Abstract
Gene therapy, which involves replacement of a defective gene with a functional, healthy copy of that gene, is a potentially beneficial cancer treatment approach particularly over chemotherapy, which often lacks selectivity and can cause non-specific toxicity. Despite significant progress pre-clinically with respect to both enhanced targeting and expression in a tumor-selective manner several hurdles still prevent success in the clinic, including non-specific expression, low-efficiency delivery and biosafety. Various innovative genetic approaches are under development to reconstruct vectors/transgenes to make them safer and more effective. Utilizing cutting-edge delivery technologies, gene expression can now be targeted in a tissue- and organ-specific manner. With these advances, gene therapy is poised to become amenable for routine cancer therapy with potential to elevate this methodology as a first line therapy for neoplastic diseases. This review discusses recent advances in gene therapy and their impact on a pre-clinical and clinical level.
Gene therapy in a global context involves correction of a genetic defect by introducing a normal version of a defective or missing gene thereby correcting an underlying disorder (Friedmann, 1992). Milestones on the path of developing gene therapies are presented in Figure 1. This concept was first advanced in the 1960s after observing that viruses could cause malignant transformation in cells by integrating their genetic information into the genomes of infected cells. In 1966, Edward Tatum proposed the use of viruses in the genetic manipulation of somatic cells and its possible therapeutic applications (Tatum, 1966). A few years later, initial proof-of-concept for gene therapy was demonstrated using tobacco mosaic virus as a vector to introduce a polyadenylate stretch to viral RNA (Rogers and Pfuderer, 1968). Encouraged by these results, gene therapy was attempted in the 1970s to correct a urea cycle disorder by administering wild type Shope papilloma virus, encoding the arginase gene, to two severely handicapped young girls suffering from hyperarginemia (Rogers et al., 1973; Terheggen et al., 1975). Unfortunately, the desired outcome was not achieved. The first successful therapeutic application of this gene therapy approach was evident in 1990 when a retrovirus-vector was used to mediate transfer of the gene encoding adenosine deaminase (ADA) into the T-cells of two children suffering from severe combined immunodeficiency (SCID) (Rosenberg et al., 1990). The response was positive for only one of the patients; however, debate arose since the patient simultaneously received enzyme replacement therapy alongside gene therapy. Another study was conducted in an 18-year-old patient suffering from ornithine transcarbamylase deficiency, a relatively mild form of nitrogen metabolism disorder. However, the application of gene therapy in patients was temporarily halted following the death of a patient due to vector-associated toxicity (Stolberg, 1999). In spite of the public backlash from this incident, in the last decades a growing body of evidences supported by positive human efficacy data confirmed that gene therapy could be used to correct certain debilitating conditions including Leber’s congenital amaurosis (Maguire et al., 2009), β-thalassemia (Cavazzana-Calvo et al., 2010), X-linked severe combined immunodeficiency (SCID-X1) (Hacein-Bey-Abina et al., 2010) and cancer (Wirth et al., 2013). Additionally, an increased understanding of the genetic basis of many diseases, improvement of vectors to minimize unwanted toxicity, advances in approaches for manipulating DNA expression and delivery in a target-specific manner has raised expectations that a clinical breakthrough may be imminent. As a consequence, there have been over 1800 clinical trials already conducted or currently ongoing worldwide (Wirth et al., 2013).
Fig. 1.
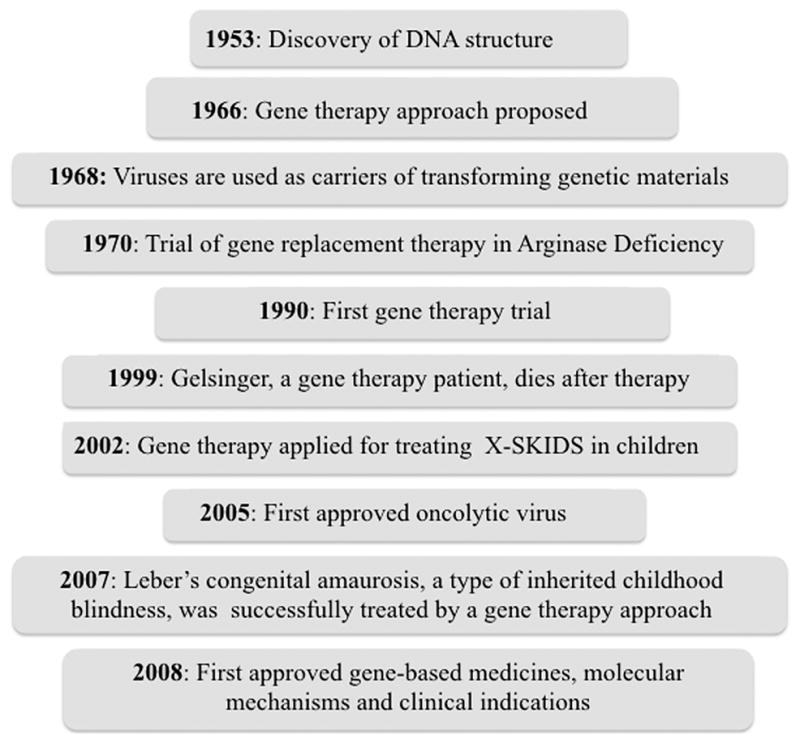
The timelines and milestones in developing gene therapy approaches: from conception to clinical applications.
Initially, gene therapy focused on rare (orphan) diseases mediated by detrimental monogenetic defects. However, with advances in the field, various chronic and progressive diseases such as heart failure, neurodegeneration or metabolic disorders were also evaluated using gene therapy approaches. These studies indicated that gene therapy techniques have broad potential applications, although cancer comprises over 60% of all ongoing clinical gene trials (Wirth et al., 2013). In this review, we focus on various gene therapy strategies that are currently employed, roadblocks and challenges in the field of cancer gene therapy, and a brief discussion of a few current successful applications of gene therapy for cancer.
Strategies in cancer gene therapy
Although the biological and clinical trial endpoints in monogenetic disease are well defined, technological and scientific advances over the past decades coupled with promising clinical data have made cancer one of the major disease targets for gene therapy. There are several prospective strategies currently being used for targeting cancer using gene therapy including: (a) expressing a gene to induce apoptosis or enhance tumor sensitivity to conventional drug/radiation therapy; (b) inserting a wild type tumor suppressor gene to compensate for its loss/deregulation; (c) blocking the expression of an oncogene by using an antisense (RNA/DNA) approach; and (d) enhancing the immunogenicity of the tumor to stimulate immune cell recognition. These strategies are briefly discussed below.
Deficient/defective apoptotic signaling stemming from mutations or imbalances in the expression of pro- and anti-apoptotic genes resulting in resistance to apoptosis in malignant cells provides the rationale for gene therapy to target the apoptotic machinery. Induction of apoptosis either by introducing genes encoding an inducer, mediator or executioner of apoptosis is among the most common approaches employed in cancer gene therapy (Lebedeva et al., 2003). TNF-related apoptosis inducing ligand (TRAIL) is an example of an apoptosis-inducer used in cancer treatment that has been found to kill a wide variety of tumor cells with minimal toxic effects on normal cells (Griffith et al., 2009). Melanoma differentiation associated gene-7 (mda-7), also known as Interleukin-24 (IL-24), a member of IL-10 gene family, is another example of an apoptosis-inducer, which selectively provokes apoptosis in various cancers without showing any detrimental effect on corresponding normal tissue (Fisher, 2005). Apoptosis executioner genes, like caspases are effective on cancer cells regardless of the status of the apoptosis machinery (Jia et al., 2012). Selective siRNA or microRNA-mediated silencing of anti-apoptotic genes in malignant cells represents a strategy to sensitize them to pro-apoptotic reagents or radiotherapy, which are frequently used therapeutic approaches in cancer gene therapy (Jia et al., 2012).
There is now overwhelming evidence based on familial, epidemiological and cytogenetic studies supporting the hypothesis that cancer is a genetic disease which develops via a multistage process in which inherited and somatic mutations in two classes of genes—proto-oncogenes and tumor suppressor genes play major contributing roles (Heredity and Cancer, www.cancer.org). A large number of tumor suppressor genes including p53 (which regulates cell cycle and apoptosis) (Matlashewski et al., 1984; Vogelstein et al., 2000), retino-blastoma gene Rb (which regulates cell cycle and differentiation) (Wiman, 1993), p16INK/CDKN2 (which regulates cell cycle), PTEN (which regulates cell survival) have been identified and numerous attempts have been made to deliver these genes specifically to cancer cells to restore normal functions (Shanker et al., 2011). In 1996, retroviral vectors expressing human p53 under the control of an actin promoter were used to treat non-small cell lung carcinoma (Roth et al., 1996). The biological activity of oncogenes can also be modulated and suppressed either at an RNA or DNA level and can be used for treating cancer. MYC, a transcription factor that regulates a variety of cellular processes is often deregulated in a wide range of human cancers (Henriksson and Luscher, 1996; Vita and Henriksson, 2006). Retardation in cell growth rate was achieved in melanoma cells treated with antisense oligonucleotides targeting the c-myc gene (Putney et al., 1999). Mutations in K-Ras, a member of the RAS gene family (H-Ras, K-Ras, N-Ras) commonly occurs in human colon cancers (about 40–60%) (Krens et al., 2010) and disruption of K-ras by antisense RNA leads to apoptosis and tumor growth suppression both in vitro and in vivo in animal models (Fleming et al., 2005). The apparent role of oncogenic microRNAs, a 20–22 small nucleotide that regulates specific genes post-transcriptionally, in tumor progression provides an entry point for gene therapy (Esquela-Kerscher and Slack, 2006). Recent advances in using antisense molecules to target oncomirs, which have been validated in both in vitro and in vivo animal studies, have established possible applications in the clinic (Liu et al., 2007; Broderick and Zamore, 2011).
Conversion of pro-drugs into active compounds to exert in situ cytotoxic effects by introducing genes that encode the converting enzyme is an effective approach in cancer gene therapy. The most common example of this approach is the delivery of Herpes Simplex Virus (HSV)-thymidine kinase (TK), followed by treatment with ganciclovir (Freeman et al., 1993). This approach has translated into the clinic and several Phase I clinical trials are now ongoing to treat patients with prostate cancer (Nasu et al., 2007) or malignant glioma (Colombo et al., 2005; Natsume and Yoshida, 2008). Development of chemoresistance during the therapeutic process is one of the major factors causing failure of many forms of chemotherapy (Gottesman, 2002). Unraveling the genetic basis and selectively silencing proto-oncogenes by gene therapy might help override such resistance. These approaches are well established on a pre-clinical level (Akada et al., 2005; Hegi et al., 2005; Modok et al., 2006), however technical innovations are necessary for effective translation into the clinic.
Despite the significant improvements made in targeting strategies, cancer gene therapy is still in its infancy, although during the past few years substantial successes have been achieved in other diseases such as severe immunodeficiency, lipid storage disorders, hereditary blindness, haemophilia B, hypercholesterolemia, etc. To appreciate the causes for this delay in achieving the full potential of gene therapy in the context of cancer, it is important to understand the challenges that impede success, and the approaches being used to overcome these impediments, which will be discussed in this review.
Challenges in gene therapy
To achieve the ultimate potential of gene therapy, that is, long-term therapeutic benefit or optimally a cure, a detailed understanding of the impediments to therapeutic intervention and developing approaches to circumvent these obstacles are essential. Optimal transgene expression for suppressing a cancer-associated gene or delivery of a cancer-therapeutic gene to diseased tissue at efficacious doses is a major barrier for successful cancer gene therapy. Identification of an appropriate therapeutic gene(s) capable of inhibiting disease progression will also influence success. Various approaches, which are discussed in the following sections, are being used to overcome these hurdles to effective gene therapy.
Optimal transgene expression
For efficacious gene therapy, it is essential to precisely regulate the expression of therapeutic transgenes based on clinical need and to also restrict any possible unwanted negative effects of the therapy. Additionally, it is important to switch gene expression off completely if adverse side effects should occur. Promoters and enhancers are two critical components that define the duration of and level of optimal transgene expression in specific cells or tissues. There are two types of promoters: constitutive and inducible. Constitutive promoters allow continuous transcription of its associated gene. Some constitutive promoters are tissue specific (Nettelbeck et al., 2000; Scanlon, 2004), such as tyrosinase for melanoma (Bentley et al., 1994), prostate specific antigen (PSA) for prostate cancer (Pang et al., 1995; Gotoh et al., 1998), while others retain functionality in a broad range of cancers, such as hTERT (Fujiwara et al., 2007) and progression elevated gene-3 (PEG-3) (Su et al., 1997, 2005b). Inducible promoters display transient expression and can be induced to express transgenes with hormones or small molecules that are provided exogenously. Both the specificity of a promoter and its’ strength are relevant in order to obtain sufficient expression of effector proteins. This may be achieved by using enhancers either from different genes or a single gene. Using this strategy, Nettelbeck and others have developed a construct where a 200 bp enhancer element was inserted upstream of pigment cell specific human tyrosinase promoter to target malignant melanoma (Nettel-beck et al., 2002). Using Prostate Specific Membrane Antigen (PSMA), an enhancer fragment placed upstream of the E1A transcription initiation site/TATA, generated a prostate cancer-specific adenovirus (Lee et al., 2002; Li et al., 2005). To limit expression in healthy cells, silencers with restricted expression in normal cells, can be used to maintain the vector in an inactive state in healthy normal cells (Xie et al., 2013). However, the regulation of transgene expression may not be required for every gene and is dependent on the nature of the encoded product and the cell’s requirements. For example, a number of clinical trials have been conducted with Adenoviruses expressing p53 with very limited side effects because of non-toxic effects when overexpressed in healthy normal cells. However, inducible controlled systems become preferable to enhance the intensity of expression over constitutive expression particularly when delivering a potentially toxic gene product.
Delivery of therapeutic genes
The clinical utility of any treatment modality is dependent on safety and efficacy. Tumor/target organ-restricted therapeutic gene expression provides a well-suited approach to maximize therapeutic outcomes. Accordingly, targeted delivery is one of the major goals for successful applications of gene therapy. There are two main targeting strategies. The first is physical targeting that allows gene delivery using catheters, gene guns, etc. These topics are extensively described (for example in Jain, 2009) and will not be discussed in this review. The second strategy uses viral or non-viral carriers of genes that are modified in such a way that they can selectively bind to tumor cells and not normal cells. In this review, we will discuss some of the strategies that are commonly used to achieve cell-specific targeting. In addition, we will also provide an overview of the approaches that are currently being evaluated in pre-clinical animal models and which hold promise for future potential clinical applications.
Viral-based gene delivery
Viral-based gene delivery provides one of the most efficient means of gene transfer relative to other current methods. However, toxicity of viral proteins to normal tissues, the possibility of random integration, host-induced neutralizing antibody or tissue-inflammation against viral vectors and adenovirus-associated liver toxicity are some challenging issues that compromise successful gene transfer (Larocca and Schlom, 2011). On the positive side, the robust expression of transgenes in diseased tissues, availability of methods for large-scale preparation, and comparative ease of genetic manipulation to make the vectors safe and non-immunogenic make them suitable for therapeutic applications. Adenoviruses (Ad), Retroviruses, Adeno-associated viruses (AAV) and Lentiviruses are the most commonly used therapeutic viral vectors, with specific advantages and disadvantages (reviewed in Das et al., 2012) as briefly described in Table I. The choice of a particular vector depends on various factors including packaging capacity, host specificity, gene expression profile, and tendency to elicit immune responses particularly when repeated administrations are required (reviewed in Das et al., 2012). In general, viral vectors fall into one of two main categories: integrating vectors (such as Retroviral and Lentiviral vectors), which insert into the recipient’s genome; and non-integrating vectors (Adenoviral vectors and Adeno-associated vectors). As, Ads are among the most popular viral vectors used for gene therapy (Jia et al., 2012), our review will focus on recent progress made in the area of Ad gene therapy.
TABLE I.
Overview of general characteristics of viral vectors commonly used in cancer gene therapy
| Virus | Family | Genome/insert size/particle size/envelope | Advantages | Disadvantages |
|---|---|---|---|---|
| Adenovirus | Adenoviridae | dsDNA 36 kb/7.5 kb/90 nm/No | Infects dividing and non-dividing cells; low toxicity to host cells; efficient gene transfer; high-viral titers achievable. | Small insert size; immune response decrease infectivity of desired target cells. |
| Herpes simplex virus | Herpesviridae | dsDNA, 152 kb/40–50 kb/120–200 nm/Yes | Infects dividing and non-dividing cells; prolonged gene expression achievable; large insert size for transgene; high-viral titers achievable; sensitive to acyclovir/ganciclovir. | Immune response of desired target cells; potential for herpes encephalitis. |
| Retrovirus | Retroviridae | ssRNA, 7–1 kb/8 kb/10 nm/Yes | High-transduction efficiency: easy to design; chronic infection; integration into host genome resulting in prolonged gene expression; viral proteins not expressed in the host, thereby limiting the chances of reconstitution of replication-competent viruses. | Infects dividing cells only; low titers; random site of integration; possibility of latent diseases, including cancer and immune-mediated diseases. |
| Adeno-associated virus | Paroviridae | ssDNA, 5 kb/5 kb/20 nm/No | Infects dividing and non-dividing cells; lack of pathogenicity; nontoxic to host cells; stable integration into the host cells at a specific site in human chromosome 19. | Low insert size; provokes humoral immunity on infection; large-scale production is labor intensive. |
Reprinted from Das et al., 2012, with permission from Elsevier.
Vector-associated immune responses: Overcoming immune barriers
A major hurdle for viral vectors, including Ads, is pre-existing immunity to specific Ad serotypes in the human population either due to natural infection or vaccination. Pre-existing antibodies towards wild type Ad viral vectors are problematic for systemic administration. However, this problem can potentially be overcome by using a heterologous prime-boosting regimen, an approach where similar antigens are delivered through different vectors (Fournillier et al., 2013). Additionally, neutralizing antibody responses following repeated viral vector administrations can also reduce therapeutic efficiency (Kaufmann and Nettelbeck, 2012; Choi and Chang, 2013). This problem can be ameliorated by temporary immunosuppression (Ungerechts et al., 2007). In an alternative approach, use of Ad capsid chimeras, developed by pseudotyping the adenoviral type 5 (Ad.5) vector with fibers from less-immunogenic Ads, such as Ad serotype 45, may be employed to reduce serological recognition (Parker et al., 2009). In addition to humoral responses, virus-specific memory immune reactions are also evident in most individuals as a result of previous exposure to the peptide H910–924, a major capsid protein, which is highly conserved in different Ad serotypes, and might hamper the efficacy of Ad-based therapies (Olive et al., 2002; Tang et al., 2004). Hexon chimeric vectors generated via replacement of immunodominant hypervariable regions (HVRs) have been developed and the immunogenicity and safety of these vectors are currently under investigation (www.Clinicaltrails.gov; NCT00695877).
Another strategy to ablate the innate immune response to vectors is by using synthetic polypeptides such as polyethylene glycol (PEG) incorporated in the capsid, which reduces vector uptake by tissue macrophages and Kupffer cells (Abel et al., 2009). Similar to PEGylation, coating with polylytic-glycolic acid (PLGA), poly-[N-(2-hydroxypropyl) methacrylamide], has also been shown to de-target the Ads and reduce host-vector interactions. Complement activation by viral particles is one of the barriers for viral-mediated gene delivery. Incorporation of host proteins within membranes helps to prevent complement activation (Vanderplasschen et al., 1998). The secretion of viral proteins such as Vaccinia complement control protein (VCP) binds and inactivates C4b and C3b, thus inhibiting the classic and alternative pathways of complement activation. Pushpakumar and colleagues have successfully used VCP to perturb complement activation and established the potential of using VCP in combination with other vectors to block complement activation (Pushpakumar et al., 2011). Shielding Ads with polyethylene glycol or genetic modification to express soluble CD59 are used to prevent deposition of the membrane-attack complex (Gandhi et al., 2011).
Initially, the first generation of therapeutic Ads was developed by deleting the early E1 genes (rendering the virus replication deficient) without or with partial E3 deletion (Das et al., 2012). To make these Ads non-immunogenic or less immunogenic, the next generation of “gutless” Ad vectors have been created by removing all viral coding sequences, which showed reduced cytotoxic T-lymphocyte and/or cytokine-mediated inflammatory responses (Alba et al., 2005). Use of organ-specific promoters that reduce expression of the transgene in professional antigen presenting cells (APCs) may also be helpful to minimize inflammatory responses (Nayak and Herzog, 2010b).
Other vector systems such as Lentiviruses and Adeno-associated viruses are inherently less inflammatory and immunogenic than Ads (Thomas et al., 2003). Indeed, T-cell responses may be elicited against the transgene product, in particular when the vectors transduce cells that are robust for antigen presentation, including dendritic cells. However, the degree of the response depends on the target organ, route of administration and dosing schedule. Several immune suppression protocols, such as the prophylactic use of an immunosuppressant drug such as rapamycin in combination with IL-10 (Nayak et al., 2009) have been successfully used in various in vivo animal models to block immune responses to the transgene product. Similar to Ad vectors, use of specific promoters also reduces the risk of immune responses to transgene products (Wang et al., 2006). Incorporation of several repeats of a target sequence is highly effective in blocking transgene expression in professional APCs (Nayak and Herzog, 2010a).
Clinically, the severity and risk of eliciting harmful side effects as well as failure to produce anticipated biological activity is intimately connected with target-specificity of vectors. Indeed, during natural infection, wild type viruses are restricted to particular organs, however, recombinant viruses are not subject to similar physical restraints when systemically delivered. Even following local administration, viral vectors may disseminate to other tissues due to the leaky vasculature of tumor tissue and promote undesirable outcomes. In this context, emphasis has been placed on developing approaches to restrict the vectors to the target site either by modifying the viral vector (Transductional or Transcriptional targeting) or by strategies designed to enhance delivery.
Targeted Transduction: Reducing the risk of non-specific delivery
Generally, virus-mediated gene therapy relies on high-affinity binding of viral fiber proteins with strain/serotype-specific host receptors. Low levels or absence of receptor expression in target tissue results in reduced infection efficiency. Additionally, one of the safety concerns for using viruses as vectors are their natural tropism that results in the uptake of therapeutic genes by non-targeted cells (Beatty and Curiel, 2012; Curiel and Fisher, 2012). To overcome these limitations, transductional retargeting, which involves modification of viral surface proteins to express particular ligands that bind to receptors preferentially or exclusively expressed on tumor cells, is a common practice in viral-mediated gene therapy. For example, Ad5 vectors, the most commonly used serotype in the clinic, employ coxsackie and adenovirus receptors (CAR) (Philipson and Pettersson, 2004; Coyne and Bergelson, 2006) to infect target cells, which are not well-expressed in human cancer cells thereby reducing efficacy of a variety of cancer therapeutic strategies. To ensure CAR-independent cell entry, tropism-modified Ads have been engineered by inserting an integrin-binding RGD (Arg-Gly-Asp) motif on their HI loop. An enhanced transductional efficacy (2-to 3-orders of magnitude) and gene delivery was observed in ovarian cancer cell lines and primary tumors (Dmitriev et al., 1998) suggesting possible utility of this Ad in the clinic. In a different strategy, a chimeric adenovirus vector was developed containing the non-CAR targeting serotype Ad3 fiber proteins incorporated in an Ad5 capsid (Krasnykh et al., 1996). The resulting virions (Ad.5/3) have shown great transductional efficacy in a variety of Ad.5-refractory cell types with low CAR expression including melanoma, ovarian cancer, renal cancer, squamous cell carcinoma, glioma, prostate, pancreatic and colorectal cancer (Haviv et al., 2002; Kanerva et al., 2002; Kawakami et al., 2003; Rein et al., 2005; Dash et al., 2010b; Eulitt et al., 2010; Hamed et al., 2010; Dash et al., 2011a; Park et al., 2011; Azab et al., 2012; Hamed et al., 2013a,b; Sarkar et al., 2013; Azab et al., 2014) and enhanced cytopathogenicity towards primary melanoma cells (Volk et al., 2003; Rivera et al., 2004). To prevent CAR-dependent native tropism while simultaneously achieving target-specific infection, Kashentseva et al. (2002) engineered Ads expressing a bi-specific adapter protein comprised of sCAR (soluble CAR) and single-chain fragment variable region (scFv) against the c-erbB-2 oncoprotein, highly expressed in breast cancer. The resulting protein in addition to limiting Ad infection to CAR-expressing cells also binds to cellular c-erbB-2 oncoprotein and therefore, enables Ad-targeting via a CAR-independent pathway. However, cancers do not comprise a homogenous population. As such, targeting a single cellular surface receptor may not achieve maximum benefit. Thus, developing Ad-targeting strategies that utilize multiple ligands within the same virion is clearly advantageous. One of the first vectors reported was an Ad.5 containing both an RGD motif and a polylysine ligand, which targets both cell surface integrin and heparin sulfate proteoglycans (Wu et al., 2002). Following that scheme, using multiple fibers within the same Ad virion, Pereboeva et al. (2004) developed an Ad vector composed of both a wild-type fiber and a knob-less fiber (fibritin) presenting a 6-His motif, which showed both CAR- and artificial 6-His receptor-specific gene transduction. Insertion of a cellular-specific targeting peptide (such as SY-GYLPLP, vascular endothelial cell-targeting peptide) or “affibody,” a small antibody mimetic, in the HI loop has also provided increased transduction in a variety of cancer cell lines. To target epithelial cell adhesion molecule (EpCAM), present in tumor cells, a neutralizing anti-fiber antibody conjugated to an anti-EpCAM antibody-expressing Ad was developed (Haisma et al., 1999). This dual-specific antibody successfully mediated gene transfer to primary human colon cancer cells (Haisma et al., 1999).
Non-specific uptake of viral vectors by the liver and spleen is a principal problem resulting in a reduction of therapeutic virus availability, when viruses are delivered systemically. With respect to Ads, up to 90% of Ad.5 is sequestered from the blood by Kupffer cells in the liver, thus reducing the systemic availability of Ads. To avoid uptake by Kupffer cells, pretreatment with warfarin followed by multiple doses of replication-defective Ads is helpful and this strategy enhanced the antitumor efficacy in pre-clinical animal models (Shashkova et al., 2008). Development of liver-off profile by modifying the Ad5 hexon with Ad serotype 48 hexon, which only weakly binds factor X, is another way to reduce liver uptake (Zhang et al., 2011).
Tumor-specific expression by transcriptional targeting
The most important consideration when using viral vectors to deliver transgene(s) is to ensure tumor-specific expression. In the context of oncolytic viruses, this property needs to be strictly regulated. To achieve this objective, a number of distinct strategies are currently being evaluated (reviewed in Bhatia et al., 2013). Utilization of tumor- or cancer- selective promoters that restrict expression to specific cellular subsets is an approach currently being evaluated in vitro and in vivo. The critical step for this approach is to identify an appropriate tumor-selective/specific promoter. Recent advances in molecular biology and bioinformatics including sequence analysis tools and database searches have proven useful in identifying such promoters.
Currently, various promoters are being used to maintain viral replication or viral-mediated gene expression in a tumor-selective manner. For example, prostate-specific antigen (PSA) promoter/enhancer element was inserted upstream of E1A to create a prostate-specific oncolytic virus (Rodriguez et al., 1997). Human telomerase reverse transcriptase (hTERT) expression and strong promoter activity is observed in different cancer cells, which rationalize the use of the hTERT promoter to generate replication-competent recombinant viruses (Fujiwara et al., 2007). These viruses show restricted replication in telomerase-positive tumor cells and efficiently lyse these target tumor cells. The use of hTERT for tumor-selective gene silencing was also reported in various cancer cells (Huang et al., 2003; Kim et al., 2003; Lanson et al., 2003; Wirth et al., 2003; Irving et al., 2004; Fujiwara et al., 2011). A limitation of this approach is that some tumors do not express hTERT where this promoter-transduction effect is not evident. Moreover, the survivin promoter for glioma (Van Houdt et al., 2006), the β-catenin responsive promoter for colorectal and liver cancer (Fuerer and Iggo, 2002), and the tyrosinase promoter for melanoma (Nettelbeck et al., 2002) are a few examples of promoters that have been used to drive viral replicating genes in specific cancers. Similarly, a cyclooxygenase-2 promoter driven oncolytic virus was used to target cervical, ovarian and pancreatic cancer cells (Hoffmann and Wildner, 2006). Other cancer-selective promoters such as MUC1/DF3 (Fukazawa et al., 2010), alpha-fetoprotein (AFP) (Hallenbeck et al., 1999), and squamous cell carcinoma antigen-2 to target squamous cell carcinoma (Hamada et al., 2010), glial fibrillary acidic protein promoter for GBM (McKie et al., 1998) were also validated at the pre-clinical level and found to be effective in diverse animal models.
Another strategy for optimizing tumor selectivity for oncolytic viruses is to modify gene functions that are critical for viral replication in normal cells, but not required in tumor cells. Wild type Ad contains E1B-55kd gene, which is responsible for p53-binding and p53-inactivation. A mutant Ad with deletion of E1B-55kd (dl1520) was generated to facilitate viral replication in tumor cells where p53 activity is compromised (Bischoff et al., 1996). Although this modification showed promising effects, recent findings demonstrated that loss of E1B-55kd-mediated late viral RNA export, rather than p53 inactivation, is important for restricting viral replication in normal cells (O’Shea et al., 2004).
Although target-specific oncolytic viruses have shown efficacy in clinical trials, the addition of a second therapeutic arm with a therapy agent in the same virus has shown additional significant clinical benefit. Ad.TKRC, an E1B-55k deleted Ad with HSV-TK, showed a better outcome in a colon xenograft model in the presence of ganciclovir (Wildner et al., 1999). Various structural permutations and combinations with diverse therapeutic agents were developed and validated for their efficacy in preclinical studies. Development of a Cancer Terminator Virus or CTV (Fisher, 2005; Sarkar et al., 2005a,b, 2006a,b, 2007b, 2008; Greco et al., 2010; Das et al., 2012; Hamed et al., 2013b; Sarkar et al., 2013; Azab et al., 2014), is an example of this approach. In one type of CTV, the Ad employs the rodent promoter from progression elevated gene-3, PEG-Prom (Su et al., 1997), to regulate oncolysis and simultaneously express a cancer-specific therapeutic cytokine gene, mda-7/IL-24 (Jiang and Fisher, 1993; Jiang et al., 1995). Significant therapeutic potential of the CTV was established in various cancer models including, breast (Sarkar et al., 2005a), prostate (Sarkar et al., 2007b; Azab et al., 2014) and melanoma (Sarkar et al., 2008) supporting its broad potential applications for treating human cancers. To obtain improved viral transduction and anti-tumor efficacy, a serotype chimera CTV, Ad.5/3-CTV, was developed and shown to have enhanced activity in low CAR human tumor cells, while retaining high activity in high CAR human tumor cells. The enhanced activity of the Ad.5/3-CTV was documented in human xenograft studies in nude mice employing human prostate (Azab et al., 2014), pancreatic (Sarkar et al., 2013) and GBM (Hamed et al., 2013b) tumor cells. In a complementary strategy, a human interferon gamma-producing CTV was generated in an Ad5 background and found to have profound activity against therapy-resistant human pancreatic cancer cells using an in vivo nude mouse human pancreatic cancer xenograft model (Sarkar et al., 2005b).
Delivery of viral vectors: Need for safety and efficiency
Even with considerable success in retargeting vectors for enhanced activity in tumor cells, non-specific uptake by other organs such as the liver or spleen can still result in unwanted side effects or loss of efficacy, principally when the viruses are delivered systemically. Vector neutralization, nonspecific adhesion, sequestration, and inability of vectors to locate tumor cells behind endothelial-matrix barriers are some common factors that limit positive clinical outcomes. Considering these impediments, appropriate methods are required that allow delivery of an effective dose into diseased tissue. However, this is challenging, particularly for organs that are potentially inaccessible for localized catheter-based methods or operative bed injections (Howard et al., 2006). In such cases, systemic administration is the only option for efficient target site delivery.
Microbubbles (MBs), small gas-filled microspheres used as contrast agents for ultrasound, provide a safe method for delivering therapeutic agents, including viral vectors, transgenes, inhibitory siRNA/shRNA and therapeutic proteins, into target cells (Lawrie et al., 2000; Ng and Liu, 2002; Larina et al., 2005). MBs are filled with a heavy-molecular-weight gas such as perflurocarbon or sulfur hexafluoride, which decreases their solubility, thus improving longevity and allowing passage through the microcirculation. MBs are metabolically inert, i.e., they do not cause an immune response in the host, and since viral vectors/transgenes/drugs are inside the bubbles this strategy permits the “stealth” delivery of therapeutics systemically. Moreover, ultrasound exposure generates microstreams or microjets, resulting in shear stress to cells thereby intensifying cellular permeability and enhancing transfection/transduction efficiency. Howard et al. (2006) initially evaluated the feasibility of microbubbles for site-specific gene delivery using an Ad vector carrying GFP marker gene in an in vivo system. Further studies experimentally confirmed that this approach could also be used to specifically deliver therapeutic viruses in both xenograft and spontaneous tumor models (Greco et al., 2010; Dash et al., 2011a).
MB size and the amplitude of ultrasound are two critical parameters that influence the behavior of MBs. The size of MBs should be within a range of 2.5 to 4 μm in diameter with adequate carrying capacity to entrap the viral vectors and prevent entrapment within the pulmonary capillary bed. The frequencies employed need to be optimized based on the target organ in order to obtain enhanced transfer efficiency and to avoid tissue damage. Recently, an approach was developed to generate target-specific MBs or decorated MBs by addition of ligands to the surface of the MBs, usually an antibody via a biotin-streptavidin spacer or direct chemical coupling, for specific receptors of target tissues or organs (Ellegala et al., 2003; Anderson et al., 2010). For example, to target the tumor vasculature, single-chain VEGF molecules attached to the MB surface assures successful targeting to the tumor vasculature that overexpresses VEGFR2. The surface molecule VCAM-1, is also used to achieve targeted imaging in murine tumor models in vivo. The utility of this decorated MB to deliver therapeutic viruses is currently being evaluated (Unpublished data; Fisher lab, VCU).
Other vectors
Mesenchymal stem cells (MSCs), usually isolated from human bone marrow or adipose tissue are an alternative delivery vehicle for tumor-targeted gene therapy. Ease of generation, high proliferative capacity and better homing capacity to the tumor microenvironment are a few advantages of MSC-mediated gene delivery. Studeny et al. (2002) used hMSCs as vehicles to deliver Interferon-β to treat melanoma xenograft model. Subsequently, diverse therapeutic genes including apoptosis inducing agent TRAIL, chemokines CXCL1, iNOS, HSV-TK, and mda-7/IL-24 have been engineered into MSCs to allow a targeted release in models of melanoma, breast cancer, glioblastoma, cervical cancer and prostate cancer, and have shown efficacy in inhibiting local tumor growth, suppressing metastasis and prolonging animal survival (Chan and Lam, 2013). Along with MSCs, neural stem cells (NSCs) also have been considered as vehicles for delivery of anticancer therapeutics (Hingtgen et al., 2012). Although remarkable progress has been made at the pre-clinical level, many questions remain before this approach becomes clinically applicable. Lack of a suitable method for ex-vivo expansion and poorly understood in vivo kinetics restricts MSCs and NSCs as a general platform for gene delivery.
Other methods for gene delivery
Despite accumulating data on improved viral vectors, issues related to insertional-transformation associated with the use of integrating vectors or viral-associated immunogenicity and toxicity have raised safety concerns in the clinic. Alternatively, chemical methods have attracted considerable attention in the area of gene therapy. These approaches have numerous advantages over their viral counterparts such as greater control of molecular composition, flexibility in the size of the transgene to be delivered, relatively lower immunogenicity and ease of generation. In this system, purified DNA is delivered with a complex with either cationic lipids (Lipoplexes) or cationic polymers (Polyplexes) and due to the anionic nature of the plasma membrane of mammalian cells, the complex is absorbed via electrostatic interactions. The efficient packaging of DNA before delivery into target cells is the first step for this process. Protonation is a common method of increasing the net positive charge of cationic polymers that facilitates DNA binding. However, strong binding does not ensure higher efficacy. Maintaining the natural DNA structure is critical during packaging. Serum stability, endocytosis by cells, endosomal escape, dissociation from lipo/polyplexes and nuclear internalization are other key factors that influence this approach. Among various cationic polymers, such as diethylaminoethyl dextran, poly(l-lysine) (PLL), gelatin, polyamidoamine dendrimer, polybrene, poly(vinyl imidazole), poly(l-histidine-g-poly(l-lysine)), poly(β-amino ester), and chitosan that are commonly used for gene delivery purposes (Aied et al., 2013), polyethyleneimine (PEI) is one the most potent polymeric vectors because of its high pH-buffering capacity and endosomal escape abilities (Boussif et al., 1995; Aied et al., 2013). A number of next-generation PEIs with degradable cross-linkers for intracellular degradation, hydrolysis at low endosomal pH, enzymatic degradation, and cytosol-specific reduced degradation by glutathione have been developed. These agents displayed high transfection efficiency and low cytotoxicity as a result of their rapid in situ degradation of the polymer into small molecular weight and water-soluble fragments, which are processed easily and removed by the cells.
Binding to serum albumin and other proteins owing to the aggregation and accumulation of the complexes in fine capillary beds and clearing by phagocytic cells is an unwanted consequence of the chemical approach (Dash et al., 1999). Modification by addition of hydrophilic monomers/polymers such as PEG prevents aggregation of the complexes, increases complex stability and improves biocompatibility (Lai et al., 2012). To confer tissue-specificity, targeting-moieties, such as sugars, antibodies, peptides, and folates are conjugated to the complex. For instance, attachment of amino galactose to chemically modified poly β-amino esters results in a carrier having a ligand which targets the asialoglycoprotein receptor expressed on the surface of HepG2 cells (human hepatocarcinoma) and liver cells (hepatocytes) (Li et al., 2008).
Presently, as a chemical-based vector, gold nanoparticles, a non-oxidized state of Au (Aurum), which exhibits unique physiochemical properties including surface plasmon resonance (SPR), ability to bind amine and thiol groups, allowing surface modifications represent superior targeted transfection efficiency (Jain et al., 2012; Ahmad et al., 2013; Vigderman and Zubarev, 2013; Conde et al., 2014). Easy excretion through urine, insignificant toxicity on cells, excellent stability in the circulation, high gene carrying capacity represent some of the major advantages (Delong et al., 2010; Jain et al., 2012). Although significant success in both in vitro cell culture and in vivo pre-clinical studies have been reported (reviewed by Jain et al. (2012); the application of nanoparticles remains relatively unexplored in clinical trails. CYT-6091, a citrate coated gold-nanoparticle carrying TNF-alpha (Paciotti et al., 2004; Farma et al., 2007; Goel et al., 2009) was used in an early phase clinical trial, which showed only partial response in one patient. Further enhancement in therapeutic approaches either by combination with a second clinical modality or using better therapeutics is under consideration for future applications of nanoparticles.
Identification of therapeutic genes
For successful gene therapy, selection of an appropriate therapeutic gene to maximize therapeutic efficacy while minimizing toxicity is critical. The therapeutic gene families presently being utilized in clinical trials are listed in Table II. Currently, research is focused on identifying novel genes that are differentially expressed in cancer cells and potentially regulate transformed properties. In this respect, cancer genomic data is a powerful tool to distinguish molecular changes in cancer cells. In these contexts, the ability to perform comprehensive molecular profiling of tumors, which facilitates the identification of target genes (Chin et al., 2011), has potential to identify novel targets for potential therapeutic intervention. Indeed, large-scale genome characterization and appropriate data interrogation are frequent bottlenecks of this approach. However, improvements in high-throughput technologies (Kircher and Kelso, 2010), such as whole-genome sequencing and array-based gene expression profiling (Sokhi et al., 2013, 2014), are improving the quality of the information generated and when combined with new computational tools is leading to meaningful and interpretable data. Additionally, the Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo/), which is a repository of high-throughput gene expression data for various types of cancers, provides a resource with potential to lead to the discovery of novel genes for cancer treatment.
TABLE II.
Different gene types delivered in gene therapy clinical trials
| Gene type | % of Gene therapy trials |
|---|---|
| Antigen | 20.5 |
| Cytokine | 18.4 |
| Tumor suppressor | 8.3 |
| Suicide | 8.1 |
| Deficiency | 8 |
| Growth factor | 7.5 |
| Receptor | 7.2 |
| Replication inhibitor | 4.3 |
| Marker | 2.9 |
| Other categories | 14.7 |
MDA-7/IL-24: A potential “magic bullet” for multiple cancers
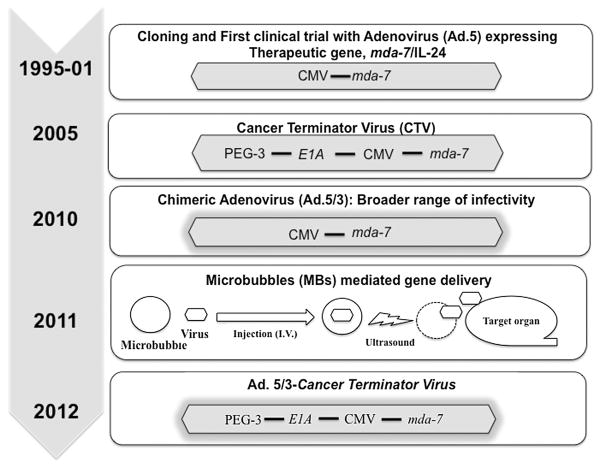
As the process of cellular de-differentiation is important in oncogenesis (Leszczyniecka et al., 2001), it was postulated that genes upregulated during reactivation of defective differentiation programs might display therapeutic potential. Based on this hypothesis, Jiang and Fisher (1993) used subtraction hybridization to define genes upregulated when cancer cells, specifically melanoma cells, were induced to lose cancerous potential and terminally differentiate. Using this strategy, Jiang et al. (1995) identified and cloned melanoma differentiation associated gene-7 (mda-7) as a gene whose expression was upregulated following growth arrest and terminal differentiation in HO-1 human melanoma cells following treatment with recombinant fibroblast interferon and the protein kinase C activator mezerein (Fisher et al., 1985). During the last two decades following its discovery, research on MDA-7 (also known as Interleukin 24 (IL-24), a member of IL-10 gene family (Sauane et al., 2003; Pestka et al., 2004; Chada et al., 2006), referred to as MDA-7/IL-24, has expanded at an exponential rate resulting in an enhanced understanding of the mechanism underlying its’ tumor suppressing activity (reviewed in Lebedeva et al., 2007; Emdad et al., 2009; Dash et al., 2010a; Menezes et al., 2014), improving strategies to enhance therapeutic outcomes (Fisher, 2005; Bhatia et al., 2013) and developing innovative approaches for cancer-specific gene delivery (Dash et al., 2011b; Das et al., 2012; Bhatia et al., 2013) and expression (Su et al., 2005b; Bhang et al., 2011). In this section, we discuss improvements in using mda-7/IL-24 as a therapeutic for cancer based on overcoming the challenges that gene therapists are currently encountering (Fig. 2).
Fig. 2.
Progression timelines for mda-7/IL-24 development as a therapeutic gene: from “bench to bedside.” After discovery, multiple approaches were used to enhance therapeutic outcome of mda-7/IL-24-mediated gene therapy of cancer.
Properties that make mda-7/IL-24 a unique and effective cancer therapeutic gene
Genetic conditions or disorders that arise from mutations in a single gene are ideal candidates for gene therapy. Unfortunately, cancer is a multigenic disorder and restoration or manipulating a single gene may not produce a desirable outcome. In this context, tumor suppressor gene(s) may provide a more general means of inhibiting cancer through inhibition of functions that go awry as result of induction of the cancerous phenotype. An ideal therapeutic gene would be one which selectively promotes killing of cancer cells without harming normal cells and is capable of attacking cancer on multiple levels (Fisher, 2005; Sarkar et al., 2007a). A critical component for an effective tumor suppressor gene would be an ability to be secreted and exert both direct and distant “bystander” anti-tumor effects (Fisher, 2005; Su et al., 2005a; Sarkar et al., 2007a).
The first demonstrations of cancer growth suppressive properties of MDA-7/IL-24 came from experiments in which mda-7/IL-24 was ectopically expressed in human melanoma and other human and rodent cancer cells (Jiang et al., 1995, 1996). These studies also provided suggestive evidence that this novel cytokine did not harm normal cells. Differential activity of mda-7/IL-24 in killing cancer vs. normal cells was shown definitively by delivering mda-7/IL-24 using a serotype 5 replication incompetent adenovirus (Ad.5.mda-7) (Su et al., 1998). By the first decade after discovery the therapeutic potential of MDA-7/IL-24 was confirmed in a wide array of solid tumors, both in vitro and in vivo, including melanoma, malignant glioma, carcinomas of the breast, cervix, colorectum, liver, lung, ovary and prostate (reviewed in Fisher et al., 2003; Fisher, 2005; Lebedeva et al., 2007; Emdad et al., 2009). This led to its entry into the clinic where intratumoral injection of a replication incompetent adenovirus expressing mda-7/IL-24 (Ad.mda-7; ING241) in patients with advance cancers resulted in clinically significant responses in 44% of treated patients (Fisher et al., 2003, 2007; Cunningham et al., 2005; Tong et al., 2005).
Elimination of a primary tumor is rarely adequate to obtain a complete cancer cure, since during cancer progression malignant tumor cells can spread and localize in multiple sites throughout the body rather than in a single tissue. Due to the limitation of diagnostic approaches to locate metastatic lesions (especially when in internal organs) and difficulties in efficient and selective drug delivery, metastatic disease is the most intractable clinical problem and a major cause of cancer-associated deaths. Ideally, a secretory therapeutic protein, a cytokine, which displays autocrine/paracrine functions and cancer-specific targeted toxicity, can remedy this situation (Pestka et al., 2004). The most intriguing property of MDA-7/IL-24 is its ability to evoke an autocrine/paracrine loop that allows it to be secreted from a primary target cell, normal or cancer, where it was introduced and to induce growth inhibition and apoptosis selectively in surrounding and in distant non-injected cancer cells (Fisher, 2005; Su et al., 2005a; Sauane et al., 2006, 2008). A series of experiments were performed in which tumor xenografts were implanted in both flanks and adenoviruses carrying mda-7/IL-24 were delivered in tumors in a single flank (Emdad et al., 2009; Dash et al., 2010a; Menezes et al., 2014). Initial pre-clinical tumor xenograft studies were extremely provocative, since both the treated tumors and non-treated tumors significantly regressed indicating potent “bystander” anti-tumor activity in vivo (Fisher, 2005). In addition to direct cancer-specific killing and “bystander” antitumor activities, mda-7/IL-24 also inhibits tumor angiogenesis (Ramesh et al., 2003; Lebedeva et al., 2007) and promotes tumor immune responses (Gao et al., 2008), making it a very attractive therapeutic gene for cancer therapy (Menezes et al., 2014).
Obstacles to selective cancer therapy
It is now considered axiomatic that a combinatorial approach is more favorable as compared to a single therapy in achieving a clinically beneficial effect. Accordingly, to maximize MDA-7/IL-24-mediated therapeutic outcomes, we have applied two strategies: (a) conditionally cancer-specific oncolytic viruses armed with mda-7/IL-24, known as “Cancer Terminator Viruses” or CTV (Sarkar et al., 2005a, 2006, 2007b, 2008; Das et al., 2012); and (b) by using mda-7/IL-24 gene therapy in combination with conventional chemo- and radio-therapy (Sarkar et al., 2006; Dent et al., 2010; Menezes et al., 2014). In the CTV, cancer-specific oncolysis is stringently maintained using a cancer-specific promoter derived from progression elevated gene-3 (PEG-3) (Su et al., 1997, 2005b), and maximum production of MDA-7/IL-24 is ensured by using an efficient ubiquitously expressing promoter CMV (Sarkar et al., 2006). Pre-clinical animal modeling studies demonstrated the efficacy of the CTV in inducing tumor-specific oncolysis with MDA-7/IL-24-mediated anti-tumorigenic and “bystander” anti-cancer activity in different solid human tumors including breast (Sarkar et al., 2005a), prostate (Sarkar et al., 2007b), melanoma (Sarkar et al., 2008) and glioblastoma multiforme (Hamed et al., 2013b). The most important finding was a complete elimination of the primary treated tumor as well as the distant non-treated tumor (established in the opposite flank), thereby implementing a cure in these immune incompetent animals. In the second approach, where mda-7/IL-24 gene therapy was combined with conventional therapy including radio/chemo-therapy, synergistic effects were evident. For example, in non-small cell lung carcinoma, MDA-7/IL-24 enhanced the efficacy of ionizing radiation and induced cell death (Kawabe et al., 2002; Nishikawa et al., 2004a,b). In another study, Chada et al. (2006) demonstrated that this combination improved the overall sensitivity of breast cancer cells to chemotherapy, biologic therapies and radiotherapy. In addition to radiation therapy, various chemotherapeutic agents are also efficiently used in conjunction with mda-7/IL-24. For example, Ad.mda-7 when delivered with gefitinib, an EGFR inhibitor enhanced apoptotic death in non-small cell lung cancer by increasing the expression of a downstream effector molecule, RNA-activated protein kinase (PKR) (Emdad et al., 2007). Combinatorial effects were also observed when MDA-7/IL-24 was combined with Temozolomide in melanoma (Zheng et al., 2008) and glioblastoma (Germano et al., 2010) or HDAC inhibitors in renal carcinoma (Hamed et al., 2013a) and glioblastoma multiforme (Hamed et al., 2013b). In pancreatic cancer, perillyl alcohol (POH) and D-limonene enhanced conversion of mda-7/IL-24 mRNA into protein resulting in significant cancer cell death both in vitro and in vivo (Su et al., 2001; Lebedeva et al., 2008a,b; Sarkar et al., 2013). Effective combinations were also demonstrated recently using MDA-7/IL-24 with a MCL-1-targeted small molecule inhibitor Sabutoclax in prostate and colon cancer (Dash et al., 2010c, 2011a; Azab et al., 2014) and an AKT inhibitor in breast cancer (Pal et al., 2014).
Methodologies ensuring enhanced cancer-specific delivery and expression
Despite successes using non-viral delivery of mda-7/IL-24 (Ramesh et al., 2004; Hingtgen et al., 2012; Gu et al., 2014), the majority of MDA-7/IL-24-related research has employed viral vectors, particularly Adenovirus serotype 5 (Ad.5). As discussed previously, the utility of Ad.5 serotype is limited in the clinic due to its dependency on surface expressed CAR for virus uptake, which often are not abundantly expressed in tumors. To overcome this obstacle and expand the host range and enhance efficiency of infectivity, Dash et al. (2010b) produced a chimeric tropism modified adenovirus (a chimeric Ad.5 with an Ad.3 modified fiber, Ad.5/3) to deliver mda-7/IL-24. This modified virus transduced mda-7/IL-24 more efficiently in low CAR human prostate cancer cells grown as tumor xenografts (Dash et al., 2010b) and in a spontaneous Hi-myc prostate cancer transgenic animal model (Dash et al., 2011a). Subsequent studies confirmed the superiority of Ad.5/3.mda-7 over Ad.5. mda-7 in other cancer cells with low CAR including glioblastoma multiforme (Hamed et al., 2013b), renal carcinoma (Eulitt et al., 2010; Park et al., 2011), colorectal carcinoma (Azab et al., 2012) and breast carcinoma (Pal et al., 2014). Additionally, this modification enhanced the infectivity of the CTV, where the tropism modified CTV (Ad.5/3-CTV) displayed better transduction efficiency at a lower multiplicity of infection (m.o.i.) in various low CAR human cancer xenografts than the first generation Ad.5-CTV (Sarkar et al., 2013; Hamed et al., 2013b; Azab et al., 2014).
Even though tropism modified adenoviral-mediated mda-7/IL-24 can induce apoptosis in diverse human cancers, it is apparent that the applicability of this therapeutic virus is restricted in a clinical context. Although the Ad.5/3-CTV displays enhanced efficacy and infectivity against a broad spectrum of human tumors, its applications clinically are limited when used systemically because of non-specific trapping in the liver, immune clearance and the limited options to efficiently deliver the virus to internal organs such as prostate, pancreas, kidney, etc. The multiple obstacles in using the Ad.5/3-CTV systemically are not unique to this virus, since these same problems are endemic to many viral-based therapeutic approaches. To circumvent these hurdles, a potentially useful approach employs MBs and ultrasound, i.e., ultrasound-targeted microbubble-destruction (UTMD) (Dash et al., 2011b; Das et al., 2012; Bhatia et al., 2013). This approach limits trapping of viruses in liver, shields the viruses from the immune system and permits release of the viruses in the tumor microenvironment and directly in internal organs as well as malignant tissues (Greco et al., 2010; Dash et al., 2011a,b; Das et al., 2012; Bhatia et al., 2013). UTMD was employed to target the release of a therapeutic virus (Ad.5/3.mda-7) at the tumor site (prostate gland) in immunocompetent Hi-Myc mice, a spontaneous prostate cancer transgenic mouse model which develops localized adenocarcinoma by 6 month of age (Dash et al., 2011a). MB loaded Ad.5/3.mda-7 was injected intravenously three times per week for four weeks and ultrasound was used to ensure prostate-specific delivery. Target-specific delivery was confirmed by demonstrating the presence of MDA-7/IL-24 protein in tumor sections by immunohistochemistry (Dash et al., 2011a). Additionally, the study also emphasized the therapeutic potential of mda-7/IL-24 gene therapy in combination with chemotherapy, i.e., an MCL-1 inhibitor (Sabutoclax). In a follow up study, Azab et al. (2014) used the UTMD approach to deliver Ad.5/3-CTV to Hi-Myc mice and confirmed and expanded on the previous findings of Dash et al. (2011b) using Ad.5/3-mda-7 (a non-replicating adenovirus expressing mda-7/IL-24). These studies suggest that the UTMD approach can be used to selectively and efficiently deliver therapeutic genes (e.g., mda-7/IL-24) in internal target organs.
Gene therapy: Successes in the clinic
In the past two decades, fundamental and seminal advances in molecular biology and genetic engineering have provided opportunities for developing various gene therapies, however, in the vast majority of cases cancer is not cured using these approaches. However, a couple of successes resulting in long-term survival provide optimism that this approach might still fulfill its promise in the future. Below, we briefly describe a few gene therapy concepts that have successfully translated from the bench to the bedside.
Gene therapies based on p53, a gene that is defective in many cancers, have been and are currently the subject of various clinical trials worldwide. In 2001, ONYX-015, also known as dl1520, a genetically modified Ad lacking a 55 kd gene in the E1B region (to restrict viral replication to p53 mutant cells), was tested in patients with non-small cell lung carcinoma (Ahrendt et al., 2003). In Phase I clinical trials, the virus was well tolerated and partially effective in promoting tumor shrinkage, however the response rate was not as profound as anticipated from pre-clinical studies. Three out of fifteen patients showed a partial response. In a follow up Phase II clinical trial, two cycles of intratumoral injections resulted in a 28% response rate in the treatment cohort as compared to 12% response rate in the control group. In the Phase III clinical trial, ONYX-015 administered alone or in combination with a chemotherapeutic, showed a 72.7% response rate as compared to a 40.4% response in the control group. In a different clinical study, a prostate specific therapeutic adenovirus, CG7870 developed by regulating viral replication under a prostate cancer-specific promoter, was used. However, no objective clinical responses in terms of tumor regression were observed although five patients showed 25–49% reduction in their serum PSA levels (Reid et al., 2002).
The first commercialized gene therapy, Gendicine™, a recombinant adenovirus engineered to express wild type-p53 under a Rous sarcoma virus promoter was used to treat head and neck squamous cell cancer (Raty et al., 2008). In a Phase III clinical trail (132 patients were enrolled), 64% of patients responded with complete regression when the virus was used in combination with radiotherapy. However, the published details of this clinical trial are limited. A similar product, Advexin® (INGN 201; originally produced by Introgen Therapeutics, Inc., Austin, TX) was also evaluated for clinical efficacy in various cancers and the results were reviewed (Gabrilovich, 2006).
In addition to p53, other tumor suppressor genes such as tumor necrosis factor alpha, TRAIL, interleukin-2, and mda-7/IL-24 genes have also shown some promise in clinical trials. In 2005, studies published by Cunningham et al. (Cunningham et al., 2005) and Tong et al. (Tong et al., 2005), reviewed in Lebedeva et al. (Lebedeva et al., 2005) and Fisher et al. (Fisher et al., 2007), demonstrated that a replication incompetent adenovirus expressing mda-7/IL-24, Ad.mda-7 (INGN-241), was well tolerated clinically and effective in patients with advanced cancers (both carcinomas and melanomas). Multiple intratumoral injections of Ad.mda-7 induced apoptosis, which was closely correlated with MDA-7/IL-24 expression, and resulted in clinically significant responses (greater than 50% shrinkage of injected lesion) in 44% (2 patients out of 5 patients) without provoking any toxicities suggesting that this therapeutic is safe as well as efficacious. Interestingly, apoptosis demonstrated in patient tumors receiving MDA-7/IL-24 was significantly greater than what was observed following treatment with Ad.p53 in non-small-cell lung cancer. The most dramatic response was observed in a 64-year old woman with metastatic melanoma. A less dramatic response was seen in another patient with squamous cell carcinoma of the penis in which one of several skin nodules were injected. Similar to preclinical studies (Fisher, 2005; Su et al., 2005a; Gao et al., 2008; Sauane et al., 2008), potent “bystander” antitumor and immune modulatory activity was also evident in clinical trials.
Gene directed enzyme pro-drug therapy relies on the conversion of non-toxic substances (pro-drug) into physiologically active agents. Most frequently, HSV-TK has been used to convert the pro-drug ganciclovir into the cytotoxic triphosphate ganciclovir. Utilizing this approach, the Anglo Finnish Company Ark Therapeutics developed Cerepro, an adenovirus containing HSV-TK under cytomegalovirus promoter to treat malignant glioma. Cerepro demonstrated a significant survival benefit when compared with patients treated with retroviral therapy or standard therapy. The adverse effects were mostly limited to transient fever. The findings from a Phase III clinical trial using Cerepro (sitimagene, ceradenovec) have recently been published (Westphal et al., 2013).
Concluding remarks
Currently, the success of cancer gene therapy is lagging behind that obtained in the treatment of monogenetic diseases. Although cancer is a genetic disorder, the abnormalities are generally polygenic and genetic variation between individuals or even tumors at different sites within the same patient are substantial. The major reasons for failure or limited success of cancer gene therapy are not only technical, which we have discussed in this review, but also related to ethical, policy and financial issues. The fear of insertional mutagenesis also raises further health risk concerns. The possibility of passing the genetic changes onto offspring is an argument used against human cancer gene therapy involving approaches that result in integration into the genome. Unlike chemotherapies, gene therapies are only effective in a subset of patients with any given cancer, making each gene therapy agent an orphan drug. Moreover, failure to target metastatic cells, the major driver of cancer-associated mortality has limited the general utility of cancer gene therapy. Targeting of metastases using gene therapy approaches has proven inefficient for many reasons including genetic and epigenetic heterogeneity, development of resistance and difficulty in locating metastases, which can frequently be disseminated throughout the body. Ideally, gene therapy should provide a means of treating primary and disseminated tumors, with a minimal effect on normal cells. This problem can be resolved by using a gene product with the ability to induce its own translation in a cancer cell-specific manner or stimulate the immune system to prevent colonization to a distant site. We are optimistic that as the field of gene therapy continues to advance, current impediments to effective systemic therapy, such as non-specific targeting, trapping in organs such as the liver, and neutralization by the immune system, will be overcome leading to further triumphs in treating cancer. Moreover, combinatorial approaches using gene therapy with chemotherapy, small molecule inhibitor therapy, radiation therapy and/or immunotherapy will lead to further incremental increases in the efficacy of this approach for the treatment of diverse human cancers.
Acknowledgments
Contract grant sponsor: National Institutes of Health;
Contract grant numbers: P01 CA104177, R01 CA097318, R01 CA127641, R01 CA168517.
Contract grant sponsor: Department of Defense synergy;
Contract grant number: W81XWH-10-PCRP-SIDA.
Contract grant sponsor: The James S. McDonnell Foundation.
Contract grant sponsor: National Cancer Institute;
Contract grant number: R01 CA138540-01A1.
Support for our laboratory was provided in part by National Institutes of Health grants P01 CA104177 (P.B.F.), R01CA097318 (P.B.F.), R01 CA127641 (P.B.F.), R01 CA168517 (Maurizio Pellecchia, P.B.F.); Department of Defense synergy grant W81XWH-10-PCRP-SIDA (P.B.F., X.Y.W.); the National Foundation for Cancer Research (P.B.F.); the Samuel Waxman Cancer Research Foundation (P.B.F., D.S.); an A. David Mazzone Prostate Cancer Foundation Challenge Award (Martin G. Pomper, P.B.F., George Sguoros); NCI Cancer Center Support Grant to VCU Massey Cancer Center (P.B.F., X.Y.W., D.S.); and VCU Massey Cancer Center developmental funds (P.B.F.); The James S. McDonnell Foundation and National Cancer Institute Grant R01 CA138540-01A1 (D.S.). D.S. is Harrison Scholars in the VCU Massey Cancer Center. P.B.F. and D.S. are SWCRF Investigators. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Literature Cited
- Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad MZ, Akhter S, Rahman Z, Akhter S, Anwar M, Mallik N, Ahmad FJ. Nanometric gold in cancer nanotechnology: current status and future prospect. J Pharm Pharmacol. 2013;65:634–651. doi: 10.1111/jphp.12017. [DOI] [PubMed] [Google Scholar]
- Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, Wu L, Sidransky D. P53 mutations and survival in stage I non-small-cell lung cancer: results of a prospective study. J Natl Cancer Inst. 2003;95:961–970. doi: 10.1093/jnci/95.13.961. [DOI] [PubMed] [Google Scholar]
- Aied A, Greiser U, Pandit A, Wang W. Polymer gene delivery: Overcoming the obstacles. Drug Discov Today. 2013;18:1090–1098. doi: 10.1016/j.drudis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Akada M, Crnogorac-Jurcevic T, Lattimore S, Mahon P, Lopes R, Sunamura M, Matsuno S, Lemoine NR. Intrinsic chemoresistance to gemcitabine is associated with decreased expression of BNIP3 in pancreatic cancer. Clinical Cancer Research. 2005;11:3094–3101. doi: 10.1158/1078-0432.CCR-04-1785. [DOI] [PubMed] [Google Scholar]
- Alba R, Bosch A, Chillon M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Therapy. 2005;12:S18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Rychak JJ, Backer M, Backer J, Ley K, Klibanov AL. ScVEGF microbubble ultrasound contrast agents: A novel probe for ultrasound molecular imaging of tumor angiogenesis. Invest Radiol. 2010;45:579–585. doi: 10.1097/RLI.0b013e3181efd581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab B, Das SK, Bhutia SK, Sarkar S, Shen X-N, Quinn BA, Dent P, Igor P, Dmitriev IP, Wang X-Y, Curiel DT, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Enhanced prostate cancer gene transfer and therapy using a novel serotype chimera cancer terminator virus (Ad.5/3-CTV) J Cell Physiol. 2014;229:34–43. doi: 10.1002/jcp.24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab B, Dash R, Das SK, Bhutia SK, Shen X-N, Quinn BA, Sarkar S, Wang X-Y, Hedvat M, Dmitriev IP, Curiel DT, Grant S, Dent P, Reed JC, Pellecchia M, Sarkar D, Fisher PB. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2012;227:2145–2153. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty MS, Curiel DT. Adenovirus strategies for tissue-specific targeting. Adv Cancer Res. 2012;115:39–67. doi: 10.1016/B978-0-12-398342-8.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14:7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang H-C, Gabrielson KL, Latera J, Fisher PB, Pomper MG. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nature Med. 2011;17:123–129. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S, Menezes ME, Das SK, Emdad L, Dasgupta S, Wang XY, Sarkar D, Fisher PB. Innovative approaches for enhancing cancer gene therapy. Discov Med. 2013;15:309–317. [PubMed] [Google Scholar]
- Bischoff JR, Kim DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo-polyethylenimine. Proc Natl Acad of Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chretien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chretien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, Vorburger SA, Pataer A, Swisher SG, Ramesh R, Kawase K, Meyn RE, Hunt KK. Mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: Correlation with expression of bcl-2 family members. Cancer Gene Ther. 2006;13:490–502. doi: 10.1038/sj.cgt.7700915. [DOI] [PubMed] [Google Scholar]
- Chan JKY, Lam PYP. Human mesenchymal stem cells and their paracrine factors for the treatment of brain tumors. Cancer Gene Therapy. 2013;20:539–543. doi: 10.1038/cgt.2013.59. [DOI] [PubMed] [Google Scholar]
- Chin L, Hahn WC, Getz G, Meyerson M. Making sense of cancer genomic data. Genes Dev. 2011;25:534–555. doi: 10.1101/gad.2017311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Chang J. Viral vectors for vaccine applications. Clin Exp Vaccine Res. 2013;2:97–105. doi: 10.7774/cevr.2013.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo F, Barzon L, Franchin E, Pacenti M, Pinna V, Danieli D, Zanusso M, Palu G. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- Conde J, Larguinho M, Cordeiro A, Raposo LR, Costa PM, Santos S, Diniz MS, Fernandes AR, Baptista PV. Gold-nanobeacons for gene therapy: Evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotoxicology. 2014;8:521–532. doi: 10.3109/17435390.2013.802821. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Curiel DT, Fisher PB, editors. Applications of viruses for cancer therapy. Adv Cancer Res. 2012;115:1–334. [Google Scholar]
- Das SK, Sarkar S, Dash R, Dent P, Wang X-Y, Sarkar D, Fisher PB. Cancer terminator viruses and approaches for enhancing therapeutic outcomes. Adv Cancer Res. 2012;115:1–38. doi: 10.1016/B978-0-12-398342-8.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Azab B, Quinn BA, Shen XN, Wang X-Y, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, Dmitriev IP, Curiel DT, Grant S, Wu BN, Stebbins JL, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Pro Natl Acad Sci USA. 2011a;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Azab B, Shen X-N, Sokhi UK, Sarkar S, Su ZZ, Wang XY, Claudio PP, Dent P, Dmitriev IP, Curiel DT, Grant S, Sarkar D, Fisher PB. Developing an effective gene therapy for prostate cancer: New technologies with potential to translate from the laboratory into the clinic. Discov Med. 2011b;11:46–56. [PMC free article] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang X-Y, Sarkar D, Grant S, Dent P, Curiel DT, Fisher PB. Mda-7/IL-24: A unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010a;21:381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010b;17:447–456. doi: 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- Dash R, Read ML, Barrett LB, Wolfert MA, Seymour LW. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 1999;6:643–650. doi: 10.1038/sj.gt.3300843. [DOI] [PubMed] [Google Scholar]
- Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, Curiel DT, Grant S, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010c;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong RK, Reynolds CM, Malcolm Y, Schaeffer A, Severs T, Wanekaya A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol Sci Appl. 2010;3:53–63. doi: 10.2147/NSA.S8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, Sarkar D, Gupta P, Emdad L, Lebedeva IV, Sauane M, Su ZZ, Rahmani M, Broaddus WC, Young HF, Lesniak M, Grant S, Curiel DT, Fisher PB. MDA-7/IL-24 as a cancer therapeutic: From bench to bedside. Anticancer Drugs. 2010;21:725–731. doi: 10.1097/CAD.0b013e32833cfbe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, Sklenar J, Lindner JR. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v) beta3. Circulation. 2003;108:336–341. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, Fisher PB. Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy. J Cell Physiol. 2007;210:549–559. doi: 10.1002/jcp.20906. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D, Fisher PB. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol & Ther. 2009;8:391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Eulitt PJ, Park MA, Hamed HA, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther. 2010;10:1290–1305. doi: 10.4161/cbt.10.12.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farma JM, Puhlmann M, Soriano PA, Cox D, Paciotti GF, Tamarkin L, Alexander HR. Direct evidence for rapid and selective induction of tumor neovascular permeability by tumor necrosis factor and a novel derivative, colloidal gold bound tumor necrosis factor. Int J Cancer. 2007;120:2474–2480. doi: 10.1002/ijc.22270. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopal krishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. Mda-7/IL, a novel cancer selective apoptosis inducing cytokine gene: From the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- Fisher PB, Prignoli DR, Hermo H, Jr, Weinstein IB, Pestka S. Effects of combined treatment with interferon and mezerein on melanogenesis and growth in human melanoma cells. J Interferon Res. 1985;5:11–22. doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, Senzer N, Nemunaitis J. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): Novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224:300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: Justification for K-ras-directed therapy. Mol Cancer Res. 2005;3:413–423. doi: 10.1158/1541-7786.MCR-04-0206. [DOI] [PubMed] [Google Scholar]
- Fournillier A, Frelin L, Jacquier E, Ahlen G, Brass A, Gerossier E, Holmstrom F, Broderick KE, Sardesai NY, Bonnefoy JY, Inchauspe G, Sallberg M. A heterologous prime/boost vaccination strategy enhances the immunogenicity of therapeutic vaccines for hepatitis C virus. J Infect Dis. 2013;208:1008–1019. doi: 10.1093/infdis/jit267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The bystander effect-tumor-regression when a fraction of the tumor mass is genetically-modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- Friedmann T. A brief history of gene therapy. Nat Genet. 1992;2:93–98. doi: 10.1038/ng1092-93. [DOI] [PubMed] [Google Scholar]
- Fuerer C, Iggo R. Adenoviruses with Tcf binding sites in multiple early promoters show enhanced selectivity for tumour cells with constitutive activation of the wnt signalling pathway. Gene Therapy. 2002;9:270–281. doi: 10.1038/sj.gt.3301651. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Shirakawa Y, Kagawa S. Telomerase-specific oncolytic virotherapy for human gastrointestinal cancer. Expert Rev Anticancer Ther. 2011;11:525–532. doi: 10.1586/era.10.200. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Urata Y, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr Cancer Drug Targets. 2007;7:191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Matsuoka J, Yamatsuji T, Maeda Y, Durbin ML, Naomoto Y. Adenovirus-mediated cancer gene therapy and virotherapy (Review) Int J Mol Med. 2010;25:3–10. [PubMed] [Google Scholar]
- Gabrilovich DI. INGN 201 (Advexin): Adenoviral p53 gene therapy for cancer. Expert Opin Biol Ther. 2006;6:823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- Gandhi J, Cashman SM, Kumar-Singh R. Soluble CD59 Expressed from an adenovirus in vivo is a potent inhibitor of complement deposition on murine liver vascular endothelium. Plos ONE. 2011;6:e21621. doi: 10.1371/journal.pone.0021621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, Fisher PB, Wang X-Y. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68:3890–3898. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano IM, Emdad L, Qadeer ZA, Binello E, Uzzaman M. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis and radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer Gene Ther. 2010;17:664–674. doi: 10.1038/cgt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine (Lond) 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh A, Ko SC, Shirakawa T, Cheon J, Kao C, Miyamoto T, Gardner TA, Ho LJ, Cleutjens CB, Trapman J, Graham FL, Chung LW. Development of prostate-specific antigen promoter-based gene therapy for androgen-independent human prostate cancer. J Urol. 1998;160:220–229. [PubMed] [Google Scholar]
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, Sarkar D, Dent P, Curiel DT, Fisher PB, Claudio PP. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18:295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TS, Stokes B, Kucaba TA, Earel JK, Jr, VanOosten RL, Brincks EL, Norian LA. TRAIL gene therapy: From preclinical development to clinical application. Curr Gene Ther. 2009;9:9–19. doi: 10.2174/156652309787354612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Chen X, Xin H, Fang X, Sha X. Serum-resistant complex nanoparticles functionalized with imidazole-rich polypeptide for gene delivery to pulmonary metastatic melanoma. Int J Pharm. 2014;461:559–569. doi: 10.1016/j.ijpharm.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH, Leiva L, Sorensen R, Debre M, Casanova JL, Blanche S, Durandy A, Bushman FD, Fischer A, Cavazzana-Calvo M. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisma HJ, Pinedo HM, Rijswijk A, der Meulen-Muileman I, Sosnowski BA, Ying W, Beusechem VW, Tillman BW, Gerritsen WR, Curiel DT. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Ther. 1999;6:1469–1474. doi: 10.1038/sj.gt.3300969. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PL, Chang YN, Hay C, Golightly D, Stewart D, Lin J, Phipps S, Chiang YL. A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Hum Gene Ther. 1999;10:1721–1733. doi: 10.1089/10430349950017725. [DOI] [PubMed] [Google Scholar]
- Hamada K, Zhang T, Desaki J, Nakashiro K, Itoh H, Tani K, Koyama Y, Hamakawa H. Carrier cell-mediated cell lysis of squamous cell carcinoma cells by squamous cell carcinoma antigen 1 promoter-driven oncolytic adenovirus. J Gene Med. 2010;12:545–554. doi: 10.1002/jgm.1467. [DOI] [PubMed] [Google Scholar]
- Hamed HA, Das SK, Sokhi UK, Park MA, Cruickshanks N, Archer K, Ogretmen B, Grant S, Sarkar D, Fisher PB, Dent P. Combining histone deacetylase inhibitors with MDA-7/IL-24 enhances killing of renal carcinoma cells. Cancer Biol Ther. 2013a;14:1039–1049. doi: 10.4161/cbt.26110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Archer K, Das SK, Sarkar D, Grant S, Fisher PB, Dent P. Histone deacetylase inhibitors interact with melanoma differentiation associated-7/interleukin-24 to kill primary human glioblastoma cells. Mol Pharmacol. 2013b;84:171–181. doi: 10.1124/mol.113.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, Curiel DT, Fisher PB, Dent P. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18:1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haviv YS, Blackwell JL, Kanerva A, Nagi P, Krasnykh V, Dmitriev I, Wang M, Naito S, Lei X, Hemminki A, Carey D, Curiel DT. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 2002;62:4273–4281. [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Luscher B. Proteins of the Myc network: Essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- Hingtgen S, Kasmieh R, Elbayly E, Nesterenko I, Figueiredo JL, Dash R, Sarkar D, Hall D, Kozakov D, Vajda S, Fisher PB, Shah K. A first-generation multi-functional cytokine for simultaneous optical tracking and tumor therapy. PLoS ONE. 2012;7:e40234. doi: 10.1371/journal.pone.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Wildner O. Restriction of adenoviral replication to the transcriptional intersection of two different promoters for colorectal and pancreatic cancer treatment. Mol Cancer Ther. 2006;5:374–381. doi: 10.1158/1535-7163.MCT-05-0374. [DOI] [PubMed] [Google Scholar]
- Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA, Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol. 2006;209:413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SL. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003;10:1241–1247. doi: 10.1038/sj.gt.3301987. [DOI] [PubMed] [Google Scholar]
- Irving J, Wang Z, Powell S, O’Sullivan C, Mok M, Murphy B, Cardoza L, Lebkowski JS, Majumdar AS. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Therapy. 2004;11:174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- Jain K. Pharmacology-VolII-Gene therapy. 2009:331–359. [Google Scholar]
- Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85:101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia LT, Chen SY, Yang AG. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat Rev. 2012;38:868–876. doi: 10.1016/j.ctrv.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Jiang H, Fisher PB. Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Mol Cell Different. 1993;1:285–299. [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, Barker SD, Straughn M, Barnes MN, Alvarez RD, Hemminki A, Curiel DT. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- Kashentseva EA, Seki T, Curiel DT, Dmitriev IP. Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res. 2002;62:609–616. [PubMed] [Google Scholar]
- Kaufmann JK, Nettelbeck DM. Virus chimeras for gene therapy, vaccination, and oncolysis: Adenoviruses and beyond. Trends Mol Med. 2012;18:365–376. doi: 10.1016/j.molmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Kawabe S, Nishikawa T, Munshi A, Roth JA, Chada S, Meyn RE. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53-independent mechanisms. Mol Ther. 2002;6:637–644. [PubMed] [Google Scholar]
- Kawakami Y, Li H, Lam JT, Krasnykh V, Curiel DT, Blackwell JL. Substitution of the adenovirus serotype 5 knob with a serotype 3 knob enhances multiple steps in virus replication. Cancer Res. 2003;63:1262–1269. [PubMed] [Google Scholar]
- Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J, Sohn JH, Kim H, Yun CO. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther. 2003;14:1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- Kircher M, Kelso J. High-throughput DNA sequencing–concepts and limitations. Bioessays. 2010;32:524–536. doi: 10.1002/bies.200900181. [DOI] [PubMed] [Google Scholar]
- Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krens LL, Baas JM, Gelderblom H, Guchelaar HJ. Therapeutic modulation of k-ras signaling in colorectal cancer. Drug Discov Today. 2010;15:502–516. doi: 10.1016/j.drudis.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Lai TC, Kataoka K, Kwon GS. Bioreducible polyether-based pDNA ternary polyplexes: Balancing particle stability and transfection efficiency. Colloids Surf B Biointerfaces. 2012;99:27–37. doi: 10.1016/j.colsurfb.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanson NA, Jr, Friedlander PL, Schwarzenberger P, Kolls JK, Wang G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res. 2003;63:7936–7941. [PubMed] [Google Scholar]
- Larina IV, Evers BM, Ashitkov TV, Bartels C, Larin KV, Esenaliev RO. Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation. Technol Cancer Res Treat. 2005;4:217–226. doi: 10.1177/153303460500400211. [DOI] [PubMed] [Google Scholar]
- Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17:359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, Barral P, Lee SG, Wang D, Vozhilla N, Park ES, Chatman L, Boukerche H, Ramesh R, Inoue S, Chada S, Li R, De Pass AL, Mahasreshti PJ, Dmitriev IP, Curiel DT, Yacoub A, Grant S, Dent P, Senzer N, Nemunaitis JJ, Fisher PB. Mda-7/IL-24, novel anticancer cytokine: Focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review) Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, Fisher PB. mda-7/IL-24: Exploiting cancer’s Achilles’ heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Fisher PB. Restoring apoptosis as a strategy for cancer gene therapy: Focus on p53 and mda-7. Semin Cancer Biol. 2003;13:169–178. doi: 10.1016/s1044-579x(02)00134-7. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis. Mol Cancer Ther. 2008a;7:2042–2050. doi: 10.1158/1535-7163.MCT-08-0245. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol. Cancer Res. 2008b;68:7439–7447. doi: 10.1158/0008-5472.CAN-08-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kim HS, Yu R, Lee K, Gardner TA, Jung C, Jeng MH, Yeung F, Cheng L, Kao C. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002;6:415–421. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- Leszczyniecka M, Roberts T, Dent P, Fisher Grant. Differentiation therapy of cancer: Basic science and clinical applications. Pharmacol Ther. 2001;90:105–156. doi: 10.1016/s0163-7258(01)00132-2. [DOI] [PubMed] [Google Scholar]
- Li YHG, Diakur J, Wiebe LI. Targeted delivery of macromolecular drugs: asialoglycoprotein receptor (ASGPR) expression by selected hepatoma cell lines used in antiviral drug development. Curr Drug Deliv. 2008;5:4–10. doi: 10.2174/156720108785915069. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang YP, Kim HS, Bae KH, Stantz KM, Lee SJ, Jung C, Jimenez JA, Gardner TA, Jeng MH, Kao C. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65:1941–1951. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- Liu G, Wong-Staal F, Li QX. Development of new RNAi therapeutics. Histol Histopathol. 2007;22:211–217. doi: 10.14670/HH-22.211. [DOI] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JIW, Hauck B, Zelenaia O, Zhu XS, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun JW, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlashewski G, Lamb P, Pim D, Peacock J, Crawford L, Benchimol S. Isolation and characterization of a human p53 cDNA clone: Expression of the human p53 gene. EMBO J. 1984;3:3257–3262. doi: 10.1002/j.1460-2075.1984.tb02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie EA, Graham DI, Brown SM. Selective astrocytic transgene expression in vitro and in vivo from the GFAP promoter in a HSV RL1 null mutant vector-potential glioblastoma targeting. Gene Ther. 1998;5:440–450. doi: 10.1038/sj.gt.3300621. [DOI] [PubMed] [Google Scholar]
- Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, Dent P, Wang X-Y, Sarkar D, Fisher PB. MDA-7/IL-24: Multifunctional cancer killing cytokine. Adv Exp Med Biol. 2014;818:127–153. doi: 10.1007/978-1-4471-6458-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modok S, Mellor HR, Callaghan R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol. 2006;6:350–354. doi: 10.1016/j.coph.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F, Manabe D, Thompson TC, Kumon H. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther. 2007;15:834–840. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- Natsume A, Yoshida J. Gene therapy for high-grade glioma: Current approaches and future directions. Cell Adh Migr. 2008;2:186–191. doi: 10.4161/cam.2.3.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost. 2009;7:1523–1532. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Herzog RW. Progress and prospects: Immune responses to viral vectors. Gene therapy. 2010a;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Herzog RW. Progress and prospects: Immune responses to viral vectors. Gene Ther. 2010b;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettelbeck DM, Jerome V, Muller R. Gene therapy: Designer promoters for tumour targeting. Trends Genet. 2000;16:174–181. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- Nettelbeck DM, Rivera AA, Balague C, Alemany R, Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: Specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–4670. [PubMed] [Google Scholar]
- Ng KY, Liu Y. Therapeutic ultrasound: Its application in drug delivery. Med Res Rev. 2002;22:204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Munshi A, Story MD, Ismail S, Stevens C, Chada S, Meyn RE. Adenoviral-mediated mda-7 expression suppresses DNA repair capacity and radiosensitizes non-small-cell lung cancer cells. Oncogene. 2004a;23:7125–7131. doi: 10.1038/sj.onc.1207917. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004b;9:818–828. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, Shen YQ, Habets G, Ginzinger D, McCormick F. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- Pal I, Sarkar S, Rajput S, Dey KK, Chakraborty S, Dash R, Das SK, Sarkar D, Barile E, De SK, Pellecchia Fisher PB, Mandal M. BI-69A11 enhances susceptibility of colon cancer cells to mda-7/IL-24-induced growth inhibition by targeting Akt. Br J Cancer. 2014;111:101–111. doi: 10.1038/bjc.2014.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Taneja S, Dardashti K, Cohan P, Kaboo R, Sokoloff M, Tso CL, Dekernion JB, Belldegrun AS. Prostate tissue specificity of the prostate-specific antigen promoter isolated from a patient with prostate cancer. Hum Gene Ther. 1995;6:1417–1426. doi: 10.1089/hum.1995.6.11-1417. [DOI] [PubMed] [Google Scholar]
- Park MA, Hamed HA, Mitchell C, ruickshanks C, Dash N, Allegood R, Dmitriev J, Tye IP, Ogretmen G, Spiegel B, Yacoub S, Grant A, Curiel S, Fisher DT, Dent PB. A serotype 5/3 adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes toxicity of agents that increase ROS and ceramide levels. Mol Pharmacol. 2011;79:368–380. doi: 10.1124/mol.110.069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AL, Waddington SN, Buckley SM, Custers J, Havenga MJ, van Rooijen N, Goudsmit J, McVey JH, Nicklin SA, Baker AH. Effect of neutralizing sera on factor X-mediated adenovirus serotype 5 gene transfer. J Virol. 2009;83:479–483. doi: 10.1128/JVI.01878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereboeva L, Komarova S, Mahasreshti P, Curiel DT. Fiber-mosaic adenovirus as a novel approach to design genetically modified adenoviral vectors. Virus Res. 2004;105:35–46. doi: 10.1016/j.virusres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Philipson L, Pettersson RF. The coxsackie-adenovirus receptor—A new receptor in the immunoglobulin family involved in cell adhesion. Curr Top Microbiol Immunol. 2004;273:87–111. doi: 10.1007/978-3-662-05599-1_3. [DOI] [PubMed] [Google Scholar]
- Pushpakumar SB, Perez-Abadia G, Soni C, Wan R, Todnem N, Patibandla PK, Fensterer T, Zhang Q, Barker JH, Maldonado C. Enhancing complement control on endothelial barrier reduces renal post-ischemia dysfunction. J Surg Res. 2011;170:e263–e270. doi: 10.1016/j.jss.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney SD, Brown J, Cucco C, Lee R, Skorski T, Leonetti C, Geiser T, Calabretta B, Zupi G, Zon G. Enhanced anti-tumor effects with microencapsulated c-myc antisense oligonucleotide. Antisense Nucl Acid Drug Dev. 1999;9:451–458. doi: 10.1089/oli.1.1999.9.451. [DOI] [PubMed] [Google Scholar]
- Ramesh R, Ito I, Saito Y, Wu Z, Mhashikar AM, Wilson DR, Branch CD, Roth JA, Chada S. Local and systemic inhibition of lung tumor growth after nanoparticle-mediated mda-7/IL-24 gene delivery. DNA Cell Biol. 2004;23:850–857. doi: 10.1089/dna.2004.23.850. [DOI] [PubMed] [Google Scholar]
- Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, Grimm EA, Renauld JC, Kotenko S, Chada S. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- Raty JK, Pikkarainen JT, Wirth T, Yla-Herttuala S. Gene therapy: The first approved gene-based medicines, molecular mechanisms and clinical indications. Curr Mol Pharmacol. 2008;1:13–23. doi: 10.2174/1874467210801010013. [DOI] [PubMed] [Google Scholar]
- Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein DT, Breidenbach M, Kirby TO, Han T, Siegal GP, Bauerschmitz GJ, Wang M, Nettelbeck DM, Tsuruta Y, Yamamoto M, Dall P, Hemminki A, Curiel DT. A fiber-modified, secretory leukoprotease inhibitor promoter-based conditionally replicating adenovirus for treatment of ovarian cancer. Clin Cancer Res. 2005;11:1327–1335. [PubMed] [Google Scholar]
- Rivera AA, Davydova J, Schierer S, Wang M, Krasnykh V, Yamamoto M, Curiel DT, Nettelbeck DM. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11:1694–1702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: A selective cytotoxic for prostate-specific antigen- positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- Rogers S, Lowentha A, Terhegge Hg, Columbo JP. Induction of arginase activity with Shope papilloma virus in tissue-culture cells from an argininemic patient. J Exp Med. 1973;137:1091–1096. doi: 10.1084/jem.137.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Pfuderer P. Use of viruses as carriers of added genetic information. Nature. 1968;219:749–756. doi: 10.1038/219749a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. Gene transfer into humans—Immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Roth JA, Nguyen D, Lawrence DD, Kemp BL, Carrasco CH, Ferson DZ, Hong WK, Komaki R, Lee JJ, Nesbitt JC, Pisters KM, Putnam JB, Schea R, Shin DM, Walsh GL, Dolormente MM, Han CI, Martin FD, Yen N, Xu K, Stephens LC, McDonnell TJ, Mukhopadhyay T, Cai D. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat Med. 1996;2:985–991. doi: 10.1038/nm0996-985. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, Curiel DT, Fisher PB. Melanoma differentiation associated gene-7 (mda-7)/IL-24: A ‘magic bullet’ for cancer therapy. Expert Opin Biol Ther. 2007a;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, Curiel DT, Fisher PB. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007b;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Quinn AB, Shen BA, Dent X, Klibanov P, Emdad AL, Das L, Sarkar SK, Fisher D. Chemoprevention gene therapy (CGT) of pancreatic cancer using perillyl alcohol and a novel chimeric serotype Cancer Terminator Virus. Curr Mol Med. 2013;14:125–140. doi: 10.2174/1566524013666131118110827. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Fisher PB. Unique conditionally replication competent bipartite adenoviruses-cancer terminator viruses (CTV): Efficacious reagents for cancer gene therapy. Cell Cycle. 2006;5:1531–1536. doi: 10.4161/cc.5.14.3095. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, Fisher PB. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15:293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005a;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, Fisher PB. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005b;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Sarkar D, Su Z-z, Lebedeva IV, Dent P, Pestka S, Fisher PB. Mda-7/IL-24: Novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gupta P, Lebedeva IV, Su ZZ, Sarkar D, Randolph A, Valerie K, Gopalkrishnan RV, Fisher PB. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Res. 2006;66:11869–11877. doi: 10.1158/0008-5472.CAN-06-1887. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci U S A. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon KJ. Cancer gene therapy: Challenges and opportunities. Anticancer Res. 2004;24(2A):501–504. [PubMed] [Google Scholar]
- Shanker M, Jin J, Branch CD, Miyamoto S, Grimm EA, Roth JA, Ramesh R. Tumor suppressor gene-based nanotherapy: From test tube to the clinic. J Drug Deliv. 2011;2011:465845. doi: 10.1155/2011/465845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova EV, Doronin K, Senac JS, Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- Sokhi UK, Bacolod MD, Dasgupta S, Emdad L, Das SK, Dumur CI, Miles MF, Sarkar D, Fisher PB. Identification of genes potentially regulated by human polynucleotide phosphorylase (hPNPase(old-35)) using melanoma as a model. PLoS One 8(10) 2013;8:e76284. doi: 10.1371/journal.pone.0076284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhi UK, Bacolod MD, Emdad L, Das SK, Dumur CI, Miles MF, Sarkar D, Fisher PB. Analysis of global changes in gene expression induced by human polynucleotide phosphorylase (hPNPase0ld-35) J Cell Physiol. 2014 doi: 10.1002/jcp.24645. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolberg SG. The biotech death of Jesse Gelsinger. NY Times Mag. Nov 28;136–140:149–150. [PubMed] [Google Scholar]
- Studeny M, Champlin MF, Zompetta RE, Fidler C. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Su ZZ, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005a;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, Young CSH, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Sarkar D, Emdad L, Duigou GJ, Young CSH, Ware J, Randolph A, Valerie K, Fisher PB. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Pro Natl Acad Sci U S A. 2005b;102:1059–1064. doi: 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Shi Y, Fisher PB. Subtraction hybridization identifies a transformation progression-associated gene PEG-3 with sequence homology to a growth arrest and DNA damage-inducible gene. Proc Natl Acad Sci U S A. 1997;94:9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Olive M, Champagne K, Flomenberg N, Eisenlohr L, Hsu S, Flomenberg P. Adenovirus hexon T-cell epitope is recognized by most adults and is restricted by HLA DP4, the most common class II allele. Gene Therapy. 2004;11:1408–1415. doi: 10.1038/sj.gt.3302316. [DOI] [PubMed] [Google Scholar]
- Terheggen HG, Lowenthal A, Lavinha F, Colombo JP, Rogers S. Unsuccessful trial of gene replacement in arginase deficiency. Z Kinderheilkd. 1975;119:1–3. doi: 10.1007/BF00464689. [DOI] [PubMed] [Google Scholar]
- Tatum EI. Molecular biology, nucleic acids, and the future of medicine. Perspect Biol Med. 1966;10:19–32. doi: 10.1353/pbm.1966.0027. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ, Cattaneo R. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther. 2007;15:1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV, Lamfers ML, Rein D, Lesniak MS, Siegal GP, Dirven CM, Curiel DT, Zhu ZB. The human survivin promoter: A novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006;104:583–592. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- Vanderplasschen A, Mathew E, Hollinshead M, Sim RB, Smith GL. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc Natl Acad Sci U S A. 1998;95:7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigderman L, Zubarev ER. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv Drug Deliv Rev. 2013;65:663–676. doi: 10.1016/j.addr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Volk AL, Rivera AA, Kanerva A, Bauerschmitz G, Dmitriev I, Nettelbeck DM, Curiel DT. Enhanced adenovirus infection of melanoma cells by fiber-modification: Incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biol Ther. 2003;2:511–515. doi: 10.4161/cbt.2.5.440. [DOI] [PubMed] [Google Scholar]
- Wang J, Voutetakis A, Papa M, Rivera VM, Clackson T, Lodde BM, Mineshiba F, Baum BJ. Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands. Gene Ther. 2006;13:187–190. doi: 10.1038/sj.gt.3302647. [DOI] [PubMed] [Google Scholar]
- Westphal M, Yla-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, Kinley J, Kay R, Ram Z. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–833. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- Wiman KG. The retinoblastoma gene: Role in cell cycle control and cell differentiation. FASEB J. 1993;7:841–845. doi: 10.1096/fasebj.7.10.8393817. [DOI] [PubMed] [Google Scholar]
- Wirth T, Parker N, Yla-Herttuala S. History of gene therapy. Gene. 2013;525:162–169. doi: 10.1016/j.gene.2013.03.137. [DOI] [PubMed] [Google Scholar]
- Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, Manns M, Kubicka S, Kuhnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. [PubMed] [Google Scholar]
- Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, Curiel DT. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647–1653. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- Xie X, Mathias JR, Smith MA, Walker SL, Teng Y, Distel M, Koster RW, Sirotkin HI, Saxena MT, Mumm JS. Silencer-delimited transgenesis: NRSE/RE1 sequences promote neural- specific transgene expression in a NRSF/REST-dependent manner. BMC Biol. 2013;10:93–99. doi: 10.1186/1741-7007-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Krimmel J, Zhang Z, Hu Z, Seth P. Systemic delivery of a novel liver-detargeted oncolytic adenovirus causes reduced liver toxicity but maintains the antitumor response in a breast cancer bone metastasis model. Hum Gene Ther. 2011;22:1137–1142. doi: 10.1089/hum.2011.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Bocangel D, Ramesh R, Ekmekcioglu S, Poindexter N, Grimm EA, Chada S. Interleukin-24 overcomes temozolomide resistance and enhances cell death by down-regulation of O6-methylguanine-DNA methyltransferase in human melanoma cells. Mol Cancer Ther. 2008;7:3842–3851. doi: 10.1158/1535-7163.MCT-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]