Abstract
Objective
The mechanisms linking obesity and osteoarthritis (OA) are not fully understood and have been generally attributed to increased weight, rather than metabolic or inflammatory factors. Here, we examined the influence of dietary fatty acids, adipokines, and body weight following joint injury in mouse model of OA.
Methods
Mice were fed high-fat diets rich in various fatty acids (FAs) including saturated FAs (SFAs), ω-6 polyunsaturated FAs (PUFAs), and ω-3 PUFAs. OA was induced by destabilizing the medial meniscus. Wound healing was evaluated using an ear punch. OA, synovitis and wound healing were determined histologically, while bone changes were measured using microCT. Activity levels and serum cytokines were measured at various time-points. Multivariate models were performed to elucidate the associations of dietary, metabolic, and mechanical factors with OA and wound healing.
Results
Using weight-matched mice and multivariate models, we found that OA was significantly associated with dietary fatty acid content and serum adipokine levels, but not with body weight. Furthermore, spontaneous activity of the mice was independent of OA development. Small amounts of ω-3 PUFAs (8% by kcal) in a high-fat diet were sufficient to mitigate injury-induced OA, decreasing leptin and resistin levels. ω-3 PUFAs significantly enhanced wound repair, SFAs or ω-6 PUFAs independently increased OA severity, heterotopic ossification, and scar tissue formation.
Conclusions
Our results indicate that dietary FA content is a primary regulator of OA severity and wound regeneration with obesity, supporting the need for further studies of dietary FA supplements as a potential therapeutic approach for OA.
Keywords: post-traumatic arthritis, chondrocyte, macrophage, metabolic syndrome, cytokine
Introduction
Obesity is one of the primary risk factors for osteoarthritis (OA), although the mechanisms linking these conditions are not fully understood.[1] While it has been believed that increased loading on the joints due to weight gain is responsible for accelerated OA with obesity, mechanical factors alone do not account for the higher incidence of OA in non-weight bearing joints, such as the hands.[2] Furthermore, studies have shown that morbidly obese mice do not develop OA when fed standard (low-fat) chow.[3] These findings suggest that factors other than adiposity or body weight – such as dietary content or the circulating levels of adipokines - must contribute to OA in obesity.
Cellular stress due to obesity induces lipolysis of adipocytes,[4] increasing the levels of circulating free fatty acids (FAs). These FAs can serve as pro- or anti-inflammatory molecules in metabolic signalings. Saturated FAs (SFAs) can activate macrophages to secrete tumor necrosis factor (TNF)-α and interleukin (IL)-1.[5] Furthermore, the derivatives of ω-6 polyunsaturated FAs (PUFAs) are involved in joint pain.[6, 7] Conversely, ω-3 PUFAs have been reported to reduce spontaneous OA in animals fed a low-fat diet.[8] These findings imply that dietary or metabolic factors may play a more direct role in joint degeneration. Furthermore, little is known regarding the effects of dietary FAs on motor function, pain perception and wound healing in obesity.
The goal of this study was to determine the effects of obesity and dietary fatty acid content in injury induced OA and to identify the primary factors linking obesity and OA. Mice fed various high-fat diets rich in SFA, ω-6, or ω-3 PUFA underwent surgery to destabilize the medial meniscus (DMM) to induce OA.[9] We also investigated the effect of FAs on wound regeneration and behavioral activity.
Methods
Animals
All procedures were approved by the Duke University IACUC. Beginning at 4 weeks of age, mice were fed a control low-fat diet or one of the three high-fat diets: SFA-rich, ω-6 PUFA-rich, or ω-3 PUFA rich (Table S1). At 16 weeks of age, mice underwent DMM surgery to induce knee OA in the left hind-limb,[9] and ears were punched using 1-mm (right) and 1.5-mm (left) diameter ear punches to examine wound healing responses. To determine how diet affected behavior and activity levels, mice were monitored at 6, 14 and 24 weeks of age. The study design is presented in Fig. S1.
Evaluation of bone structure, OA and synovitis
Bone volume (BV), bone fraction (bone volume/total volume, BV/TV), bone mineral density (BMD), and heterotopic ossification (HO) of the limbs was analyzed using microCT (Bruker Skycan 1176).[10] After standard histological processes, the joints were stained for evaluation of OA and synovitis.
Statistics
Statistical analyses are described in each figure caption. Analyses were performed using SPSS Statistics, with significance reported at the 95% confidence level.
Detailed materials and method are provided in the online supplementary document.
Results
Weight analysis
At 28 weeks of age, SFA and ω-6 mice were heavier than Control and ω-3 mice (Fig. 1A). Although animals slightly lost weight at 8, 17, and 24 weeks, likely due to the mild stress of behavioral testing, they maintained the trend of gaining weight. To describe the influence of weight on joints over time, the area under the weight-versus-time curves (AUC) was calculated (Fig. 1B).[11] All high-fat diet fed mice had higher AUC4-28wk versus Control mice.
Figure 1.
(A) The influence of diets on body weight over time. (B) To precisely describe the effect of weight on knee joint over time, the areas under weight curve of different diets were calculated for the period from 4 to 28 weeks (AUC4-28wk) and from 17 to 28 weeks (AUC17-28wk), respectively. The Control mice had lower AUC4-28wk values as compared to the mice fed high-fat diets. (C and D) SFA and ω-6 mice had increased percentages of body fat, but decreased body BMD relative to Control mice at 28 weeks of age. (E) The ω-6 mice had significantly increased percentages of fat in the inguinal and epididymal depots relative to body weight compared to Control mice. (F) SFA and ω-6 mice showed increased F4/80+ macrophage infiltration (red arrowheads) into adipose tissues (Sub: subcutaneous fat; Vis: visceral fat). Infiltrated macrophages into the visceral fat pads in SFA and ω-6 mice also showed “crown-like” structure (green square). Scale bar = 100 μm. (G) Analysis of F4/80, CD11c. IL-6, TNF-α, and MCP-1 gene expression in visceral adipose tissue in the mice at 28 weeks of age. For gene expression, n = 4 mice/diet. For other figures, n = 11–14 mice/diet. Different letters are significantly different, p < 0.05, from each other. (A, C and D) Statistical significance was determined by two-way repeated measures ANOVA using age and diet as factors. (B, E and G) Statistical significance was determined by one-way ANOVA using diet as factor. ANOVA was then followed by Tukey’s post-hoc test. All data are presented as mean ± SEM.
Body composition and gene expression
At 28 weeks of age, SFA and ω-6 mice had increased body fat percentage but decreased BMD versus Control mice. The ω-3 mice displayed lower BMD but no difference in body fat percentage versus Control mice (Fig. 1C,D). The SFA and ω-6 mice had a higher percentage of epididymal fat relative to body weight, and only ω-6 mice had a significantly higher percentage of inguinal fat than Control mice (Fig. 1E).
F4/80+ macrophages staining in adipose tissue indicated that SFA and ω-6 PUFA diets were associated with massive macrophage infiltration (Fig. 1F). Gene expression of epididymal fat showed that SFA and ω-6 PUFA diets trended toward higher expression of IL-6 with no difference in MCP-1 expression versus those of the ω-3 and Control mice (Fig. 1G). All the high-fat diet fed mice had higher expression of F4/80 and CD11c versus Control mice.
Bone structure
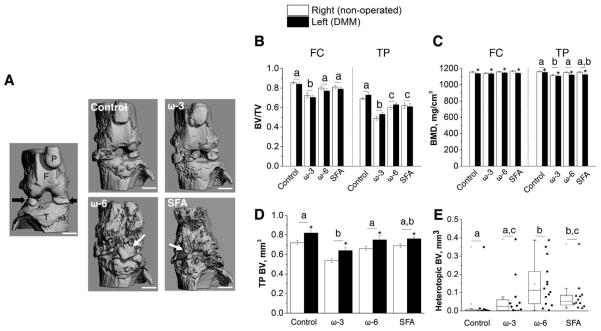
MicroCT imaging of the joints (Fig. 2A) showed that the ω-3 mice had a lower BV/TV of the femoral condyle as compared to the other diets (Fig. 2B). Surgery, but not diet, decreased the BMD of the femoral condyle (Fig. 2C). For the tibial epiphysis, ω-3 mice had lower BV and BMD versus the mice treated with the other diets. For all mice, surgery increased BV but decreased the BMD of the tibial epiphysis (Fig. 2C,D). Diet affected the incidence of heterotopic ossification (HO) in the DMM-operated joint (Table S2). The Control and ω-3 mice had a lower incidence of HO. The SFA and ω-6 mice had high BV of HO fragments, while Control mice had the lowest (Fig. 2E).
Figure 2.
(A) 3D reconstruction of MicroCT of limbs at 28 weeks of age. Right (non-operated) joint from SFA mice showed intact bone structure (F = femur, T = tibia, P = patella; black arrows = partially calcified menisci). Left (DMM) joints of ω-6 and SFA mice had increased heterotopic ossification (white arrows) relative to the other groups. (B) Cancellous bone fraction (bone volume/total volume, BV/TV) for femoral condyle (FC), and cancellous and cortical BV/TV for tibial epiphysis (TP). ω-3 mice had significantly lower BV/TV in FC and TP as compared to the mice fed other diets. (C) Bone mineral density (BMD) of FC and TP. All the mice showed significantly decreased BMD after DMM surgery and the ω-3 mice also showed relatively low BMD to the mice fed other diets. (D and E) BV of TP and heterotopic ossification of DMM joints. All the mice had significantly increased BV in TP after DMM. ω-3 mice exhibited low heterotopic BV among the mice fed other high-fat diets. n = 11–14 mice/diet. * p < 0.05 versus corresponding right (non-operated) joints. Different letters are significantly different, p < 0.05, from each other. (B–D) Statistical significance was determined by two-way repeated measures ANOVA using right (non-operated) joints as the contralateral control. (E) The line inside the box represents the median of each diet group and the length of the box indicates the interquartile range. Statistical significance was determined by Kruskal-Wallis test with Mann-Whitney U test and Holm–Bonferroni correction. Except for (E), all data are presented as mean ± SEM.
ω-3 PUFAs mitigate injury-induced OA in obese mice
A modified Mankin grading scheme was used to determine OA severity. Severe cartilage loss was observed in the DMM-operated joints of SFA and ω-6 mice; the operated joints of Control and ω-3 mice showed surface fibrillation and moderate loss of proteoglycan (Fig. 3A). Three out of fourteen ω-6 mice had severe subchondral bone erosions (Fig. S2). All operated joints had higher OA scores than their corresponding non-operated joints. Notably, the operated joints of SFA and ω-6 mice exhibited the most severe OA versus the operated joints of Control and ω-3 mice. The non-operated joints did not differ among diet groups.
Figure 3.
(A) Safranin-O (glycosaminoglycans) and fast green (bone and tendon) histology for the DMM-operated joint (F = femur, M = meniscus, T = tibia). Severe cartilage loss (yellow arrowheads) was found in ω-6 and SFA mice. The DMM-operated joints from Control and ω-3 mice had significantly lower OA scores compared to those from ω-6 mice and SFA mice. (B) Accumulative counts of osteophyte diseases-stages of the DMM-operated joint. The ω-6 and SFA mice had more mature osteophytes relative to Control and ω-3 mice. (C) OA severity was positively correlated with osteophyte disease stages. (D) H&E histology of the medial femoral condyle of DMM-operated joints (S = synovium). Thickened synovium from ω-6 and SFA mice with high density of infiltrated cells was observed (black arrows). The DMM-operated joints from ω-3 mice had significantly lower synovial inflammation than those from ω-6 mice. (E) F4/80+ macrophage IHC (red arrowheads) of each quadrant of the DMM-operated joint and its quantification (medial femoral condyle, MF; medial tibial plateau, MT; lateral femoral condyle, LF; lateral tibial plateau, LT). The ω-6 mice also exhibited high macrophage scores at medial side of the joint. Levels of serum (F) insulin, (G) leptin, (H) adiponectin, and (I) resistin at various time-points; (J) PGE2 and (K) the Active/Total TGF-β1 ratio were measured at 28 weeks of age. n = 11–14 mice/diet. * p < 0.05 for regression analysis. # p < 0.05, versus all the other diets. Different letters are significantly different, p < 0.05, from each other. (A and D) Statistical significance was determined by two-way repeated measures ANOVA using right (non-operated) joints as the contralateral control. (B) Statistical significance was determined by Kruskal-Wallis H Test, p = 0.07. (F–I) Statistical significance was determined by two-way repeated measures ANOVA using age and diets as factors, while (E, J and K) statistical significance was determined by one-way ANOVA using diet as the factor. ANOVA was then followed by Tukey’s post-hoc test. All data are presented as mean ± SEM.
Osteophyte formation
Osteophytes were present primarily in the operated joints (Table S3). SFA and ω-6 mice trended toward greater osteophyte severity than Control and ω-3 mice (Table S4 and Fig. 3B,). Osteophyte score also correlated positively with OA (Fig. 3C).
ω-3 PUFAs decrease synovitis in obese mice
Synovitis was determined by previously established grading scheme consisting of assessment of stromal cell density and lining layer thickness.[12] Compared to the non-operated joints, the DMM-operated joints of all diet groups had increased synovial lining hyperplasia (Fig. 3D). Operated joints of SFA and ω-6 mice exhibited a thicker synovium with a higher number of infiltrating cells than those of mice fed Control or ω-3 diets. The joints of ω-3 mice had less synovitis versus those of ω-6 mice.
Macrophage distribution in synovial tissue
At 12 weeks post-surgery, macrophages were still present within the synovium of the operated joints from all groups (Fig. 3E). However, for Control and ω-3 mice, macrophages appeared mostly in the synovial lining layer, while for SFA and ω-6 mice, macrophages were either scattered throughout the synovial stroma or were contained in the follicle-like lymphocytic infiltrates. The ω-6 mice exhibited increased frequency of macrophages in the synovium (Table S5). The ω-6 mice had the highest macrophage score in the medial side of the joint versus Control and ω-3 mice (Fig. 3E).
Serum cytokines
The SFA and ω-6 mice had the highest insulin concentrations versus Control and ω-3 mice at 23 weeks of age. Although the insulin levels of all mice decreased at 28 weeks of age (potentially due to the time of serum collection on the day of euthanasia was different from that of other time-points), ω-6 mice still exhibited higher insulin concentrations than Control mice (Fig. 3F). At 23 and 28 weeks of age, SFA and ω-6 mice had elevated leptin concentrations versus Control and ω-3 mice, while ω-3 mice exhibited higher adiponectin levels (Fig. 3G,H). Compared with other mice, ω-3 mice had lower levels of resistin (Fig. 3I). Surprisingly, ω-3 mice also exhibited the highest concentrations of prostaglandin E2 (PGE2) relative to other mice (Fig. 3J). While there were no differences in the active form of TGF-β1 among the mice, the ω-3 mice showed the highest latent TGF-β1 concentration (Fig. S3) but the lowest active-to-total TGF-β1 ratio (Fig. 3K). To investigate the relationships between each cytokines and OA, we performed bivariate models (Table S6) and found leptin and resistin had a positive association with OA.
The relationships among mechanical factors, metabolic factors, and OA
A potential confounding variable in evaluating the links between metabolic factors and OA is the effect of body mass. To control for body weight, we first examined weight-matched mice from each group. In weight-matched mice, SFA and ω-6 mice still demonstrated significantly higher OA severity than the mice fed the other diets, indicating that the link between OA and dietary FAs is independent of body mass (Fig. S4). Multivariate models were then performed to further validate these associations (Table 1). Only diet and metabolic factors leptin, and resistin, but not body mass (AUC4-28 or AUC17-28) were significantly associated with OA.
Table 1.
Multivariate regression analyses for variables predicting OA severity
| Parameters | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
|
|
|
|||||
| β | β | β | β | β | β | |
| Biochemical | ||||||
| Dieta | 0.60** | 0.58** | ||||
| Leptin | 0.37** | 0.31 (p = 0.08) | ||||
| Resistin | 0.29* | 0.29* | ||||
| Biomechanical | ||||||
| AUC4-28 | −0.03 | 0.02 | 0.23 | |||
| AUC17-28 | 0.01 | 0.12 | 0.29* | |||
|
| ||||||
| Whole model (r2) | 0.34** | 0.31** | 0.15* | 0.16* | 0.14* | 0.18* |
Ordinal variable: Control and ω-3 = 0, SFA and ω-6 = 1
β = standardized coefficient
p values less than 0.05 shown in bold.
p < 0.05,
p < 0.01
Behavioral activity
The data at 6 and 14 weeks of age were used to examine how diet affected changes in biomechanical and neurobehavioral functions of the mice. Results at 24 weeks of age were used to determine whether diets altered mouse activity levels after OA induction.
At 14 and 24 weeks of age, diet did not significantly influence voluntary activity or rotarod performance (Fig. 4A,B). To determine the effect of high-fat feeding, we combined all high-fat diet groups together and compared to the low-fat diet group. Six to 14 week-old mice subjected to high-fat feeding had lower motor function but maintained similar spontaneous activities versus Control mice (Table S7). With age, high-fat feeding decreased forelimb grip strength, but had no effect on hind-limb grip strength. (Fig. 4C,D). Rotarod performance was positively associated with forelimb grip strength, indicating that musculoskeletal strength is related to motor function (Fig. S5)[13].
Figure 4.

Diets supplemented with different types of FAs did not significantly affect (A) spontaneous locomotion activity, (B) rotarod performance, (C) forelimb and (D) hind-limb grip strength prior to and post-surgery. For nociception, diet significantly influenced (E) the tail flick but not (F) the hot plate latency. However, if mice fed the 60% kcal high-fat diets were pooled together and were compared to the mice fed the 10% kcal Control low-fat diet, high-fat feeding significantly decreased rotarod performance and forelimb grip strength (main effect, p < 0.05). Nonetheless, no effects on spontaneous locomotion activity were observed. n = 11–14 mice/diet. * p < 0.05, versus ω-6 mice. # p < 0.05, high-fat feeding versus age-matched low-fat feeding. For 6 and 14 weeks of age, statistical significance was determined by two-way repeated measures ANOVA using age and diet as factors. For 24 weeks of age, statistical significance was determined by one-way ANOVA using diet as factor. ANOVA was then followed by Fisher’s post-hoc test. All data are presented as mean ± SEM.
The effect of diet on thermal hyperalgesia was evaluated using the hot plate and tail-flick tests, which investigate nociceptive reflexes that are associated with supraspinal and spinal pathways, respectively (Fig. 4E,F).[14] There was no significant difference in the hot plate latency among the mice at any time-points. However, after 10 weeks of feeding, ω-3 and SFA mice had decreased tail-flick latency versus Control mice. At 24 weeks, a significant difference in tail-flick latency was observed between ω-6 and SFA mice, but not between other diet groups. High-fat feeding decreased tail-flick latency in the period from 6 to 14 weeks of age.
Using bivariate models, we examined whether activity levels correlated with weight gain or OA (Table S8). Spontaneous locomotion did not correlate with either weight gain or OA, while rotarod latency was negatively associated with weight gain but not with OA. Grip strength was negatively associated with weight gain, but only forelimb grip strength was negatively associated with OA. However, neither hot plate nor tail-flick latency correlated with either weight gain or OA.
ω-3 PUFAs accelerate wound repair in obese mice
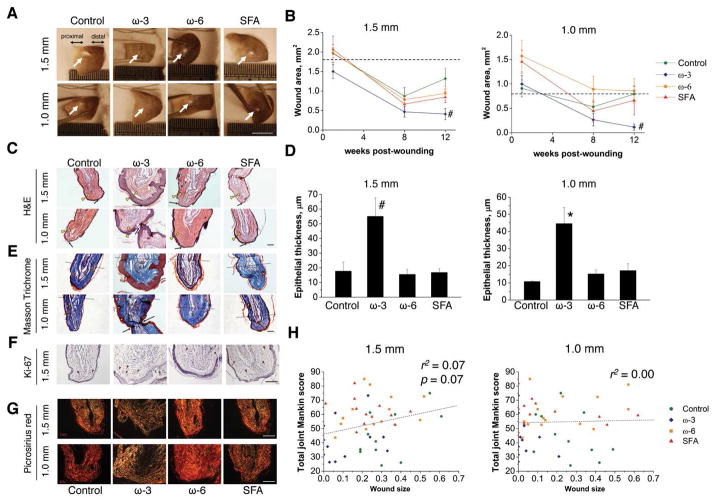
ω-3 mice had a significantly improved regenerative response (Fig. 5A,B), which was characterized by increased epithelial thickness (Fig. 5C,D). Three out of eleven ω-3 mice had complete epithelial fusion of the proximal and distal wound margins (Fig. S6). All the mice demonstrated some features of regeneration including re-epithelialization and formation of sebaceous glands (Fig. 5E). We next evaluated whether the cells in the wound margins were in a proliferative stage using Ki-67 marker; however, no obvious differences among the mice were observed (Fig. 5F). Picrosirius red staining was then used to investigate the matrix composition at the wound site. The ω-3 mice showed less collagen type I (COLI) deposition, while the other mice had densely packed COLI fibers (Fig. 5G). Ear wound size showed a trend toward positive association with OA in the 1.5-mm model (Fig. 5H).
Figure 5.
The ω-3 mice demonstrated enhanced ear wound healing capacity. (A) Representative images of ear hole (white arrows) at 12 weeks post-wounding. (B) The ω-3 mice had the smallest wound area as compared to the mice fed other diets. (C) H&E stained images showed that ω-3 mice had a thickened epithelium (yellow arrowheads). Black arrows indicated the wound edge of each sample. (D) ω-3 mice had significantly thicker epithelia as compared to other mice. (E) Masson’s Trichrome indicated that all mice exhibited healing features such as regeneration of sebaceous glands (yellow arrowheads) and new cartilage islands (green arrowheads); however, only the ω-3 mice had several new cartilage condensations. (F) No difference in cell proliferation marker (Ki-67) among the diets was observed. (G) Picrosirius red staining indicated that the ω-3 mice had less deposition of collagen type I fibers in the wound area, suggesting less scar formation. (H) Wound healing capacity exhibited a trend towards a negative relationship with OA severity in the more severe ear wounding (1.5-mm punch) model, but not in the less severe (1.0-mm punch) model. n = 11–14 mice/diet. (A) The scale bar = 5 mm, and for others images the scale bar = 100 μm. (B) Statistical significance was determined by two-way repeated measures ANOVA. * p < 0.05, versus all the other groups. (D) Statistical significance was determined by one-way ANOVA. * p < 0.05 and # p < 0.01, versus all the other groups. All data are presented as mean ± SEM.
Discussion
The findings of this study showed that a small amount of ω-3 PUFA supplementation was sufficient to mitigate the effects of obesity on injury-induced OA and to accelerate would repair. Conversely, SFA and ω-6 PUFA independently acted as a detrimental factor in OA, increasing osteophyte formation, HO, synovitis as well as increasing infiltration of macrophages into synovial tissue. By examining multivariate models and weight-matched mice from different diet groups, we found that OA was only associated with dietary content and serum levels of pro-inflammatory adipokines, but not with body mass or activity levels. Our results indicate that dietary and metabolic factors plays a more significant role than mechanical factors in the link between obesity and post-traumatic OA.
To investigate the specific effects of SFAs on OA, we maintained the same ω-6 to ω-3 PUFA ratio in the low-fat and SFA rich high-fat diet. We found that SFAs exacerbated OA as compared to a low-fat diet with the same PUFA ratio. This result is in agreement with several animal studies showing that a high-fat diet rich in SFA increases the severity of injure-induced arthritis.[10, 15] Although the specific effect of SFAs on chondrocytes is less well characterized, SFAs can activate synovial macrophages to secrete IL-1 and TNF-α that are involved in cartilage degradation.[16] The Western diet is characterized by a high ratio of ω-6 to ω-3 PUFAs.[17] While maintaining the same PUFA content but altering ω-6 and ω-3 ratio in PUFA rich high-fat diets, we found that the ω-6 mice developed severe OA and synovitis with elevated systemic inflammation. In contrast to ω-6 PUFAs, the beneficial effect of ω-3 PUFAs in OA and rheumatoid arthritis has been reported in animal models.[8, 18] However, most of these studies supplemented ω-3 PUFAs in regular chow (i.e., low-fat diet) and thus the investigation of ω-3 PUFAs in the context of a high-fat diet is a novel aspect of this study. Here we discovered that even a relatively small amount of supplementation (only 8% kcal of the energy provided), ω-3 PUFAs provided protective effects on arthritic changes of the joint, while reducing leptin and resistin levels. In addition, ω-3 mice had high levels of adiponectin.[19] The influence of adiponectin on chondrocytes is not fully understood, and some evidence suggests that adiponectin is associated with cartilage matrix breakdown.[20] Nevertheless, adiponectin may indirectly benefit cartilage by reducing inflammation through polarizing macrophages toward anti-inflammatory phenotypes.[21]
Despite having relatively higher AUC values than Controls, obese mice supplemented with ω-3 PUFAs showed similar OA scores as Control mice, suggesting that factors other than body weight are responsible for OA severity in this model. In examining weight-matched mice from different diet groups, or using multivariate models, we showed that OA was significantly associated with diet and pro-inflammatory adipokines, but it was not with body mass. These results emphasize the potential significance that systemic metabolic factors may play in exacerbating injury-induced OA.
Another significant finding was that ω-3 obese mice showed superior wound healing capacity. However, the cell proliferation marker did not differ among the diet groups, likely due to the fact that the skin wound of rodents enters the maturation phase 14 days post-injury,[22] with reduced Ki-67 expression.[23] The ω-3 mice also contained low levels of COLI fibers in the wounding area, suggesting less scar formation. In addition, adiponectin has been shown to accelerate wound repair by promoting keratinocyte proliferation.[24, 25] Furthermore, low ratio of active/total TGF-β1 in ω-3 mice may further prevent them from developing scar tissue because active TGF-β1 is involved in excessive matrix deposition.[26]
Interestingly, we observed that the healing capacity of the ear wound tended to be negatively associated with OA. These findings are consistent with a recent study demonstrated that ear wound closure and cartilage regeneration may share a common heritable genetic basis that is associated with OA severity.[27] Our findings suggest that the effects of diet may similarly reflect associations between wound healing and OA via epigenetic changes, potentially in the body’s stem cell populations.[28]
ω-3 mice exhibited a low incidence of mature osteophytes, potentially due to their low active/total TGF-β1 ratio systemically, as TGF-β1 is potent inducer of osteophyte.[29] Furthermore, ω-3 mice showed lower levels of HO, which is associated with surgical trauma during DMM. Tendon mineralization has been reported in patients with tendon rupture, and could be a cause of chronic pain.[30] Studies have indicated that PGE2, a lipid derivative from ω-6 PUFAs, enhances osteogenesis of tendon stem cells, providing a potential explanation for the greater HO in ω-6 mice. As anticipated, PGE2 concentrations were relatively higher in the ω-6 mice than those in Control mice, although not statistically significant. To our surprise, however, ω-3 mice had the highest PGE2 levels. The reason for this phenomenon remains unclear but may be related to their high adiponectin levels, as a recent study reported that adiponectin stimulates PGE2 production in cells in a dose-dependent manner.[31]
Although the whole body BMD did not differ among the obese mice, ω-3 mice had lower BMD and BV in the epiphyseal region of the both limbs. Increased subchondral bone density is a hallmark of OA, and an inverse relationship between osteoporosis and OA has been observed in humans.[32] Our observations of lower BMD, BV, and less HO in the joints of the ω-3 mice do not support the protective role of ω-3 PUFAs on bone measures in OA,[33] but are in agreement with a recent study demonstrating that mice with up-regulated adiponectin expression had decreased osteocalcin levels and displayed low-bone-mass phenotypes.[34] Nevertheless, further studies are required to determine the long-term effects of ω-3 PUFAs on bone metabolism in obese mice.
The fact that high-fat feeding did not alter spontaneous locomotor activity is consistent with our prior animal studies and those of others,[13, 35, 36] suggesting that weight gain in obese mice is not associated with lower voluntary activity or energy expenditure. In humans, a study conducted with lean and obese individuals indicates similar levels of spontaneous physical activity.[37] Furthermore, spontaneous locomotion was independent of OA in our obese model, consistent with studies showing that recreational activities do not contribute to knee OA in normal and overweight individuals.[38, 39]
Our results indicate that dietary FAs differentially influence the development of OA, contributing a more critical role in arthritic changes of the joint than does mechanical factor in obesity. The progress of OA and wound repair could be explained by regulation of obesity-associated inflammation (Fig. S7). Our findings have significant implications on the mechanisms of OA and wound healing, and provide a path toward clinical studies of dietary FA supplements to modify the course of OA.
Supplementary Material
Fig. S1. Study design. Animals received the prescribed diets at 4 weeks of age until the end of the study at 28 weeks of age. DMM surgery and ear punch were performed at 16 weeks of age (green). Behavioral measurements were assessed at 6, 14 and 24 weeks of age (brown). Sera were collected at 8, 12, 17, 23, and 28 weeks of age (red).
Fig. S2. Three out of 14 ω-6 mice had severe subchondral erosion through the medial tibial plateau on the DMM-operated joint. Ectopic bone formation (green arrowheads, and chondrogenesis and massive deposition of proteoglycans (yellow arrowheads) in the ligaments were also observed in these joints. (F) femoral condyle. (T) tibial plateau. (M) menicus. (OM) original location of menicus (black dashed line). (AB) abnormal fusion of cartilage, menisuc and subchondral bone. (GP) growth plate. (SB) remaining subchondral bone. (OS) osteophyte. (ACL/PCL) anterior/posterior cruciate ligament.
Fig. S3. (A) Active and (B) latent form of serum TGF-β1 measured at 28 weeks of age. The ω-3 mice had the highest levels of the latent TGF-β1 as compared to the mice fed other diets. n = 11–14 mice/diet. # p < 0.01, versus other diets. Mean ± SEM.
Fig. S4. Weight-matched strategy for analyzing the relationship between diet and OA severity (A) at 28 weeks of age. The mice whose weights were in the range of 31 to 45 grams were used for OA analysis. The line the box indicates median and the length of the box represents interquartile range. (B) Left (DMM-operated) to right (non-operated) joint OA score ratio of the weight-matched mice. The ω-6 mice had a significantly higher OA ratio score compared to the ω-3 and Control mice. Different letters are significantly different (p < 0.05) from each other. Mean ± SEM. n = 5–9 mice/diet.
Fig. S5. Rotarod performance showed a positive association with forelimb grip strength, suggesting musculoskeletal strength is associated with motor function.
Fig. S6. Representative images of complete ear wound fusion of ω-3 PUFA fed mice. (A) H&E staining reveals regenerated matrix between two developing cartilage ends (dashed line). Regenerative features including chondrogenesis (green arrowhead), adipogenesis (blue arrowhead), sebaceous gland formation (yellow arrowheads), and folliculogenesis (orange arrowhead) were observed in the restored tissue region (magnified in B and C). Scale bar = 100 μm
Fig. S7. Cytokine levels in the mice fed different diets and their potential effects on various disease conditions. The oval size corresponds to the concentrations of cytokines or the degree of disease/healing conditions. The solid line indicates a strong stimulus effect, while the dashed line represents weak induction. It is important to note that SFA and ω-6 mice still had different cytokine levels and disease scores. However, since these differences were minor, the mice fed the SFA and ω6 high-fat diets were grouped together in this diagram. OA: osteoarthritis; HO: heterotopic ossification.
Fig. S8: Representative images for osteophyte grading. 0 point = normal periosteal surface, 1 point = early stage (cells in the periosteum and synovial lining layer start to proliferate), 2 points = middle stage (cells in the periosteum and synovial lining layer show massive proliferation and hypertrophic chondrocytes can be observed), 3 points = mature stage (osteophyte integrated with the subchondral bone with the presence of bone marrow cavities). Scale bar = 100 μm
Table S1. Composition of experimental diets
Table S2. Incidence, bone volume and bone mineral density of heterotopic bone
Table S3. Incidence of osteophyte formation
Table S4. Osteophyte disease stage of joint
Table S5. Incidence of macrophages at the specific site of DMM-operated (left) joints
Table S6. Bivariate regression analyses for variables predicting OA severity
Table S7. The effect of low- and high-fat feeding on biomechanical and neurobehavioral functions
Table S8. Bivariate regression analysis for variables predicting biomechanical and neurobehavioral activity#
Table S9. PCR primers
Acknowledgments
The authors thank Mr. Steve Johnson of the Duke Orthopedic Research Laboratories for assistance with animal handling. This study was supported in part by NIH grants AR50245, AG15768, AR48852, AR48182, AG46927, a Taiwan GSSA graduate fellowship, the North Carolina Biotechnology Center, and the Arthritis Foundation.
References
- 1.Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):65–8. doi: 10.1038/nrrheum.2010.123. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Chaisson CE. Understanding the relationship between body weight and osteoarthritis. Baillieres Clinical Rheumatology. 1997;11(4):671–81. doi: 10.1016/S0950-3579(97)80003-9. [DOI] [PubMed] [Google Scholar]
- 3.Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis and rheumatism. 2009;60(10):2935–44. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of biological chemistry. 2007;282(48):35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 6.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1751–6. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pincus T, Koch G, Lei H, et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Annals of the rheumatic diseases. 2004;63(8):931–39. doi: 10.1136/ard.2003.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knott L, Avery NC, Hollander AP, Tarlton JF. Regulation of osteoarthritis by omega-3 (n-3) polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2011;19(9):1150–7. doi: 10.1016/j.joca.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Louer CR, Furman BD, Huebner JL, Kraus VB, Olson SA, Guilak F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis and rheumatism. 2012;64(10):3220–30. doi: 10.1002/art.34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins C, Kulseng B, King N, Holst JJ, Blundell J. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1609–16. doi: 10.1210/jc.2009-2082. [DOI] [PubMed] [Google Scholar]
- 12.Diekman BO, Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Current opinion in rheumatology. 2013;25(1):119–26. doi: 10.1097/BOR.0b013e32835aa28d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin TM, Fermor B, Huebner JL, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis research & therapy. 2010;12(4):R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langerman L, Zakowski MI, Piskoun B, Grant GJ. Hot plate versus tail flick: evaluation of acute tolerance to continuous morphine infusion in the rat model. Journal of pharmacological and toxicological methods. 1995;34(1):23–27. doi: 10.1016/1056-8719(94)00077-h. [DOI] [PubMed] [Google Scholar]
- 15.Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis research & therapy. 2011;13(6):R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid–induced insulin resistance. Journal of Clinical Investigation. 2006;116(11):3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & pharmacotherapy. 2002;56(8):365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang M-j, Wang L, Zhang Z-m, et al. Enhancement of the synthesis of n-3 PUFAs in fat-1 transgenic mice inhibits mTORC1 signalling and delays surgically induced osteoarthritis in comparison with wild-type mice. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2013-203231. [DOI] [PubMed] [Google Scholar]
- 19.Neschen S, Morino K, Rossbacher JC, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55(4):924–8. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 20.Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis research & therapy. 2011;13(6):R184. doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. The Journal of biological chemistry. 2010;285(9):6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabol F, Dancakova L, Gal P, et al. Immunohistological changes in skin wounds during the early periods of healing in a rat model. Veterinarni Medicina. 2012;57(2):77–82. [Google Scholar]
- 23.Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489(7417):561–5. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata S, Tada Y, Asano Y, et al. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. Journal of immunology. 2012;189(6):3231–41. doi: 10.4049/jimmunol.1101739. [DOI] [PubMed] [Google Scholar]
- 25.Salathia NS, Shi J, Zhang J, Glynne RJ. An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. The Journal of investigative dermatology. 2013;133(3):812–21. doi: 10.1038/jid.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. International Journal of Burns and Trauma. 2012;2(1):18. [PMC free article] [PubMed] [Google Scholar]
- 27.Rai MF, Hashimoto S, Johnson EE, et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis and rheumatism. 2012;64(7):2300–10. doi: 10.1002/art.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Diekman B, Jain D, Guilak F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fad pad: the effects of free fatty acids. International Journal of Obesity. 2012 doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakker A, van de Loo F, Van Beuningen H, et al. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis and Cartilage. 2001;9(2):128–36. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 30.Camillieri G, Di Sanzo V, Ferretti M, Calderaro C, Calvisi V. Patellar tendon ossification after anterior cruciate ligament reconstruction using bone--patellar tendon--bone autograft. BMC musculoskeletal disorders. 2013;14(1):164. doi: 10.1186/1471-2474-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusunoki N, Kitahara K, Kojima F, et al. Adiponectin stimulates prostaglandin E2 production in rheumatoid arthritis synovial fibroblasts. Arthritis & Rheumatism. 2010;62(6):1641–49. doi: 10.1002/art.27450. [DOI] [PubMed] [Google Scholar]
- 32.Zupan J, Van’t Hof RJ, Vindisar F, et al. Osteoarthritic versus osteoporotic bone and intra-skeletal variations in normal bone: Evaluation with microCT and bone histomorphometry. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(7):1059–66. doi: 10.1002/jor.22318. [DOI] [PubMed] [Google Scholar]
- 33.Reinwald S, Li Y, Moriguchi T, Salem N, Watkins BA. Repletion with (n-3) Fatty Acids Reverses Bone Structural Deficits in (n-3)–Deficient Rats. The Journal of nutrition. 2004;134(2):388–94. doi: 10.1093/jn/134.2.388. [DOI] [PubMed] [Google Scholar]
- 34.Kajimura D, Lee HW, Riley KJ, et al. Adiponectin Regulates Bone Mass via Opposite Central and Peripheral Mechanisms through FoxO1. Cell metabolism. 2013;17(6):901–15. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada-Goto N, Katsuura G, Ochi Y, et al. Impairment of fear-conditioning responses and changes of brain neurotrophic factors in diet-induced obese mice. Journal of neuroendocrinology. 2012;24(8):1120–5. doi: 10.1111/j.1365-2826.2012.02327.x. [DOI] [PubMed] [Google Scholar]
- 36.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis & Rheumatism. 2012;64(2):443–53. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. International journal of obesity. 1982;6(1):23. [PubMed] [Google Scholar]
- 38.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis and rheumatism. 2007;57(1):6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 39.Ageberg E, Engstrom G, Gerhardsson de Verdier M, Rollof J, Roos EM, Lohmander LS. Effect of leisure time physical activity on severe knee or hip osteoarthritis leading to total joint replacement: a population-based prospective cohort study. BMC Musculoskelet Disord. 2012;13:73. doi: 10.1186/1471-2474-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Study design. Animals received the prescribed diets at 4 weeks of age until the end of the study at 28 weeks of age. DMM surgery and ear punch were performed at 16 weeks of age (green). Behavioral measurements were assessed at 6, 14 and 24 weeks of age (brown). Sera were collected at 8, 12, 17, 23, and 28 weeks of age (red).
Fig. S2. Three out of 14 ω-6 mice had severe subchondral erosion through the medial tibial plateau on the DMM-operated joint. Ectopic bone formation (green arrowheads, and chondrogenesis and massive deposition of proteoglycans (yellow arrowheads) in the ligaments were also observed in these joints. (F) femoral condyle. (T) tibial plateau. (M) menicus. (OM) original location of menicus (black dashed line). (AB) abnormal fusion of cartilage, menisuc and subchondral bone. (GP) growth plate. (SB) remaining subchondral bone. (OS) osteophyte. (ACL/PCL) anterior/posterior cruciate ligament.
Fig. S3. (A) Active and (B) latent form of serum TGF-β1 measured at 28 weeks of age. The ω-3 mice had the highest levels of the latent TGF-β1 as compared to the mice fed other diets. n = 11–14 mice/diet. # p < 0.01, versus other diets. Mean ± SEM.
Fig. S4. Weight-matched strategy for analyzing the relationship between diet and OA severity (A) at 28 weeks of age. The mice whose weights were in the range of 31 to 45 grams were used for OA analysis. The line the box indicates median and the length of the box represents interquartile range. (B) Left (DMM-operated) to right (non-operated) joint OA score ratio of the weight-matched mice. The ω-6 mice had a significantly higher OA ratio score compared to the ω-3 and Control mice. Different letters are significantly different (p < 0.05) from each other. Mean ± SEM. n = 5–9 mice/diet.
Fig. S5. Rotarod performance showed a positive association with forelimb grip strength, suggesting musculoskeletal strength is associated with motor function.
Fig. S6. Representative images of complete ear wound fusion of ω-3 PUFA fed mice. (A) H&E staining reveals regenerated matrix between two developing cartilage ends (dashed line). Regenerative features including chondrogenesis (green arrowhead), adipogenesis (blue arrowhead), sebaceous gland formation (yellow arrowheads), and folliculogenesis (orange arrowhead) were observed in the restored tissue region (magnified in B and C). Scale bar = 100 μm
Fig. S7. Cytokine levels in the mice fed different diets and their potential effects on various disease conditions. The oval size corresponds to the concentrations of cytokines or the degree of disease/healing conditions. The solid line indicates a strong stimulus effect, while the dashed line represents weak induction. It is important to note that SFA and ω-6 mice still had different cytokine levels and disease scores. However, since these differences were minor, the mice fed the SFA and ω6 high-fat diets were grouped together in this diagram. OA: osteoarthritis; HO: heterotopic ossification.
Fig. S8: Representative images for osteophyte grading. 0 point = normal periosteal surface, 1 point = early stage (cells in the periosteum and synovial lining layer start to proliferate), 2 points = middle stage (cells in the periosteum and synovial lining layer show massive proliferation and hypertrophic chondrocytes can be observed), 3 points = mature stage (osteophyte integrated with the subchondral bone with the presence of bone marrow cavities). Scale bar = 100 μm
Table S1. Composition of experimental diets
Table S2. Incidence, bone volume and bone mineral density of heterotopic bone
Table S3. Incidence of osteophyte formation
Table S4. Osteophyte disease stage of joint
Table S5. Incidence of macrophages at the specific site of DMM-operated (left) joints
Table S6. Bivariate regression analyses for variables predicting OA severity
Table S7. The effect of low- and high-fat feeding on biomechanical and neurobehavioral functions
Table S8. Bivariate regression analysis for variables predicting biomechanical and neurobehavioral activity#
Table S9. PCR primers