Abstract
Canine atopic-like dermatitis (ALD) is suspected to be associated with food allergies, particularly those mediated by lymphocytes. In this study, 54 cases were included as ALD dogs, based on the negative IgE test results. In the dogs, the percentage of activated cells in helper-T lymphocytes was measured by flow cytometry using cultured peripheral lymphocytes under food allergen stimulation. We observed that 49 of the 54 ALD dogs (90.7%) had positive lymphocyte reactions against one or more food allergens. The most common food allergen was soybean, showing positive results in 21 dogs (42.9%), while the allergen to cause the lowest number of reactions was catfish (only 5 dogs, 10.2%). These results may be useful in considering elimination diets for ALD dogs.
Keywords: canine, canine atopic-like dermatitis, food allergen, food allergy, lymphocyte
Chronic and recurrent pruritic dermatitis, such as canine atopic dermatitis (AD) and atopic-like dermatitis (ALD), is a common manifestation of these allergy-induced dermatological signs. Canine AD is typically associated with immunoglobulin E (IgE)-mediated hypersensitivity mostly against environmental allergens, such as mites, pollens and molds [9]. On the other hand, ALD, while having clinical features similar to those of canine AD, does not have detectable IgE in allergy tests [3]. Moreover, the pathogenesis of ALD remains speculative, and there have been no diagnostic tests reported in the literature, making it difficult for veterinary clinicians to diagnose and treat ALD. Since, currently, two different canine allergy tests using peripheral blood are available, one of which involves quantitatively measuring the serum IgE levels (type I hypersensitivity) [2], while the second tests lymphocyte proliferation (type IV hypersensitivity) [8]. Since both type I and type IV hypersensitivities have been associated with severe cutaneous signs [4], the combined use of the quantitative IgE assay and the lymphocyte proliferation test has already successfully been used to avoid food allergens in a number of dogs with food allergy [2]. A possible association between ALD and lymphocyte-mediated food allergies was suspected; however, because of the low number of ALD dogs investigated in this previous study, a tendency of food allegens in ALD dogs was not discussed [6]. Thus, in this study, we sought to examine 54 additional dogs, all diagnosed with ALD, using the lymphocyte proliferation test in order to know the suspected association between ALD and lymphocyte-mediated reaction against food allergens.
A total of 319 clinical cases with recurrent and chronic skin diseases accompanied by pruritus were examined in this study. It was found that all the dogs showed atopic dermatitis, based on the criteria previously reported [1]. All the dogs were referred to a private animal hospital owned by two of the authors from 2008 to 2011. Subjects with parasite infestation were excluded from this study using routine dermatological examination. Further, dogs with fungal infections were also excluded following the observation of skin lesions and/or ultraviolet light examination. Pyoderma was treated with antibiotics for at least 2 weeks, if necessary. All the blood samples used for the allergy tests described below were obtained when the dog displayed dermatological signs.
The quantitative antigen-specific IgE test was carried out using a commercially available fluorometric ELISA (Animal Allergy Clinical Laboratories, Inc., Sagamihara, Japan), according to the previous report [2]. Similar to the previous report [2], a threshold of 100 ng/ml of serum IgE was used in this study, and IgE titers greater than or equal to this were identified as positive for allergen-induced dermatitis. The allergens in this test were 40 kinds including mites, pollens, fungi and foods.
According to previously reported methodology [2, 10], lymphocyte proliferation was examined on 18 kinds of food allergens, including beef, pork, chicken, egg yolk, egg white, milk, corn, soybean, lamb, turkey, duck, salmon, cod fish, catfish, capelin, potato, wheat and rice. After the peripheral blood mononuclear cells were cultured with the allergens, CD4+/CD25low lymphocytes were measured with FACSCanto II flow cytometer and FACSDiva software (BD). The percentage of activated CD4+/CD25low cells among the CD4+ lymphocytes was calculated. The cut-off value of the lymphocyte proliferation test was set at 1.1% in the clinical cases with food allergy [2].
All the data were analyzed with descriptive statistics. The percentage of dogs with food allergies was calculated for the lymphocyte proliferation test. Further, we also examined which the food allergens were more frequently detected with the test.
Among the 319 dogs with AD, 54 dogs (16.9%) met the ALD criteria used, whereby all of the allergens examined were negative in the IgE test. Of the 54 ALD dogs, the most frequent breed was Toy Poodle (8 cases, 14.8%), followed by French Bulldog (6 cases, 11.1%), Papillion (5 cases, 9.3%), Miniature Dachshund (4 cases, 7.4%), Mongrel (4 cases, 7.4%), Labrador Retriever (3 cases, 5.6%), Miniature Pinscher (3 cases, 5.6%), Shiba Inu (2 cases, 3.7%), Beagle (2 cases, 3.7%), Miniature Schnauzer (2 cases, 3.7%) and West Highland White Terrier (2 cases, 3.7%). Only 1 case (1.9%) was observed for each of the following breeds: Australian Shepherd, Chihuahua, Pembroke Welsh Corgi, Pomeranian, Pug, Shih Tzu, Wire Fox Terrier and Yorkshire terrier. Of the ALD dogs, 2 Miniature Dachshunds, 1 Chihuahua, 1 Labrador retriever and 1 Pug did not have a positive allergy response against any of the food allergens examined in the lymphocyte proliferation test.
Further, there was no gender bias in the 54 ALD dogs, and the prevalence of the disease was equal between the males (27 total; intact, 17 dogs; neutered, 10 dogs) and females (27 total; intact, 14 dogs; spayed, 13 dogs). The age of each dog at the onset of clinical signs could be determined for 42 of the 54 ALD dogs. Clinical onset was observed before 1 year of age in 59.5% (25 out of 42 dogs).
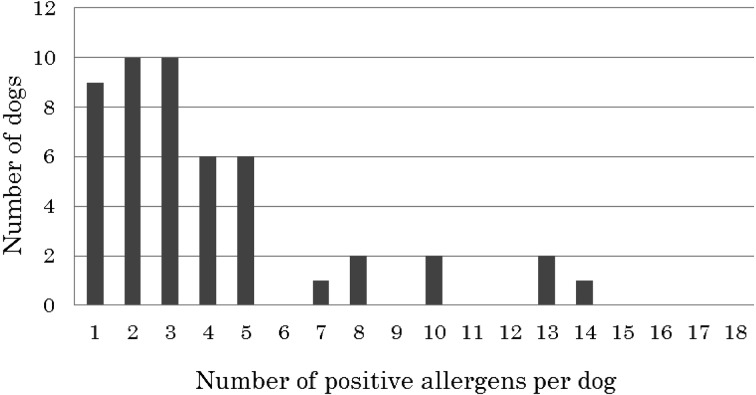
Among the 49 ALD dogs that had positive results in the lymphocyte proliferation test (90.7%), the number of positive allergens ranged from 1 to 15 (Fig. 1). The majority of the dogs displayed one to five positive food allergens, with nine of the dogs being positive to only one allergen, ten being positive to two, ten being positive to three, six being positive to four and six being positive to five allergens. The number of dogs with more than five positive food allergens was rare in this study. However, we did observe one or two dogs with positive results for 7, 8, 10, 13 or 14 food allergens.
Fig. 1.
Number of dogs positive for one or more allergens in the lymphocyte proliferation test.
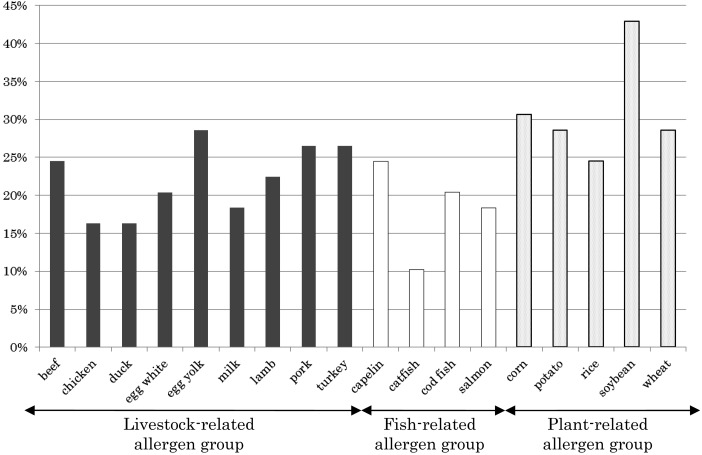
Among the allergens examined in this study, the most typical allergen detected as positive was soybean (21 dogs, 42.9%), while catfish was the least likely to be detected as positive (5 dogs, 10.2%) (Fig. 2). Typically, the likelihood of an allergen to cause dermatitis ranged between 10 and 20% in the 49 dogs examined. Thus, there did not seem to be a specific allergen associated with ALD in this study. However, we did observe a higher number of allergens for the plant-related allergens (e.g., soybean, corn, wheat) than the livestock- and fish-related allergens, the latter of which caused the lowest number of allergens.
Fig. 2.
Percentage of ALD dogs (n=49) positive for each food allergen.
It has recently been reported that ALD may be associated with food allergies mediated by type IV hypersensitivity [6]. Therefore, we sought to determine what kinds of food allergens could induce allergic reactions in dogs with ALD. In this study, soybean was most frequently found in the dogs with ALD, showing positive results in 42.9% of the subjects in the lymphocyte proliferation test. This was not altogether that surprising as a previous study using the lymphocyte proliferation test to examine dogs for allergies also had a higher number of subjects with soybean-induced allergies [6]. Taken together, these data indicate that use of soybean and soybean-related products as ingredients in dog food should be minimized in order to avoid possible allergic reactions in dogs. It was not yet clear how or why soybean was related to such a high number of lymphocyte-mediated allergic responses in the dogs; however, in humans, lymphocytes reactive to Gly m 4, one of major allergen of soybean, were also found to be cross-reactive with other plant allergens, such as birch [5]. This cross-reactivity can occur among a number of different allergens. We suspect that lymphocyte cross-reactivity can also occur between soybean and the other plant allergens in dogs, and is likely causing the higher perceived number of soybean-related allergies in the lymphocyte proliferation test compared to the other allergens.
Notably, many of the ALD dogs in this study showed lymphocytes reactive to multiple food allergens. We observed that an individual dog typically had between one and five food allergens, corresponding to the results in a previous report investigating dogs with AD [2]. It is difficult to explain why the dogs had such a range in the number of positive allergens. However, we again suspect that the immunological recognition of allergens may be to blame, which may be, in part, due to the allergen cross-reactivity. A dog acquiring one kind of food allergy will have lymphocyte reaction to the other allergens possibly cross-reactive to the original one, resulting in increasing the number of positive food allergens in lymphocyte proliferation test. Other than allergen cross-reactivity, it may be also considered to step-by-step acquiring food allergies against several kinds of foods. Therefore, as a dog owner or veterinary practitioner, it is important to understand what type and the number of protein sources used in the dog’s diets and to monitor the animal for any allergies in order to prevent multiple food allergies.
Further, more than 90% of the ALD dogs examined in this study had increased levels of lymphocyte proliferation in response to food allergens, suggesting that type IV hypersensitivity-mediated food allergies might involve in dermatitis in dogs, as previously suggested [6]. Unfortunately, diet restriction based on the test results was not completed for each case (data not shown), and it is still unknown whether such food allergens are the primary cause of ALD signs. In a previous study using a small number of dogs with ALD (n=12), the clinical signs of ALD were relieved with the elimination of the allergens from the animal’s diet based on the results of lymphocyte proliferation test [6]. Furthermore, the age of onset was also particularly important during the diagnosis of food allergies [12], whereby almost 60% of the dogs with ALD in this study showed clinical signs at less than 1 year of age, which is typical of food allergy in dogs. Taken together, ALD can be considered one of the clinical manifestations of type IV hypersensitivity-mediated food allergies.
We have also found that ALD often affects various kinds of small-sized breeds. However, we cannot rule out the possibility of a size bias as the data in this study were collected at a single animal hospital, which may be located in an area of Japan that prefers small dog breeds. In a previous report examining 138 dogs with allergic skin diseases, including canine AD and ALD, Miniature Dachshund, Toy Poodle, French Bulldog and Shih Tzu, were frequently seen in the dog population examined [6]. In the present study, we also frequently identified allergies in Toy Poodles, French Bulldogs and Miniature Dachshunds, indicating that these dog breeds may be more sensitive to food allergens. In a previous study, the genetic factors of the West Highland White Terrier, a well-documented breed predisposed to canine AD, were analyzed, but no conclusive risk factors were identified [13]. It is likely that examining one breed is not enough to determine the universal genetic factors of canine allergies. Therefore, before conducting additional genetic analyses, it is necessary to use strict inclusion criteria, such as family history, pattern of clinical manifestation and strength of allergic reaction, even in a single breed.
Identification of food allergens causing a low number of positive tests, for example, catfish, is also important as they will aid in the selection of appropriate food ingredients for each dog. Currently, many kinds of commercial dog foods contain poultry or mammalian meat as the main source of protein. However, in this study, the fish-related allergen group seemed to show the lowest relative number of positive allergy tests than the other allergen groups examined, indicating that a diet using fish as the major component may be much safer in terms of allergic reactions. It is still important that the allergenicity of the specific fish be further examined in each dog prior to a change in diet, as high levels of cross-reactivity, particularly to the fish allergen parvalbumin, among different fish species have been reported for humans [11].
It is still possible that ALD may be caused by a type of hypersensitivity other than type I and type IV. This is particularly interesting as five dogs with ALD did not show lymphocyte proliferation in response to any of the food allergens examined in this study. In a human patient, type III hypersensitivity was shown to mediate food allergies based on the detection of casein and bovine serum albumin immune complexes [7]. Thus, it would be interesting to further examine type III hypersensitivity with regard to the pathogenesis of food allergy-related canine ALD, particularly in the dogs that had negative results for type I and type IV hypersensitivity tests.
Here, we have reported the possible role of offending food allergens in the development of ALD in dogs. Soybean, for example, was found to be a high-risk food allergen, while the fish-related allergens, especially catfish, were considered to be relatively safe with regard to allergenicity in ALD dogs. Our results are, to our knowledge, the first to focus on identification of offending food allergens in ALD dogs and should be used to help select diet ingredients during the treatment of canine ALD.
REFERENCES
- 1.DeBoer D. J., Hillier A.2001. The ACVD task force on canine atopic dermatitis (XV): fundamental concepts in clinical diagnosis. Vet. Immunol. Immunopathol. 81: 271–276. doi: 10.1016/S0165-2427(01)00312-9 [DOI] [PubMed] [Google Scholar]
- 2.Fujimura M., Masuda K., Hayashiya M., Okayama T.2011. Flow cytometric analysis of lymphocyte proliferative responses to food allergens in dogs with food allergy. J. Vet. Med. Sci. 73: 1309–1317. doi: 10.1292/jvms.10-0410 [DOI] [PubMed] [Google Scholar]
- 3.Halliwell R.2006. Revised nomenclature for veterinary allergy. Vet. Immunol. Immunopathol. 114: 207–208. doi: 10.1016/j.vetimm.2006.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Ishida R., Masuda K., Kurata K., Ohno K., Tsujimoto H.2004. Lymphocyte blastogenic responses to inciting food allergens in dogs with food hypersensitivity. J. Vet. Intern. Med. 18: 25–30. doi: 10.1111/j.1939-1676.2004.tb00131.x [DOI] [PubMed] [Google Scholar]
- 5.Jahn-Schmid B., Radakovics A., Lüttkopf D., Scheurer S., Vieths S., Ebner C., Bohle B.2005. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J. Allergy Clin. Immunol. 116: 213–219. doi: 10.1016/j.jaci.2005.04.019 [DOI] [PubMed] [Google Scholar]
- 6.Kawano K., Oumi K., Ashida Y., Horiuchi Y., Mizuno T.2013. The prevalence of dogs with lymphocyte proliferative responses to food allergens in canine allergic dermatitis. Pol. J. Vet. Sci. 16: 735–739. [DOI] [PubMed] [Google Scholar]
- 7.McCrory W. W., Becker C. G., Cunningham-Rundles C., Klein R. F., Mouradian J., Reisman L.1986. Immune complex glomerulopathy in a child with food hypersensitivity. Kidney Int. 30: 592–598. doi: 10.1038/ki.1986.226 [DOI] [PubMed] [Google Scholar]
- 8.Okayama T., Matsuno Y., Yasuda N., Tsukui T., Suzuta Y., Koyanagi M., Sakaguchi M., Ishii Y., Olivry T., Masuda K.2011. Establishment of a quantitative ELISA for the measurement of allergen-specific IgE in dogs using anti-IgE antibody cross-reactive to mouse and dog IgE. Vet. Immunol. Immunopathol. 139: 99–106. doi: 10.1016/j.vetimm.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Olivry T.International Task Force of Canine Atopic Dermatitis.2010. New diagnostic criteria for canine atopic dermatitis. Vet. Dermatol. 21: 123–126. doi: 10.1111/j.1365-3164.2009.00776.x [DOI] [PubMed] [Google Scholar]
- 10.Olivry T., Kurata K., Paps J. S., Masuda K.2007. A blinded randomized controlled trial evaluating the usefulness of a novel diet (aminoprotect care) in dogs with spontaneous food allergy. J. Vet. Med. Sci. 69: 1025–1031. doi: 10.1292/jvms.69.1025 [DOI] [PubMed] [Google Scholar]
- 11.Perez-Gordo M., Cuesta-Herranz J., Maroto A. S., Cases B., Ibáñez M. D., Vivanco F., Pastor-Vargas C.2011. Identification of sole parvalbumin as a major allergen: study of cross-reactivity between parvalbumins in a Spanish fish-allergic population. Clin. Exp. Allergy 41: 750–758. doi: 10.1111/j.1365-2222.2011.03721.x [DOI] [PubMed] [Google Scholar]
- 12.Proverbio D., Perego R., Spada E., Ferro E.2010. Prevalence of adverse food reactions in 130 dogs in Italy with dermatological signs: a retrospective study. J. Small Anim. Pract. 51: 370–374. doi: 10.1111/j.1748-5827.2010.00951.x [DOI] [PubMed] [Google Scholar]
- 13.Salzmann C. A., Olivry T. J., Nielsen D. M., Paps J. S., Harris T. L., Olby N. J.2011. Genome-wide linkage study of atopic dermatitis in West Highland White Terriers. BMC Genet. 12: 37. doi: 10.1186/1471-2156-12-37 [DOI] [PMC free article] [PubMed] [Google Scholar]