Abstract
To investigate the possible circulation of arboviruses in South Korea, nationwide surveillance of five arbovirues was conducted in sentinel calves during 2009−2012. We used serum neutralization tests to investigate the presence of antibodies for the Aino virus, Akabane virus, bovine ephemeral fever virus, Chuzan virus and Ibaraki virus. In 2009, 2011 and 2012, the seropositive rates for these five arboviruses were all less than 14.1%. In 2010, however, the seropositive rates for Aino virus and Akabane virus were 33.2% and 40.2%, respectively. High seropositive rates were also associated with a large-scale outbreak of Akabane viral encephalomyelitis in cattle in southern Korea in 2010. Continued seroprevalence surveillance will be useful for monitoring natural arboviral diseases.
Keywords: arbovirus, seroprevalence, South Korea
Arthropod-borne viruses (arboviruses) are transmitted by blood-sucking arthropods, such as mosquitoes, Culicoides biting midges and ticks [21]. Arboviral infections generally cause not only reproductive disorders, such as abortion, stillbirth and congenital malformation, but also acute febrile disease, resulting in economic losses to the ruminant industry [10]. Aino virus (AINOV) and Akabane virus (AKAV), in the family Bunyaviridae, are among the arboviruses that cause disease outbreaks in cattle [1, 19]. Bovine ephemeral fever virus (BEFV) is classified into the family Rhabdoviridae and is known to cause an acute febrile disease called “three day sickness” [20]. Chuzan virus (CHUV) and Ibaraki virus (IBAV) belong to the family Reoviridae and cause reproductive disorders, fever, anorexia and deglutitive disorder [5, 21].
Isolation and antibody surveys of arboviruses, including AINOV, AKAV, BEFV, CHUV and IBAV, have previously been reported in Australia, Asia, Africa and the Middle East [3, 4, 10, 12, 18, 23]. Outbreaks of the diseases caused by these viruses have been isolated, identified and studied epidemiologically in South Korea [6, 16]. Since 2007, Akabane viral encephalitis has been reported in calves, and the genetic and pathogenic characteristics of AKAV isolated from cattle with encephalomyelitis have also been described [9, 15]. In a 2007 study of thoracic fluids from aborted calves, virus neutralization assays indicated positive rates for AINOV, AKAV and CHUV of 11%, 14.2% and 22.8%, respectively [10]. Serosurveillance of AINOV, AKAV and CHUV in Korean native goats (Capra hircus) revealed positive rates of 13.3%, 5.5% and 2.0%, respectively [22].
Live vaccines for AKAV and BEFV have been commercially available in South Korea since the 1980s. Additionally, an AINOV, AKAV and CHUV trivalent vaccine was developed in 2011 [8]. However, rates of inoculation with the AKAV and BEFV vaccines are low in Korean native cattle, and the AINOV, AKAV and CHUV trivalent vaccine has received little use in the field. Therefore, it is difficult to distinguish between vaccinated and unvaccinated Korean cattle. In this study, serological monitoring of five arboviruses was conducted during 2009−2012 using a total of 4,000 blood samples, with samples collected from 500 unvaccinated sentinel cattle twice each year. This study represents the first nationwide serological survey of AINOV, AKAV, BEFV, CHUV and IBAV among sentinel cattle conducted by neutralization antibody tests.
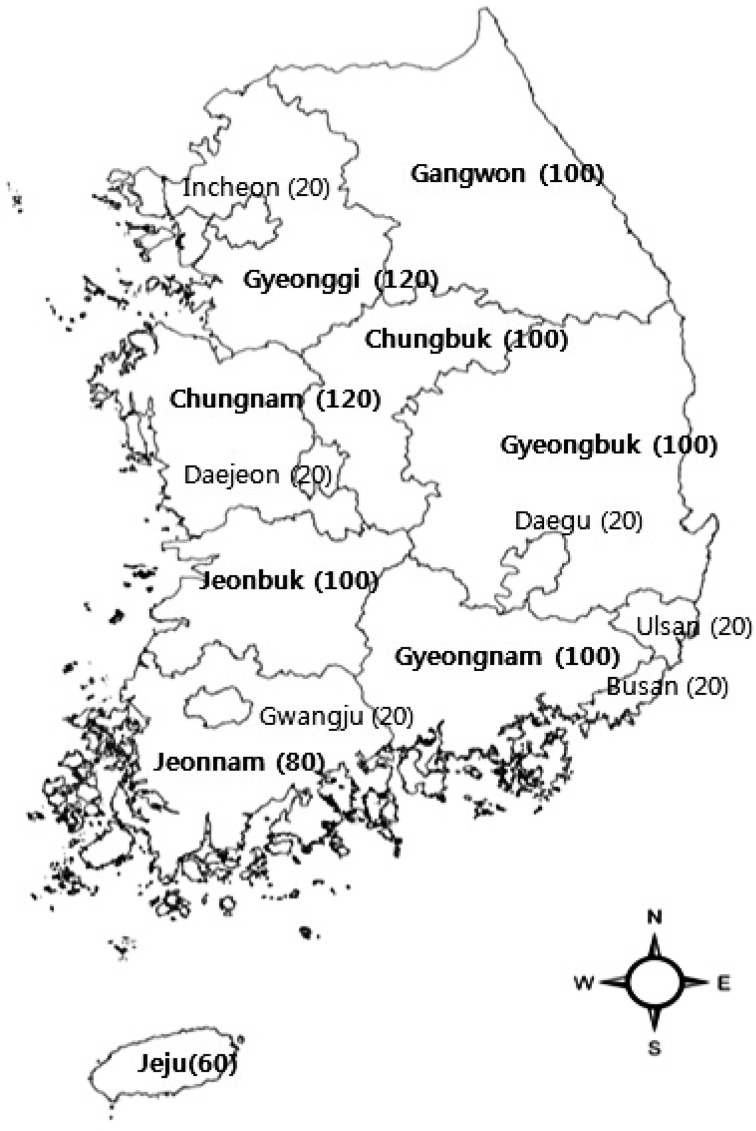
The study was carried out in all provinces and metropolitan cities of the Republic of Korea. In May and October of each year from 2009 through 2012, blood samples were collected from 500 unvaccinated Korean calves, yielding 1,000 blood samples annually. In May, the sampled cattle were 6−7 months of age. Samples were again collected in October of each year, following the season of vector activity from June to August. These samples were compared to the samples collected from the same individuals in May of that year. The necessary sample size was calculated using Win Episcope 2.0: 475 cattle were required to analyze the nationwide arboviral prevalence, based on an error of 4.5%, 95% confidence and 50% expected prevalence. Sampling locations are shown in Fig. 1.
Fig. 1.
Number of cattle sampled in each province of South Korea. Serum was collected from same individual in May and October of each year from 2009 to 2012 for serosurveillance of viruses. Numbers in parentheses represent sample size. Bold characteristics represent the province of South Korea.
The AINOV strain KSA 9910 (Korea Veterinary Culture Collection (KVCC)-VR64), AKAV strain 93FMX (KVCC-VR63), BEFV strain TongRae (KVCC-VR41), CHUV strain YongAm (KVCC-VR66) and IBAV strain 08220 (KVCC-VR65) were used for serum neutralization tests (SNT) [8, 10, 13]. Vero cells (ATCC, C-1586) were maintained in alpha-minimum essential medium (Gibco, Grand Island, NY, U.S.A.) containing 5% fetal bovine serum and antimycotic-antibiotics (Gibco). SNT against AINOV, AKAV, BEFV, CHUV and IBAV were performed in flat-bottomed 96-well plates. Briefly, two-fold serial dilutions of sera were mixed with equal volumes of virus containing 200 TCID50/0.1 ml and inoculated with Vero cells [8, 11]. The plates were microscopically examined after 3 and 5 days for evidence of virus-specific cytopathic effects (CPE). Antibody titers were expressed as the reciprocal of the highest serum dilution at which CPE was inhibited. A titer of 1:4 or greater was considered to be positive.
Seropositive animals were those that were antibody-negative in May, but antibody-positive in October of the same year. Therefore, the seroprevalences for the five viruses in this study represent the proportions of the 500 sampled individuals that tested positive for the antibody in each year. Collecting serum samples from the same individuals before and after the vector season provided reliable epidemiological data for arboviral infection.
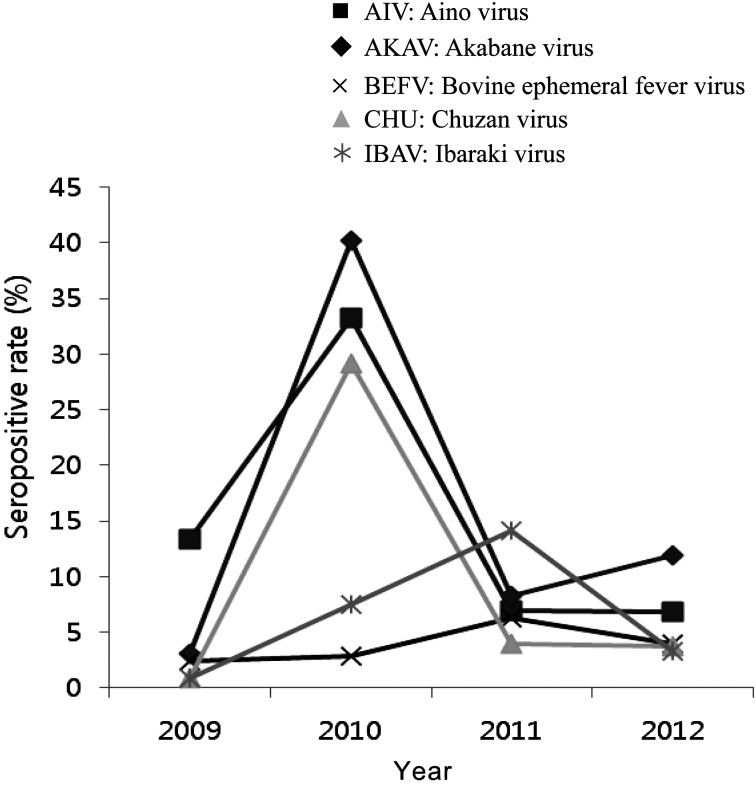
Among the regions and years studied, Gwangju had the highest seropositivity for AINOV and AKAV at 100% and 66.7%, respectively, in 2010. Gyeongnam had the highest seropositivity for CHUV at 61% in 2010. The highest seropositivity rate for BEFV was 35.7% in Daegu, while that for IBAV was 33.3% on Jeju Island, both in 2011. In 2009, the first year of monitoring, the same individuals could not be sampled a second time in Chungbuk and Jeonnam (Table 1). Therefore, the seropositivity rates for these provinces are not shown in Table 1. No significant association was found between seropositivity and region. Figure 2 shows the seropositivity rates for each virus in each year. In 2009, no virus had a seropositivity rate greater than 13.3%. However, in 2010, the overall seropositivity rates were 33.2% for AINOV, 40.2% for AKAV, 2.9% for BEFV, 29.1% for CHUV and 7.5% for IBAV. In 2011 and 2012, all seropositivity rates were less than 14.1% (Fig. 2). We found a high seropositivity rate for AKAV in 2010, suggesting that AKAV infection may have caused disease outbreaks in cattle. Indeed, a large-scale outbreak of Akabane viral encephalomyelitis in cattle aged 4−72 months occurred in the southern part of Korea from late summer to late autumn in 2010. Most of the affected cattle were found in Jeonbuk and Jeonnam provinces [14]. These data were supported by a previous report indicating that arboviral disease has been suspected to be sporadic outbreaks [17]. Although elevated antibody prevalence also against AINOV in 2010 was founded, AINOV which was associated with the large-scale outbreak including samples of the southern part was not detected.
Table 1. Percentage of seropositivity from Korean sentinel cattle to each five arboviruses in 2009−2012, presented by region.
| Virus | Aino virus |
Akabane virus |
Bovine ephemeral fever virus |
Chuzan virus |
Ibaraki virus |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region\Year | ’09 | ’10 | ’11 | ’12 | ’09 | ’10 | ’11 | ’12 | ’09 | ’10 | ’11 | ’12 | ’09 | ’10 | ’11 | ’12 | ’09 | ’10 | ’11 | ’12 |
| Busan | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 30.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Chungbuk | − | 15.4 | 3.4 | 3.6 | − | 20.8 | 11.9 | 10.9 | − | 4.0 | 3.4 | 0.0 | − | 34.0 | 10.2 | 9.1 | − | 14.3 | 13.6 | 3.6 |
| Chungnam | 43.6 | 20.0 | 17.8 | 7.4 | 0.0 | 39.3 | 13.3 | 13.0 | 0.0 | 2.0 | 6.7 | 13.0 | 0.0 | 21.9 | 0.0 | 5.6 | 1.8 | 11.6 | 11.1 | 3.7 |
| Daegu | 0.0 | 27.3 | 0.0 | 27.3 | 0.0 | 14.3 | 0.0 | 0.0 | 0.0 | 0.0 | 35.7 | 0.0 | 0.0 | 10.0 | 0.0 | 9.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Daejeon | 10.0 | 75.0 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 10.0 | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Gangwon | 2.3 | 40.0 | 2.9 | 0.0 | 2.3 | 25.0 | 5.7 | 5.9 | 0.0 | 0.0 | 2.9 | 5.9 | 0.0 | 15.0 | 0.0 | 2.0 | 0.0 | 5.4 | 14.3 | 0.0 |
| Gwangju | 0.0 | 100.0 | 0.0 | 14.3 | 0.0 | 66.7 | 6.7 | 14.3 | 0.0 | 0.0 | 13.3 | 0.0 | 0.0 | 37.4 | 0.0 | 0.0 | 10.0 | 0.0 | 13.3 | 0.0 |
| Gyeongbuk | 0.0 | 36.0 | 8.0 | 6.0 | 2.0 | 24.0 | 2.0 | 5.0 | 10.0 | 12.8 | 6.0 | 0.0 | 0.0 | 34.1 | 0.0 | 2.0 | 4.0 | 5.0 | 18.0 | 2.0 |
| Gyeonggi | 12.7 | 40.0 | 10.7 | 8.0 | 3.2 | 44.1 | 21.4 | 20.0 | 0.0 | 1.7 | 3.6 | 0.0 | 1.6 | 20.8 | 7.1 | 2.0 | 1.6 | 7.7 | 10.7 | 4.0 |
| Gyeongnam | 22.5 | 51.9 | 8.0 | 8.0 | 11.3 | 31.7 | 8.0 | 8.6 | 2.8 | 5.4 | 8.0 | 0.0 | 1.4 | 61.0 | 4.0 | 2.0 | 1.4 | 11.5 | 20.0 | 2.0 |
| Incheon | 0.0 | 28.6 | 0.0 | 0.0 | 0.0 | 36.3 | 0.0 | 7.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 7.7 |
| Jeonbuk | 9.1 | 46.2 | 2.4 | 3.3 | 0.0 | 34.8 | 9.5 | 5.0 | 0.0 | 0.0 | 0.0 | 6.7 | 3.0 | 28.1 | 2.4 | 1.7 | 0.0 | 3.2 | 21.4 | 3.3 |
| Jeonnam | − | 42.3 | 16.3 | 11.4 | − | 56.3 | 2.3 | 8.6 | − | 0.0 | 16.3 | 17.1 | − | 38.5 | 11.6 | 8.6 | − | 5.6 | 7.0 | 0.0 |
| Jeju | 10.0 | 26.7 | 3.7 | 3.3 | 3.3 | 14.3 | 11.1 | 26.7 | 0.0 | 0.0 | 0.0 | 6.7 | 3.3 | 0.0 | 7.4 | 3.3 | 3.3 | 7.4 | 33.3 | 3.3 |
| Ulsan | 0.0 | 66.7 | 0.0 | 10.0 | 0.0 | 20.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 |
− ; Chungbuk and Jeonnam had no the same individual’s data to analyze.
Fig. 2.
Nationwide seropositivity rates for five arboviruses in South Korea from 2009 through 2012.
Significant seroconversions against AKAV, AINOV and CHUV were observed in 2010. However, no clinical cases were reported in the field for any virus, except for AKAV during the study period. Many other factors may be involved in this result, including characteristics of subclinical signs in most arbovirus-infected cattle. In AINOV and CHUV infections of cattle, in utero infections of pregnant cows are common during outbreaks. Therefore, laboratory-confirmed cases are rare in many countries. Additionally, a sporadic outbreak in the field may not be reported. Although unlikely, cross reactivity with other related arboviruses in the virus neutralization test cannot be eliminated as a factor. Therefore, further studies are necessary to reveal the relationship between disease incidence and the seroconversion of sentinel animals.
In this study, we established a statistically reliable sampling strategy for the serological surveillance of arboviruses in South Korea. The sample size used in this study was optimal for the surveillance of herd immunity to arboviruses across the country.
Baseline serological studies of animals, such as the current study, can be used to determine antibody seropositivity rates. The determination of seropositivity rates often leads to an understanding of virus circulation dynamics and is useful in the formulation of disease control measures [2]. Therefore, quarantines and the summer control of vectors could be better implemented in those provinces with elevated seropositivity rates. Indeed, after the large-scale outbreak in 2010, the Animal and Plant Quarantine Agency (QIA) recommended preventive vaccination in all regions of South Korea.
In South Korea, Shin et al. monitored bovine arboviral diseases using a method for detecting viral genes from arthropod vectors, such as Culicoides species, in 2006−2008 [17]. However, as data from the continued monitoring of arthropod vectors have not been reported since 2009, we are unable to speculate regarding the epidemiological relationship between the viral genes from these arthropod vectors and the seropositivity rates observed in the present study. The species of the biting midge of the genus Culicoides (Diptera: Ceratopogonidae) most commonly collected on cattle farms was Culicoides (C.) punctatus, followed by C. arakawae [13]. This result is consistent with those of previous seasonal abundance observations of cowsheds conducted in the southern part of the Republic of Korea [7]. However, the isolation of bovine arboviruses from Culicoides biting midges and the identification of the main vector species remain subjects for a future study. The results of this seroprevalence study may serve as a basis for future epidemiological studies of arboviral infection.
Acknowledgments
This work was financially supported by a grant from the QIA, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
REFERENCES
- 1.Akashi H., Kaku Y., Kong X. G., Pang H.1997. Sequence determination and phylogenetic analysis of the Akabane bunyavirus S RNA genome segment. J. Gen. Virol. 78: 2847–2851. [DOI] [PubMed] [Google Scholar]
- 2.Batista P. M., Andreotti R., Chiang J. O., Ferreira M. S., Vasconcelos P. F.2012. Seroepidemiological monitoring in sentinel animals and vectors as part of arbovirus surveillance in the state of Mato Grosso do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 45: 168–173. doi: 10.1590/S0037-86822012000200006 [DOI] [PubMed] [Google Scholar]
- 3.Jagoe S., Kirkland P. D., Harper P. A.1993. An outbreak of Akabane virus-induced abnormalities in calves after agistment in an endemic region. Aust. Vet. J. 70: 56–58. doi: 10.1111/j.1751-0813.1993.tb15139.x [DOI] [PubMed] [Google Scholar]
- 4.Jun Q., Qingling M., Zaichao Z., Kuojun C., Jingsheng Z., Minxing M., Chuangfu C.2012. A serological survey of Akabane virus infection in cattle and sheep in northwest China. Trop. Anim. Health Prod. 44: 1817–1820. doi: 10.1007/s11250-012-0168-3 [DOI] [PubMed] [Google Scholar]
- 5.Jusa E. R., Inaba Y., Kadoi K., Kurogi H., Fonseca E., Shope R. E.1994. Identification of Kagoshima and Chuzan viruses of Japan as Kasba virus, an orbivirus of the Palyam serogroup. Aust. Vet. J. 71: 57. doi: 10.1111/j.1751-0813.1994.tb06155.x [DOI] [PubMed] [Google Scholar]
- 6.Kang W. C., Kim E. J., Hyun K. J., Cheon C. I., Kim H. S., Lee D. S.2000. Isolation, identification and epidemiological study of akabane virus on Jeju-do. Korea. J. Vet. Serv. 12: 93–102. [Google Scholar]
- 7.Kim H. C., Bellis G. A., Kim M. S., Chong S. T., Lee D. K., Park J. Y., Yeh J. Y., Klein T. A.2012. Seasonal abundance of biting midges, Culicoides spp. (Diptera: Ceratopogonidae), collected at cowsheds in the southern part of the Republic of Korea. Korean J. Parasitol. 50: 127–131. doi: 10.3347/kjp.2012.50.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y. H., Kweon C. H., Tark D. S., Lim S. I., Yang D. K., Hyun B. H., Song J. Y., Hur W., Park S. C.2011. Development of inactivated trivalent vaccine for the teratogenic Aino, Akabane and Chuzan viruses. Biologicals 39: 152–157. doi: 10.1016/j.biologicals.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Lee J. K., Kim J. H., Park B. K., Yoo H. S., Lee B. C., Kim D. Y.2007. Akabane viral encephalitis in calves in South Korea. Vet. Rec. 161: 236–238. doi: 10.1136/vr.161.7.236 [DOI] [PubMed] [Google Scholar]
- 10.Lim S. I., Kweon C. H., Tark D. S., Kim S. H., Yang D. K.2007. Sero-survey on Aino, Akabane, Chuzan, bovine ephemeral fever and Japanese encephalitis virus of cattle and swine in Korea. J. Vet. Sci. 8: 45–49. doi: 10.4142/jvs.2007.8.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim S. I., Kweon C. H., Yang D. K., Tark D. S., Kweon J. H.2005. Apoptosis in Vero cells infected with Akabane, Aino and Chuzan virus. J. Vet. Sci. 6: 251–254. [PubMed] [Google Scholar]
- 12.Metselaar D., Robin Y.1976. Akabane virus isolated in Kenya. Vet. Rec. 99: 86. doi: 10.1136/vr.99.5.86-a [DOI] [PubMed] [Google Scholar]
- 13.Oem J. K., Chung J. Y., Kwon M. S., Kim T. K., Lee T. U., Bae Y. C.2013. Abundance of biting midge species (Diptera: Ceratopogonidae, Culicoides spp.) on cattle farms in Korea. J. Vet. Sci. 14: 91–94. doi: 10.4142/jvs.2013.14.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oem J. K., Lee K. H., Kim H. R., Bae Y. C., Chung J. Y., Lee O. S., Roh I. S.2012. Bovine epizootic encephalomyelitis caused by Akabane virus infection in Korea. J. Comp. Pathol. 147: 101–105. doi: 10.1016/j.jcpa.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 15.Oem J. K., Yoon H. J., Kim H. R., Roh I. S., Lee K. H., Lee O. S., Bae Y. C.2012. Genetic and pathogenic characterization of Akabane viruses isolated from cattle with encephalomyelitis in Korea. Vet. Microbiol. 158: 259–266. doi: 10.1016/j.vetmic.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 16.Park B. K., Lee J. C., An S. H., Moon H. K., Kim K. S., Son D. S.1993. An outbreak of Chuzan disease in Korea and the immunogenicity of binary ethylenimine-treated Chuzan virus vaccine in cattle. Kor. J. Vet. Publ. Hlth. 17: 301–305. [Google Scholar]
- 17.Shin Y. K., Oem J. K., Yoon S. R., Hyun B. H., Cho I. S., Yoon S. S., Song J. A.2009. Monitoring of Five Bovine Arboviral Diseases Transmitted by Arthropod Vectors in Korea. J. Bacteriol. Virol. 39: 353–362. doi: 10.4167/jbv.2009.39.4.353 [DOI] [Google Scholar]
- 18.Taylor W. P., Mellor P. S.1994. The distribution of Akabane virus in the Middle East. Epidemiol. Infect. 113: 175–185. doi: 10.1017/S0950268800051591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchinuno Y., Noda Y., Ishibashi K., Nagasue S., Shirakawa H., Nagano M., Ohe R.1998. Isolation of Aino virus from an aborted bovine fetus. J. Vet. Med. Sci. 60: 1139–1140. doi: 10.1292/jvms.60.1139 [DOI] [PubMed] [Google Scholar]
- 20.Uren M. F., St George T. D., Murphy G. M.1992. Studies on the pathogenesis of bovine ephemeral fever in experimental cattle. III. Virological and biochemical data. Vet. Microbiol. 30: 297–307. doi: 10.1016/0378-1135(92)90017-N [DOI] [PubMed] [Google Scholar]
- 21.Yanase T., Kato T., Kubo T., Yoshida K., Ohashi S., Yamakawa M., Miura Y., Tsuda T.2005. Isolation of bovine arboviruses from Culicoides biting midges (Diptera: Ceratopogonidae) in southern Japan: 1985–2002. J. Med. Entomol. 42: 63–67. doi: 10.1603/0022-2585(2005)042[0063:IOBAFC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Yang D. K., Hwang I. J., Kim B. H., Kweon C. H., Lee K. W., Kang M. I., Lee C. S., Cho K. O.2008. Serosurveillance of viral diseases in Korean native goats (Capra hircus). J. Vet. Med. Sci. 70: 977–979. doi: 10.1292/jvms.70.977 [DOI] [PubMed] [Google Scholar]
- 23.Yeruham I., Van Ham M., Stram Y., Friedgut O., Yadin H., Mumcuoglu K. Y., Braverman Y.2010. Epidemiological investigation of bovine ephemeral Fever outbreaks in Israel. Vet. Med. Int. 2010: 1–5. doi: 10.4061/2010/290541 [DOI] [PMC free article] [PubMed] [Google Scholar]