores are an indication of NCI. NIS scores less than 30T were considered low, scores between 30T and 50T were considered average, scores between 50T and 60T were considered high, and any score above 60T was considered very high.31
Table 1.
Subscale scores provided by the Neuropsychological Impairment Scale (NIS).
| Three summary scores | |
| GMI | Global measure of impairment: Provides best general index of neuropsychological functioning. |
| TIC | Total number of symptoms endorsed. |
| SIM | Symptom intensity measure: ratio of GMI and TIC. |
| Seven clinical subscales | |
| COG | Cognitive efficiency: Assesses general symptoms of NCI (eg, “I get confused easily”). |
| CRIT | Critical items: Assesses patient’s history of neurological illness or injury (eg, “part of my body feels numb”). |
| ATT | Attention: Assesses patient’s ability to attend and concentrate (eg, “I have trouble concentrating”). |
| MEM | Memory: Assesses patient’s memory (eg, “I have trouble remembering important things”). |
| L-V | Learning-verbal: Assesses learning and expressive speech (eg, “I have trouble learning new things”). |
| FRU | Frustration tolerance: Assesses irritability, anger and temper (eg, “I feel easily annoyed and irritable”). |
| ACD | Academic skills: Assesses ability to carry out daily activities involving computing and reading (eg, “I have trouble understanding what I read”). |
| Three scales measuring test attitude | |
| DEF | Defensiveness: Provides indication of test-taking attitude (eg, “I like everyone I know”). |
| AFF | Affective disturbance: Provides an estimation of emotional state of the subject at the time of the test (eg, “I often feel sad and blue”). |
| INC | Internal inconsistency: Identifies inconsistent response pairs. |
Cocaine-use measures
For the purpose of this study, we used the constructs related to cocaine-use variables. For example, “What has been your primary method of cocaine use in the past 6 months?”, “For how many years have you been using cocaine?”, “To what extent were you experiencing cocaine craving in the past week?” “Do you plan to stop using cocaine completely in the next three months?”, and “When was the last time you used cocaine?” Cocaine-use items required the participants to categorize and quantify their use. Quantity and frequency data of cocaine use was collected for the past week. For example, “How many days did you use cocaine in the past week?” and “How much cocaine did you use in total in the past week?”
Procedure
Both the NIS and drug use measurements were assessed using the Audio Computer-Assisted Self-Interview (ACASI).24,31 For the purpose of this study, the NIS measurements – including seven impairment subscales, three validity scores, three summary scores (Table 1), and cocaine-use measurements – were taken only from the baseline assessment. All subjects were reimbursed for the time and effort needed to participate in the study, and this study was approved by the institution’s IRB.
Data analysis
Data analysis proceeded as follows: 1) T-tests (on continuous variables) and chi-square analyses (on categorical variables) were performed to determine whether the sex of participants were well-matched on relevant sociodemographic and drug use variables.) A t-test revealed that there was a difference between men and women in terms of age and years of cocaine use. Therefore, the effects of these characteristics as well as other variables including methadone dose, years in the MMT program, use of opiate in the past six months, and duration of opiate use were adjusted in all of our analyses. NIS summary scores, clinical subscale scores, and NIS test-taking attitude scores were then compared by sex using ANCOVA. 3) Multiple regression analysis was conducted to explore the relative contributions of cocaine-use variables and sex on self-reported NCI. 4) Regression analysis was conducted between NIS subscale scores and cocaine-use variables by sex in order to further explore sex-related differences between male and female cocaine users. Both subjective tests (visual examination of the standardized residual scatterplots) and objective tests (Shapiro—Wilk test) satisfied all assumptions of normality, linearity, and homoscedastic ity between the predicted dependent variable scores and errors of prediction. Furthermore, the Durbin–Watson test statistic revealed no correlation in adjacent residuals, and the variance inflation factor (VIF) and tolerance statistic suggested no problem with multicollinearity. P-Values were set at <0.05 for all our planned multiple measures.32–34 Effect sizes are reported as bivariate standardized beta coefficients in order to distinguish between different results with a clinical meaning.35 The effect sizes can be classified following Cohen’s classification as small, medium, and large effect size – 0.1, 0.3, and 0.5, respectively – for bivariate standardized regression coefficients.36,37 There were no missing values in the dataset. SPSS software, version 20.0, was used for all statistical analyses.
Results
Comparison between male and female participants on sociodemographics and drug-use variables
Aside from age and years of cocaine use, there was no significant difference between male and female participants. Men tended to be older and to have used cocaine for a longer time as compared to women (Tables 2 and 3).
Table 2.
Comparison between male and female participants on sociodemographic variables.
| VARIABLES | MALE | FEMALE | P |
|---|---|---|---|
| N | 98 (49.2%) | 101 (50.8%) | |
| Age | 35.8 (±10.1) | 32.1 (±8.7) | 0.005* |
| Ethnicity | 71 (%) | 0.222 | |
| White | 6 (%) | 75 (%) | |
| African-American | 19 (%) | 12 (%) | |
| Hispanic | 2 (%) | 11 (%) | |
| Other | 3 (%) | ||
| Employment | 0.217 | ||
| Working | 11 (%) | 4 (%) | |
| Disability | 8 (%) | 13 (%) | |
| Unemployed | 77 (%) | 79 (%) | |
| Student | 1 (%) | 2 (%) | |
| Other | 1 (%) | 3 (%) | |
| Income | 0.11 | ||
| 0–$10,999 | 80 (%) | 94 (%) | |
| $11,000–$20,999 | 5 (%) | 2 (%) | |
| $21,000–$30,000 | 9 (%) | 3 (%) | |
| > $30,000 | 4 (%) | 2 (%) | |
| Educational Level | 12.1 (±1.7) | 11.9 (±2.0) | 0.218 |
| Marital status | 0.617 | ||
| Married | 9 (%) | 9 (%) | |
| Never married | 69 (%) | 66 (%) | |
| Separated | 6 (%) | 9 (%) | |
| Divorced | 14 (%) | 15 (%) | |
| Widowed | 0 (%) | 2 (%) | |
| Number of children | 1.1 (±1.4) | 1.4 (±1.5) | 0.168 |
Notes: Values in columns represent n (percent in sample) for categorical variables and mean (±SD) for continuous measures.
Significance P < 0.05.
Table 3.
Comparison between male and female participants on drug use variables.
| VARIABLES | MALE | FEMALE | P |
|---|---|---|---|
| Methadone dose | 57.5 (±25.7) | 57.1 (±24.4) | 0.913 |
| Years using cocaine | 13.3 (±9.7) | 10.5 (±7.6) | 0.029* |
| Route of cocaine administration | 22 (%) | 21 (%) | 0.633 |
| Intravenous | 52 (%) | 60 (%) | 0.085 |
| Smoke | 24 (%) | 20 (%) | 0.149 |
| Snort | 55 (%) | 62 (%) | 0.716 |
| Cocaine craving (Past week) | 24 (%) | 12 (%) | 0.344 |
| Not at all | 13 (%) | 15 (%) | 0.187 |
| Slightly | 5 (%) | 6 (%) | |
| Moderately | 1 (%) | 6 (%) | |
| A lot | 2 (%) | 0 (%) | |
| Extremely | 96 (%) | 101 (%) | |
| Plan to stop using cocaine | 31.3 (±41.6) | 33.5 (±42.4) | |
| No | 0.9 (±1.4) | 1.1 (±1.9) | |
| Yes | 3.0 (±9.3) | 5.3 (14.7) | |
| Last used cocaine | |||
| Cocaine use in past week | |||
| Days | |||
| Grams/week | |||
Notes: Values in columns represent n (percent in sample) for categorical variables and mean (±SD) for continuous measures.
Significance P < 0.05.
Comparison between male and female participants on the NIS summary scores, clinical scales, and test-taking attitude scales
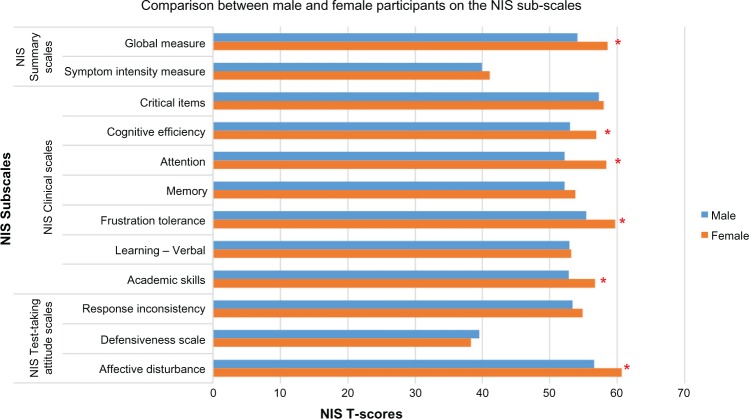
NIS T-scores on each subscale were coded as “high” if they were within the range of the normative data provided in the NIS manual and used in prior study (ie, 50T–60T).25,31 The degree of impairment on all of the NIS subscales, except for symptom intensity measure (SIM) and defensiveness (DEF), was found to be “high” (ie, ranged from 50T to 60T) among all participants in our sample relative to the general population norm. Furthermore, women had significantly higher scores on one of the three NIS summary scores: global measure of impairment (GMI) [F (165) = 6.1, P < 0.015]. Similarly, women had significantly higher scores on four of the seven clinical subscales scores: cognitive efficiency (COG) [F (165) = 5.6, P < 0.019], attention (ATT) [F (165) = 5.6, P < 0.019], frustration tolerance (FRU) [F (165) = 9.8, P < 0.002], and academic skills (ACD) [F (165) = 5.1, P < 0.025]. On the validity subscales assessing test-taking attitudes, female patients had higher affective disturbance scores (AFF) [F (165) = 6.5, P < 0.012] as compared to males (Fig. 1; Table 4).
Figure 1.
Comparison between male and participants on the NIS summary scores, clinical scales, and test-taking attitude scales.
Note: *Significance P < 0.05.
Table 4.
Comparison between male and female participants on the NIS summary scores, clinical scales, and test-taking attitude scales.
| VARIABLES | MALE (n = 98) | FEMALE (n = 101) | SIGNIFICANCE (165) df |
|---|---|---|---|
| NIS summary scales | |||
| Global measure | 54.1 (±12.9) | 58.6 (±10.4) | F = 6.1, P < 0.015* |
| Total items circled | 39.9 (±11.2) | 41.1 (±11.5) | F =.4, P < 0.522 |
| Symptom intensity measure NIS clinical scales | |||
| Critical items | 57.3 (±11.8) | 58.0 (±10.4) | F =.8, P < 0.359 |
| Cognitive efficiency | 53.0 (±12.2) | 56.9 (±10.1) | F = 5.6, P < 0.019* |
| Attention | 52.2 (±12.9) | 58.4 (±10.3) | F = 9.8, P < 0.002* |
| Memory | 52.2 (±12.3) | 53.8 (±10.7) | F = 1.0, P < 0.314 |
| Frustration tolerance | 55.4 (±11.8) | 59.7 (±9.9) | F = 4.6, P < 0.034* |
| Learning-verbal | 52.9 (±11.8) | 53.2 (±10.1) | F =.023, P < 0.880 |
| Academic skills | 52.8 (±12.1) | 56.7 (±9.2) | F = 5.1, P < 0.025* |
| NIS Test-taking attitude scales | |||
| Response inconsistency | 53.4 (±10.1) | 54.9 (±7.6) | F = 1.7, P < 0.197 |
| Defensiveness scale | 39.5 (±6.3) | 38.3 (±5.6) | F = 1.7, P < 0.189 |
| Affective disturbance | 56.6 (±11.3) | 60.7 (±8.2) | F = 6.5, P < 0.012* |
Notes: Values in columns represent mean (±SD).
Significance P < 0.05.
The mean value of the response inconsistency (INC) score (male: 53.4T and female: 54.9T), which evaluates the individual’s ability to respond in a coherent and consistent manner, does not reflect any unusual inconsistency in the participants’ NIS responses. The mean DEF score (male: 39.5T and female: 38.3T) suggests that participants had a positive and open attitude in completing the assessment and did not appear to have any unusual difficulty making social judgments. A marginally elevated affective disturbance score (male: 56.6T and female: 60.7T) suggested some degree of anxiety, depression, or poor stress tolerance among participants at the time of testing, which is not unusual for this patient population. Further clinical examination is recommended to determine the nature and extent of affective disturbance.25
Multiple regression analysis: prediction of NCI among participants
The following variables, together with the NIS summary and clinical subscales, were entered simultaneously into a standard multiple regression analysis that allowed each variable to be evaluated for its ability to add to the prediction of NCI among participants: sex (0/1: female/male), cocaine craving (0/1; not at all/yes), years of cocaine use, frequency (day) of cocaine use in the previous week, amount (in grams) of cocaine use in the previous week, plan to stop using cocaine (0/1; no/yes), and cocaine last used (day).
The full model accounted for between 32.4% and 38.6% of the variance in each of the NIS summary scales and between 17% and 37.8% of the variance in each of the clinical subscales. Out of the nine NIS subscales, four showed a statistically significant and unique relationship with sex, namely GMI, COG, ATT, and ACD, thus indicating a greater level of impairment for men vs women (Table 5).
Table 5.
Prediction of neurocognitive impairment among participants.
| NIS SUBSCALE SCORES | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GMI | SIM | CRIT | COG | ATT | MEM | FRU | L-V | ACD | |
| Explanatory variables | |||||||||
| Sex | −0.186* | −0.038 | −0.061 | −0.179* | −0.226* | −0.071 | −0.152 | −0.009 | −0.181* |
| Cocaine craving | 0.220* | 0.238* | 0.193* | 0.259* | 0.187* | 0.198* | 0.156 | 0.181* | 0.239* |
| Years of cocaine use | 0.216* | 0.211* | 0.261 | 0.220* | 0.189 | 0.073 | 0.187 | 0.223* | 0.117 |
| Cocaine use (day) in past week | −0.058 | −0.044 | −0.047 | −0.028 | −0.109 | −0.046 | −0.041 | −0.026 | −0.101 |
| Cocaine use (g) in past week | 0.168 | 0.239 | 0.151 | 0.184 | 0.194 | 0.159 | 0.090 | 0.210 | 0.174 |
| Plan to stop using cocaine | 0.129 | 0.094 | 0.154* | 0.108 | 0.191* | 0.121 | 0.198* | −0.002 | 0.079 |
| Cocaine last used (day) | −0.064 | 0.060 | −0.075 | −0.031 | −0.056 | −0.007 | −0.022 | −0.040 | −0.080 |
Notes: Sex coded 0/1 (female/male); cocaine craving 0/1 (not at all/yes); plan to stop using cocaine 0/1 (no/yes). Values in columns represent standardized beta coefficients.
Significance P < 0.05.
Exploration of associations between NCI and cocaine-use variables by sex
Regression analyses were conducted between NIS subscale scores and cocaine-use variables (ie, those found to be significant in regression model) by sex in order to further explore differences between men and women. Interestingly, cocaine craving contributed significantly to all NIS summary scores, and all clinical subscales except FRU for men. Similarly, the plan to use cocaine in the next three months was significantly associated with the cognitive impairment of men as measured by FRU. In contrast, for women, only one of the nine NIS subscales, namely SIM, was found to have a significant association with cocaine craving. No significant association was found between any of the other NIS subscales and cocaine-use variables among women (Table 6).
Table 6.
Associations between neurocognitive impairment and cocaine-use variables by sex.
| MALE (n = 98) COCAINE-USE VARIABLESa |
FEMALE (n = 101) COCAINE-USE VARIABLESa |
|||||
|---|---|---|---|---|---|---|
| CRAVING | YEARS | PLAN | CRAVING | YEARS | PLAN | |
| GMI | 0.312* | 0.073 | 0.068 | 0.173 | 0.035 | −0.055 |
| SIM | 0.267* | 0.037 | 0.109 | 0.232* | 0.001 | −0.042 |
| CRIT | 0.321* | 0.198 | −0.029 | 0.118 | 0.125 | −0.091 |
| COG | 0.384* | 0.050 | 0.066 | 0.198 | 0.103 | −0.051 |
| MEM | 0.285* | 0.031 | 0.207 | 0.121 | 0.037 | −0.067 |
| ATT | 0.232* | −0.027 | 0.179 | 0.162 | −0.017 | −0.047 |
| FRU | 0.116 | 0.057 | 0.317* | 0.205 | −0.122 | −0.014 |
| L-V | 0.317* | 0.022 | 0.021 | 0.141 | −0.034 | 0.120 |
| ACD | 0.304* | 0.033 | 0.046 | 0.184 | 0.021 | −0.026 |
Note: Craving, craving for cocaine in last 7 days week (0/1: not at all/yes). Years, number of years using cocaine. Plan, plan to stop using cocaine (0/1: no/yes). Values in columns represent standardized beta coefficients.
Include only significant cocaine-use variables.
Significance *P < 0.05.
Discussion
Over the past decade, studies have reported significant levels of neuropsychological impairment among individuals who repeatedly use cocaine.2–5 Consistent with other studies with this target population, relatively high levels of neurocognitive impairment was evident in this group of participants, as indicated by average scores between 50T and 60T for most of the NIS subscales [ie, GMI, critical items (CRIT), COG, ATT, MEM, FRU, learning-verbal (L-V), ACD, INC, and AFF]. In addition, the current study assessed the potential sex-related differences in self-reported NCI among cocaine users receiving clinical care. This is the first study, to our knowledge, to employ the NIS assessment to study sex-related differences in self-reported neurocognitive impairment among cocaine users enrolled in methadone-maintained treatment program.
Our sample of women reported more impairment than men, as indicated by significantly higher scores on GMI, COG, ATT, FRU, ACD, and AFF (after controlling for age, years of cocaine use, methadone dose, years in MMT program, use of opiate in the past six months, and duration of opiate use). The female participants’ significantly higher score on GMI scale (P < 0.015) indicates a stronger likelihood of neuropsychological impairment as compared to their male counterparts. This difference in elevated GMI score may have been partly explained by a history of neurological illness or injury, as suggested by a higher CRIT score among women as compared to men, although it was not significantly different (Table 4)25. Also, a significantly higher AFF score among women (P <.012) suggests that general psychological distress may have contributed more to this differential cognitive difficulty compared to men.
In addition, women scored significantly higher than men on COG score (P < 0.019), which suggests a higher degree of cognitive impairment, such as slowness of mentation of praxis, fatigue, confusion, and mental efficiency, among women as compared to men. A significantly higher score on ATT scale among women (P < 0.002) signifies greater memory complaints and symptoms of general cognitive impairment. The FRU, which was also found to be significantly higher among women (P < 0.034), suggests the presence of a higher degree of symptoms, consistent with “organic personality disorder”, such as irritability, anger, or temper. Furthermore, higher score on the ACD scale among women (P < 0.025), as compared to men, indicates higher degree of cognitive deficits associated with neurological impairment.
These findings provide support to the notion that sex-related differences in neurocognitive deficit exist among cocaine users, which may be both theoretically and clinically meaningful. This was consistent with our central prediction that NCI among cocaine users is sex-specific – with women having significantly higher degree of neurocognitive impairments than men. The results are also consistent with a previous finding, which reported significantly more executive impairment in women addicted to cocaine as compared to their male counterparts.22 The higher degree of cognitive impairment among female cocaine users is accompanied by higher patterns of psychiatric, medical, social, family, and employment problems compared to that of male cocaine users.22
The sex-related differences in neurocognitive deficits among cocaine users may have been moderated to a degree by neurobiological development through hormonal influences, though this is well beyond the scope of this study. Estradiol, which is known to influence many drug-related behaviors, has been linked to greater sensitization to cocaine use.38,39 Studies have shown that women have higher extra-striatal dopamine receptor binding in the frontal cortex, temporal cortex, and thalamus40,41 and higher striatal dopamine transporter levels in the caudate nucleus and putamen.42 Likewise, neuroimaging studies have confirmed an increase in D2 receptor levels in the frontal cortex of female cocaine users.40,43,44 Thus, the effects of higher doses of circulating estradiol in the neural circuits may lead to an increased vulnerability to cocaine use among women.45 Overall, this evidence – along with findings from the current study – point to a differential sex-related interaction on NCI among cocaine users. Furthermore, it provides a platform for further and more exhaustive research to investigate sex-related association of neurocognitive deficits among male and female cocaine users.
One unexpected and intriguing finding was that cocaine craving significantly predicted NCI among men but not among women, as indicated by the significant association between cocaine craving and all NIS subscales, except for the FUS scale, among male cocaine users. This may suggest that impairment in this sample of men may be relatively temporary and moderated by cocaine craving. Similar relationships have been reported between self-reported impairment and cocaine craving among HIV-seronegative, cocaine-dependent, methadone-maintained patients.46 Previous studies have shown that craving for food and nicotine has a significant impact on cognitive functioning, but the specific impact of cocaine craving, especially the differential impact between men and women, has not been investigated to date.47,48 Future studies will need to be conducted to examine the effects of repeated cocaine use and cocaine craving on neuropsychological impairment – as well as the directionality of these effects – among cocaine-dependent patients at different stages of clinical care.
Limitations and future research
Several limitations of our study must be acknowledged. First, the symptoms of NCI were assessed using the NIS, which is a self-reported screening instrument. This instrument does not provide a comprehensive assessment of NCI and does not measure all possible cognitive domains. It is, however, an easy-to-administer assessment tool that provides a quick and accurate picture of neuropsychological symptoms, eliciting relevant diagnostic information, and has been used widely among this patient population.28–31,46 Second, we relied on the self-report assessment approach, which may have constrained our ability to precisely detect some variables due to the participants’ reluctance to report socially undesirable behaviors, such as cocaine use and NCI components. This may have been moderated, however, by the use of the ACASI assessment approach, which provided participants with a high level of privacy. Third, this study does not address directionality of the association between cognitive impairments and cocaine use – whether cognitive impairments represent predisposing traits or consequences of cocaine use. Such knowledge could further inform the development of tailored treatment approaches. Fourth, a number of studies have demonstrated that pharmacological treatments (ie. methadone maintenance treatment) for substance-abuse disorders can lead to neurocognitive defi-cits.49–52 Thus, the level of cognitive deficit seen among our sample may have been interfered due to use of methadone. We did, however, account for the effect of methadone on differences in NCI within our sample by controlling for “methadone use” and “years in MMT program”.
Conclusion and Recommendations
The present study contributes to the notion that cocaine-dependent, methadone-maintained women and men may enter into substance-abuse treatment with a range of cognitive impairments. Furthermore, a sex-related difference of neurocognitive deficits – with cocaine-addicted women having significantly more impaired decision-making ability than cocaine-addicted men – may contribute to differential treatment outcomes among male and female cocaine users. Indeed, there is evidence that tailoring behavioral interventions – based on demographics such as sex – can be more efficacious as opposed to using a one-size-fits-all approach. Surprisingly, there have been very few randomized trials of addiction treatment programs that include additional services such as neuropsychological testing services and sex-specific treatment programs.53 Future work in this area is essential, as it may have direct implications for the development of specifically tailored interventions designed to optimally reduce adverse health outcomes in these high-risk, cognitively impaired patients.
Footnotes
Author Contributions
Conceived and designed the study: RS, TBH, MC. Analyzed the data: RS, TBH. Wrote the first draft of the manuscript: RS. Contributed to the writing of the manuscript: RS, TBH, MC. Agree with manuscript results and conclusions: RS, TBH, MC. Jointly developed the structure and arguments for the paper: RS, TBH, MC. Made critical revisions and approved final version: RS, TBH, MC. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Gregory Stuart, Editor in Chief
FUNDING: Funding to support the parent study was provided by a National Institute on Drug Abuse (NIDA) Grants (R01-DA022122; K02DA033139) to MC. NIDA had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration . Results From the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. (No. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795)). [Google Scholar]
- 2.Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3257–66. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134(pt 7):2013–24. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27(2):189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 5.Woicik PA, Moeller SJ, Alia-Klein N, et al. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34(5):1112–22. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst T, Chang L, Oropilla G, Gustavson A, Speck O. Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Res. 2000;99(2):63–74. doi: 10.1016/s0925-4927(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 7.Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51(2):134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein RZ, Leskovjan AC, Hoff AL, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–58. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Lim KO, Wozniak JR, Mueller BA, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92(1–3):164–72. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15(3):358–66. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makris N, Gasic GP, Kennedy DN, et al. Cortical thickness abnormalities in cocaine addiction – a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008;60(1):174–88. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sim ME, Lyoo IK, Streeter CC, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32(10):2229–37. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- 13.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 14.National Advisory Committee on Drugs . Drug Use in Ireland and Northern Ireland 2006/2007 Drug Prevalence Survey: Cocaine Results. Dublin, IE: National Advisory Committee on Drugs & Public Health Information and Research Branch; 2008. [Google Scholar]
- 15.Van Etten ML, Anthony JC. Comparative epidemiology of initial drug opportunities and transitions to first use: marijuana, cocaine, hallucinogens and heroin. Drug Alcohol Depend. 1999;54(2):117–25. doi: 10.1016/s0376-8716(98)00151-3. [DOI] [PubMed] [Google Scholar]
- 16.Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend. 2007;86(2–3):191–8. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Haas AL, Peters RH. Development of substance abuse problems among drug-involved offenders. Evidence for the telescoping effect. J Subst Abuse. 2000;12(3):241–53. doi: 10.1016/s0899-3289(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 18.Ramoa CP, Doyle SE, Naim DW, Lynch WJ. Estradiol as a mechanism for sex differences in the development of an addicted phenotype following extended access cocaine self-administration. Neuropsychopharmacology. 2013;38(9):1698–705. doi: 10.1038/npp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falck RS, Wang J, Siegal HA, Carlson RG. The prevalence of psychiatric disorder among a community sample of crack cocaine users: an exploratory study with practical implications. J Nerv Ment Dis. 2004;192(7):503–7. doi: 10.1097/01.nmd.0000131913.94916.d5. [DOI] [PubMed] [Google Scholar]
- 20.Najavits LM, Lester KM. Gender differences in cocaine dependence. Drug Alcohol Depend. 2008;97(1–2):190–4. doi: 10.1016/j.drugalcdep.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CJ, Badger GJ, Sigmon SC, Higgins ST. Examining possible gender differences among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2002;10(3):316–23. doi: 10.1037//1064-1297.10.3.316. [DOI] [PubMed] [Google Scholar]
- 22.van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31(6):706–19. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maremmani I, Pani PP, Mellini A, et al. Alcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment program. J Addict Dis. 2007;26(1):61–70. doi: 10.1300/J069v26n01_08. [DOI] [PubMed] [Google Scholar]
- 24.Copenhaver MM, Lee IC, Baldwin P. A randomized controlled trial of the community-friendly health recovery program (CHRP) among high-risk drug users in treatment. AIDS Behav. 2013;17(9):2902–13. doi: 10.1007/s10461-013-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell WE, Reynolds D. Neuropsychological Impairment Scale (NIS) Manual. Annapolis, MD: Annapolis Neuropsychological Services; 1983. [Google Scholar]
- 26.Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25(5):561–75. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- 27.Errico AL, Nixon SJ, Parsons OA, Tassey J. Screening for neuropsychological impairment in alcoholics. Psychol Assess. 1990;2(1):45–50. [Google Scholar]
- 28.O’Donnell WE, Reynolds DM, De Soto CB. Neuropsychological impairment scale (NIS): Initial validation study using trailmaking test (A & B) and WAIS digit symbol (scaled score) in a mixed grouping of psychiatric, neurological, and normal patients. J Clin Psychol. 1983;39(5):746–8. doi: 10.1002/1097-4679(198309)39:5<746::aid-jclp2270390517>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell WE, De Soto CB, Reynolds DM. Sensitivity and specificity of the neuropsychological impairment scale (NIS) J Clin Psychol. 1984;40(2):553–5. doi: 10.1002/1097-4679(198403)40:2<553::aid-jclp2270400229>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell WE, Reynolds DM, De Soto CB. Validity and reliability of the neu-ropsychological impairment scale (NIS) J Clin Psychol. 1984;40(2):549–53. doi: 10.1002/1097-4679(198403)40:2<549::aid-jclp2270400228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Ezeabogu I, Copenhaver MM, Potrepka J. The influence of neurocognitive impairment on HIV treatment outcomes among drug-involved people living with HIV/AIDS. AIDS Care. 2012;24(3):386–93. doi: 10.1080/09540121.2011.608794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y. Simultaneous and selective inference: current successes and future challenges. Biom J. 2010;52(6):708–21. doi: 10.1002/bimj.200900299. [DOI] [PubMed] [Google Scholar]
- 33.Miller RG. Simultaneous Statistical Inference. USA: Springer; 1966. [Google Scholar]
- 34.Rodger RS, Roberts M. Comparison of power for multiple comparison procedures. J Methods Meas Soc Sci. 2013;4(1):20–47. [Google Scholar]
- 35.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. London: Routledge Academic; 2013. [Google Scholar]
- 37.Vacha-Haase T, Thompson B. How to estimate and interpret various effect sizes. J Couns Psychol. 2004;51(4):473. [Google Scholar]
- 38.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–9. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68(4):641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 40.Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiatry. 2001;158(2):308–11. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- 41.Sun WL, Festa ED, Jenab S, Quinones-Jenab V. Sex differences in dopamine D2-like receptor-mediated G-protein activation in the medial prefrontal cortex after cocaine. Ethn Dis. 2010;20(1 suppl 1):S1–88. [PubMed] [Google Scholar]
- 42.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158(9):1492–9. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 43.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function and chemistry. Biol Psychiatry. 2007;62(8):847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riccardi P, Zald D, Li R, et al. Sex differences in amphetamine-induced displacement of [18F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163(9):1639–41. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- 45.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2004;29(1):81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 46.Avants SK, Margolin A, McMahon TJ, Kosten TR. Association between self-report of cognitive impairment, HIV status, and cocaine use in a sample of cocaine-dependent methadone-maintained patients. Addict Behav. 1997;22(5):599–611. doi: 10.1016/s0306-4603(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 47.Kemps E, Tiggemann M. A cognitive experimental approach to understanding and reducing food cravings. Curr Dir Psychol Sci. 2010;19(2):86–90. [Google Scholar]
- 48.Sayette MA, Schooler JW, Reichle ED. Out for a smoke: the impact of cigarette craving on zoning out during reading. Psychol Sci. 2010;21(1):26–30. doi: 10.1177/0956797609354059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95(5):687–95. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- 50.Rapeli P, Fabritius C, Alho H, Salaspuro M, Wahlbeck K, Kalska H. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5. doi: 10.1186/1472-6904-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapeli P, Fabritius C, Kalska H, Alho H. Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: stability and correlates. BMC Clin Pharmacol. 2011;11:13. doi: 10.1186/1472-6904-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdejo A, Toribio I, Orozco C, Puente KL, Perez-Garcia M. Neuropsycho-logical functioning in methadone maintenance patients versus abstinent heroin abusers. Drug Alcohol Depend. 2005;78(3):283–8. doi: 10.1016/j.drugalcdep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Greenfield SF, Trucco EM, McHugh RK, Lincoln M, Gallop RJ. The women’s recovery group study: a stage I trial of women-focused group therapy for substance use disorders versus mixed-gender group drug counseling. Drug Alcohol Depend. 2007;90(1):39–47. doi: 10.1016/j.drugalcdep.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]