Abstract
Objective
We explored the feasibility of shear wave speed (SWS) estimates to detect differences in cervical softening pre- and post-ripening in women undergoing induction of labor.
Methods
Subjects at 37–41 weeks undergoing cervical ripening prior to induction of labor were recruited (n=20). Examinations, performed prior to misoprostol administration and 4 hours later, included Bishop score, transvaginal cervical length (TVCL), and 10 replicate SWS measurements using a Siemens S2000 ultrasound system and prototype transducer (128 element, 3mm diameter, 14mm aperture) attached to the clinician’s hand. Measurements were compared via individual paired hypotheses tests and the linear mixed model, and the latter was also used to compare groups. Spearman’s rank correlation coefficient was used to compare SWS to Bishop score. The linear mixed model provides more robust analysis of results by incorporating multiple variables into one model.
Results
The Wilcoxon signed-rank paired test established a significant difference in pre- vs. post-ripening SWS, with mean SWS estimates 2.53±0.75 and 1.54±0.31 m/s, respectively (p<0.001) in the Not in Labor group (decrease in stiffness) and 1.58±0.33 m/s and 2.35±0.65 for the Marked Progression group (increase in stiffness). The linear mixed model corroborated significant differences in pre- and post-ripening measurements in individual subjects (p<0.001) as well as between groups (p<0.0001). SWS estimates were significantly correlated with digitally-assessed cervical softness and marginally correlated with Bishop score via Spearman’s rank correlation coefficient.
Conclusions
In vivo SWS estimates detected stiffness differences before and after misoprostol-induced softening in term pregnancy. This ultrasonic shear elasticity imaging technique shows promise for assessing cervical softness.
Introduction
As delivery nears, the cervix ripens (shortens and softens). Transvaginal cervical length (TVCL) objectively assesses shortening, and its value for preterm birth risk assessment is indisputable, albeit inadequate, because most women with a midtrimester short cervix do not deliver preterm, and most preterm births in low risk women occur in those with a normal midtrimester TVCL.1 In addition, TVCL is not useful in term pregnancy. 2
Well before the cervix shortens, it softens.3–10 In the late 1800s, prior to the development of tests to detect beta-HCG in blood and urine, pregnancy was diagnosed via digital cervical examination because clinicians recognized that the cervix softened by around 6 weeks of gestation (the “Hegar sign”). To this day, clinical assessment of cervical softness remains subjective; the clinician denotes the cervix “soft, medium or firm” based on digital examination alone. Softness is a component of a numerical score (the Bishop score) that also includes dilation, effacement, station and position, and is used to predict labor induction success.11 Its ability to determine eligibility for cesarean section after failed induction is low12, 13, 14 however, and it is not useful for assessing risk of preterm birth prediction.
Emerging technologies, such as strain elastography and shear wave elastography may objectively describe softness.15 Soft tissue deforms more easily than stiff tissue, a principle exploited by strain elastography, which compares relative deformation between neighboring areas of tissue before and after a compression.16–19 Assessing overall cervical stiffness and standardizing the compressive force have proven challenging, calling into question its utility.16–20 For shear wave elastography, a speed (shear wave speed, SWS) is estimated for a shear wave generated with acoustic radiation force, and this quantitative speed is related to softness because shear waves travel more slowly in softer tissue29. The technique is less relative than strain elastography; although the applied force cannot be discounted (nonlinear elastic response should be avoided), measurement does not depend on comparing pre- and post-compression values. SWS has been demonstrated to work well in tissues such as liver that are isotropic (same material properties in all orientations) and homogeneous.21–24 Unfortunately, the cervix is anisotropic, heterogenous, and comprised of layers of collagen that remodel differently throughout gestation.25 We have demonstrated with SWS that the ex vivo human cervix has considerable spatial variability, however, fortunately, pre- and post-ripening changes can be reliably assessed using our experimental methods. 26
To our knowledge, this is the first study to evaluate the feasibility of in vivo measurement of SWS in the human pregnant cervix.
Materials and Methods
Patient Population
This study was approved by the Institutional Review Boards at Intermountain Healthcare and University of Wisconsin, and each subject provided written informed consent. Patients scheduled for cervical ripening prior to induction of labor at 37–41 weeks were recruited (n=20). Sample size calculations based on our previous study of the ex vivo cervix suggested that 10 subjects with pre- and post-ripening measurements obtained under nearly identical conditions were required. “Nearly identical conditions” meant that the cervix that was long enough (≥1cm) for adequate coupling of the transducer to the cervix for SWS measurement, and that contractions were no closer than 3 minutes apart (allowing enough time to obtain measurements during a contraction-free interval). The latter is important because meaningful comparison demanded similar conditions during data acquisition, and the cervix palpably stiffens during a uterine contraction. (Also, additional dosing of misoprostol is contraindicated if contractions are less than 3 minutes apart; this is a sign of progression into labor.) We therefore continued enrollment until we had 10 subjects in whom we acquired both adequate pre- and post-ripening measurements, requiring total enrollment of 20 subjects. Pre-ripening measurements were obtained in all 20 subjects (18 nulliparous and 2 multiparous). Subjects were divided into two groups based on cervical evaluation 4 hours after administration of misoprostol. Those who required a second dose were considered “Not in Labor”; in this group, the post-ripening measurements were possible under the same conditions as the pre-ripening (cervix ≥1cm long and contractions >3 minutes apart). All other subjects were considered “Marked Progression” (cervix <1cm long and/or contractions <3 minutes apart); these subjects did not require additional ripening with misoprostol.
Data Acquisition and Processing
All examinations were done by the same clinician (STR) and acquisitions supervised by the same engineer (LCC) in order to reduce inter-observer variability. Scanning was performed using a Siemens Acuson S2000 Ultrasound system (Siemens Healthcare, Ultrasound Business Unit, Mountain View, CA, USA). A prototype catheter transducer (128 elements, 14 mm aperture, 3 mm diameter), operated in linear array mode to allow for alignment of ultrasound waves with cervical structure, was used to acquire shear wave speeds. The probe was attached to the clinician’s index finger in order to minimize contact force by the operator. (Pressing on tissue causes it to stiffen and would bias shear wave speed estimates. To minimize bias caused by stiffening due to contact force, before obtaining measurements, we assessed for minimal tissue displacement via observation of the B-mode image after the transducer was acoustically coupled to the tissue.) At each examination, Bishop score and transvaginal cervical length (TVCL, using EV-9C4 endovaginal probe) were recorded. The prototype transducer was then secured to the index finger of the clinician’s hand with the active aperture on her fingertip, and then placed in a sterile glove filled with gel for acoustic coupling (Figure 1). The clinician’s finger was placed on the anterior surface of the cervix, roughly parallel to the endocervical canal in the mid-position along the length of the canal (verified by B-mode ultrasound). This location was chosen based on ex vivo studies in human cervix.26
Figure 1.
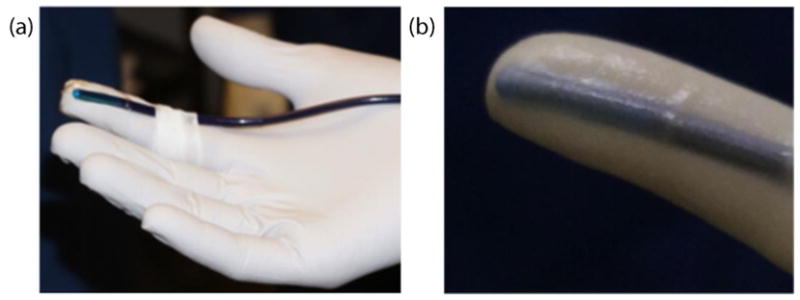
Transducer was attached to the clinicians hand (a) and inserted into a sterile glove filled with gel (b).
Shear wave data were acquired via the Siemens Virtual Touch Tissue Quantification software package. Imaging parameters are summarized in Table 2. A 5x5 mm region of interest (ROI) was placed mid-thickness through the anterior half of the cervix, 5–6 mm from the outer surface of the cervix in order to avoid the first 2 mm of inner and outer boundaries (where shear wave behavior is unpredictable). Ten replicate measurements were made at this location (mid-length between external and internal os) before misoprostol was administered (pre-ripening) and 4 hours afterwards (post-ripening).
Table 2.
Summary of acoustic parameters. F-number is defined as the ratio of focal depth to active aperture width and describes the imaging system focal properties. These parameters are necessary for generation of acoustic force and accurate displacement tracking for shear wave estimation.
| Parameter | Value |
|---|---|
| Probe | P128 |
| Push Frequency (MHz) | 6.15 |
| Track Frequency (MHz) | 7.27 |
| Push Cycles | 400 |
| Pulse Duration | 65μs |
| Track Pulse Repetition | 10330Hz |
| Frequency | |
| Push F-number | 1.0 |
| Track F-number | 1.5 |
| Push focal depth | 0.6cm |
| MI | 1.9 |
All data processing was performed offline in the MATLAB (The MathWorks) environment. Tissue displacement was estimated using the Loupas Method on IQ data.27 A threshold of 0.98 was used for complex correlation coefficient magnitude to remove less reliable displacement estimates compromised by excessive motion artifact and other sources of noise. The SWS was estimated using the RANSAC algorithm described by Wang et al.28 This method involves selection of data points within the 5x5mm ROI where the percentage of points included (% inliers) in the estimate is a measure of data quality. Inliers (those displacement estimates consistent with the model) are chosen based on an error function threshold. Estimates that had less than 30% inliers were removed from analysis (e.g. the subject who was removed from analysis due to poor data quality). Data sets with 30–40% inliers were evaluated based on visual inspection of exploratory plots for data inclusion.
Statistical Analysis
Shear wave speed estimates were analyzed to evaluate (1) changes pre- and post-ripening and (2) correlation to Bishop score and the softness component of Bishop score. To compare pre- and post-ripening measurements in the same subjects, we used the Wilcoxon signed-rank paired test in R (The R Foundation for Statistical Computing ) with the probability of equal medians (P value) less than 0.05 (two-sided) as a criterion for statistical significance. This test was chosen because it is non-parametric, and thus does not rely on an assumption of normality, which was important because SWS measurements in the cervix are not yet established enough to make assumptions about normality. Continuous variables were summarized as mean±SD [median, quartile 1 (Q1) - quartile 3 (Q3)] and were represented graphically with box and whisker plots. The notches correspond to the 95% confidence interval of the median. Tests performed on individual hypotheses can only take into account one variable at a time and therefore we compared results to a linear mixed model, which provides more robust analysis of results by incorporating multiple variables into one model. In addition, it accounts for relationships between variables (covariance), unequal sample sizes, and random effects (e.g. variability within same patient). Analysis was performed using R with the ‘nmle’ package. The linear mixed model tested for significant differences in SWS estimates in individuals pre- vs. post-ripening, among groups, and the softness component score of the Bishop score. All variables were found to be significant using a likelihood ratio F-test with a 0.05 significance level30. The Spearman rank correlation was used to test correlation between SWS estimates and Bishop score. The ratings of ‘soft’, ‘medium’ and ‘firm’ of the Bishop score were given a score 2 = ‘soft’, 1 = ‘medium’ and 0 = ‘firm’ in the model to determine changes in softness between pre- and post-ripening examinations. Results were considered as marginally significant if 0.05 < P < 0.15.
Results
In total, there were 11 subjects in the Not in Labor group, 1 of whom declined an additional vaginal examination, and therefore 10 subjects were included in the final analysis of pre- vs post-ripening measurements. All of these subjects were nulliparous. The remaining 9 subjects were all in the Marked Progression group, but post-ripening measurement (during contraction) was possible in only 4 because the cervix was too short (<1cm) in 4 of these subjects (including both multiparous subjects) and the data quality too poor in 1. Pre- vs. post-ripening measurements were analyzed in the 4 subjects in the Marked Progression group for interest and in order to develop hypotheses for future studies, but interpreted with caution because of low numbers. Table 1 summarizes the final data inclusion and group assignment.
Table 1.
Numbers of subjects in each group for pre-ripening and post-ripening examinations. Reasons for exclusion of post-ripening examinations are in parentheses.
| Group | Pre | Post |
|---|---|---|
| Not in Labor | 11 | 10 (refused = 1) |
| Marked Progression | 9 | 4 (cervix <1cm = 4, poor quality data = 1) |
Figure 2 shows results of all SWS estimate replicates for each subject in the Not in Labor and Marked Progression groups. The interior lines display the medians, the edges of the boxes are the upper and lower quartiles (25th and 75th percentiles) of the SWS estimates, and the bars (‘whiskers’) display the maxima and minima [within 1.5*interquartile range (IQR)]. Outliers are specified as >1.5*IQR. For the Not in Labor group, all mean SWS estimates for each patient decreased from the pre- to the post-ripening examination.
Figure 2.
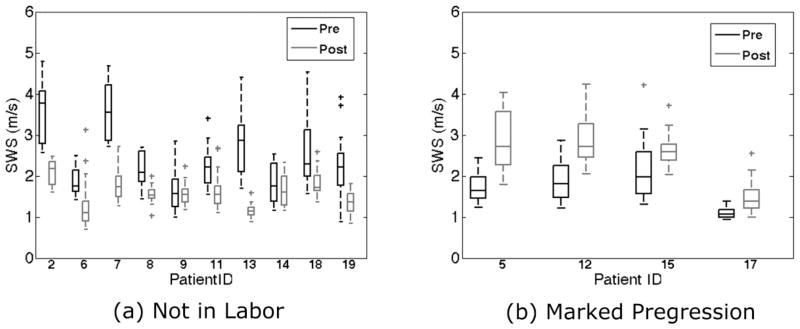
Boxplot of replicate shear wave speed (SWS) estimates for each patient ID for the Not In Labor and Marked Progression groups. The boxes represent interquartile range (IQR), the interior line is the median, whiskers are maxima and minima within 1.5*IQR, and cross markers show outliers beyond the 1.5*IQR, and cross markers show outliers beyond the 1.5*IQR.
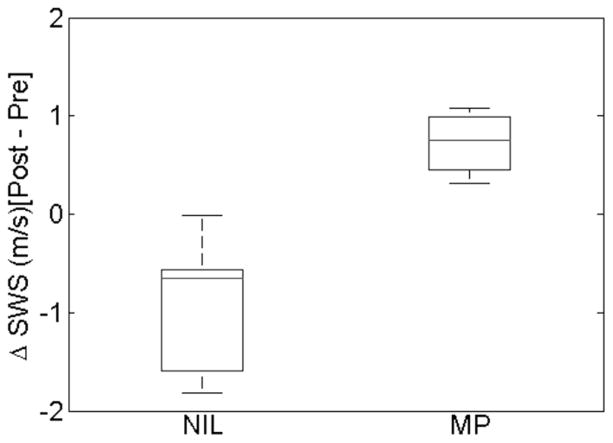
Results from the individual paired hypotheses tests showed significant differences in pre- vs. post-ripening median SWS estimates in the Not in Labor group (p = 0.002). The means were 2.53±0.75 [median = 2.22, 2.13–3.17] m/s for the pre-ripening examination and 1.54±0.31 [median = 1.55, 1.40–1.69] m/s for the post-ripening examination, respectively. The corresponding mean SWS for the Marked Progression group were 1.58±0.33 [median = 1.63, 1.36–1.84] m/s and 2.35±0.65 [median = 2.65, 2.28–2.71] m/s for pre- and post-ripening examinations, respectively. Figure 3 shows a box plot of change from pre- to post-ripening SWS medians for all patients in each group. For all patients in the Not in Labor group, the SWS measurements decreased (ΔSWS < 0, cervix was the same or softer) and it increased (ΔSWS > 0, cervix was same or stiffer) for all patients in the Marked Progression group. The linear mixed model agreed with significant differences between pre- and post-ripening examinations (p<0.001) for both groups. In addition, it found a covariance between scan (pre- vs. post-ripening) and group (Not in Labor vs Marked Progression, p<0.0001), suggesting that the SWS estimates for pre- vs. post-ripening depend on group. The pre- and post-ripening measurements for the Marked Progression group were marginally significant (p ≈ 0.12), likely due to small sample size.
Figure 3.
Boxplot of Post-Pre SWS medians for each patient for the Not in Labor (NIL) and Marked Progression (MP) groups. For the NIL group, all SWS medians decreased for each patient indicated by values < 0 and all patients in the MP group had an increase in SWS median from pre to post measurement.
Tables 3 and 4 show pre- and post-ripening Bishop score and digitally assessed cervical softness for each subject in the Not in Labor and Marked Progression, respectively. As expected, the Bishop score increased for all 15 subjects between the pre- and post-ripening examinations. In the Not in Labor group, the softness rating was unchanged (e.g., soft → soft) in 5 subjects, and increased (e.g., firm → medium) in 5 subjects. Similarly, in the Marked Progression group, the softness rating was unchanged in 2 subjects and decreased (e.g., soft → medium) in 2 subjects. As noted above, SWS estimates decreased (indicating softer tissue) in all subjects in the Not in Labor group, and increased in all subjects in the Marked Progression group. SWS were significantly correlated with digitally assessed softness ratings (2=soft, 1=medium, 0=firm) in both groups, and marginally correlated with Bishop score. Results from the Marked Progression group must be interpreted with caution due to low numbers because the study was not designed to analyze such a group.
Table 3.
Summary of Bishop score and softness component of Bishop score (with points assigned for analysis in model) for all subjects in Not in Labor group.
| Subject number | Pre-Ripening Bishop score/softness (points) | Post-Ripening Bishop score/softness (points) |
|---|---|---|
| 2 | Bishop score = 3/firm (0) | Bishop score = 8/medium (1) |
| 6 | Bishop score = 4/soft (2) | Bishop score = 7/soft (2) |
| 7 | Bishop score = 0/firm (0) | Bishop score = 5/soft (2) |
| 8 | Bishop score = 3/soft (2) | Bishop score = 4/soft (2) |
| 9 | Bishop score = 5/soft (2) | Bishop score = 7/soft (2) |
| 11 | Bishop score = 2/soft (2) | Bishop score = 7/soft (2) |
| 13 | Bishop score = 1/firm (0) | Bishop score = 5/soft (2) |
| 14 | Bishop score = 1/medium (1) | Bishop score = 4/medium (1) |
| 18 | Bishop score = 0/firm (0) | Bishop score = 5/medium (1) |
| 19 | Bishop score = 4/medium (1) | Bishop score = 7/soft (2) |
Table 4.
Summary of Bishop score and softness component of Bishop score (with points assigned for analysis in model) for all subjects in Marked Progression group.
| Subject number | Pre-Ripening Bishop score/softness (points) | Post-Ripening Bishop score/softness (points) |
|---|---|---|
| 5 | Bishop score = 6/soft (2) | Bishop score = 9/soft (2) |
| 12 | Bishop score = 3/soft (2) | Bishop score = 4/soft (2) |
| 15 | Bishop score = 4/soft (2) | Bishop score = 5/medium (1) |
| 17 | Bishop score = 5/soft (2) | Bishop score = 6/medium (1) |
For the Not in Labor group, statistical significance was found between the change in softness and change in SWS for each subject (ρ = 0.76, p = 0.01) and marginal significance found between change in Bishop score and change in SWS (ρ = −0.58, p = 0.08). The linear mixed model found the softness component of Bishop score to be significantly associated with SWS (p<0.0001) but the complete Bishop score did not improve the model (p = 0.67), suggesting that SWS are more sensitive to the softness component of Bishop score than total Bishop score. The Marked Progression group was not analyzed due to the low number of patients.
Discussion
Our results show that carefully acquired and interpreted SWS estimation may be promising for objective, quantitative description of softening in the in vivo pregnant human cervix. Specifically, SWS changes reflected cervical softening after ripening prior to induction of labor in nulliparous women.
SWS detected differences in softening pre- and post-cervical ripening. As shown in Figure 2, there are clear differences in SWS measurements between pre- and post-ripening examinations shown by minimal overlap of box/whiskers of individual subjects in both groups. For the Not in Labor group, the post-ripening examinations showed a decrease in variance, suggesting more homogeneous tissue, as would be expected for the microstructural disorganization that is known to occur with ripening. For the Marked Progression group, the post-ripening cervix was deemed stiffer or unchanged per digital examination than the pre-ripened cervix, which is not unexpected given the presence of uterine contractions during the examination (contraction of uterine muscle and pressure of the fetal head causes the cervix to stiffen). In other words, increases in SWS are consistent with digitally-assessed increase in stiffness.
Significant differences were confirmed between pre- and post-ripening in SWS estimates for both groups using paired tests, shown in Figure 3 by no values crossing the ΔSWS = 0 line. The linear mixed model, a robust statistical approach because it simultaneously accounts for low unequal sample sizes and multiple variables, found statistical significance between pre- and post-ripening examinations for both groups. In addition, the model found a significant covariance between scans and group – SWS decreases for Not in Labor and increases for Marked Progression groups. All results supported clinical observations. Specifically, the SWS increased or decreased as expected; when the cervix felt either the same or softer after ripening, the associated SWS were slower, and when it felt the same or stiffer due to frequent contractions, the associated SWS were faster.
One limitation of our study was the inability to find the exact location along the length of the cervix in the same patient between scans. This is because in preparation for labor, the cervix shortens in most patients. In a previous study on hysterectomy specimens, a significant gradient in SWS vs. location along the length of the cervix was found for both ripened and unripened cervices, meaning that location is important.26. However the gradient difference is less pronounced for ripened cervices, likely because of microstructural disorganization which causes tissue to become more homogenous. The pregnant cervix is more closely related to ‘ripened’ group in which the gradient was much less (0.202 m/s*cm for ripened anterior) and therefore small deviations in location (±1 cm) are unlikely to cause large changes in SWS estimates9. We are therefore confident that changes in SWS estimates pre- vs. post-ripening are due to actual changes in softness.
Another limitation of this study is the need for a special transducer for SWS estimation. The elastic nonlinearity in all tissues causes them to stiffen when they are deformed. To minimize that stiffening, we used an intravascular prototype transducer attached to the clinician’s finger so tactile sensing could be used to judge ‘barely in contact’. We specifically avoided using a standard endovaginal curved linear array in this study because simply placing such a large inflexible transducer next to the cervix likely causes a deformation on the cervix and vaginal wall without tactile clinician feedback, thereby causing an increase in stiffening (due to initial pre-load). In other words, the standard transvaginal transducer is unlikely to be suitable for accurate shear wave speed estimates because of the inevitable pre-stiffening it would induce.
Much work remains to confirm these preliminary findings in a larger sample (study ongoing) and to develop a more ergonomic transducer. However, it is encouraging that SWS measurements detected an increase in softening from the pre- to post-ripening examination in all individuals as well as grouped pre- vs post-ripening examinations in the Not in Labor group, which was consistent with the clinician-determined softness component of the Bishop score. Although numbers were too small in the Marked Progression group to make appropriate statements about clinical prediction, it is encouraging that SWS measurements increased in all subjects whose post-ripening measurement was taken during uterine contraction, again consistent with the digitally determined cervical stiffness. In summary, SWS estimation may be a promising methodology for objective, quantitative determination of cervical softness.
Acknowledgments
This work was supported by NIH Grants R21HD061896, R21HD063031 and R01HD072077 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, T32CA009206 and the Intermountain Research & Medical Foundation. The authors would also like to recognize Peggy Reed, Tonya Edvalson in the Maternal Fetal Medicine Department at Intermountain Medical Center (IMC) for their invaluable assistance, and the obstetrician-gynecologists and Labor and Delivery unit RNs at IMC for providing access to their patients for this study. We are also grateful to Siemens Healthcare Ultrasound division for an equipment loan and technical support.
References
- 1.Grobman W. Randomized controlled trial of progesterone treatment for preterm birth prevention in nulliparous women with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;206(suppl):S367. [Google Scholar]
- 2.Gonen R, Degani S, Ron A. Prediction of successful induction of labor: comparison of transvaginal ultrasonography and the Bishop score. European journal of ultrasound. 1998;7:183–187. doi: 10.1016/s0929-8266(98)00042-1. [DOI] [PubMed] [Google Scholar]
- 3.Iams JD, Goldenberg RL, Meis PK, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–573. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 4.Akins M, Luby-Phelps K, Mahendroo M. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Opt. 2010;15:026020. doi: 10.1117/1.3381184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breeveld-Dwarkasing V, de Boer-Brouwer M, Tekoppele J, Bank R, van der Weijden C, Taverne M, van Dissel-Emiliani F. Regional differences in water content, collagen content, and collagen degradation in the cervix of nonpregnant cows. Biol Reprod. 2003;69:1600–1607. doi: 10.1095/biolreprod.102.012443. [DOI] [PubMed] [Google Scholar]
- 6.Breeveld-Dwarkasing V, teKoppele J, Bank R, van der Weijden C, Taverne M, van Dissel Emiliani F. Changes in water content, collagen degradation, collagen content and concentration on repeat biopsies of the cervix of pregnant cows. Biol Reprod. 2003;69:1608–1614. doi: 10.1095/biolreprod.102.012534. [DOI] [PubMed] [Google Scholar]
- 7.Aspden RM. Collagen organization in the cervix and its relation to mechanical function. Coll Relat Res. 1988;8:103–112. doi: 10.1016/s0174-173x(88)80022-0. [DOI] [PubMed] [Google Scholar]
- 8.Danforth D. The morphology of the human cervix. Clin Obstet Gynecol. 1983;26:7–13. doi: 10.1097/00003081-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Feltovich H, Janowski J, Delance N, Moran C, Chien E. The effects of nonselectgive PGE2 receptor agonists on cervical tensile strength and cervical collagen content and structure. Am J Obstet Gynecol. 2006;92:753–760. doi: 10.1016/j.ajog.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 10.Maul H, Mackay L, Garfield RE. Cervical ripening: biochemical, molecular, and clinical considerations. Clin Obstet Gynecol. 2006;49:551–563. doi: 10.1097/00003081-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Bishop E. Pelvic scoring for elective induction. Obstet and Gynecol. 1964;24:266–268. [PubMed] [Google Scholar]
- 12.Kolkman DG, Verhoeven CJ, Brinkhorst SJ, van der Post JA, Pajkrt E, Opmeer BC, Mol BW. The bishop score as a predictor of labor induction success: a systematic review. Am J Perinatol. 2013 Sep;30(8):625–30. doi: 10.1055/s-0032-1331024. [DOI] [PubMed] [Google Scholar]
- 13.Verhoeven CJ, Oudenaarden A, Hermus MA, Porath MM, Oei SG, Mol BW. Validation of models that predict Cesarean section after induction of labor. Ultrasound Obstet Gynecol. 2009 Sep;34(3):316–21. doi: 10.1002/uog.7315. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen PE, Howard BC, Crabtree T, Batig AL, Pates JA. The distribution and predictive value of Bishop scores in nulliparas between 37 and 42 weeks gestation. J Matern Fetal Neonatal Med. 2012 Mar;25(3):281–5. doi: 10.3109/14767058.2011.573831. [DOI] [PubMed] [Google Scholar]
- 15.Feltovich H, Hall TJ, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am J Obstet Gynecol. 2012;207:345–54. doi: 10.1016/j.ajog.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swiatkowska-Freund M, Preis L. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 17.Molina FM, Gomez LF, Florido J, Padilla MC, Nicolaides KH. Quantification of cervical elastography: a reproducibility study. Ultrasound Obstet Gynecol. 2012;39:685–689. doi: 10.1002/uog.11067. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Andrade E, Hassan SS, Ahn H, Korzeniewski SJ, Yeo L, Chaiworapongsa T, Romero R. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet Gynecol. 2013;41:152–161. doi: 10.1002/uog.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fruscalzo A, Steinhard J, Londero AP, Fröhlich C, Bijnens B, Klockenbusch W, Schmitz R. Reliability of quantitative elastography of the uterine cervix in at-term pregnancies. J Perinat Med. 2013;7:1–7. doi: 10.1515/jpm-2012-0180. [DOI] [PubMed] [Google Scholar]
- 20.Feltovich H, Hall TJ. Quantitative imaging of the cervix: setting the bar. Ultrasound Obstet Gynecol. 2013;41:121–128. doi: 10.1002/uog.12383. [DOI] [PubMed] [Google Scholar]
- 21.Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Nonivasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011 Sep;55(3):666–72. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotemberg V, Byram B, Palmeri M, Wang M, Nightingale K. Ultrasonic characterization of the nonlinear properties of canine livers by measuring shear wave speed and axial strain with increasing portal venous pressure. J Biomech. 2013 Jul 26;46(11):1875–81. doi: 10.1016/j.jbiomech.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchard RR, Dahl JJ, Hsu SJ, Palmeri ML, Trahey GE. Image quality, tissue heating, and frame rate trade-offs in acoustic radiation force impulse imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:63–76. doi: 10.1109/TUFFC.2009.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma AC, Soo MS, Trahey GE, Nightingale KR. Acoustic radiation force impulse imaging of in vivo breast masses. IEEE Ultrason Symp. 2004;1:728–731. [Google Scholar]
- 25.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21:353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, Munoz Del Rio A, Hall TJ. Shear wave speed estimation in the human uterine cervix. Ultrasound Obstet & Gynecol. 2013;43(4):452–458. doi: 10.1002/uog.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinton GF, Dahl JJ, Trahey GE. Rapid tracking of small displacements with ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53:1103–1117. doi: 10.1109/tuffc.2006.1642509. [DOI] [PubMed] [Google Scholar]
- 28.Wang MH, Palmeri ML, Rotemberg VM, Rouze NC, Nightingale KR. Improving the robustness of time-of-flight based shear wave speed reconstruction methods using RANSAC in human liver in vivo. Ultrasound Med Biol. 2010;36:802–813. doi: 10.1016/j.ultrasmedbio.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarvazyan OV, Rudenko OV, Swanson SD, Fowlkes J, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Bio. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 30.Carlson LC, Feltovich H, Palmeri ML, Munoz del Rio A, Hall TJ. Statistical Analysis of Shear Wave Speed in the Uterine Cervix. IEEE Trans Ultrason Ferroelectr Freq Control. 2014 doi: 10.1109/tuffc.2014.006360. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]