Abstract
Sepsis is a major cause of mortality in intensive care medicine. Propofol, an intravenous general anesthetic, has been suggested to have anti-inflammatory properties and able to prevent sepsis induced by Gram-positive and Gram-negative bacteria by down-regulating the gene expression of pro-inflammatory cytokines. However, propofol’s anti-inflammatory effects upon canine peripheral blood mononuclear cells (PBMCs) have not yet been clarified. Here, we isolate canine PBMCs and investigate the effects of propofol on the gene expressions of both lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α and upon the production of nitric oxide (NO). Through real-time quantitative PCR and the Griess reagent system, we found that non-cytotoxic levels of propofol significantly inhibited the release of NO and IL-6 and TNF-α gene expression in LPS-induced canine PBMCs. Western blotting revealed that LPS does significantly increase the expression of inducible NO synthase (iNOS) protein in canine PBMCs, while pretreatment with propofol significantly decreases the LPS-induced iNOS protein expression. Propofol, at concentration of 25 µM and 50 µM, also significantly inhibited the LPS-induced nuclear translocation of nuclear factor (NF)-κB p65 protein in canine PBMCs. This diminished TNF-α, IL-6 and iNOS expression, and NO production was in parallel to the respective decreased NF-κB p65 protein nuclear translocation in the LPS-activated canine PBMCs pretreated with 25 µM and 50 µM propofol. This suggests that non-cytotoxic levels of propofol pretreatment can down-regulate LPS-induced inflammatory responses in canine PBMCs, possibly by inhibiting the nuclear translocation of the NF-κB p65 protein.
Keywords: canine, LPS, nuclear factor-κB, propofol, sepsis
Sepsis, as a systemic inflammatory response syndrome, is a major cause of mortality in intensive care medicine [11, 20]. The strong immune response elicited by Lipopolysaccharide (LPS), one of the structural components of the outer membrane of Gram-negative bacteria, is considered to be the major factor that contributes to the pathogenesis of sepsis [27, 28]. During inflammation, LPS is released into the circulation and, from there, proceeds to progressively and extensively act on a number of host cells, mainly monocytes and macrophages, triggering release of a variety of pro-inflammatory cytokines which amplify the inflammatory response and can induce tissue damage [3, 8, 19].
Monocytes and macrophages make up a major cell population of both the innate and adaptive immune system. They constitute the first line of defense in cell-mediated immunity against bacterial invasion and play key roles in the killing of bacteria through their phagocytic and bactericidal action [4, 37]. They are actively involved in inflammatory processes and produce a number of pro-inflammatory cytokines, each of which modulates the immune response in turn. It is well demonstrated that interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are particularly important monocyte- or macrophage-derived cytokines, which act on various target tissues. Multiple studies indicate that the excessive monocyte-release of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, is an important propagating factor in severe sepsis and may contribute to multiple organ failure [7]. Modulating these inflammatory factors by using anti-inflammatory agents may therefore have some benefit for the suppression of the LPS-induced septic pathogenesis and towards the protection of cells from LPS-induced cellular injury.
Propofol (2,6-diisopropylphenol), a potent intravenous hypnotic agent, has more-stable hemodynamics than many other anesthetic drugs and is widely used for the induction and maintenance of anesthesia during surgical procedures or for sedation of intensive care patients [32]. In addition to its anesthetic properties, there is increasing evidence from both animal and human studies, suggesting that propofol exerts protective effects during acute inflammatory processes. The focus upon this interesting additional benefit of propofol is increasing [14, 18]. Propofol, for example, in both murine and human studies, has been shown to decrease the production of pro-inflammatory cytokines, alter the production of nitric oxide (NO) and inducible nitric oxide synthase (iNOS) and inhibit neutrophil function and platelet aggregation, leading to alleviation of the inflammatory response [14, 18, 35].
The molecular mechanisms for the anti-inflammatory properties of propofol have been widely investigated. Increasing evidence suggests that propofol causes a decrease in the expression of genes encoding various pro-inflammatory cytokines by the inhibition of the nuclear factor (NF)-κB, which plays an important role in transcriptional regulation of many pro-inflammatory genes, such as IL-1β, IL-6, TNF-α and iNOS [13]. It has been reported that mortality in patients with sepsis can be predictable by measuring the increased NF-κB activity of the patient’s blood mononuclear cells [2]. Although many studies have demonstrated the anti-inflammatory roles of propofol relating to murine and human monocytes and macrophages, none of them have yet addressed propofol’s similar immunomodulatory effect relating to canine monocytes and macrophages. In the present study, we test the anti-inflammatory properties of propofol through analyzing the effect of propofol treatment upon the production of IL-6, TNF-α, NO and iNOS in LPS-stimulated canine peripheral blood mononuclear cells (PBMCs). To examine the potential mechanism responsible for the immunomodulatory effects of propofol, its effect upon nuclear translocation of the NF-κB p65 protein was also evaluated.
MATERIALS AND METHODS
Reagents and antibodies: Propofol was obtained from AstraZeneca (Diprivan; Basiglio, MI, Italy). LPS from Escherichia coli 055:B5 was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). NF-κB p65 rabbit polyclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Histone H3 rabbit polyclonal antibody and β-actin mouse monoclonal antibody were purchased from Abcam (San Diego, CA, U.S.A.), and iNOS rabbit polyclonal antibody was obtained from Bioss Inc. (Woburn, MA, U.S.A.).
Cell culture and stimulation: Venous blood samples were collected from six healthy beagles which were being used for another hemorrhagic shock experiment. All procedures related to the animals and their care conformed to the internationally accepted principles as found in the Guidelines for Keeping Experimental Animals issued by the government of China. The PBMCs were purified from the sterile heparinized whole blood sample using a Ficoll-Hypaque density gradient separation centrifugation (GE Healthcare, Buckinghamshire, UK) as described previously [35]. Contaminated red blood cells were lysed by ammonium chloride. After centrifugation, the PBMCs were washed three times with PBS and then cultured in RPMI 1640 medium supplemented with 10% heat inactivated fetal calf serum (FCS; Hyclone, UT, U.S.A.). One hundred U/ml of penicillin and 100 µg/ml of streptomycin were added into the culture media, and cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2.
To determine the effects of propofol on LPS-induced NF-κB activation and cytokine production in canine PBMCs, the cells were pretreated with two final concentrations of propofol (25 µM and 50 µM) for 6 hr. Thereafter, the cells were stimulated with 100 ng/ml LPS for 24 hr. Stimulated and non-stimulated cells and the supernatants from those cultures were used in different experiments.
Cell viability assay: The PBMCs were seeded into 96-well plates at 5 × 104 cell per well. Following treatment with propofol and/or LPS for the indicated time periods, cell viability was evaluated by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, the medium was refreshed after different treatments, and the cells were incubated with 0.5 mg/ml MTT for a further 3 hr. The blue formazan products in the macrophages were dissolved in DMSO and spectrophotometrically measured at a wavelength of 490 nm.
Real-time quantitative PCR (RT-PCR) analysis: After treatment, cells were harvested by centrifugation (380×g at 4°C for 10 min) and washed with ice-cold PBS. Total RNA was extracted using Trizol® reagent (Invitrogen Corporation, San Diego, CA, U.S.A.) according to the manufacturer’s instructions. The RNA preparations were treated with RNase-free DNase I (TaKaRa Bio Technology Co., Ltd., Dalian, China) to remove any possible contaminated DNA, quantified by an A260 measurement on a spectrophotometer (Eppendorf, Hamburg, Germany) and stored at −86°C. Reverse transcription was performed by mixing 1 µg of RNA with 5 µl of iScript reagent (Bio-Rad, Hercules, CA, U.S.A.) in a DEPC-treated tube. Nuclease-free water was added to a final volume of 20 µl. The reaction conditions for reverse transcription were performed according to the manufacturer’s protocols.
RT-PCR was performed on an ABI 7500 (PE Applied Biosystems, Grand Island, NY, U.S.A.) using a SYBR premix TaqTM (TaKaRa Bio Technology Co., Ltd.). The housekeeping gene GAPDH was used to correct for minor variations in the amount of RNA and the synthesis efficacy of cDNA in subsequent quantitative PCR assays. IL-6 primers were as follows: forward primer 5′-GGC TAC TGC TTT CCC TAC CC-3′ and reverse primer 5′-TTT TCT GCC AGT GCC TCT T-3′. TNF-α primers were as follows: forward primer 5′-TCT CGA ACC CCA AGT GAC AAG-3′ and reverse primer 5′-GGA GCT GCC CCT CAG CTT-3′. The sequence of the GAPDH primers was as follows: forward primer 5′-GTG ATG CTG GTG CTG AGT ATC-3′ and reverse primer 5′-GTG ATG GCA TGG ACK GTG G-3′.
Amplification was carried out in a total volume of 20 µl containing 2 µl of cDNA template, 1 µl of form primer, 1 µl of reverse primer, 6 µl of nuclease-free water, 0.4 µl of ROX and 10 µl of SYBR premix TaqTM(2×; TaKaRa). The following experimental run protocol was used: denaturation program (95°C for 5 min), amplification and quantification program repeated for 36 cycles (95°C for 25 sec and 62°C for 40 sec), and the melting curve program (95°C for 25 sec and 60°C for 1 min). The relative quantification between samples was achieved by the 2-ΔΔCt method and calculated by the software REST 2005 (provided by Eppendorf Co., Germany). Relative cytokines mRNAs levels were normalized to mRNAs levels of the GAPDH housekeeping gene.
Detection of NO production: As nitrite is the primary and nonvolatile breakdown product of NO, production of NO was assessed in the culture according to the accumulation of nitrite supernatants, using a colorimetric reaction with the Griess reagent (Promega, Madison, WI, U.S.A.) as described previously [13]. Absorbance was measured at 550 nm, and the nitrite concentration was determined using sodium nitrite as a standard.
Western blot analysis: The PBMCs were pretreated with 25 or 50 µM propofol for 6 hr and then stimulated with 100 ng/ml LPS for either 1 or 24 hr. After stimulation, the cells were washed with cold PBS. The total protein and nuclear protein were extracted using M-PER Mammalian Protein Extraction Reagent Kit (Pierce, Rockford, IL, U.S.A.) and NE-PERTM Nuclear Extraction Reagent Kit (Pierce), respectively. The protein concentrations of the extracts were measured by the BCA protein assay (Pierce) according to the manufacturer’s instructions. The samples with 50 µg of protein were mixed with the sample buffer, boiled for 5 min and then separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto a nitrocellulose membrane (Millipore, Billerica, MA, U.S.A.) that was then soaked in a blocking solution containing 10% (w/v) Skimmed Milk in phosphate-buffered saline containing 0.05% Tween 20 (PBST) for 1 hr at room temperature. The soaked membrane was incubated with primary antibodies (NF-κB p65, phosphor-specific NF-κB p65 or iNOS antibody) overnight at 4°C, followed by HRP-conjugated secondary antibodies. To normalize protein loading, the membrane was simultaneously incubated with antibody against β-actin or Histone H3 at a dilution of 1:1,000. Immunoblotting was examined by SuperSignal West Pico Chemiluminescent Substrate (Pierce) according to the manufacturer’s instruction, and the membrane was exposed to X-ray films (Kodak, Rochester, NY, U.S.A.) which were developed with an Alpha Imager (Alpha Inntech, San Leandro, CA, U.S.A.). All of the experiments were repeated three times.
Statistical analysis: Results were expressed as mean ± standard deviation. The significance of variability among the experimental groups was determined by one-way ANOVA with Tukey’s multiple comparison tests for post hoc analysis using Graphad Prism® Version 5.0 software (San Diego, CA, U.S.A.). Differences among experimental groups were considered statistically significant at P<0.05 (*indicates P<0.05).
RESULTS
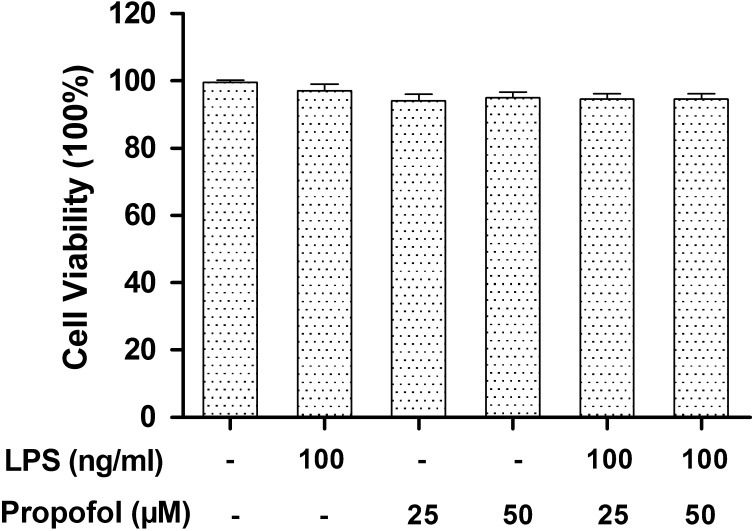
Toxicity of propofol and LPS to canine PBMCs: To avoid any cytotoxic effects caused by propofol, we investigated the effects of propofol on cell survival and cytotoxicity in canine PBMCs. Exposure of canine PBMCs to propofol (25 or 50 µM) in the presence or absence of 100 ng/ml LPS for 24 hr did not cause any significant change in MTT absorbance (Fig. 1). Under the experimental conditions described above, cell viability was determined to be>95% in each group. These data indicated that 25 or 50 µM of propofol was non-cytotoxic to canine PBMCs and excluded the possibility that any cytotoxic action of propofol was present to influence the following experiments.
Fig. 1.
Effects of propofol on the cell viability of canine PBMCs after LPS treatment. Canine PBMCs were pretreated for 6 hr with different concentrations of propofol (0, 25 or 50 µM), and then, cells were treated with LPS (100 ng/ml) for 24 hr. Cell viability was measured by MTT assay. Results are expressed as means ± S.D. from three independent experiments.
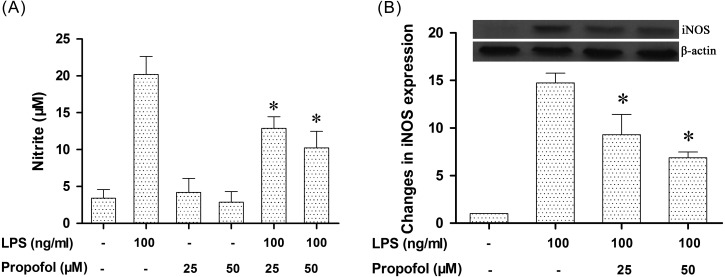
Propofol inhibits the production of NO and iNOS in LPS-stimulated canine PBMCs: It is well known that LPS stimulation typically promotes iNOS/NO biosynthesis and increases the production of pro-inflammatory cytokines in immune cells. To investigate the anti-inflammatory activity of propofol, we evaluated the expressions of NO and iNOS using a Griess reaction and Western blotting, respectively. Results showed that exposure of canine PBMCs to 100 ng/ml LPS for 24 hr significantly enhanced the levels of NO in the culture supernatants. However, after pretreatment with 25 or 50 µM propofol for 6 hr, the LPS-upregulated NO production was significantly decreased by 36% and 47%, respectively (Fig. 2A). Consistent with the NO production, the result of Western blot analysis showed that LPS significantly increased the expression of iNOS protein in canine PBMCs, while pretreatment with propofol significantly decreased the LPS-induced iNOS protein expression (Fig. 2B). These results show that non-cytotoxic levels of propofol suppress LPS-induced iNOS/NO biosynthesis in canine PBMCs.
Fig. 2.
Inhibition of nitrite production and iNOS protein expression by propofol in LPS-stimulated canine PBMCs. (A) Propofol inhibits NO production in LPS-stimulated canine PBMCs. (B) Propofol inhibits iNOS protein expression in LPS-stimulated canine PBMCs. Canine PBMCs were pretreated with different doses of propofol (0, 25 or 50 µM) for 6 hr and then stimulated with LPS (100 ng/ml) for 24 hr. The concentration of NO in the culture medium was determined by using a Griess assay. Cell lysates were extracted, and the protein levels of iNOS were detected by Western blotting. β-actin was used as a loading control. Densitometry was normalized to β-actin and graphed as the mean ± S.D. *indicates P<0.05 (compared with LPS stimulation alone). Similar results were obtained from three independent experiments.
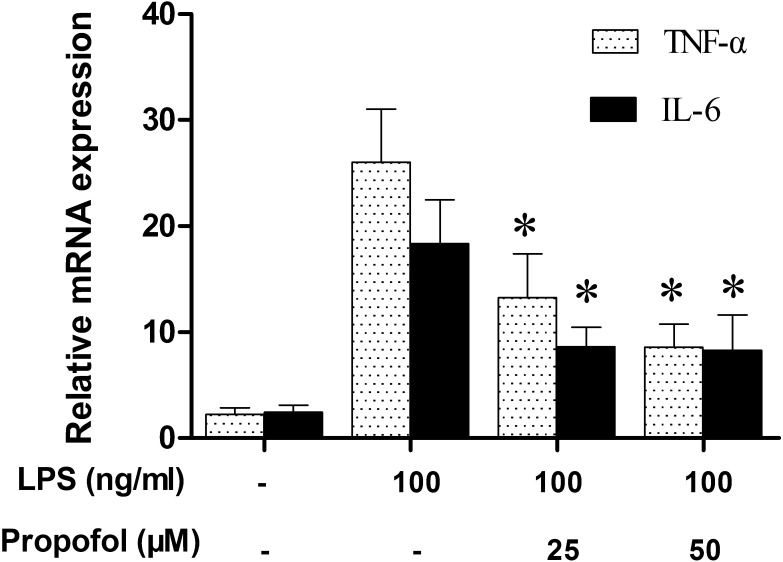
Propofol modulates the expression of TNF-α and IL-6 in canine PBMCs: Several previous reports have shown that LPS stimulation can up-regulate TNF-α and IL-6 expression in various species’ mononuclear cells and that the induction of TNF-α and IL-6 plays a crucial role in mediating LPS-induced sepsis [7]. However, to our knowledge, it had remained unclear if LPS could increase TNF-α and IL-6 gene expressions in canine PBMCs. The following experiment was carried out to test, if co-treatment with LPS could lead to any change in the expression of TNF-α and IL-6 at the transcriptional level in canine PBMCs. RNA expression levels for TNF-α and IL-6-specific genes in canine PBMCs were measured by RT-PCR. Results showed that low levels of TNF-α and IL-6 were detected in none-LPS stimulated canine PBMCs. As reported in other species [14, 35], a co-treatment of mononuclear cells with LPS for 24 hr resulted in significant increases in TNF-α and IL-6 expression in canine PBMCs (Fig. 3). To determine the effect of propofol on pro-inflammatory cytokine gene expression in LPS-stimulated canine PBMCs, the RNA levels of TNF-α and IL-6 in propofol pretreated canine PBMCs were also detected. Results showed that pretreatment of canine PBMCs with propofol (25 or 50 µM) alone did not affect the expression of TNF-α and IL-6 (data not shown). However, pretreatment with 25 or 50 µM propofol significantly reduced LPS-stimulated levels of TNF-α mRNA by 49% and 67% and IL-6 mRNA by 51% and 53%, respectively. These results show that non-cytotoxic levels of propofol suppress LPS-induced inflammatory responses in canine PBMCs as measured by TNF-α and IL-6 expression.
Fig. 3.
Effect of propofol on LPS-induced TNF-α and IL-6 expression. Canine PBMCs were pretreated with different doses of propofol (0, 25 or 50 µM) for 6 hr and then stimulated with LPS (100 ng/ml) for 24 hr. The levels of TNF-α and IL-6 mRNA were measured by RT-PCR. The amount of mRNA was normalized by comparison with the amount of mRNA for GAPDH. Results were reported as the mean ± S.D. *indicates P<0.05 (compared with LPS stimulation alone). Similar results were obtained from three independent experiments.
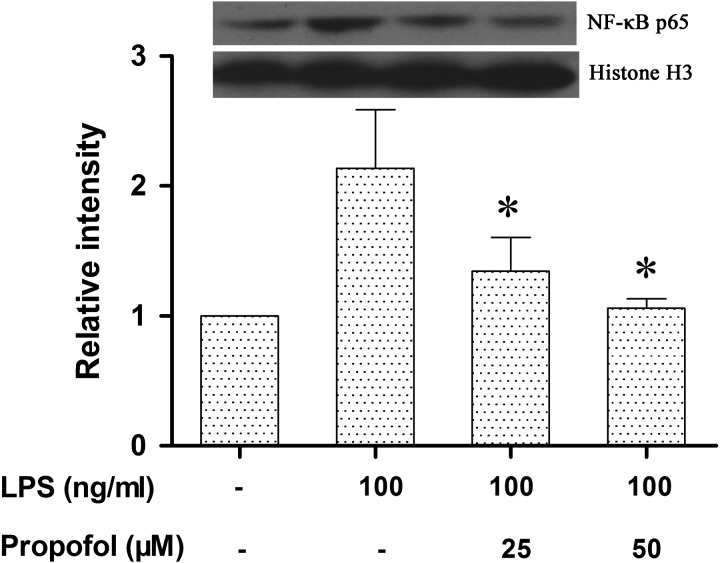
Propofol reduces LPS-induced activation of NF-κB in canine PBMCs: NF-κB activation is essential in the transcriptional regulation of many pro-inflammatory cytokines, including TNF-α and IL-6. The above results indicated that propofol inhibited the expression of TNF-α and IL-6 mRNA in LPS-stimulated canine PBMCs. Therefore, we next investigated whether the upstream NF-κB p65 nuclear translocation was also involved. Using Western blot, we found that the translocation of NF-κB p65 protein into the nucleus was significantly increased in canine PBMCs stimulated with LPS. However, propofol at concentration of 25 µM and 50 µM significantly inhibited the LPS-induced nuclear translocation of the NF-κB p65 protein. The diminished TNF-α, IL-6 and iNOS expression and NO production (Figs. 2 and 3) were in parallel to the decrease in NF-κB p65 protein nuclear translocation in the LPS-activated canine PBMCs pretreated with 25 µM and 50 µM propofol (Fig. 4). These results suggest that propofol pretreatment probably reduces LPS-induced inflammatory responses in canine PBMCs by inhibiting NF-κB activation.
Fig. 4.
Propofol inhibits the nuclear translocation of the NF-κB p65 protein in LPS-stimulated canine PBMCs. Canine PBMCs were pretreated with different doses of propofol (0, 25 or 50 µM) for 6 hr and then stimulated with LPS (100 ng/ml) for 1 hr. The NF-κB p65 protein was analyzed by Western blotting. Histone H3 was used as a loading control. Densitometry was normalized to Histone H3 and graphed as the mean ± S.D. *indicates P<0.05 (compared with LPS stimulation alone). Similar results were obtained from three independent experiments.
DISCUSSION
Increasing evidence indicates that mononuclear cells are the most important effector cells in endotoxin-induced sepsis. They play a critical role in initial recognition of microbial invasion and contributing to downstream immune responses by secreting a variety of pro-inflammatory cytokines [4, 7, 37]. Among these cytokines, overwhelming increase in IL-6 and TNF-α seems to play a major role in modulating the inflammatory response and in the pathogenesis and progression of LPS-induced sepsis shock [3, 7]. Many previous studies have reported that the intravenous anesthetic propofol can inhibit the production of various pro-inflammatory cytokines, such as IL-6 and TNF-α, and NO, in murine and human mononuclear cells. However, the anti-inflammatory effects of propofol upon canine PBMCs remain unclear. In the present study, we provide the first evidence that in canine PBMCs, non-cytotoxic levels of propofol can suppress LPS-induced IL-6 and TNF-α expression. We show that this may be mediated through suppression of NF-κB p65 nuclear translocation. Our results also indicate that propofol can decrease NO biosynthesis through inhibition of the iNOS protein expression in LPS-stimulated canine PBMCs.
A previous study suggested that decreases in the levels of IL-6 and TNF-α in macrophages can lead to immunomodulation [31]. In rats injected with endotoxin, propofol was shown to attenuate septic syndrome through down-regulating IL-6 and TNF-α biosyntheses at the protein level [14]. Propofol has also been shown to inhibit the release of inflammatory factors, such as IL-6, IL-1β and TNF-α, in murine or human mononuclear cells [13, 14, 18, 35]. Consistent with previous studies, our results showed that propofol pretreatment significantly inhibited LPS-induced IL-6 and TNF-α expression in vitro in canine PBMCs. This suggests that propofol treatment leads to down-regulation of the inflammatory response.
The induction of inflammatory cytokines by LPS in immune cells has been reported to be via the NF-κB-mediated signal transduction pathway [2]. NF-κB is a common transcription factor which participates in the regulation of the expressions of a diverse number of inflammatory genes. In the process of NF-κB activation, the translocation of this transcription factor from the cytoplasm to nuclei is important for the regulation of the expressions of certain pro-inflammatory genes. For example, previous studies have shown that NF-κB translocation participates in the up-regulation of LPS-induced iNOS, IL-6 and TNF-α expression [2, 18]. Thus, the blocking of the initial nuclear translocation of NF-κB could prevent the development of sepsis [33]. Recent studies in murines have shown that propofol inhibits the nuclear translocation of NF-κB in LPS-induced macrophages, leading to transcriptional suppression of a large number of downstream genes [13]. In the current study, we showed that propofol down-regulated the inflammatory response via suppression of IL-6 and TNF-α expression in canine PBMCs. However, whether the anti-inflammatory effects of propofol in canine PBMCs are achieved through the inhibition of the nuclear translocation of the NF-κB protein awaited further confirmation. In accordance with previous studies in other species, Western blotting analysis showed that non-cytotoxic levels of propofol pretreatment significantly inhibited the nuclear translocation of the NF-κB p65 subunit in canine PBMCs stimulated by LPS. In parallel with the down-regulation of NF-κB p65 translocation, levels of IL-6 and TNF-α mRNA expression were significantly attenuated by propofol in LPS-activated canine PBMCs. These results suggest that propofol can suppress LPS-induced IL-6 and TNF-α expression, partially through the inhibition of NF-κB p65 translocation in canine PBMCs.
In immune responses, iNOS-derived NO expression plays an important role in protecting mononuclear cells against microbial infection. However, overproduction of NO is known to be correlated with a variety of diseases, leading to septic shock, atherosclerosis, tissue injury and other conditions [5, 12, 16]. Excess production of NO has also been shown to play a critical role in LPS-induced hypotension, which is one of the major symptoms of LPS-induced septic shock [26]. The generation of large quantities of NO may also cause a maldistribution of regional blood flow, the formation of a diffusion barrier for oxygen and the inhibition of mitochondrial respiration [30]. Mitaka et al. [22] suggested that the endotoxin-induced hypotension, lactic acidosis and NO overproduction were significantly attenuated by iNOS inhibition in a canine endotoxic shock model. Furthermore, NO overproduction is shown to induce cell apoptosis which can aggravate tissue injury [5, 17]. Therefore, down-regulation of NO is an important target in the treatment of LPS-induced septic shock. In the present study, we found that exposure of canine PBMCs to LPS significantly increased the levels of NO in the culture medium, while co-treatment with propofol and LPS significantly attenuated this LPS-induced increase in NO. In parallel with the decrease in LPS-induced NO production, propofol at 25 or 50 µM significantly inhibited iNOS protein expressions in LPS-stimulated canine PBMCs. These data suggested that propofol inhibits NO biosynthesis by down-regulating iNOS protein expression.
Clinically relevant plasma concentrations of propofol for anesthesia in dogs have been reported previously; however, large differences were found between the minimum and maximum values. For example, Musk et al. [23] suggested that a target of 3.0–3.5 µg/ml(equivalent to approximately 16.8–19.6 µM) propofol in plasma provided adequate reflex suppression to achieve successful induction of anesthesia. However, Nolan and Reid [25] suggested that tracheal intubation should not be performed when the average propofol blood concentration is below 5.4 µg/ml(30.3 µM). Previous studies have also suggested that, despite high doses of propofol administered as a single anesthetic in dogs, such dosages have been able to produce an increase in anesthetic depth and decrease in hemodynamic parameters where the heart rate and mean arterial pressure remained within normal physiological limits even when the maximum plasma propofol concentration was achieved at 7.78 µg/ml (43.6 µM), 11.5 µg/ml (64.5 µM) or 15.27 µg/ml (85.7 µM) in these different studies [24, 29, 34]. Therefore, the concentration of propofol at 25 µM and 50 µM which was chosen as the dosages administrated in the present study can be considered to be within the clinically relevant range.
Despite considerable progress in general healthcare over the past decades, sepsis continues to be a major life-threatening condition with a mortality rate of about 35% [6]. Therefore, it has been widely accepted that pre-emptive therapy of high-risk patients would be a better approach than treatment of sepsis. Our present study demonstrates that pretreatment with propofol significantly attenuates LPS-induced inflammatory response in canine PBMCs. Consistent with our findings, propofol has been reported to exert potent pre-conditioning protection against LPS-induced inflammatory damage in various types of cells, including mouse macrophages [13, 21], rat alveolar epithelial cells [39], mouse cardiomyocytes [38] and rat hepatocytes [15]. Recent evidence also indicates that pretreatment with propofol significantly reduces the mortality rate of rats in LPS- or polymicrobial induced sepsis models and protects various important organs, such as the liver, kidney, lung and heart, in rats with sepsis [1, 9, 10, 36]. Interestingly, Gao et al. [9, 10] also observed that early propofol treatment (including both pretreatment and simultaneous treatment) significantly attenuates LPS-induced acute lung injuries and dramatically improves the survival rates of septic rats. In contrast, these beneficial effects were blunted in the rats receiving propofol post LPS treatment. Moreover, although propofol has been often used to sedate septic patients in the intensive care unit, it might be also possible to give propofol in advance to critically ill patients who are predisposed to sepsis, such as severe burn injury, persisting ileus and major trauma. In this context, we believe that our study, which investigated the effect of propofol pretreatment on LPS-induced canine PBMCs, has clinical relevance and implications.
The MTT assay showed that exposure of canine PBMCs to propofol, LPS or a combination of these two drugs, under such concentrations as administered herein, did not affect cell viability. This is consistent with previous studies [13, 18, 35, 40]. Thus, sub-toxic administration of propofol can downregulate LPS-induced IL-6, TNF-α and NO expression through inhibition of NF-κB and iNOS protein expression, rather than via a death mechanism. Taken together, the current study demonstrates that non-cytotoxic levels of propofol can inhibit the LPS-induced inflammatory response in canine PBMCs, possibly through suppression of the biosynthesis of iNOS/NO and by inhibiting the nuclear translocation of NF-κB p65 and its downstream of pro-inflammatory cytokine expressions (IL-6 and TNF-α). These findings suggest that propofol possesses anti-inflammatory and anti-oxidative action and it may be used as not only a general anesthetic, but also as an effective therapeutic agent for sepsis. However, it remains of note that the present study was carried out with in vitro cell cultures and the results should be further confirmed by in vivo studies.
Acknowledgments
The authors thank Dr. Yan Mao, Veterinary Teaching Hospital of Zhejiang University, for critical reading of the manuscript. This study was supported by Specialized Research Fund for the Doctoral Program of Higher Education of China (SRFDP, No. 20110101120089) and the National Natural Science Foundation of China (NSFC, No. 31201960).
REFERENCES
- 1.Bao H. G., Li S.2011. Effects of propofol on the outcomes of rats with sepsis. J. Surg. Res. 168: e111–e115. doi: 10.1016/j.jss.2010.12.034 [DOI] [PubMed] [Google Scholar]
- 2.Böhrer H., Qiu F., Zimmermann T., Zhang Y., Jllmer T., Männel D., Böttiger B. W., Stern D. M., Waldherr R., Saeger H. D., Ziegler R., Bierhaus A., Martin E., Nawroth P. P.1997. Role of NFkappaB in the mortality of sepsis. J. Clin. Invest. 100: 972–985. doi: 10.1172/JCI119648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrelli E., Roux-Lombard P., Grau G. E., Girardin E., Ricou B., Dayer J., Suter P. M.1996. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 24: 392–397. doi: 10.1097/00003246-199603000-00006 [DOI] [PubMed] [Google Scholar]
- 4.Calandra T., Roger T.2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3: 791–800. doi: 10.1038/nri1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung H. T., Pae H. O., Choi B. M., Billiar T. R., Kim Y. M.2001. Nitric oxide as a bioregulator of apoptosis. Biochem. Biophys. Res. Commun. 282: 1075–1079. doi: 10.1006/bbrc.2001.4670 [DOI] [PubMed] [Google Scholar]
- 6.Daniels R.2011. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective). J. Antimicrob. Chemother. 66 Suppl 2: ii11–ii23. doi: 10.1093/jac/dkq515 [DOI] [PubMed] [Google Scholar]
- 7.Froidevaux C., Roger T., Martin C., Glauser M. P., Calandra T.2001. Macrophage migration inhibitory factor and innate immune responses to bacterial infections. Crit. Care Med. 29 Suppl: S13–S15. doi: 10.1097/00003246-200107001-00006 [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto Y., Nakatani N., Kubo T., Semi Y., Yoshida N., Nakajima H., Iseri T., Azuma Y. T., Takeuchi T.2012. Adenosine and ATP affect LPS-induced cytokine production in canine macrophage cell line DH82 cells. J. Vet. Med. Sci. 74: 27–34. doi: 10.1292/jvms.11-0228 [DOI] [PubMed] [Google Scholar]
- 9.Gao J., Zeng B. X., Zhou L. J., Yuan S. Y.2004. Protective effects of early treatment with propofol on endotoxin-induced acute lung injury in rats. Br. J. Anaesth. 92: 277–279. doi: 10.1093/bja/aeh050 [DOI] [PubMed] [Google Scholar]
- 10.Gao J., Zhao W. X., Xue F. S., Zhou L. J., Xu S. Q., Ding N.2010. Early administration of propofol protects against endotoxin-induced acute lung injury in rats by inhibiting the TGF-beta1-Smad2 dependent pathway. Inflamm. Res. 59: 491–500. doi: 10.1007/s00011-009-0110-y [DOI] [PubMed] [Google Scholar]
- 11.Grandel U., Grimminger F.2003. Endothelial responses to bacterial toxins in sepsis. Crit. Rev. Immunol. 23: 267–299. doi: 10.1615/CritRevImmunol.v23.i4.20 [DOI] [PubMed] [Google Scholar]
- 12.Hobbs A. J., Higgs A., Moncada S.1999. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu. Rev. Pharmacol. Toxicol. 39: 191–220. doi: 10.1146/annurev.pharmtox.39.1.191 [DOI] [PubMed] [Google Scholar]
- 13.Hsing C. H., Lin M. C., Choi P. C., Huang W. C., Kai J. I., Tsai C. C., Cheng Y. L., Hsieh C. Y., Wang C. Y., Chang Y. P., Chen Y. H., Chen C. L., Lin C. F.2011. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS ONE 6: e17598. doi: 10.1371/journal.pone.0017598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu B. G., Yang F. L., Lee R. P., Peng T. C., Chen H. I.2005. Effects of post-treatment with low-dose propofol on inflammatory responses to lipopolysaccharide-induced shock in conscious rats. Clin. Exp. Pharmacol. Physiol. 32: 24–29. doi: 10.1111/j.1440-1681.2005.04155.x [DOI] [PubMed] [Google Scholar]
- 15.Jawan B., Kao Y. H., Goto S., Pan M. C., Lin Y. C., Hsu L. W., Nakano T., Lai C. Y., Sun C. K., Cheng Y. F., Tai M. H., Eng H. L., Wang C. S., Huang C. J., Lin C. R., Chen C. L.2008. Propofol pretreatment attenuates LPS-induced granulocyte-macrophage colony-stimulating factor production in cultured hepatocytes by suppressing MAPK/ERK activity and NF-kappaB translocation. Toxicol. Appl. Pharmacol. 229: 362–373. doi: 10.1016/j.taap.2008.01.044 [DOI] [PubMed] [Google Scholar]
- 16.Komeno M., Akimoto A., Fujita T., Aramaki T., Aoki M., Shimada T., Ohashi F.2004. Role of nitric oxide in hemodialysis-related hypotension in an experimental renal dysfunction dog model. J. Vet. Med. Sci. 66: 53–57. doi: 10.1292/jvms.66.53 [DOI] [PubMed] [Google Scholar]
- 17.Li C. Q., Wogan G. N.2005. Nitric oxide as a modulator of apoptosis. Cancer Lett. 226: 1–15. doi: 10.1016/j.canlet.2004.10.021 [DOI] [PubMed] [Google Scholar]
- 18.Ma X., Hu Y. W., Zhao Z. L., Zheng L., Qiu Y. R., Huang J. L., Wu X. J., Mao X. R., Yang J., Zhao J. Y., Li S. F., Gu M. N., Wang Q.2013. Anti-inflammatory effects of propofol are mediated by apolipoprotein M in a hepatocyte nuclear factor-1α-dependent manner. Arch. Biochem. Biophys. 533: 1–10. doi: 10.1016/j.abb.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Maeda K., Sakonju I., Kanda A., Suzuki T., Kakuta T., Shimamura S., Okano S., Takase K.2010. Priming effects of lipopolysaccharide and inflammatory cytokines on canine granulocytes. J. Vet. Med. Sci. 72: 55–60. doi: 10.1292/jvms.08-0494 [DOI] [PubMed] [Google Scholar]
- 20.Mayr F. B., Yende S., Angus D. C.2014. Epidemiology of severe sepsis. Virulence 5: 4–11. doi: 10.4161/viru.27372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng T., Yu J., Lei Z., Wu J., Wang S., Bo Q., Zhang X., Ma Z., Yu J.2013.Propofol reduces lipopolysaccharide-induced, NADPH oxidase (NOX 2) mediated TNF- α and IL-6 production in macrophages. Clin. Dev. Immunol. 2013: 325481. doi: 10.1155/2013/325481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitaka C., Hirata Y., Narumi Y., Yokoyama K., Makita K., Katsuyama K., Imai T.2005. Blockade of nuclear factor-kappaB activation prevents hypodynamic shock and gastric hypoperfusion induced by endotoxin in anesthetized dogs. Intensive Care Med. 31: 718–723. doi: 10.1007/s00134-005-2617-1 [DOI] [PubMed] [Google Scholar]
- 23.Musk G. C., Pang D. S., Beths T., Flaherty D. A.2005. Target-controlled infusion of propofol in dogs—evaluation of four targets for induction of anaesthesia. Vet. Rec. 157: 766–770. [DOI] [PubMed] [Google Scholar]
- 24.Nakaigawa Y., Akazawa S., Shimizu R., Ishii R., Yamato R.1995. Effects of graded infusion rates of propofol on cardiovascular haemodynamics, coronary circulation and myocardial metabolism in dogs. Br. J. Anaesth. 75: 616–621. doi: 10.1093/bja/75.5.616 [DOI] [PubMed] [Google Scholar]
- 25.Nolan A., Reid J.1993. Pharmacokinetics of propofol administered by infusion in dogs undergoing surgery. Br. J. Anaesth. 70: 546–551. doi: 10.1093/bja/70.5.546 [DOI] [PubMed] [Google Scholar]
- 26.Petros A., Bennett D., Vallance P.1991. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet 338: 1557–1558. doi: 10.1016/0140-6736(91)92376-D [DOI] [PubMed] [Google Scholar]
- 27.Raetz C. R., Ulevitch R. J., Wright S. D., Sibley C. H., Ding A., Nathan C. F.1991. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 5: 2652–2660. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran G.2014. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence 5: 213–218. doi: 10.4161/viru.27024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro L. M., Ferreira D. A., Bressan N. M., Nunes C. S., Amorim P., Antunes L. M.2008. Brain monitoring in dogs using the cerebral state index during the induction of anaesthesia via target-controlled infusion of propofol. Res. Vet. Sci. 85: 227–232. doi: 10.1016/j.rvsc.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 30.Sarti P., Arese M., Forte E., Giuffrè A., Mastronicola D.2012. Mitochondria and nitric oxide: chemistry and pathophysiology. Adv. Exp. Med. Biol. 942: 75–92. doi: 10.1007/978-94-007-2869-1_4 [DOI] [PubMed] [Google Scholar]
- 31.Scott D. L., Kingsley G. H.2006. Tumor necrosis factor inhibitors for rheumatoid arthritis. N. Engl. J. Med. 355: 704–712. doi: 10.1056/NEJMct055183 [DOI] [PubMed] [Google Scholar]
- 32.Sebel P. S., Lowdon J. D.1989. Propofol: a new intravenous anesthetic. Anesthesiology 71: 260–277. doi: 10.1097/00000542-198908000-00015 [DOI] [PubMed] [Google Scholar]
- 33.Senftleben U.2003. NF-kappaB in critical diseases: a bad guy? Intensive Care Med. 29: 1873–1876. doi: 10.1007/s00134-003-1932-7 [DOI] [PubMed] [Google Scholar]
- 34.Silva A., Ribeiro L. M., Bressan N., Oliveira P., Ferreira D. A., Antunes L. M.2011. Dogs mean arterial pressure and heart rate responses during high propofol plasma concentrations estimated by a pharmacokinetic model. Res. Vet. Sci. 91: 278–280. doi: 10.1016/j.rvsc.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 35.Song H. K., Jeong D. C.2004. The effect of propofol on cytotoxicity and apoptosis of lipopolysaccharide-treated mononuclear cells and lymphocytes. Anesth. Analg. 98: 1724–1728. doi: 10.1213/01.ANE.0000112317.68730.B0 [DOI] [PubMed] [Google Scholar]
- 36.Song X. M., Wang Y. L., Li J. G., Wang C. Y., Zhou Q., Zhang Z. Z., Liang H.2009. Effects of propofol on pro-inflammatory cytokines and nuclear factor kappaB during polymicrobial sepsis in rats. Mol. Biol. Rep. 36: 2345–2351. doi: 10.1007/s11033-009-9456-z [DOI] [PubMed] [Google Scholar]
- 37.Takahashi Y., Isuzugawa K., Murase Y., Imai M., Yamamoto S., Iizuka M., Akira S., Bahr G. M., Momotani E., Hori M., Ozaki H., Imakawa K.2006. Up-regulation of NOD1 and NOD2 through TLR4 and TNF-alpha in LPS-treated murine macrophages. J. Vet. Med. Sci. 68: 471–478. doi: 10.1292/jvms.68.471 [DOI] [PubMed] [Google Scholar]
- 38.Tang J., Hu J. J., Lu C. H., Liang J. N., Xiao J. F., Liu Y. T., Lin C. S., Qin Z. S.2014. Propofol inhibits lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression through decreasing the generation of superoxide anion in cardiomyocytes. Oxid. Med. Cell. Longev. 2014: 157376. doi: 10.1155/2014/157376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei L., Matsumoto H., Yamaguchi H.2013. Propofol attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production through p38 MAPK and SAPK/JNK in alveolar epithelial cells. J. Anesth. 27: 366–373. doi: 10.1007/s00540-012-1539-7 [DOI] [PubMed] [Google Scholar]
- 40.Wu G. J., Chen T. L., Chang C. C., Chen R. M.2009. Propofol suppresses tumor necrosis factor-alpha biosynthesis in lipopolysaccharide-stimulated macrophages possibly through downregulation of nuclear factor-kappa B-mediated toll-like receptor 4 gene expression. Chem. Biol. Interact. 180: 465–471. doi: 10.1016/j.cbi.2009.05.003 [DOI] [PubMed] [Google Scholar]