Abstract
Purpose
Fatigue is the most common and distressing symptom reported by cancer patients during and after treatment. Tumor growth increases oxidative stress and cytokine production, which causes skeletal muscle wasting and cardiac dysfunction. The purpose of this study was to determine whether treatment with the antioxidant ubiquinol improves muscle mass, cardiac function, and behavioral measures of fatigue in tumor-bearing mice.
Method
Adult female mice were inoculated with colon26 tumor cells. Half the control and tumor-bearing mice were administered ubiquinol (500 mg/kg/day) in their drinking water. Voluntary wheel running (i.e., voluntary running activity [VRA]) and grip strength were measured at Days 0, 8, 14, and 17 of tumor growth. Cardiac function was measured using echocardiography on Day 18 or 19. Biomarkers of inflammation, protein degradation, and oxidative stress were measured in serum and heart and gastrocnemius tissue.
Results
VRA and grip strength progressively declined in tumor-bearing mice. Muscle mass and myocardial diastolic function were decreased, and expression of proinflammatory cytokines was increased in serum and muscle and heart tissue on Day 19 of tumor growth. Oxidative stress was present only in the heart, while biomarkers of protein degradation were increased only in the gastrocnemius muscle. Ubiquinol increased muscle mass in the tumor-bearing and control animals but had no effect on the expression of biomarkers of inflammation, protein degradation, or oxidative stress or on behavioral measures of fatigue.
Keywords: cancer-related fatigue, ubiquinol, tumor growth, mice, colon26 adenocarcinoma, oxidative stress
Cancer patients report that the symptom of fatigue is more distressing than that of pain, nausea, or vomiting (Berger et al., 2010). Fatigue can be present at diagnosis, increases during treatment, and can persist for years after treatment is completed. Cancer-related fatigue (CRF) impairs the patient’s functional ability and affects employment status (Amir, Wilson, Hennings, & Young, 2012; Minton et al., 2013; Munir, Yarker, & McDermott, 2009) and, in patients with persistent tumor disease, is associated with loss of lean body (muscle) mass and reduced effort tolerance (Martin et al., 2013; Weber et al., 2009). The causes of CRF are not clearly understood, and treatment is largely intuitive (Dantzer, Meagher, & Cleeland, 2013; Dodson et al., 2011).
Disturbances in regulation of the hypothalamic–pituitary–adrenal axis, serotonin synthesis, and circadian rhythms (Barsevik et al., 2010; Silverman, Heim, Nater, Marques, & Sternberg, 2010) have all been implicated in the pathobiology of CRF. There is also a significant body of literature implicating increased host or tumor production of proinflammatory cytokines (Liu et al., 2012; Saligan & Kim, 2012; Wang et al., 2012) and reactive oxygen species (ROS; Gilliam & St. Clair, 2011; Reid & Moylan, 2011) in the pathobiology of CRF, particularly in tumor-induced skeletal muscle wasting (Suzuki, Asakawa, Amitani, Nakamura, & Inui, 2013; van Hall, 2012) and cardiac dysfunction (Marin-Corral et al., 2010; Mazevet et al., 2013). Tumor necrosis factor α (TNF-α) acts via the type I receptor for TNF-α (TNFR1) to activate intracellular pathways involved in muscle wasting, including the ubiquitin proteosome ATP-dependent pathway (UPP) and the autophagy–lysosome system (ALS; Fearon, Glass, & Guttridge, 2012; Sandri, 2013). MuRF1 and MAFbx are two ligases in the UPP that ubiquinate myofibrillar proteins to be degraded in the proteasome (Foletta, White, Larsen, Leger, & Russell, 2011). BNIP3 is involved in the formation of phagosomes and increased activity of the ALS (Bellot et al., 2009). Skeletal muscle expression of MAFbx, MuRF1, and BNIP3 messenger RNA (mRNA) is increased in tumor-bearing mice (Tian, Asp, Nishijima, & Belury, 2011; Xu et al., 2011), though evidence from muscle biopsies of cancer patients is inconsistent (D’Orlando et al., 2014; Sun, Ye, Qian, Xu, & Hu, 2012; V. E. Baracos to D.O.M., personal communication, October 11, 2013).
Proinflammatory cytokines also increase the production of ROS in target tissues (Reid & Moylan, 2011). Increased oxidant activity is associated with increased expression of MuRF-1, MAFbx, and BNIP3 mRNA and skeletal muscle wasting (Suzuki et al., 2013). Researchers have also described evidence of oxidative damage to proteins affecting contraction, glycolysis, and mitochondrial metabolism in the heart as well as in the skeletal muscle of tumor-bearing animals (Marin-Corral et al., 2010; Tian et al., 2010). These studies suggest that oxidative stress may be a potential target for reducing skeletal muscle wasting and perhaps CRF.
Ubiquinone (CoQ10) is an endogenously synthesized, lipid-soluble antioxidant. Tissue levels of CoQ10 are decreased in patients with lung, melanoma, and cervical cancer compared to healthy controls (Cobanoglu et al., 2011; Rusciani et al., 2006). Treatment with CoQ10 has been shown to reduce chemotherapy-induced cardiotoxicity and nephrotoxicity in healthy mice (El-Sheikh, Morsy, Mahmoud, Rifaai, & Abdelrahman, 2012; Fouad, Al-Sultan, Refaie, & Yacoubi, 2010). The effects of CoQ10 or ubiquinol, the reduced form of CoQ10, on tumor-induced oxidative stress, muscle wasting, and myocardial dysfunction have not been studied. The purpose of the present study was to determine the effects of ubiquinol treatment on skeletal muscle mass, myocardial function, expression of biomarkers of inflammation, oxidative stress, protein degradation, and behavioral measures of fatigue in a mouse model of CRF.
Method
A total of 64 adult female CD2F1 mice (Charles River) were randomly assigned to one of four groups (tumor/no drug, tumor + drug, control/no drug, and control + drug). A power analysis using data from pilot work indicated that 16 mice per group were needed to detect a moderate effect size of ubiquinol on muscle mass. Three experiments were conducted using 18–24 animals in each experiment, and the data were pooled. All procedures were approved by the Institutional Animal Care and Use Committee of the Ohio State University and were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
All mice were maintained on a 12-hr light–dark cycle and housed individually. Tumor growth was induced using the colon26 murine tumor cell line. This tumor cell line secretes interleukin 6 (IL-6) and TNF-α (Graves, Ramsay, & McCarthy, 2006) and does not metastasize when injected subcutaneously (Okayama et al., 2009). It is frequently used to model the systemic effects of tumor growth on skeletal muscle, heart, gut, and skin (Cosper & Leinwand, 2011; Murphy, Chee, Trieu, Naim, & Lynch, 2012; Redon et al., 2010; Shadfar et al., 2011; Tian et al., 2011). The gastrocnemius is most often used to study the effects of tumor growth on skeletal muscle because it contains both slow- and fast-twitch fibers.
On Day 0, a total of 32 mice were injected subcutaneously between the scapulae with 5 × 105 of colon26 cells suspended in 0.2 ml of saline, as previously described (Xu et al., 2011), and 32 control animals were injected with 0.2 ml saline alone. On Day 1, half of the control (n = 16) and half of the tumor-bearing mice (n = 16) received a 2% solution of ubiquinol (Kaneka Nutrients L.P., Pasadena, CA) in their drinking water; the other half of the tumor-bearing and control mice received a control solution provided by the manufacturer in their drinking water. The concentration was based on delivering a dose of 500 mg/kg/day in a volume of 4–5 ml, the average volume of fluid consumed by 20-g mice. This dose was previously shown to reduce inflammatory gene transcripts in a mouse model of aging (Schmelzer et al., 2010). Fluid intake was measured every 3 days by weighing the sipper bottles before and after placement of fresh food and water.
Fatigue was modeled as reduced voluntary running activity (VRA; Wood, Nail, Gilster, Winters, & Elsea, 2006; Zombeck, Fey, Lyng, & Sonis, 2013). Running wheels (4 in.) were placed inside each cage and connected to a computer and data processor (Columbus Instruments, Columbus, OH). Mice were acclimated to the running wheels for 1 week prior to tumor cell injection. Total wheel turns were recorded for 24 hr on Days 0, 8, 14, and 17 of tumor growth to capture the onset of changes in VRA in relation to tumor growth.
Weakness was modeled as reduced forelimb grip strength (Murphy et al., 2012) using an automated grip strength meter (Columbus Instruments). Grip strength was measured on Days 0, 8, 14, and 17 of tumor growth. Each mouse was lowered vertically by its tail until the forelimbs rested on the grid and then pulled horizontally until it dislodged from the grid. Three measurements were taken and averaged to determine the grams of force required to dislodge the mouse from the grid. Because smaller mice would be expected to generate less grip strength, this measure was normalized to body weight (Murphy et al., 2012).
Echocardiography was performed on Day 18 or 19 using a VisualSonics Vivo 2100 Ultra High Resolution In Vivo Imaging System (VisualSolics, Toronto, Ontario, Canada), as previously described (Tournoux et al., 2011; Xu et al., 2011). Scanning was performed at a frequency of 20 MHz, and three total measures were averaged from different points within the cardiac cycle according to the standards set forth by the American Society for Echocardiography. M-mode images were obtained at the level of the papillary muscles to assess left ventricular systolic diameter (LVSD), LV diastolic diameter (LVDD), isovolumetric relaxation (IVRT), and posterior wall thickness (PWT). M-mode calculations were used to derive fractional shortening (FS = LVDD − LVSD/LVDD).
Following echocardiography, the mice were deeply anesthetized by injecting 2 mg of 1% ketamine/xylazine. Blood was drawn from the submandibular vein and placed into a micro-centrifuge tube containing 100 μl of 0.1 M EDTA. The heart, right and left gastrocnemius muscles, and tumor were dissected and weighed. The muscles and heart were snap frozen in liquid nitrogen and stored at −80°C until biochemical analyses. Blood samples were centrifuged, and the serum was collected and stored at −20°C until analyses of cytokine levels. To control for variations in dissected muscle weights, the weight of the left and right gastrocnemius muscles were averaged to determine gastrocnemius muscle weight. The heart and gastrocnemius muscle weights were also normalized to body weight and expressed as heart or gastrocnemius muscle mass (mg/g). Weights of these tissues were also normalized to carcass weight (body weight – tumor weight).
Serum concentrations of IL-6 and the TNFR1 were measured using commercially available enzyme-linked immunosorbent assay kits for murine TNFR1 and IL-6 (Meso Scale Discovery, Rockville, MD) according to the manufacturer’s instructions. All samples were tested in duplicate, and the results are expressed as picograms protein per milliliter of serum (pg/ml). Glutathione exists in cells in reduced sulfhydryl (GSH) and oxidized (GSSG) forms (Owen & Butterfield, 2010). Cardiac and gastrocnemius muscle tissue (30 mg each) were homogenized to measure GSH and GSSG concentrations as described by Rahman, Kode, and Biswas (2006). Results are reported as the GSH/GSSG ratio. A decreased ratio indicates oxidative stress.
Expression of BNIP3, MAFbx, MuRF1, IL-6, and TNFR1 mRNA in gastrocnemius and heart tissue were measured using real-time polymerase chain reaction (PCR). Total RNA was extracted from 30 mg of frozen tissue using the Fibrous Tissue Mini Kit per manufacturer’s instructions (Qiagen, Netherlands). RNA quantity and purity were checked using the Nano-Drop (Thermo Scientific, Waltham, MA), and quality was verified on an agarose gel. The RNA was reverse transcribed, and the complementary DNA was probed for expression of genes of interest using PCR with primer pairs specific for those genes as previously described (Xu et al., 2011). Expression of each gene was normalized to expression of glyceraldehyde-3-phosphate dehydrogenase.
Data were analyzed using two-way analysis of variance (ANOVA) to determine the main effects and potential interaction effects of tumor growth and ubiquinol treatment. Repeated-measures ANOVA was used to examine the effects of tumor growth and ubiquinol on VRA and grip strength on Days 0, 8, 14, and 17 or 18, followed by Bonferroni post hoc tests. Independent samples t-test was performed to compare tumor weights. Statistical significance was set a priori at α = .05.
Results
Tumor growth was palpable by Day 7 and did not exceed 5% of body weight at the time of sacrifice on Days 18–19 of tumor growth. As shown in Table 1, there was no effect of ubiquinol on tumor growth. All four groups of mice drank an average of 4 ml of water per day over the course of the study, and there was no effect of tumor growth or ubiquinol treatment on water or food intake. Tissue analysis was completed on all 64 animals, but only 42 animals were utilized for echocardiography due to scheduling issues and 46 for wheel running due to equipment issues.
Table 1.
Effects of Tumor Growth and Ubiquinol on Tissue Weights.
| Tissue | Control n = 16 M (SD) |
Control + drug n = 16 M (SD) |
Tumor n = 16 M (SD) |
Tumor + drug n = 16 M (SD) |
Test statistic |
|---|---|---|---|---|---|
| Body (g) | 19.5 (1.4) | 19.5 (1.4) | 18.6 (1.8) | 18.7 (1.5) | F(1, 64) = 5.5*, a |
| Carcass (g) | 19.5 (1.4) | 19.5 (1.4) | 17.9 (1.9) | 17.9 (1.7) | F(1, 64) = 17.5***, a |
| Gastrocnemius (mg) | 96.8 (12.6) | 104.4 (8.4) | 78.1 (16.3) | 83.0 (12.5) |
F(1, 64) = 41.8***, a F(1, 64) = 4.0*,b |
| Gastrocnemius/body weight (mg/g) | 5.0 (.5) | 5.4 (.32) | 4.2 (.75) | 4.4 (.5) |
F(1, 64) = 40.9***, a F(1, 64) = 5.5*,b |
| Gastrocnemius/carcass weight (mg/g) | 5.0 (.5) | 5.4 (.32) | 4.4 (.77) | 4.6 (.5) |
F(1, 64) = 24.1***, a F(1, 64) = 6.4*,b |
| Heart (mg) | 88.4 (6.3) | 88.4 (4.5) | 86.9 (9.7) | 88.2 (7.8) | ns |
| Heart/body weight (mg/g) | 4.6 (.41) | 4.6 (.29) | 4.7 (.43) | 4.7 (.38) | ns |
| Tumor (g) | – | – | .73 (.30) | .82 (.24) | ns |
Note.
Main effect of tumor.
Main effect of drug.
p < .05.
p < .01.
p < .001.
Tissue Weights
The tumor-bearing mice weighed 5% less than healthy controls by Days 18–19 of tumor growth (p < .05). Carcass weight of the tumor-bearing mice was 9% less than that of healthy controls (p < .001). As shown in Table 1, there was no effect of ubiquinol on body weight or carcass weight of the animals.
The weight of the gastrocnemius muscles from tumor-bearing mice was 20% less than muscles from control mice by Days 18–19 of tumor growth (p < .001), and muscle mass in tumor-bearing mice was 16% less than in controls (p < .001). As shown in Table 1, there was a main effect of ubiquinol treatment (p < .05) on muscle weight and muscle mass. There was, however, no interaction effect. There was no significant effect of tumor growth or ubiquinol treatment on heart weight or heart mass of any of the groups of mice.
Proinflammatory Cytokines
As shown in Table 2, IL-6 was increased in serum of tumor-bearing mice by Day 18 or 19 of tumor growth compared to healthy controls (p < .001), as was TNFR1 (p < .001). However, there was no effect of ubiquinol on serum levels of either cytokine. Expression of IL-6 mRNA was increased in the gastrocnemius (p < .05) and heart tissue (p < .001) of tumor-bearing animals compared to healthy controls. Expression of TNFR1 mRNA was also increased in the gastrocnemius (p < .001) and heart tissue (p < .001) of tumor-bearing mice. However, there was no effect of ubiquinol on expression of IL-6 or TNFR1 mRNA in the gastrocnemius muscles or heart.
Table 2.
Inflammatory Biomarkers in Serum and Tissue.
| Inflammatory biomarker | Control n = 16 M (SD) |
Control + drug n = 16 M (SD) |
Tumor n = 16 M (SD) |
Tumor + drug n = 16 M (SD) |
Test statistic |
|---|---|---|---|---|---|
| Cytokines | |||||
| Serum (pg/ml) | |||||
| IL-6 | 9.4 (9.4) | 7.9 (7.7) | 241.1 (162.8) | 240.5 (166.2) | F(1, 64) = 62.8***,a |
| TNFR1 | 690.3 (251.2) | 813.4 (204.2) | 1284.1 (595.6) | 1341.1 (405.6) | F(1, 64) = 33.8***,a |
| Heart (ddct) | |||||
| IL-6 | .95 (.69) | .50 (.35) | 5.7 (2.9) | 7.0 (5.8) | F(1, 64) = 43.6***,a |
| TNFR1 | 1.0 (.19) | .95 (2.0) | 2.2 (.60) | 3.4 (2.6) | F(1, 64) = 27.1***,a |
| Gastrocnemius (ddct) | |||||
| IL-6 | 1.1 (1.0) | .51 (.23) | 1.3 (1.0) | 1.8 (2.3) | F(1, 64) = 4.2*,a |
| TNFR1 | 1.1 (.47) | .94 (.43) | 1.7 (.72) | 1.7 (.64) | F(1, 64) = 22.4***,a |
| GSH/GSSG (μM) | |||||
| Heart | 6.8 (3.5) | 5.9 (2.1) | 3.2 (.6) | 3.3 (.78) | F(1, 60) = 34.5***,a |
| Gastrocnemius | 5.2 (1.2) | 4.9 (1.3) | 4.1 (1.3) | 4.9 (1.3) | ns |
Note. ddct = relative to expression of GAPDH; GADPH = glyceraldehyde-3-phosphate dehydrogenase; IL = interleukin; GSH/GSSG = ratio of reduced sulfhydryl glutathione to oxidized glutathione; TNFR1 = tumor necrosis factor-α type 1 receptor.
Main effect of tumor.
p < .05.
p < .01.
p < .001.
Oxidative Stress
The GSH/GSSH ratio was decreased 18% in the gastrocnemius muscle of the tumor-bearing mice but was not significantly different from that of the controls (p = .06). However, the GSH/ GSSH ratio was decreased 53% in the hearts of tumor-bearing mice and was significantly different from that in the heart tissue of control mice (p < .001). As shown in Table 2, there was no effect of ubiquinol treatment on the GSH/GSSG ratio in gastrocnemius or heart tissue.
Protein Degradation
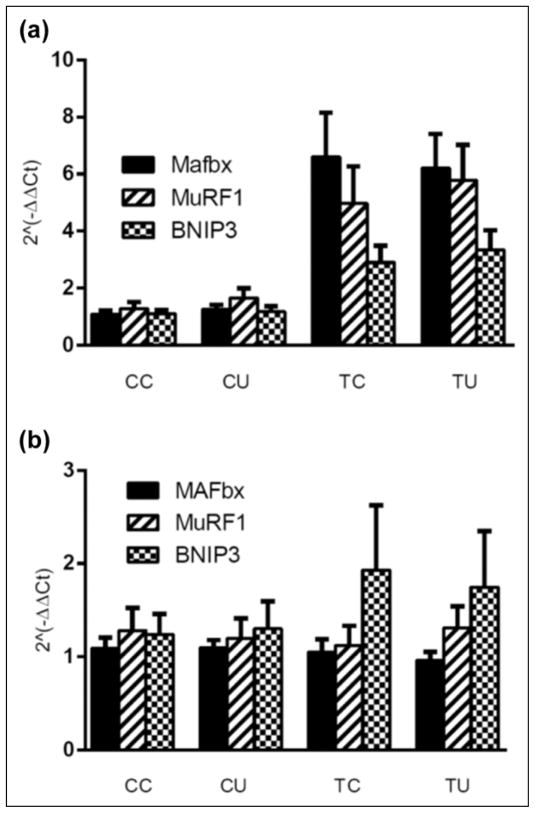
Expression of MAFbx mRNA in the gastrocnemius muscle of tumor-bearing mice was increased on Day 18 or 19 of tumor growth compared to its expression in the muscles of control animals, F(1, 64) = 22.1, p < .01, as was the expression of MuRF1 (p < .01) and BNIP3 mRNA (p < .01). In contrast, expression of MAFbx, MuRF1, and BNIP3 mRNA was not significantly increased in heart tissue of the tumor-bearing mice. In spite of the increase in muscle mass of the animals described earlier, there was no effect of ubiquinol on expression of these biomarkers of protein degradation in gastrocnemius or heart tissue. Data are shown in Figure 1.
Figure 1.
Effect of tumor growth and ubiquinol on expression of MAFbx, MuRF1, and BNIP3 mRNA in gastrocnemius muscle (a) and heart (b). Main effect of tumor in gastrocnemius, p < .01. CC = control no drug, n = 16; CU = control/ubiquinol, n = 16; TC = tumor no drug, n = 16; TU = tumor/ubiquinol, n = 16.
Myocardial Function
Neither tumor growth nor ubiquinol had a significant effect on LVDD, LVSD, PWT, or FS on Day 18 or 19 of tumor growth. However, IVRT was significantly increased in the tumor-bearing mice, F(1, 38) = 9.32, p < .01, compared to the control mice. There was no effect of ubiquinol on IVRT. Data are shown in Table 3.
Table 3.
The Effects of Tumor Growth and Ubiquinol on Myocardial Function.
| Myocardial function (mm) | Control M (SD) |
Control + drug M (SD) |
Tumor M (SD) |
Tumor + drug M (SD) |
Test statistic |
|---|---|---|---|---|---|
| LVDD | 3.5 (.30) | 2.7 (1.5) | 3.0 (1.5) | 2.8 (1.8) | ns |
| LVSD | 2.3 (.28) | 1.9 (1.1) | 2.1 (1.1) | 1.9 (1.3) | ns |
| PWT | 1.2 (.46) | .89 (.65) | .93 (.49) | 1.1 (.58) | ns |
| FS | 33.3 (11.9) | 25.1 (17.7) | 25.0 (13.5) | 25.7 (13.3) | ns |
| IVRT | .02 (.01) | .03 (.02) | .04 (.02) | .04 (.02) | F(1, 38) = 9.3**,a |
Note. LVDD = left ventricular diastolic diameter; LVSD = left ventricular systolic diameter; PWT = posterior wall thickness; FS = fractional shortening; IVRT = isovolumetric relaxation time.
Main effect of tumor.
p < .01.
Grip Strength
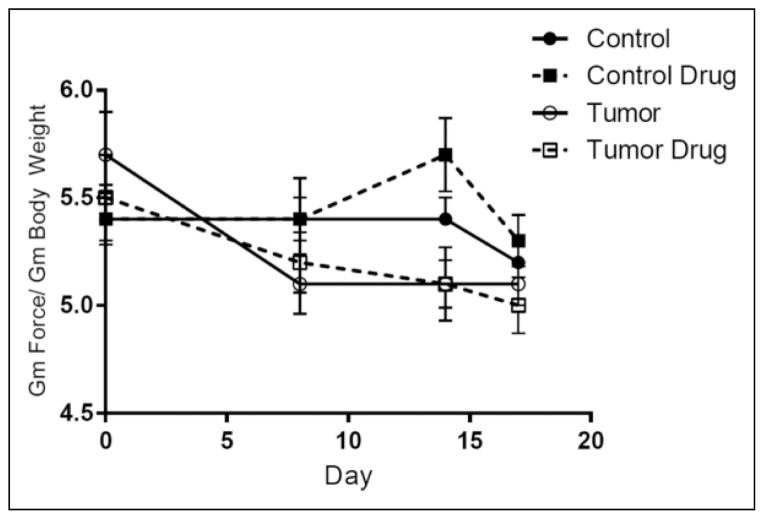
Grip strength on Days 0, 8, 14, and 17 of tumor growth was normalized to body weight and analyzed using repeated-measures ANOVA (data shown in Figure 2). There was a within-subjects effect of days on grip strength, F(2.84, 170.24) = 10.6, p < .01, and a significant interaction of days and tumor (p < .01). There was a main effect of tumor on grip strength, F(1, 60) = 3.97, p = .05, but no effect of ubiquinol treatment. Bonferroni comparisons revealed a significant difference in grip strength between tumor-bearing and control mice as early as Day 8 of tumor growth.
Figure 2.
Effect of tumor growth and ubiquinol on grip strength. Main effect of tumor, p = .05; main effect of days, p < .01; day-by-tumor interaction, p < .01. For all groups, n = 16.
VRA
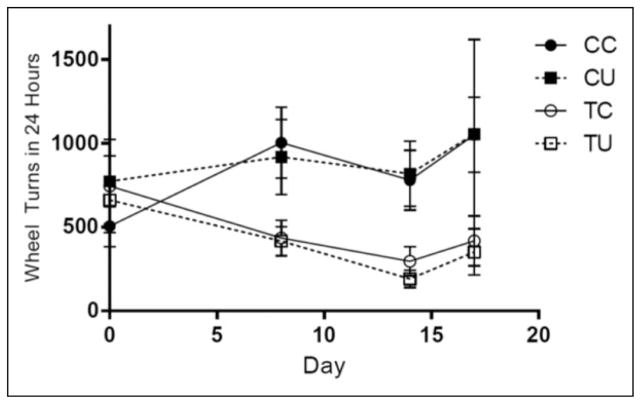
VRA on Days 0, 8, 14, and 17 of tumor growth was analyzed using repeated-measures ANOVA. There was no within-subjects effect of days on VRA, but there was a significant interaction between days and tumor, F(1.88, 78.86) = 3.39, p = .04. There was a main effect of tumor growth, F(1, 42) = 7.40, p < .01, and no effect of ubiquinol on VRA (data shown in Figure 3).
Figure 3.
Effects of tumor growth and ubiquinol on voluntary running activity. Main effect of tumor, p < .01; day-by-tumor interaction, p = .04. CC = control no drug, n = 11; CU = control/ ubiquinol, n = 12; TC = tumor no drug, n = 12; TU = tumor/ubiquinol, n = 11.
Discussion
Fatigue (CRF) is a significant problem for patients during and after treatment for cancer. The causes of CRF are not clearly understood, and no effective treatments are available. The purpose of the present study was to examine the effects of the anti-oxidant ubiquinol, the reduced form of CoQ10, on muscle wasting, myocardial function, and fatigue behaviors in adult female mice inoculated with the colon26 tumor cell line.
We report that VRA and grip strength progressively declined in tumor-bearing mice compared to healthy control animals. By Days 18–19 of tumor growth, gastrocnemius muscle mass was 20% less and body weight was 5% less in tumor-bearing animals than in healthy control animals, confirming the targeted effect of tumor growth on lean body mass (Martin et al., 2013). These changes in body and gastrocnemius muscle weight were not due to anorexia–cachexia syndrome, as food and water intake of the tumor-bearing animals did not decline during the study period, a finding supported by previous reports (Graves et al., 2006; Shadfar et al., 2011; Xu et al., 2011). However, others have observed reduced food intake and up to 20% body weight loss in this mouse model of tumor-induced muscle wasting (Murphy et al., 2012; Tian et al., 2010). This difference in findings may be due to differences in duration of tumor growth, C26 cell line variants (Murphy et al., 2012), or the use of only female mice, which have been shown to maintain their food intake and lose a smaller percentage of body weight than tumor-bearing male mice (Cosper & Leinwand, 2011). Similar sex differences in weight loss have also been noted in a rat model of cardiac cachexia (Palus, Akashi, von Haehling, Anker, & Springer, 2009).
In the present study, tumor growth increased serum, muscle, and heart levels of IL-6 and TNFR1 compared to tissues from healthy control animals. Oxidative stress was increased in heart tissue and the gastrocnemius of the tumor-bearing mice; however, levels in the gastrocnemius were not significantly different from controls. Tumor growth increased the expression of MAFbx, MuRF1, and BNIP3 mRNA only in the gastrocnemius muscle and not in the heart, as has also been described by others (Cosper & Leinwand, 2011). These data suggest that increased UPP activity may be an early driver of protein degradation in skeletal muscle but not in the heart. This idea is supported by our observation that tumor growth was associated with loss of skeletal muscle mass but had no effect on heart mass, a finding that has also been reported by others (Muhlfeld et al., 2011; Xu et al., 2011). Others have observed tumor-induced cardiac atrophy in male animals (Tian et al., 2011; Wysong et al., 2011) but not in females (Cosper & Leinwand, 2011; Muhlfeld et al., 2011). Several studies have demonstrated increased UPP activity in hearts of tumor-bearing mice (Tian et al., 2011; Wysong et al., 2011), including female mice (Shadfar et al., 2011; Xu et al., 2011). It should also be noted that, in previous studies, cardiac atrophy more often occurred in mice that had lost up to 20% of body weight, while mice in the present study had lost an average of only 5% of body weight. Further research is needed to clarify the effects of sex, UPP activity, and progressive weight loss on cardiac atrophy in tumor-bearing animals.
The negative effects of cancer treatment on myocardial function have been known for years (Curigliano et al., 2010). Evidence indicating a direct effect of tumor growth on myocar-dial function, however, has only recently been described in the literature. We and others have reported increased LVSD, decreased FS, and decreased PWT in tumor-bearing rodents (Shadfar et al., 2011; Tian et al., 2010, 2011; Wysong et al., 2011; Xu et al., 2011). Others have observed no effects of tumor growth on myocardial function in tumor-bearing mice (Cosper & Leinwand, 2011; Muhlfeld et al., 2011). While we found no evidence of systolic dysfunction in the present study, we did find evidence of increased IVRT, indicating diastolic dysfunction. This finding is supported by our prior report of prolonged relaxation in cardiomyocytes isolated from hearts of tumor-bearing mice (Xu et al., 2011). Prolonged IVRT is not normally associated with compromised oxygen status in the heart, which could affect effort tolerance such as VRA or grip strength. However, alterations in diastolic function (or diastolic failure) are known to precede systolic dysfunction, particularly in cases of diabetic cardiomyopathy (Isfort, Stevens, Shaffer, Jong, & Wold, 2014). Further research is needed to understand the role of myocardial dysfunction in CRF.
Treatment with 500 mg/kg/day of ubiquinol in the present study had no effect on expression of proinflammatory cytokines in serum, gastrocnemius muscle, or heart tissue or on biomarkers of protein degradation or oxidative stress in the gastrocnemius muscle or heart. This dose was previously shown to reduce inflammatory gene transcripts in a mouse model of aging (Schmelzer et al., 2010). Others have shown that administration of an antioxidant “cocktail” reduced subject reports of fatigue and serum measures of oxidative stress and IL-6 and/or TNF-α in cancer patients (Mantovani, Madeddu, & Maccio, 2012). Because the colon26 tumor cell line constitutively expresses IL-6, it is possible that the dose of ubiquinol used in the present study was inadequate to suppress tumor-induced oxidative stress and cytokine production in the context of progressive tumor growth.
Surprisingly, ubiquinol increased gastrocnemius muscle mass in both the tumor-bearing and healthy control mice. However, muscle mass of the tumor-bearing animals treated with ubiquinol was still 20% less than that of control animals treated with ubiquinol, suggesting the anabolic effect of ubiquinol was independent of the catabolic effects of tumor growth. The increase in muscle mass, however, was not associated with an increase in VRA or grip strength in the tumor-bearing mice. Others have reported that treatment with perindopril, an angiotensin-converting enzyme inhibitor, improved grip strength and locomotor activity of tumor-bearing mice but did not improve muscle mass (Murphy, Chee, Trieu, Naim, & Lynch, 2013). These data suggest that loss of muscle mass does not explain the tumor-induced decrease in VRA and grip strength in this mouse model of CRF.
Behavioral measures of fatigue, such as VRA and grip strength, require voluntary engagement in the behavior as well as physical ability. In this regard, proinflammatory cytokines have negative effects on mood and cognition (Haroon, Raison, & Miller, 2012; Silverman et al., 2010; Wood & Weymann, 2013). CRF often co-occurs with depressed mood (Pertl et al., 2013). A recent report described no effect of tumor growth on spontaneous movement of tumor-bearing mice in the home cage but a significant decrease in motor activity during the tail suspension test, which is used to model resignation, a depressive-like behavior in mice. The increased resignation was associated with increased cytokine expression in the hippocampus of tumor-bearing mice (Yang et al., 2014). Further research is needed to unravel the associations among decreased physical activity, effort tolerance, and negative mood in patients with CRF.
In summary, we reported that treatment with the antioxidant ubiquinol improved gastrocnemius muscle mass in both tumor-bearing and control mice but had no effect on biomarkers of protein degradation, inflammation, or oxidative stress in the gastrocnemius muscle or heart of tumor-bearing mice or on behavioral measures of fatigue. These data suggest that antioxidant treatment alone is not likely to reduce fatigue in cancer patients.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funded in part by 1R01NR012618-01A1 to D.O.M.; 1F31NR012897-01 to Y.Y.C.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Amir Z, Wilson K, Hennings J, Young A. The meaning of cancer: Implications for family finances and consequent impact on lifestyle, activities, roles and relationships. Psycho-Oncology. 2012;21:1167–1174. doi: 10.1002/pon.2021. [DOI] [PubMed] [Google Scholar]
- Barsevik A, Frost M, Zwinderman A, Hall P, Halyard M GENEQOL Consortium. I’m so tired: Biological and genetic mechanisms of cancer-related fatigue. Quality of Life Research. 2010;19:1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure N. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Molecular Cell Biology. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. doi:10.1128?MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, Wagner LI. Cancer-related fatigue. Journal of the National Comprehensive Cancer Network. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- Cobanoglu U, Demir H, Cebi A, Sayir F, Alp HH, Akan Z, Bakan E. Lipid peroxidation, DNA damage and coenzyme q10 in lung cancer patients—markers for risk assessment? Asian Pacific Journal of Cancer Prevention. 2011;12:1399–1403. [PubMed] [Google Scholar]
- Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Research. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Roila F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Annals of Oncology. 2010;23:vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature Reviews Clinical Oncology. 2013;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS. Muscle wasting in cancer cachexia: Clinical implications, diagnosis and emerging treatment strategies. Annual Review of Medicine. 2011;62:265–279. doi: 10.1146/annurev-med-061509-131248. [DOI] [PubMed] [Google Scholar]
- D’Orlando C, Marzetti E, Francois S, Lorenzi M, Conti V, di Stasio E, Bossola M. Gastric cancer does not affect the expression of atrophy-related genes in human skeletal muscle. Muscle Nerve. 2014;49:528–533. doi: 10.1002/mus.23945. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, Morsy MA, Mahmoud MM, Rifaai RA, Abdelrahman AM. Effect of coenzyme-Q10 on doxorubicin-induced nephrotoxicity in rats. Advances in Pharmacological Sciences, 2012. 2012 doi: 10.1155/2012/981461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon K, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metabolism. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Foletta VC, White LJ, Larsen AE, Leger B, Russell AP. The role and regulation of MAFbx/atrogen-1 and MuRF1 in skeletal muscle atrophy. Pflügers Archiv: European Journal of Physiology. 2011;461:325–335. doi: 10.1007/s00424-010-0919-9. [DOI] [PubMed] [Google Scholar]
- Fouad AA, Al-Sultan A, Refaie SM, Yacoubi MT. Coenzyme Q10 treatment ameliorates acute cisplatin nephrotoxicity in mice. Toxicology. 2010;274:49–56. doi: 10.1016/j.tox.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gilliam L, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxidants & Redox Signaling. 2011;15:2543–2563. doi: 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves E, Ramsay E, McCarthy DO. Inhibitors of COX activity preserve muscle mass in mice bearing the Lewis lung carcinoma, but not the B16 melanoma. Research in Nursing and Health. 2006;29:87–97. doi: 10.1002/nur.20114. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications for the impact of inflammation on behavior. Neuropsychopharmacology Reviews. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort M, Stevens SC, Shaffer S, Jong CJ, Wold LE. Metabolic dysfunction in diabetic cardiomyopathy. Heart Failure Reviews. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Mills PJ, Rissling M, Florentino L, Natarajan L, Dimsdale JE, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain, Behavior and Immunity. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani G, Madeddu C, Maccio A. Cachexia and oxidative stress: An innovative therapeutic management. Current Pharmaceutical Design. 2012;18:4813–4818. doi: 10.2174/138161212803216889. [DOI] [PubMed] [Google Scholar]
- Marin-Corral J, Fontes CC, Pascual-Guardia S, Sanchez F, Olivan M, Argiles JM, Barriero E. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxidants & Redox Signaling. 2010;12:365–380. doi: 10.1089/ars.2009.2818. [DOI] [PubMed] [Google Scholar]
- Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Baracos VE. Cancer cachexia in tht age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of Clinical Oncology. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- Mazevet M, Moulin M, Llach-Martinez A, Chargari C, Deutsch E, Gomez A, Morel E. Complications of chemotherapy, a basic science update. Presse Medicale. 2013;42:e352–e361. doi: 10.1016/j.lpm.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Minton O, Berger A, Barsevick A, Cramp F, Goedendorp M, Mitchell SA, Stone PC. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119:2124–2130. doi: 10.1002/cncr.28058. [DOI] [PubMed] [Google Scholar]
- Muhlfeld C, Das SK, Heinzel FR, Schmidt A, Post H, Schauer S, Hoefler G. Cancer induced cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLoS ONE. 2011;6:1–11. doi: 10.1371/journal.pone.0020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir F, Yarker J, McDermott H. Employment and the common cancers: Correlates of work ability during or following cancer treatment. Occupational Medicine. 2009;59:381–389. doi: 10.1093/occmed/kpg088. [DOI] [PubMed] [Google Scholar]
- Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Disease Models & Mechanisms. 2012;5:533–545. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. International Journal of Cancer. 2013;133:1234–1246. doi: 10.1002/ijc.28128. [DOI] [PubMed] [Google Scholar]
- Okayama T, Kokura S, Ishikawa T, Adachi S, Hattori T, Takagi T, Yoshikawa T. Antitumor effect of pretreatment for colon cancer cells with hyperthermia plus geranylgeranlacetone in experimental metastasis models and a subcutaneous tumor model of colon cancer in mice. International Journal of Hyperthermia. 2009;25:141–149. doi: 10.1080/02656730802631783. [DOI] [PubMed] [Google Scholar]
- Owen JB, Butterfield DA. Measurement of oxidized/ reduced glutathione ratio. Methods of Molecular Biology. 2010;648:169–277. doi: 10.1007/978-1-60761-756-3_18. [DOI] [PubMed] [Google Scholar]
- Palus D, Akashi Y, von Haehling S, Anker SD, Springer J. The influence of age and sex on disease development in a novel animal model of cardiac cachexia. International Journal of Cardiology. 2009;133:388–392. doi: 10.1016/j.ijcard.2009.01.060. [DOI] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain, Behavior, and Immunity. 2013;34:109–119. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nature Protocols. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Redon CE, Dickey JS, Nakamura AJ, Kareva IG, Naf D, Nowsheen S, Sedelnikova OA. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proceedings of the National Academy of Science (USA) 2010;107:17992–17997. doi: 10.1073/pnas.1008260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Moylan JS. Beyond atrophy: Redox mechanisms of muscle dysfunction in chronic inflammatory disease. Journal of Physiology. 2011;589:2171–2179. doi: 10.1113/jphysiol.2010.203356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusciani L, Proietti I, Rusciani A, Paradisi A, Sbordoni G, Alfano C, Lippa S. Low plasma coenzyme Q10 levels as an independent prognostic factor for melanoma progression. Journal of the American Academy of Dermatology. 2006;54:234–241. doi: 10.1016/j.jaad.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain, Behavior, and Immunity. 2012;26:830–848. doi: 10.1016/j.bbi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. International Journal of Biochemistry & Cell Biology. 2013;45:2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C, Kubo H, Mori M, Sawashita J, Kitano M, Hosoe K, Higuchi K. Treatment with the reduced form of coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-α gene expression signature in SAMP1 mice. Molecular Nutrition & Food Research. 2010;54:805–815. doi: 10.1002/mnfr.200900155. [DOI] [PubMed] [Google Scholar]
- Shadfar S, Couch ME, McKinney KA, Weinstein LJ, Yin X, Rodriguez JE, Willis M. Oral Resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutrition and Cancer. 2011;63:749–762. doi: 10.1080/01635581.2011.563032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Heim CM, Nater UM, Marques AH, Sternberg EM. Neuroendocrine and immune contributors to fatigue. PM & R. 2010;2:338–346. doi: 10.1016/j.pmrj.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YS, Ye ZY, Qian ZY, Xu XD, Hu JF. Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of gastric cancer patients. Journal of Experimental & Clinical Cancer Research. 2012;31:81. doi: 10.1186/1756-9966-31-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia: Pathophysiology and management. Journal of Gastroenterology. 2013;48:574–594. doi: 10.1007/s00535-013-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Asp ML, Nishijima Y, Belury MA. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. International Journal of Oncology. 2011;39:1321–1326. doi: 10.3892/ijo.2011.1150. [DOI] [PubMed] [Google Scholar]
- Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer-induced cachexia in mice. International Journal of Oncology. 2010;37:347–353. doi: 10.3892/ijo_00000683. [DOI] [PubMed] [Google Scholar]
- Tournoux F, Petersen B, Thibault H, Zou L, Raher MJ, Kurtz B, Scherrer-Crosbie M. Validation of noninvasive measurements of cardiac output in mice using echocardiography. Journal of the American Society of Echocardiography. 2011;24:465–470. doi: 10.1016/j.echo.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G. Cytokines: Muscle protein and amino acid metabolism. Current Opinion in Clinical Nutrition & Metabolic Care. 2012;15:85–91. doi: 10.1097/MCO.0b013e32834e6ea2. [DOI] [PubMed] [Google Scholar]
- Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao LM, Cleeland CS. Serum sTNFR1, IL6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain, Behavior, and Immunity. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MA, Krakowski-Roosen H, Schroder L, Kinscherf R, Krix M, Kopp-Schneider A, Hildebrandt W. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncology. 2009;48:116–124. doi: 10.1080/02841860802130001. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: Evidence to suggest a role of proinflammatory cytokines. Oncology Nursing Forum. 2006;33:535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Weymann K. Inflammation and neural signaling: Etiologic mechanisms of the cancer treatment-related symptom cluster. Current Opinion in Supportive and Palliative Care. 2013;7:54–59. doi: 10.1097/SPC.0b013e32835dabe3. doi:10.1097SPC.ob013e328535dabe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysong A, Couch M, Shadfar S, Li L, Rodriguez JE, Asher S, Willis MS. NF-kB inhibition protects against tumor-induced cardiac atrophy in vivo. American Journal of Pathology. 2011;178:1059–1068. doi: 10.1016/j.ajpath.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Crawford D, Hutchinson KR, Youtz DJ, Lucchesi PA, Velten M, Wold LE. Myocardial dysfunction in an animal model of cancer cachexia. Life Sciences. 2011;88:406–410. doi: 10.1016/j.lfs.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Moon C. Hippocampal dysfunction in tumor-bearing mice. Brain, Behavior, and Immunity. 2014;36:147–155. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comparative Medicine. 2013;63:491–497. [PMC free article] [PubMed] [Google Scholar]