Abstract
PURPOSE
Previous studies have demonstrated lower prostate-specific antigen (PSA) concentrations in men with type 2 diabetes (T2DM), paralleling the reported lower prevalence of prostate cancer among diabetic men. Data on PSA in men with type 1 diabetes (T1DM), in whom insulin and obesity profiles differ from those in T2DM, are lacking. The objective of this study was to examine the relationship between long-term glycemic control and PSA in men with T1DM.
MATERIALS & METHODS
Total PSA was measured at one time in 639 men in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the observational follow up of participants in the Diabetes Control and Complications Trial (DCCT). The relationship between DCCT/EDIC weighted mean HbA1c and log PSA was assessed using linear regression modeling after adjusting for age, body mass index (BMI), total testosterone, statin and thiazide medication use, diabetes duration, and DCCT randomization arm and cohort.
RESULTS
The subjects had a median age of 52 years, BMI of 28.4 kg/m2 and DCCT/EDIC time weighted HbA1c of 7.9%. Total median (interquartile range) for PSA levels was 0.64 (0.43, 1.05). PSA levels increased significantly with age (p<0.0001) and with lower time weighted HbA1c (p<0.0001). Each 10% increase in HbA1c was accompanied by an 11% reduction in PSA (p=0.0001).
CONCLUSIONS
PSA levels decrease as HbA1c increases in men with T1DM. This relationship is independent of age, BMI, androgen levels, medication use and measures of diabetes severity, which suggest that factors related to glycemia may be directly affecting PSA levels.
Keywords: Glycemic Control, Diabetes, PSA
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed malignancy and the second leading cause of cancer-related deaths among men in the United States. An estimated 238,590 new cases of PCa were diagnosed in 2013, with an associated mortality of 29,720 cases.1 Although controversial,2 elevations in PSA concentrations are commonly used as an initial step in screening for PCa.3 PSA is a glycoprotein secreted by the prostate gland, and on average, men with malignancy, inflammation, or enlargement have higher total PSA compared to men with lesser degrees of pathology.
While studies have reported that persons with diabetes may have a higher risk of specific malignancies compared to adults without diabetes,4 a recent meta-analysis demonstrated that patients with diabetes have a statistically significant decreased risk of developing PCa.5 This is paralleled by the inverse relationship reported between glucose levels and PSA. Specifically, previous studies have demonstrated lower PSA in men with type 2 diabetes mellitus (T2DM) and inverse associations between poor glycemic control, as assessed by hemoglobin A1c (HbA1c), and PSA.6–9 There are several possible explanations why PSA may be lower among men with T2DM than among men without T2DM, including greater obesity and more frequent use of medications used to treat dyslipidemia and hypertension. Men with higher BMI have lower mean total PSA than men with lower BMIs,10–12 possibly due to hemodilution from increased plasma volume in larger men,13 or lower androgen levels that characterize more obese and insulin resistant adult males.14 PSA has been reported to decrease in response to statins, which are more commonly used among persons with T2DM.15–17 PSA concentrations also are lower among men who use thiazide diuretics to treat hypertension.16 Finally, the vascular disease that characterizes diabetes might lead to prostate ischemia and subsequently lower PSA, although there is no direct evidence to support this hypothesis.18
These reports have focused upon men with T2DM and the relationship between HbA1c and PSA among men with type 1 diabetes mellitus (T1DM) has not been reported but could inform the discussion in several ways. As opposed to men with T2DM, men with T1DM have elevations in glycemic levels due to beta-cell destruction and subsequent lack of insulin secretion; obesity and insulin resistance are less common at initial diagnosis. The prevalence of other cardiovascular risk factors, particularly dyslipidemia, tends to be lower in persons with T1DM as opposed to T2DM. Thus, examination of adult males with T1DM offers the opportunity to focus upon the role of hyperglycemia in PSA elevation apart from other potential confounders.
The objective of this study was to examine the distribution of PSA concentrations in men with T1DM and the relationship between long-term glycemic control and PSA, adjusted for body size, androgen levels, diabetes treatment, and medication use. We hypothesized that poor glycemic control in T1DM may result in lower PSA.
MATERIALS & METHODS
Subjects
The DCCT randomly assigned 761 males with T1DM to intensive or conventional diabetes therapy, with a mean of 6.5 years of treatment during 1983–1993.19 At baseline, the 378 primary prevention cohort males had no retinopathy or nephropathy and 1–5 years of diabetes. The 383 secondary intervention cohort males had mild to moderate non-proliferative retinopathy, as much as 200 mg/day albuminuria, and 1–15 years of diabetes. Individuals with hypertension, symptomatic ischemic heart disease, or symptomatic peripheral neuropathy requiring treatment were excluded from participation in the DCCT.
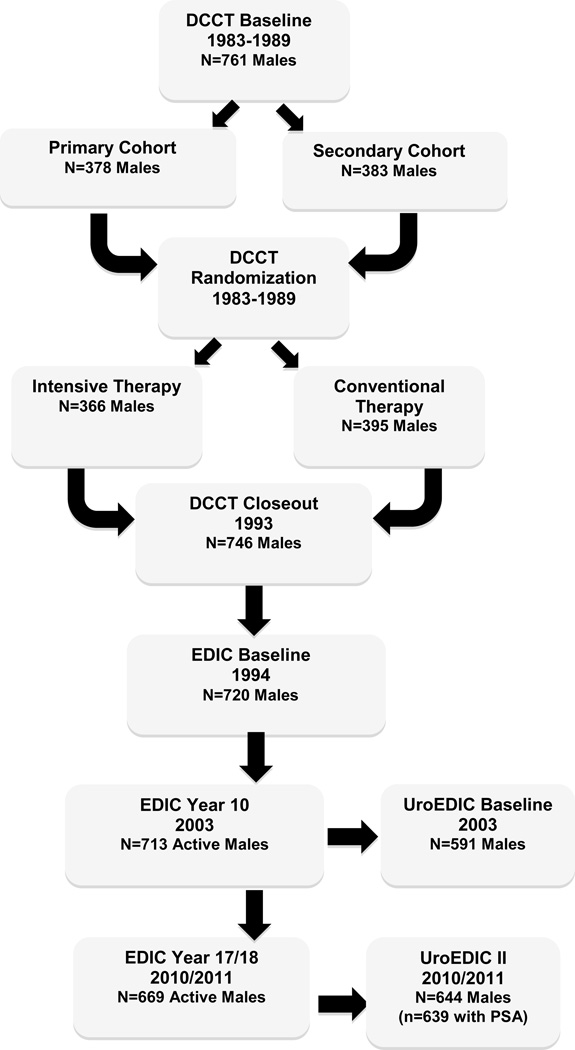
Figure 1 presents the flow of participants from enrollment into the DCCT, its longitudinal follow up in the Epidemiology of Diabetes Intervention and Complication study (EDIC), and an ancillary study to examine urological complications, UroEDIC. Of the 761 men enrolled originally in DCCT, 639 (84%) provided serum for one-time measured PSA testing in 2010/11 and are included in the current study. This sample represented 96% of the males who were active in the study during that period. The institutional review board of each participating center approved the study, and the Federal Government issued a Certificate of Confidentiality.
Figure 1.
Flow of Male Participants in UroEDIC
DCCT Intervention and Other Therapies
Intensive therapy during the DCCT was aimed at achieving glycemic levels as close to the non-diabetic range as safely possible using multiple daily insulin injections or continuous subcutaneous insulin infusion with dose adjustments based on frequent self-monitoring of glucose. Conventional therapy was aimed at maintaining clinical well-being with 1–2 daily insulin injections with no glucose targets. At the end of DCCT, conventional group subjects were trained in intensive therapy and all subjects were returned to their own physicians for diabetes management with the recommendation to implement intensive therapy. By EDIC year 10, 97% of intensive and 94% of conventional group subjects reported implementing intensive therapy. During EDIC, the levels of HbA1c merged and have been similar in the two groups.20, 21
Diabetes Measurements
HbA1c was measured at baseline, quarterly during DCCT, and annually in EDIC. Therefore, mean HbA1c over DCCT/EDIC weighted each DCCT value by 0.25, and EDIC value by 1.21 Peripheral neuropathy was defined during EDIC by >6 positive responses to the Michigan Neuropathy Screening Instrument (MNSI) or a score >2 on the examination.22 Autonomic neuropathy was defined as: 1) R-R variation with deep breathing <15 or 2) R-R variation between 15–19.9 plus either a Valsalva ratio ≤1.5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure. Retinopathy was assessed with stereoscopic fundus photography centrally graded using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale.23 During the DCCT, albumin excretion rate (AER) was measured annually with timed urine collections. During EDIC, AER was measured in half of the cohort annually. Nephropathy was defined as microalbuminuria (AER 40–300 mg/24hr) or albuminuria (AER >300 mg/24hr). Statin and thiazide medication status was defined by use of any lipid-lowering statin, any thiazide diuretic or anti-hypertensive, respectively, at EDIC Year 17.
Prostatic Specific Antigen Measurements
Total serum PSA was measured at the DCCT/EDIC central laboratory (University of Minnesota) with the Advia Centaur PSA assay, a two-site sandwich immunoassay using direct chemiluminometric technology, which uses constant amounts of two antibodies (Bayer Diagnostics, Tarrytown, New York). The limit of detection of the assay is 0.01 ng/mL to 100ng/mL. Intra- and inter-assay coefficient of variations are 4.38% and 4.05% at 0.44ng/mL; 2.09% and 4.67% at 1.83ng/mL; and 2.29% and 2.4% at 17.71ng/mL.
Statistical Analyses
Descriptive analysis examined the overall distribution of demographic and clinical characteristics in men with PSA measurements at EDIC Year 17 using medians and interquartile ranges (IQR). Differences in distributions of PSA and HbA1c at EDIC Year 17 by demographic and clinical characteristics, markers of diabetes treatment and control, and diabetes complications were tested using the Wilcoxon rank-sum test for two-category variables or the Jonchjeere-Terpstra trend test for variables with more than two categories.
PSA and HbA1c values were natural-log transformed to improve normality. Separate linear regression models were used to assess the relationship between time-weighted DCCT/EDIC log HbA1c and log PSA, stratified by attained age and current BMI. The relationships between time-weighted DCCT/EDIC mean HbA1c and current HbA1c and log PSA were also modeled using multivariable linear regressions, adjusting for attained age, continuous BMI, continuous total testosterone concentrations, statin and thiazide use, DCCT intensive vs. standard therapy, DCCT primary prevention vs. secondary intervention cohort, duration of diabetes, follow-up interval length, and eligibility HbA1c. The percent change in PSA per 10% increase in both current and time-weighted HbA1c were reported using the following equation: 100[1.1β-1]. Effects significant at p<0.05 are cited. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Overall, the 639 DCCT/EDIC male subjects had a median age of 52 years, median BMI of 28.4 kg/m2 and weighted median HbA1c 7.9%. Median (IQR) PSA was 0.64 (0.43, 1.05) ng/mL. Table 1 presents the demographic and clinical characteristics of participants at EDIC year 17.
Table 2.
Serum PSA Concentrations and HbA1c Levels by Sociodemographic, Clinical and Diabetes Characteristics in Men at EDIC Year 17 in the UroEDIC II Study
| Characteristics | Year 17 EDIC (2010) UroEDIC II PSA and HbA1c Levels (n=639) |
|||
|---|---|---|---|---|
| PSA | p-value* | HbA1c | p-value* | |
| Sociodemographic/Clinical | Median (Interquartile Range) | |||
| Attained age (yr) | ||||
| Q1 ≤47 | 0.55 (0.38–0.73) | <0.0001 | 7.90 (7.00–8.90) | 0.01 |
| Q2 48–52 | 0.63 (0.43–1.01) | 7.60 (7.20–8.40) | ||
| Q3 53–57 | 0.66 (0.45–1.17) | 7.70 (7.00–8.30) | ||
| Q4 >57 | 0.90 (0.49–1.41) | 7.55 (6.90–8.20) | ||
| Duration of diabetes (yr) | ||||
| Q1 ≤25 | 0.63 (0.43–0.97) | 0.4 | 7.80 (7.20–8.60) | 0.07 |
| Q2 26–28 | 0.60 (0.43–1.10) | 7.55 (6.85–8.30) | ||
| Q3 29–33 | 0.65 (0.41–0.97) | 7.90 (7.10–8.70) | ||
| Q4 >33 | 0.69 (0.45–1.11) | 7.50 (7.00–8.20) | ||
| BMI category | ||||
| Normal BMI<25 | 0.67 (0.43–0.95) | 0.3 | 7.50 (6.90–8.40) | 0.05 |
| Overweight BMI 25–30 | 0.68 (0.44–1.08) | 7.60 (7.00–8.40) | ||
| Obese BMI≥30 | 0.58 (0.42–1.06) | 7.80 (7.10–8.60) | ||
| BMI change since DCCT baseline (kg/m2) | ||||
| Q1 ≤2.54 | 0.61 (0.37–0.95) | 0.9 | 7.80 (7.10–8.50) | 0.9 |
| Q2 2.55–4.86 | 0.71 (0.50–1.11) | 7.50 (6.95–8.30) | ||
| Q3 4.87–7.18 | 0.60 (0.44–1.07) | 7.80 (7.10–8.50) | ||
| Q4 >7.18 | 0.60 (0.41–1.06) | 7.70 (6.90–8.60) | ||
| Total Testosterone | ||||
| Low (TT<300ng/dL) | 0.58 (0.42–0.82) | 0.1 | 8.00 (7.30–9.10) | 0.02 |
| Normal (TT≥300ng/dL) | 0.65 (0.43–1.06) | 7.60 (7.00–8.40) | ||
| Medication | ||||
| Statin use | ||||
| Yes | 0.63 (0.43–1.07) | 0.4 | 7.70 (7.00–8.40) | 0.4 |
| No | 0.65 (0.42–0.96) | 7.80 (7.00–8.60) | ||
| Thiazide Use | ||||
| Yes | 0.69 (0.53–1.09) | 0.2 | 7.55 (6.95–8.50) | 0.7 |
| No | 0.62 (0.42–1.05) | 7.70 (7.00–8.40) | ||
| Diabetes Treatment and Control | ||||
| DCCT treatment arm | ||||
| Intensive | 0.65 (0.43–1.09) | 0.6 | 7.70 (7.00–8.60) | 0.5 |
| Standard | 0.63 (0.42–1.00) | 7.60 (7.00–8.40) | ||
| DCCT cohort assignment | ||||
| Primary Prevention | 0.61 (0.41–1.03) | 0.3 | 7.70 (7.10–8.40) | 0.3 |
| Secondary Intervention | 0.66 (0.45–1.06) | 7.60 (7.00–8.40) | ||
| Current HbA1c Mean (%) | ||||
| Q1 ≤7.00 | 0.66 (0.48–1.13) | 0.004 | --- | |
| Q2 7.01–7.70 | 0.67 (0.42–1.14) | --- | ||
| Q3 7.71–8.40 | 0.71 (0.45–1.10) | --- | ||
| Q4 >8.40 | 0.56 (0.38–0.77) | --- | ||
| Time-weighted DCCT/EDIC HbA1c Mean (%) | ||||
| Q1 ≤7.25 | 0.75 (0.47–1.25) | <0.0001 | --- | |
| Q2 7.26–7.85 | 0.66 (0.47–1.10) | --- | ||
| Q3 7.86–8.57 | 0.65 (0.46–1.05) | --- | ||
| Q4 >8.57 | 0.54 (0.35–0.77) | --- | ||
| Insulin Dose (u/kg/day) | ||||
| Q1 ≤0.55 | 0.62 (0.46–0.99) | 0.6 | 7.70 (6.95–8.35) | 0.02 |
| Q2 0.56–0.69 | 0.65 (0.43–1.23) | 7.60 (7.00–8.30) | ||
| Q3 0.70–0.90 | 0.61 (0.43–0.97) | 7.60 (7.00–8.40) | ||
| Q4 >0.90 | 0.66 (0.41–1.05) | 7.90 (7.20–9.00) | ||
| Diabetes Complications | ||||
| Retinopathy‡ | ||||
| Non Proliferative or None | 0.65 (0.43–1.10) | 0.03 | 7.60 (6.90–8.30) | <0.0001 |
| Proliferative | 0.58 (0.41–0.87) | 8.20 (7.40–9.00) | ||
| Nephropathy£ | ||||
| None (AER<40) | 0.65 (0.43–1.06) | 0.5 | 7.60 (7.00–8.30) | <0.0001 |
| Microalbuminuria(40≤AER<300) | 0.64 (0.43–1.11) | 8.15 (7.40–9.00) | ||
| Albuminuria (AER≥300) | 0.60 (0.41–0.77) | 8.35 (7.50–9.60) | ||
| Sustained AER≥30 | ||||
| No | 0.65 (0.43–1.11) | 0.06 | 7.60 (7.00–8.30) | <0.0001 |
| Yes | 0.60 (0.39–0.93) | 8.30 (7.50–9.10) | ||
| eGFR<60 | ||||
| No | 0.63 (0.43–1.05) | 0.1 | 7.60 (7.00–8.30) | 0.3 |
| Yes | 0.80 (0.53–1.22) | 7.80 (7.10–8.70) | ||
| Peripheral Neuropathy§ | ||||
| No | 0.65 (0.45–1.10) | 0.1 | 7.60 (6.95–8.40) | 0.005 |
| Yes | 0.61 (0.41–1.03) | 7.80 (7.20–8.70) | ||
| Autonomic Neuropathy¶ | ||||
| No | 0.66 (0.44–1.06) | 0.09 | 7.70 (7.00–8.40) | 0.003 |
| Yes | 0.59 (0.37–1.03) | 7.90 (6.80–9.30) | ||
p-values based on the Wilcoxon rank-sum or Jonckheere-Terpstra trend test.
Defined through EDIC year 14 using the Early Treatment Diabetic Retinopathy Study on a scale of 0–23 (<12 Non Proliferative or None, ≥12 Proliferative).
Albumin Excretion Rate (mg/24hr) at EDIC years 15/16.
Defined at EDIC year 17 by the Michigan Neuropathy Screening Instrument >6 responses on the questionnaire or a score of >2 on the exam.
Autonomic testing completed in EDIC year 16/17 and abnormal finding defined as R-R variation<15 or RR variation 15–20 in combination with Valsalva Ratio ≤1.5 or a decrease of >10 mmHg in diastolic BP.
Median PSA concentrations and HbA1c levels were examined by demographic and clinical characteristics at EDIC Year 17. (Table 2) Median PSA increased significantly with age (p<0.001) and decreased with elevated current (p=0.005) as well as time-weighted (p<0.001) mean HbA1c. Lower PSA concentrations were observed among men with retinopathy and evidence of nephropathy. No significant differences in PSA were observed by total testosterone, BMI, DCCT treatment arm, DCCT cohort or presence of neuropathy. Median HbA1c levels decreased significantly with age (p=0.01). Men who were obese tended to have higher median HbA1c (7.8%) compared to their overweight (7.6%) and normal BMI (7.5%) counterparts (p=0.05). Men with low testosterone (TT<300 ng/dL) and those with diabetes complications had significantly higher HbA1c compared to those with normal testosterone and no complications.
Table 3.
Association of Time-weighted DCCT/EDIC HbA1c and log Serum PSA Concentrations in Men at EDIC Year 17 in the UroEDIC II Study
| Characteristics | Year 17 EDIC (2010) UroEDIC II PSA Levels (n=639) |
|||
|---|---|---|---|---|
| Model with DCCT/EDIC Time-weighted Mean HbA1c |
Model with EDIC Year 17 Current HbA1c |
|||
| Estimate (Std Error) |
p-value | Estimate (Std Error) |
p-value | |
| Unadjusted | ||||
| Time-weighted DCCT/EDIC HbA1c (%) | −1.218 (0.228) | <0.0001 | --- | --- |
| Current HbA1c (%) | --- | --- | −0.632 (0.185) | 0.0007 |
| Adjusted | ||||
| Attained age (yr) | 0.025 (0.004) | <0.0001 | 0.025 (0.004) | <0.0001 |
| BMI (kg/m2) | −0.005 (0.006) | 0.4 | −0.006 (0.006) | 0.3 |
| Testosterone (ng/dL) | −0.001 (0.001) | 0.5 | −0.001 (0.001) | 0.5 |
| Statin use (yes vs. no) | 0.004 (0.060) | 0.9 | −0.001 (0.060) | 0.9 |
| Thiazide use (yes vs. no) | 0.007 (0.079) | 0.9 | −0.005 (0.080) | 0.9 |
| DCCT Treatment (INT vs. CONV) | 0.005 (0.057) | 0.9 | 0.072 (0.055) | 0.2 |
| DCCT Cohort (PRIM vs. SCND) | −0.064 (0.081) | 0.4 | −0.060 (0.082) | 0.5 |
| Duration of Diabetes on Entry (yr) | −0.008 (0.010) | 0.4 | −0.006 (0.010) | 0.6 |
| Follow-up Interval Length (yr)* | 0.003 (0.017) | 0.9 | 0.005 (0.017) | 0.8 |
| Eligibility HbA1c (%) | −0.024 (0.020) | 0.2 | −0.044 (0.019) | 0.02 |
| Time-weighted DCCT/EDIC HbA1c (%) | −0.984 (0.255) | 0.0001 | --- | --- |
| Current HbA1c (%) | --- | --- | −0.382 (0.189) | 0.04 |
Data are parameter estimates (standard errors) and p-values from two separate multivariable regression models. Time-weighted DCCT/EDIC HbA1c and Current HbA1c were modeled on the log scale.
Time (in years) from randomization to PSA evaluation. The average follow-up interval was 23.99±1.69 years.
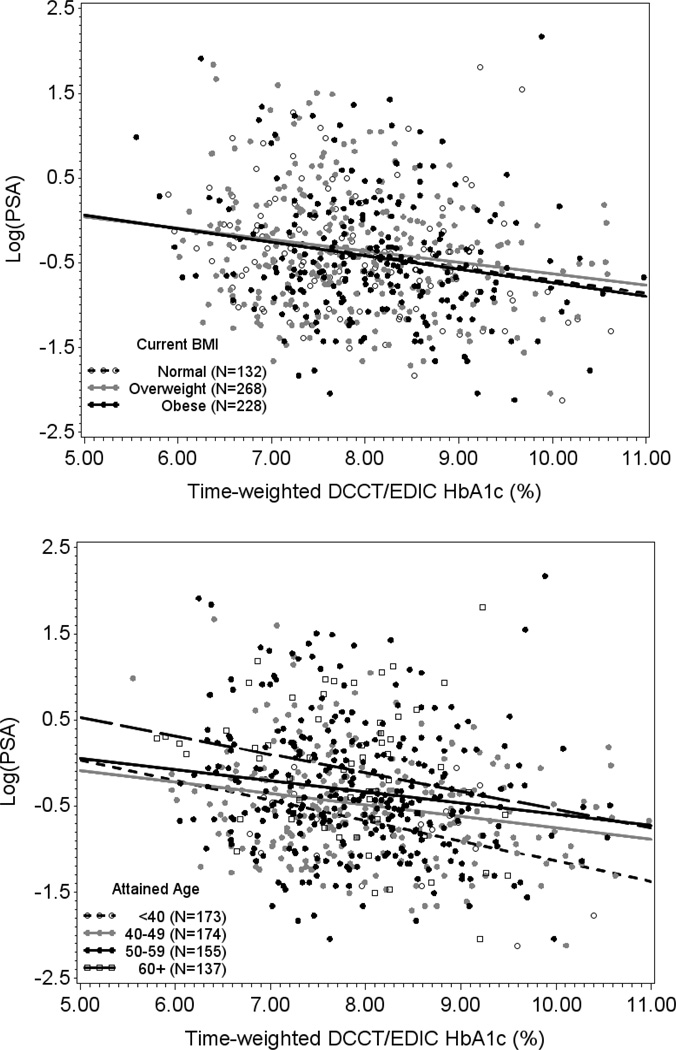
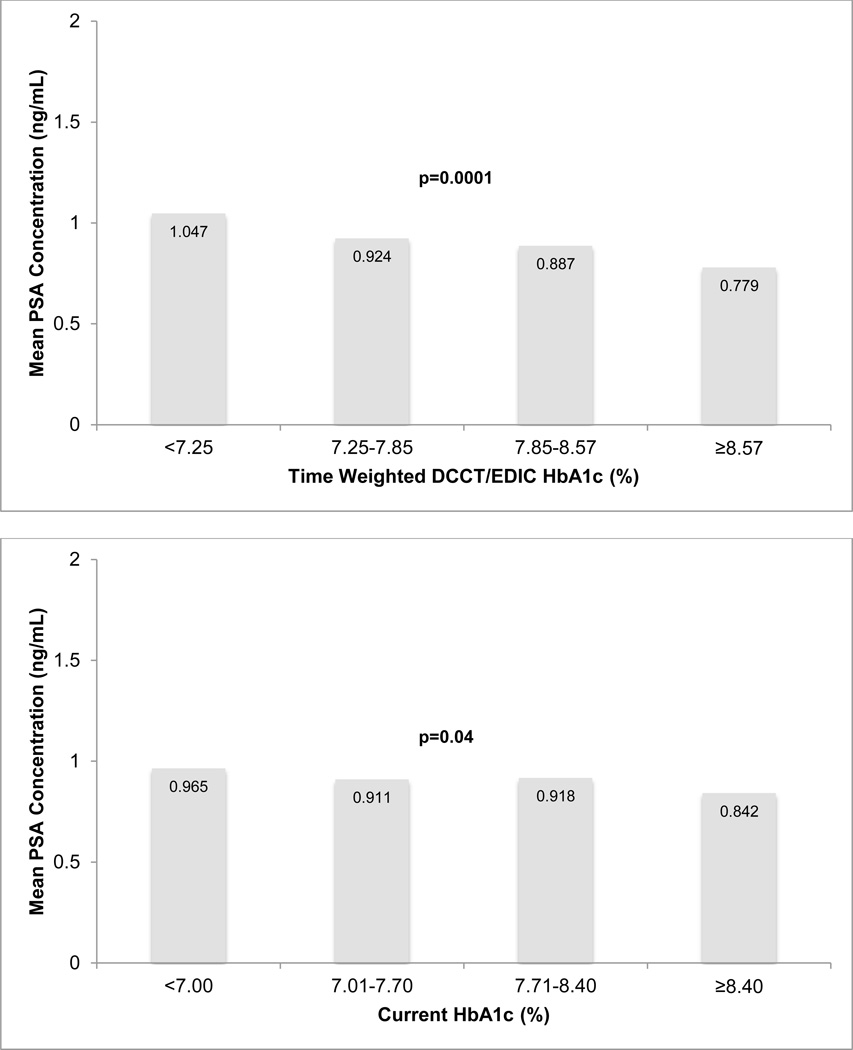
Two separate linear regression models stratified by attained age and current BMI, respectively, revealed inverse relationships between time-weighted HbA1c and PSA. (Figure 2) These relationships were not affected by age or BMI (p-value for interaction 0.8 and 0.9, respectively). Mean PSA decreased by increasing quartiles of both time weighted and current HbA1c levels after adjustment for age, BMI, total testosterone, statin and thiazide use, DCCT treatment group, DCCT cohort assignment, duration of diabetes, follow-up interval length, and eligibility HbA1c. (Figure 3) Multivariable regression analysis demonstrated an 11% decrease in PSA per 10% increase (e.g. from 7% to 7.7%) in time-weighted HbA1c (p=0.0001) after adjustment for all covariates listed above. (Table 3) A separate multivariable regression model examining current HbA1c levels revealed a 9% (p=0.04) decrease in PSA per 10% increase in current HbA1c demonstrating a stronger association with long-term glycemic control compared to contemporaneous HbA1c.
Figure 2.
Time-weighted DCCT/EDIC HbA1c Levels and Serum PSA Concentrations at EDIC Year 17 by Attained Age and Current BMI Status
Figure 3.
Adjusted* Mean PSA Concentrations by Time-Weighted and Current Hemoglobin A1c Levels**
* Mean PSA concentrations adjusted for attained age, BMI status, total testosterone concentrations, thiazide use, DCCT treatment group, and DCCT cohort assignment.
** Time-weighted DCCT/EDIC HbA1c reference HbA1c<7.25%, Current HbA1c reference HbA1c<7.00%
DISCUSSION
We observed a significant inverse relationship between HbA1c levels and PSA concentrations in men with T1DM who were enrolled in a long-term observational follow-up of a clinical trial designed to evaluate the impact of intensive vs. conventional diabetes therapy. The association was independent of age, BMI, testosterone concentrations, thiazide medication use and DCCT treatment and cohort, suggesting that glycemia itself may be affecting PSA.
Our findings that PSA concentrations were associated with elevated HbA1c levels are consistent with previous reports suggesting that PSA concentrations are lower in men with T2DM than in men without diabetes. Using the National Health and Nutrition Examination Survey, Werny and colleagues found 22% lower average serum PSA among men with T2DM after accounting for age.24 These findings were replicated by Fukui et al., who observed 10%–16% lower average PSA among Japanese men with T2DM, aged 50–79 years.25 In a population-based longitudinal cohort study, Wallner et al. demonstrated that men with diabetes had slower increases in PSA over time, which may account for the lower PSA observed in men with diabetes in cross-sectional studies.26 These studies were all focused on men with T2DM, were limited in their cross-sectional design or did not examine the effects of glycemic control independent of body size, medication use or androgen levels.
Our findings are also consistent with those studies in men with T2DM specifically examining glycemic control. Müller et al found men with HbA1c of 6.1–6.9% and ≥7% had 15% (p=0.004) and 29% (p=0.003) lower serum PSA compared to men with normal HbA1c (<6.1%).6 In a cross-sectional sample of the Southern Community Cohort Study aged 40–78 years, Fowke et al. reported decreasing mean PSA with increasing HbA1c quartiles (ptrend=0.005) among Caucasian men.7 In contrast to these cross-sectional studies and our current report, longitudinal observations in a Japanese hospital cohort found increases in HbA1c and PSA to be concordant (5.7% increase per 1-unit HbA1c change, p<0.001).27 However, when the data were analyzed in a cross-sectional manner, the authors reported small non-significant inverse relationships between HbA1c and PSA. Furthermore, this investigation was limited to a one-year follow-up whereas our report examines the effect of 23 years of mean HbA1c levels on PSA in men with T1DM. While our study demonstrated a significant effect of current HbA1c on PSA, long-term glycemic control as assessed by 23-year time-weighted HbA1c had greater impact.
Several explanations why PSA may be reduced with poor glycemic control have been described above. Despite previous reports of the effect of BMI and androgen levels on PSA, in our report, while we observed median PSA to be lower in obese men and men with low testosterone, these findings were not statistically significant. Further, the reduction in PSA with elevated HbA1c was independent of total testosterone and BMI possibly due to the relatively younger age of the cohort and the narrower distribution of androgen concentrations and their effect on obesity. Additionally, medication use may be a proxy for diabetes severity and correlated with reduced PSA.15–17 We found no significant impact, however, of duration of diabetes, diabetes complications or these medications on PSA independent of HbA1c. Thus, while the mechanisms for linking HbA1c to PSA require further investigation, the observed inverse relationships in this study are generally consistent with the previously reported complex interactions between adiposity, androgen levels and insulin sensitivity.
It is unclear whether decreased PSA observed in diabetic men relative to non-diabetic men accurately reflect a decreased risk of PCa in the diabetic population observed in previous studies.28 If smaller increases in PSA among men with diabetes results in less detection of PCa among asymptomatic cases, it might suggest a detection bias among men with diabetes, similar to that thought to exist among obese men due to their lower PSA as a result of hemodilution.29 While the younger age and lower PSA values in our cohort do not represent the average population amenable to screening, the potential impact of diabetes on PCa detection warrants further investigation.
This was the largest study to investigate PSA in a T1DM population to date. The long duration of follow-up allowed for exploration of long-term glycemic control and its impact on PSA. However, there are a number of limitations. While the cohort has been followed for many years, participants are relatively young and clinical relevance of PSA assessment in men under the age of 50 is questionable. This cohort consisted of predominantly Caucasian men. Given reported variations in PSA and diabetes related conditions in African-Americans, we are limited in our ability to generalize these findings to other racial groups. Further, this cohort may not accurately reflect the general population of middle-aged men with T1DM. DCCT/EDIC participants are a highly motivated group of individuals who have been followed for many years with a goal of tight diabetes control. There was not a control arm of men without diabetes, thus this study does not allow for a true comparative analysis. Finally, prostate volume was not measured and limits our ability to determine the impact of prostatic growth. As few participants had PSA levels in the range sufficient for referral to biopsy (n=12 men had PSA≥4.0), we were unable to evaluate the clinical significance of PSA suppression on PCa detection.
CONCLUSIONS
Our results suggest that PSA concentrations decrease as overall HbA1c increases in men with T1DM. This relationship is independent of BMI, age, testosterone concentrations, and diabetes medications, which suggest that factors directly related to glycemia may be affecting PSA. It is possible that the presence of poorly controlled T1DM may lead to fewer prostate cancers being detected owing to the reduction in PSA. As the controversy surrounding PSA screening continues and guidelines are revised for PCa, it may be prudent to consider the presence of diabetes and its severity and control.
Supplementary Material
ACKNOWLEDGEMENTS
A complete list of participants in the DCCT/EDIC research group can be found in New England Journal of Medicine, 2011;365:2366–2376.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), OmniPod® Insulin Management System (Bedford, MA) , Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN) , and Sanofi-Aventis (Bridgewater NJ).
Funding/Support: The DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982–93, 2011–2016), and contracts (1982–2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA.
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
Additional support for this DCCT/EDIC collaborative study (UroEDIC) was provided by an R01 grant (2009–2013) with the National Institute of Diabetes and Digestive and Kidney Disease (5R01DK083927-03)
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.null: Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2008;149:185. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Scosyrev E, Wu G, Golijanin D, et al. Prostate-specific antigen testing in older men in the USA: data from the behavioral risk factor surveillance system. BJU International. 2012;110:1485. doi: 10.1111/j.1464-410X.2012.11013.x. [DOI] [PubMed] [Google Scholar]
- 4.Wulaningsih W, Holmberg L, Garmo H, et al. Serum Glucose and Fructosamine in Relation to Risk of Cancer. PloS one. 2013;8:e54944. doi: 10.1371/journal.pone.0054944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Jiang H-w, Ding G-x, et al. Diabetes mellitus and prostate cancer risk of different grade or stage: A systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2013;99:241. doi: 10.1016/j.diabres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Müller H, Raum E, Rothenbacher D, et al. Association of diabetes and body mass index with levels of prostate-specific antigen: implications for correction of prostate-specific antigen cutoff values? ancer Epidemiology Biomarkers & Prevention. 2009;18:1350. doi: 10.1158/1055-9965.EPI-08-0794. [DOI] [PubMed] [Google Scholar]
- 7.Fowke JH, Matthews CM, Buchowski MS, et al. Association between prostate-specific antigen and leptin, adiponectin, HbA1c or C-peptide among African-American and Caucasian men. Prostate cancer and prostatic diseases. 2007;11:264. doi: 10.1038/sj.pcan.4501022. [DOI] [PubMed] [Google Scholar]
- 8.Fall K, Garmo H, Gudbjörnsdottir S, et al. Diabetes Mellitus and Prostate Cancer Risk; A Nationwide Case–Control Study within PCBaSe Sweden. Cancer Epidemiology Biomarkers & Prevention. 2013;22:1102. doi: 10.1158/1055-9965.EPI-12-1046. [DOI] [PubMed] [Google Scholar]
- 9.Onitilo AA, Stankowski RV, Berg RL, et al. Type 2 diabetes mellitus, glycemic control, and cancer risk. European Journal of Cancer Prevention, Publish Ahead of Print. doi: 10.1097/CEJ.0b013e3283656394. 10.1097/CEJ.0b013e3283656394, 9000. [DOI] [PubMed] [Google Scholar]
- 10.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate - specific antigen in a population based study. Cancer. 2005;103:1092. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 11.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001–2004. Cancer Epidemiology Biomarkers Prevention. 2007;16:70. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 12.Rundle A, Neugut AI. Obesity and screening PSA levels among men undergoing an annual physical exam. The Prostate. 2008;68:373. doi: 10.1002/pros.20704. [DOI] [PubMed] [Google Scholar]
- 13.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. Jama. 2007;298:2275. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 14.Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton RJ, Goldberg KC, Platz EA, et al. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 16.Chang SL, Harshman LC, Presti JC. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. Journal of Clinical Oncology. 2010;28:3951. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondul AM, Selvin E, De Marzo AM, et al. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001–2004. Cancer Causes & Control. 2010;21:671. doi: 10.1007/s10552-009-9494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Hu R. Why does diabetes offer protective effects against prostate cancer? The possible role of its microvascular complications. Medical hypotheses. 2010;74:242. doi: 10.1016/j.mehy.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 19.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 23.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98:741. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 24.Werny DM, Saraiya M, Gregg EW. Prostate-specific Antigen Values in Diabetic and Nondiabetic US Men, 2001–2002. American Journal of Epidemiology. 2006;164:978. doi: 10.1093/aje/kwj311. [DOI] [PubMed] [Google Scholar]
- 25.Fukui M, Tanaka M, Kadono M, et al. Serum prostate-specific antigen levels in men with type 2 diabetes. Diabetes Care. 2008;31:930. doi: 10.2337/dc07-1962. [DOI] [PubMed] [Google Scholar]
- 26.Wallner LP, Morgenstern H, McGree ME, et al. The effects of type 2 diabetes and hypertension on changes in serum prostate specific antigen levels: results from the Olmsted County study. Urology. 2010;77:137. doi: 10.1016/j.urology.2010.07.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohwaki K, Endo F, Muraishi O, et al. Relationship between changes in haemoglobin A<sub>1C</sub>and prostate-specific antigen in healthy men. European journal of cancer. 2011;47:262. doi: 10.1016/j.ejca.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiology Biomarkers & Prevention. 2006;15:2056. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 29.Bañez LL, Hamilton RJ, Partin AW, et al. OBesity-related plasma hemodilution and psa concentration among men with prostate cancer. JAMA. 2007;298:2275. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.