Abstract
Introduction
Rates of abnormal visual inspection with acetic acid (VIA) and prevalence of high-risk human papillomavirus (HPV) subtypes have not been well characterized in HIV-infected women in Malawi.
Methods
We performed a prospective cohort study of VIA (N=440) in HIV-infected women ages 25-59, with a nested study of HPV subtypes in first 300 women enrolled. Wilcoxon's Rank-Sum Test was used to compare continuous variables and Fisher's exact test was used to compare categorical variables between women with normal versus abnormal VIA.
Results: Of 440 women screened, 9.5% (N=42) had abnormal VIA with 69.0% (N=29) having advanced disease not amenable to cryotherapy. Of 294 women with HPV results, 39% (N=114) of women were positive for high-risk HPV infection. Only lower CD4 count (287 cells/mm3 vs. 339 cells/mm3, p=0.03) and high-risk HPV (66.7% versus 35.6%, p<0.01) were associated with abnormal VIA. The most common high-risk HPV subtypes in women with abnormal VIA were 35 (33.3%), 16 (26.7%), and 58 (23.3%).
Conclusion
Low CD4 cell count was associated with abnormal VIA and raises the importance of early ART and expanded availability of VIA. HPV vaccines targeting additional non-16/18 high-risk HPV subtypes may have greater protective advantages in countries such as Malawi.
Keywords: HIV/AIDS, cervical cancer, visual inspection with acetic acid (VIA), human papillomavirus (HPV)
Introduction
In Malawi, cervical cancer is the most common cancer among women, accounting for approximately 45% of all female cancers and resulting in over 1600 deaths per year.1, 2 The incidence of cervical intraepithelial neoplasia (CIN) is 4-5 times higher in HIV-infected compared to HIV-uninfected women3, 4 and the risk of invasive cervical cancer is 5-8 times higher.5-7 Women in Sub-Saharan Africa often do not have access to cervical cancer screening given lack of infrastructure and financial resources for Pap smears, biopsies, and colposcopy.8, 9 Visual inspection with acetic acid (VIA) has emerged as a more feasible strategy for screening compared to Pap smears, but has been associated with high rates of loss to follow-up when women have been asked to return at a later date for cryotherapy and/or other interventions.10 As a result of these challenges, many programs in resource- limited settings have implemented a “see and treat” approach using VIA and immediate cryotherapy for abnormalities. Screening programs using VIA in Africa have been found to be feasible, safe, and acceptable for both health care providers and for patients.11-14
Cervical cancer is known to be associated with infection with high-risk oncogenic Human Papillomavirus (HPV) subtypes and while at least one study has shown HPV testing followed by cryotherapy to be more efficacious at reducing CIN grade 2 and above (CIN2+) than VIA15, in many resource-limited settings, including Malawi, there is no availability for HPV testing and the introduction of direct HPV tests for screening purposes may be prohibitive due to lack of infrastructure and high cost. The use of VIA has been piloted in several centers in Malawi since 2005 and has proven to be implementable16, but has not been widely integrated into HIV care due to limitations in trained personnel, availability of supplies and equipment (such as for cryotherapy), and cost. While there has been discussion of HPV vaccination in Malawi, an HPV vaccine is currently not widely available. We implemented VIA in a large HIV clinic in Lilongwe and sought to determine the prevalence of cervical abnormalities seen with VIA, characterize HPV subtypes, and evaluate the prevalence of high-risk HPV-infection in HIV-infected women with and without abnormalities on VIA.
Methods
We performed a prospective cohort study of VIA with a nested study of HPV subtypes. The study was performed at Partners in Hope Medical Center in Lilongwe, Malawi, a free HIV/AIDS clinic providing care to approximately 5,000 patients, in a new screen-and-treat VIA program in HIV-infected women. The study took place over a 6-month period from November 2011 to April 2012. All women coming to the clinic for routine antiretroviral therapy (ART) or pre-ART care were offered VIA and given the opportunity to participate in the study. Women were eligible for the study if they were HIV-positive, 25-59 years of age, able to provide informed consent, and had a history of vaginal intercourse. Women were excluded if they were pregnant, less than 6 weeks postpartum, had a history of hysterectomy, or had prior cryotherapy or cervical cancer. The most recent CD4 cell count prior to VIA was abstracted from the patient chart. The study was approved by the Malawi National Health Sciences Research Committee (#893) and by the University of California Los Angeles Institutional Review Board (#10-001710).
Research Procedures
Participants were administered a baseline questionnaire to assess risk factors for cervical dysplasia and HPV infection. Women underwent a speculum examination with VIA which was performed using 3-5% acetic acid applied to the cervix, waiting one minute, and then examining the cervix to identify suspicious lesions. VIA lesions were classified as per the International Agency for Research on Cancer Guidelines (IARC/WHO).17 The first 300 participants underwent HPV testing. HPV samples were collected prior to VIA using standard cytobrush technique and placed in ThinPrep preservative (Hologic, Marlborough, MA). The number of women HPV tested was determined by available funding for this nested aim. HPV specimens were shipped to the University of California San Francisco in San Francisco, California for low and high-risk HPV DNA analysis by polymerase chain reaction. A crude DNA preparation was made by pelleting 1.5 ml of the Hologic cell suspension. After the pellet was allowed to dry, it was suspended in 100 μl Qiagen Specimen Transport Media with proteinase K (Invitrogen Life Technologies) at a concentration of 200 μg/ml and incubated at 56°C for 1 hour, then the proteinase K was heat inactivated. PCR was performed using a modified pool of MY09/MY11 consensus HPV L1 primers as well as primers for amplification of the human beta-globin as an indicator of specimen adequacy as described previously.18 After 40 amplification cycles, specimens were probed with a biotin-labeled HPV L1 consensus probe mixture. A separate membrane was probed with a biotin-labeled probe to the human beta-globin gene. Specimens were also typed by hybridizing to 38 different HPV types.19
Women with abnormal lesions seen on VIA that met criteria (raised and thickened white plaques or acetowhite epithelium near the squamocolumnar junction) were offered same day treatment with a standard cryotherapy procedure (two, three-minute freezing periods separated by five minutes). Women who reported increased vaginal discharge, lower pelvic pain, or were noted on exam to have abnormal discharge were give treatment for presumed STD and asked to return on a later date for cryotherapy. Individuals with lesions extending to the vaginal wall, lesions involving more than 75% of the cervix or > 2mm beyond the cryoprobe (including the tip of probe), and women suspected of having invasive cancer were referred to the central hospital in Lilong we for further evaluation. VIA and cryotherapy were performed by three experienced nurses who had completed a five-day training approved by the Ministry of Health. Quality control officials from the Ministry of Health visited eight times over six months to monitor and evaluate the nurse's clinical assessments of cervical lesions.
Statistical Analysis
This study was designed as a pilot to acquire preliminary data over a limited time period (6 months). Summary statistics were generated for basic socio-demographic information and for baseline clinical information. Wilcoxon's Rank-Sum Test was used to compare continuous variables and Fisher's exact test was used to compare categorical variables between the women who did versus those who did not undergo HPV screening, between the women with normal and abnormal VIA, and between the women with and without one or more high-risk HPV subtypes. A frequency table was also constructed to present the distribution of the various HPV subtypes in the population. All tests are two sided and all analyses were carried out using SAS v9.3 (Cary, North Carolina).
Results
Between November 2011 and April 2012 a total of 446 women were screened for the study. All women from this group were enrolled in the prospective cohort study of VIA, with the first 300 women also enrolled into the nested substudy of HPV infection. Six women were excluded from the analysis because of missing data, leaving 440 women in the cohort and 294 women with HPV results. The median age for all participants was 35.0 years (IQR 31, 42), median CD4 count was 337 cells/mm3 (IQR 215, 491), 81.8% were on antiretroviral therapy (ART), and 89.5% were undergoing VIA for the first time. Participants who were tested for HPV had a lower CD4 nadir (188 cells/mm3 vs. 232 cells/mm3, p=0.04), and were more likely to be on ART (84.7% vs. 76.0%, p= 0.04) compared to women who did not have HPV screening. Table 1 describes baseline characteristics of all women as well as comparisons between those who did versus did not receive HPV testing.
Table 1. Baseline characteristics of women in the cohort and comparisons of women who did versus did not receive HPV testing.
| Variable | All N = 440 | No HPV Testing N = 146 | HPV Testing N = 294 | P Value* |
|---|---|---|---|---|
| Median age(IQR) | 35 (31, 42) | 34.5 (31, 41) | 36 (30, 43) | 0.32 |
| Median months on ART(IQR) | 36 (12, 48) | 36 (24, 60) | 24 (12, 48) | 0.06 |
| Median months since HIV diagnosis(IQR) | 36 (24, 60) | 48 (24, 60) | 36 (24, 60) | 0.72 |
| ˆMedian CD4 Count (cells/mm3)(IQR) | 337 (215, 491) | 358.5 (257, 493) | 329.5 (200, 488) | 0.14 |
| %Median CD4 Nadir (cells/mm3)(IQR) | 203 (117, 314) | 232 (127, 333.5) | 188 (111, 275) | 0.04 |
| Median number of lifetime sex partners(IQR) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 0.44 |
| Median number of sex partners in last month(IQR) | 1 (0, 1) | 1 (0, 1) | 1 (0, 1) | 0.87 |
| Median age at first intercourse(IQR) | 18 (16, 20) | 18 (16, 20) | 18 (16, 20) | 0.20 |
| Education N, % | 0.52 | |||
| No schooling | 37 (8.4) | 10 (6.9) | 27 (9.2) | |
| Primary school completed | 251 (57.1) | 79 (54.1) | 172 (58.5) | |
| Secondary school completed | 141 (32.1) | 53 (36.3) | 88 (29.9) | |
| University completed | 11 (2.5) | 4 (2.7) | 7 (2.4) | |
| On ART N, % | 0.04 | |||
| Yes | 360 (81.8) | 111 (76.0) | 249 (84.7) | |
| No | 80 (18.2) | 35 (24.0) | 45 (15.3) | |
| Number of pregnancies N, % | 0.71 | |||
| 0 | 10 (2.3) | 3 (2.1) | 7 (2.4) | |
| 1 | 32 (7.3) | 12 (8.2) | 20 (6.8) | |
| 2 | 47 (10.7) | 12 (8.2) | 35 (11.9) | |
| 3-5 | 232 (52.7) | 82 (56.2) | 150 (51.0) | |
| 6-10 | 112 (25.5) | 36 (24.7) | 76 (25.9) | |
| More than 10 | 7 (1.6) | 1 (0.7) | 6 (2.0) | |
| Bleeding after sex N, % | 0.10 | |||
| Yes | 45 (10.2) | 20 (13.7) | 25 (8.5) | |
| No | 395 (89.8) | 126 (86.3) | 269 (91.5) | |
| History of sexually transmitted infection N, % | 0.40 | |||
| Yes | 127 (28.9) | 48 (32.9) | 79 (26.9) | |
| No | 307 (69.8) | 96 (65.8) | 211 (71.8) | |
| Not sure | 6 (1.4) | 2 (1.4) | 4 (1.4) | |
| Primary sex partner circumcised N, % | 0.53 | |||
| Yes | 103 (31.2) | 38 (33.6) | 65 (30.0) | |
| No | 227 (68.8) | 75 (66.4) | 152 (70.1) |
P-value for HPV tested versus not tested. For numeric variables, the Wilcoxon Rank Sum test was used. For categorical variables, Fisher's Exact Test was used
Median CD4 cell count was available for 420 of 440 participants (95.5%) and was collected a median of 14.6 months (IQR: 8.4, 18.0) prior to VIA
Median nadir CD4 cell count was available for 417 of 440 participants (94.8%)
Ten percent of women (N=42) had abnormal VIA. Of these, 16.7% (N=7) received same day cryotherapy, 14.3% (N=6) returned on a later date for cryotherapy, either because they required syndromic sexually transmitted infection (STI) treatment prior to cryotherapy or desired permission from a spouse before undergoing the procedure, and 69.0% (N=29) were referred to KCH. Reasons for referral to KCH included clinically suspected cervical cancer (N=4), lesions extending to the vaginal wall (N=4), lesions involving more than 75% of the cervix (N=2), and lesions more than 2mm beyond the cryoprobe (N=19) (Figure). Lower median CD4 count (287 cells/mm3 vs. 339 cells/mm3, p=0.03) and infection with one or more high-risk HPV (66.7% versus 35.6%, p<0.01) were associated with abnormal VIA. Age, duration of HIV infection, ART status and duration, median number of lifetime sex partners, and number of sex partners within the last month were not associated with abnormal VIA.
Figure. Schematic showing distribution of normal versus abnormal VIA, HPV, and outcome (cryotherapy versus referral).
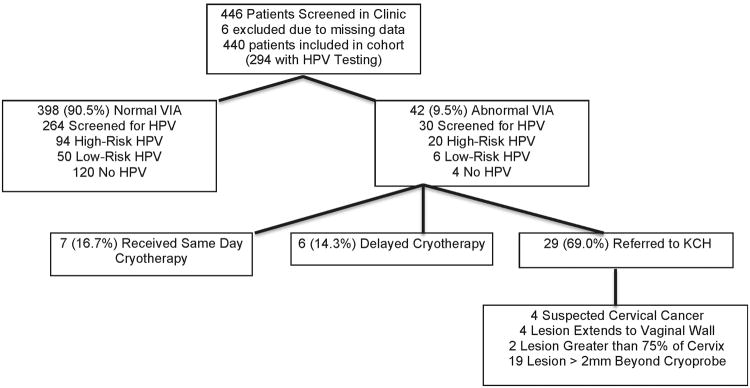
A total of 57.8% of HPV screened women (N=170) had HPV detected, with 38.8% (N=114) having at least one high-risk subtype. HPV 58 was the most frequent high-risk HPV subtype (9.5%), followed by HPV 35 (8.5%) (Table 2). Thirty women with abnormal VIA were among the subgroup of women tested for HPV infection. Of these, 66.7% (N= 20) had high-risk HPV infection with the most common subtypes being 35 (33.3%), 16 (26.7%), and 58 (23.3%). Among the 264 women with normal VIA who underwent HPV testing, 35.6% (N=94) tested positive for high-risk HPV with the most common subtypes 58 (8.0%), 35 (5.7%), and 16 (5.7%) (Table 2). Twelve percent (N=36) of women were infected with either HPV 16 or 18; however, one of these subtypes was present in 36.7% of women with abnormal VIA. Among those with abnormal VIA, the median number of high-risk HPV subtypes per woman was 1 (range 0-3) compared to 0 (range 0-1) in women with normal VIA (p<0.01).
Table 2. Distribution of high-risk HPV subtypes overall and by VIA status (N = 294).
| High-risk HPV subtype | Percent of all HPV subtypes1 | Frequency | Overall Prevalence (%)2 | Abnormal VIA N = 30 | Normal VIA N = 264 | P-value* |
|---|---|---|---|---|---|---|
| Type 58 | 5.7 | 28 | 9.5 | 7 (23.3) | 21 (8.0) | 0.01 |
| Type 35 | 5.1 | 25 | 8.5 | 10 (33.3) | 15 (5.7) | < 0.01 |
| Type 16 | 4.7 | 23 | 7.8 | 8 (26.7) | 15 (5.7) | < 0.01 |
| Type 33 | 3.4 | 17 | 5.8 | 3 (10.0) | 14 (5.3) | 0.40 |
| Type 52 | 3.4 | 17 | 5.8 | 6 (20.0) | 11 (4.2) | 0.05 |
| Type 18 | 2.6 | 13 | 4.4 | 3 (10.0) | 10 (3.8) | 0.13 |
| Type 51 | 2.4 | 12 | 4.1 | 2 (6.7) | 10 (3.8) | 0.35 |
| Type 56 | 1.8 | 9 | 3.1 | 1 (3.3) | 8 (3.0) | 1.0 |
| Type 82 | 1.8 | 9 | 3.1 | 3 (10.0) | 6 (2.3) | < 0.01 |
| Type 68 | 1.4 | 7 | 2.4 | 2 (6.7) | 5 (1.9) | 0.15 |
| Type 31 | 1.0 | 5 | 1.7 | 1 (3.3) | 4 (1.5) | 0.42 |
| Type 39 | 1.0 | 5 | 1.7 | 1 (3.3) | 4 (1.5) | 0.42 |
| Type 45 | 0.6 | 3 | 1.0 | 1 (3.3) | 2 (0.8) | 0.28 |
| Type 59 | 0.6 | 3 | 1.0 | 1 (3.3) | 2 (0.8) | 0.27 |
| Type 73 | 0.2 | 1 | 0.3 | 1 (3.3) | 0(0) | 0.10 |
Frequency divided by the total number of occurrences of all HPV subtypes (Total = 495).
Frequency divided by the number of women with HPV Testing (N = 294)
Fisher's exact test for abnormal versus normal VIA
Women with and without high-risk HPV were compared (Table 3). Shorter median duration of ART (2 years versus 3 years, p <0.01) and shorter duration since HIV diagnosis (3 years versus 4 years, p < 0.01) were associated with having at least one high-risk HPV type. While younger age was associated with infection with high-risk HPV, the age difference between the two groups was minimal (35 versus 36 years, p= 0.02).
Table 3. Comparison of women with and without high-risk HPV.
| Variable | HPV Tested N = 294 | Negative for high-risk HPV N = 180 | Positive for ≥ 1 high-risk HPV subtype N = 114 | P Value |
|---|---|---|---|---|
| Median age(IQR) | 36 (30, 43) | 36 (32, 44) | 35 (30, 42) | 0.02 |
| Median months on ART(IQR) | 24 (12, 48) | 36 (24, 60) | 24 (11, 36) | <.01 |
| Median months since diagnosis(IQR) | 36 (24, 60) | 48 (24, 72) | 36 (12, 60) | <.01 |
| Median CD4 count (cells/mm3)(IQR) | 329.5 (200, 488) | 364 (247, 534) | 256 (159, 380) | <.01 |
| Median CD4 nadir (cells/mm3)(IQR) | 188 (111, 275) | 194 (120, 288) | 180 (86, 274) | 0.20 |
| Median number of lifetime sex partners(IQR) | 3 (2, 4) | 3 (2, 3) | 3 (2, 4) | 0.48 |
| Median age at first intercourse(IQR) | 18 (16, 20) | 18 (16, 20) | 18 (16, 19) | 0.32 |
| Education N, % | 0.44 | |||
| No schooling | 27 (9.18) | 16 (8.89) | 11 (9.65) | |
| Primary school completed | 172 (58.5) | 111 (61.67) | 61 (53.51) | |
| Secondary school completed | 88 (29.93) | 50 (27.78) | 38 (33.33) | |
| University completed | 7 (2.38) | 3 (1.67) | 4 (3.51) | |
| On ART N, % | 0.87 | |||
| Yes | 249 (84.69) | 153 (85) | 96 (84.21) | |
| No | 45 (15.31) | 27 (15) | 18 (15.79) | |
| Bleeding after sex N, % | 0.67 | |||
| Yes | 25 (8.5) | 14 (7.78) | 11 (9.65) | |
| No | 269 (91.5) | 166 (92.22) | 103 (90.35) | |
| History of sexually transmitted infectionˆ N, % | 0.92 | |||
| Yes | 79 (26.87) | 49 (27.22) | 30 (26.32) | |
| No | 211 (71.77) | 129 (71.67) | 82 (71.93) | |
| Not sure | 4 (1.36) | 2 (1.11) | 2 (1.75) | |
| Primary sex partner circumcised N, % | 1 | |||
| Yes | 65 (29.95) | 39 (29.77) | 26 (30.23) | |
| No | 152 (70.05) | 92 (70.23) | 60 (69.77) |
For numeric variables, the Wilcoxon Rank Sum test was used. For categorical variables, Fisher's Exact Test was used
Discussion
Abnormal VIA in HIV-infected women in the ART-era in Malawi
Studies of HIV-infected women in Africa have shown an abnormal VIA rate between 6.5 to 55%.14, 20-22 Ten percent of women in our study had abnormal VIA, with the majority (69%) having extensive disease not amenable to cryotherapy. The high rate of those with advanced disease is likely attributable to the fact that VIA was not widely available to this population prior to this study, with the majority of women (89.5%) undergoing first-time screening. Similar data was reported by Jhpiego during the implementation of VIA in Guyana, Cote D'Ivoire, and Tanzania, with high prevalence of advanced disease in HIV-infected women screened for the first time.23
In our cohort, aside from high-risk HPV, only lower CD4 cell count was associated with abnormal VIA, with every 100 cell/mm3 gain associated with a 19% decrease in the odds of having abnormal VIA (p = 0.03). Several other studies have reported this association, as well as an association between low CD4 cell count and high-risk HPV infection and squamous intraepithelial lesions.24-26 Higher HIV viral load has also been associated with cervical dysplasia, as well as the number of lifetime sexual partners and >2 sexual partners in the last year.24, 27, 28 We did not have viral load values available to test this association in our cohort and did not find an association with the number of sex partners. Identifying features strongly associated with cervical abnormalities can help prioritize VIA and other screening strategies, particularly in settings like Malawi, where capacity for routine screening of all women remains extremely limited. Several authors included on this study participate in a large President's Emergency Plan for AIDS Relief (PEPFAR) care and treatment program in Malawi. Among sites supported in this program, VIA is offered at only 9.1% of ART sites (5/55) and is largely limited to hospitals and urban clinics. Increased efforts towards VIA implementation would have tremendous benefit, but are challenging due to limited financial and human resources.
High-risk HPV in HIV-infected women in the ART-era in Malawi
Our study had a similar rate of overall HPV infection (57.8%) compared to a large South African study (61%)20, but we found lower rates of high-risk HPV (39%) compared to most other studies from Africa, which have reported rates of 46%-90%.15, 24, 26, 27, 29 The only prior published data on cervical lesions and HPV from Malawi was done in the pre-ART era and utilized Pap smear and cervical lavage in 124 HIV-infected women. This study reported squamous intraepithelial lesions in 15 % of HIV-infected women and found high-risk HPV DNA in 60% of those with CD4 counts < 300 cells/mm3; however, the specific distribution of HPV subtypes were not described in this study.30 It is possible that ART reduces cervical abnormalities and HPV infection rates and this could explain the difference in our results compared to the prior study from the pre-ART era in Malawi. Published data about ART and HPV infection is conflicting,31 with one study showing ART to be associated with decreased prevalence and incidence of high-risk HPV32 and another demonstrating ART to be associated with increased HPV clearance in women with CIN, but not in women with normal cervical cytology.33 Eighty-two percent of participants in our study were on ART and this large number limited power to test associations between ART, cervical abnormalities, and high-risk HPV.
Normal VIA and the presence of high-risk HPV: Implications for screening strategies
In our study, 36% of women with normal VIA had at least one high-risk HPV subtype. HIV-infected women with high-risk HPV infection and normal VIA will be at higher risk for HPV persistence and development of cervical dysplasia in the future.34, 35 Several studies from Africa have explored direct tests for HPV as a screening method.15, 20 A recent study from South Africa compared Pap smear, HPV testing, and VIA and found that, as a stand-alone test for CIN2+, HPV was the most sensitive test (92% sensitivity) followed by Pap smear (76%) and VIA (65.5%); however, HPV had the lowest specificity of the three options (51%) with specificity further reduced at CD4 counts ≤ 200 cells/mm3.20 In the Malawian setting, HPV screening would require a large infusion of funds to establish the necessary infrastructure and would need to be weighed against the lower specificity of this method (51.4% compared to 68% for VIA). A more feasible approach may be forthcoming with the Care-HPV (QIAGEN/PATH)36 which can be done more easily in resource-limited settings, but implementation would need to be accompanied by expanded availability of VIA and interventions such as cryotherapy.
High-risk HPV subtypes: Implications for an HPV vaccination program
HPV 58 and 35 were the most prevalent high-risk HPV types seen in our study population, accounting for almost 20% of infections. A study done in Zambia found that 98% of HIV-infected women harbored at least one high-risk HPV subtype with a median of four subtypes per person. In this study, HPV 52 was the common (37%), followed by 16 (17%) and 18 (13%)25, while a study from Botswana showed HPV 58 to be most common (26%) followed by 39and 16 (20% each).27 Several South African studies have shown high prevalence of HPV 16 and 18.22, 37 Common themes from these studies include high rates of current HPV infection (68%-98%) and multiple HPV infection, with a median of 2-4 subtypes per woman.
Taken together, studies from HIV-infected women in Africa suggest important geographic differences in HPV infection, with a tendency toward non-16 and 18 subtypes to be most prevalent. The quadrivalent HPV vaccine (Gardisil) protects against 6/11/16/18, but is thought to offer some cross protection against subtype 31. The bivalent vaccine (Cervarix) protects against 16/18 and may offer cross protection against subtypes 31/33/45/52/58.38 Vaccines directly targeting additional HPV subtypes may have even greater protective advantages in countries such as Malawi, due to high prevalence of non-16/18 subtypes reported from these settings. Randomized clinical trials are currently examining the efficacy of a nonavalent HPV vaccine (types 6/11/16/18/31/33/45/52/58), with preliminary modeling data suggesting high potential to further reduce precancerous lesions and cervical cancer.39 In early 2013, the GAVI Alliance announced support for eight countries, including Malawi, to receive support to provide HPV vaccination to girls ages 9-13.40 The funding has been provided in order to allow countries to test their ability to develop the systems that would be needed to roll out the HPV vaccines nationally, and to inform future policy decisions. Sustainable financing for such a vaccination program in Malawi remains a major barrier to date.41
Study Limitations
Although we had a quality control officer visiting our site once every two weeks, we did not use photographs or more advanced imaging techniques for confirming VIA findings, and it is possible our study underestimates the rate of abnormal VIA. Given the large number of women on ART (82%), we had limited power to determine associations of ART with abnormal VIA and/or high-risk HPV. Participants who were tested for HPV had a lower CD4 nadir and were more likely to be on ART suggesting that the subset with HPV data may not have been representative of the entire clinical cohort and data may not be generalizable. Finally, we studied HPV among women with abnormal VIA and cannot comment specifically on subtypes most contributory to invasive cervical cancer.
Conclusions
We found high rates of advanced disease at time of VIA among this group of women undergoing screening. Low CD4 cell count was associated with abnormal VIA and raises the importance of early initiation of ART for those eligible, and expanded availability of VIA within HIV care and treatment programs in Malawi. The expansion of ART in Malawi may lead to better overall outcomes via improved clearance of HPV and reduction in CIN, although the role of ART in HPV infection remains unclear and is an important area for further research. Sustainable resources for HPV vaccination programs will be of great benefit in preventing CIN and invasive cervical cancer in Malawi and other areas of Sub-Saharan Africa.
Acknowledgments
We would like to thank the patients at Partners in Hope Center for their participation in the study. Additionally, we are grateful to the administration, providers, and staff at Partners in Hope who provided support for the study.
Funding: This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through USAID-Malawi under the terms of Grant No. 674-A-00-10-00035-00 as well as by a seed grant from the UCLA Center for AIDS Research (AI28697).
Footnotes
Declaration of Conflicting Interests: The authors have no disclosures or conflicts of interest.
References
- 1.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC research notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization and Institut Catala d'Oncologia. Geneva: 2010. [Accessed February 14, 2014]. Human Papillomavirus and Related Cancers: Malawi, Summary Report Update. Available at http://www.hpvcentre.net/statistics/reports/MWI.pdf. [Google Scholar]
- 3.Moscicki AB, Ellenberg JH, Crowley-Nowick P, Darragh TM, Xu J, Fahrat S. Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. The Journal of infectious diseases. 2004;190:1413–21. doi: 10.1086/424466. [DOI] [PubMed] [Google Scholar]
- 4.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA : the journal of the American Medical Association. 2000;283:1031–7. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. Journal of the National Cancer Institute. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 6.Serraino D, Dal Maso L, La Vecchia C, Franceschi S. Invasive cervical cancer as an AIDS-defining illness in Europe. Aids. 2002;16:781–6. doi: 10.1097/00002030-200203290-00014. [DOI] [PubMed] [Google Scholar]
- 7.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. Journal of the National Cancer Institute. 2000;92:1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 8.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;24(Suppl 3):S3/71–7. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 9.Denny L. The prevention of cervical cancer in developing countries. BJOG : an international journal of obstetrics and gynaecology. 2005;112:1204–12. doi: 10.1111/j.1471-0528.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- 10.Khozaim K, Orang'o E, Christoffersen-Deb A, et al. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2014;124:12–8. doi: 10.1016/j.ijgo.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Rajkumar R, Esmy PO, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. British journal of cancer. 2007;96:738–43. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2005;294:2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal PD, Gaffikin L, Deganus S, et al. Cervical cancer prevention: safety, acceptability, and feasibility of a single-visit approach in Accra, Ghana. American journal of obstetrics and gynecology. 2007;196:407 e1–8. doi: 10.1016/j.ajog.2006.12.031. discussion e8-9. [DOI] [PubMed] [Google Scholar]
- 14.Ramogola-Masire D, de Klerk R, Monare B, Ratshaa B, Friedman HM, Zetola NM. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. Journal of acquired immune deficiency syndromes. 2012;59:308–13. doi: 10.1097/QAI.0b013e3182426227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn L, Wang C, Tsai WY, Wright TC, Denny L. Efficacy of human papillomavirus-based screen-and-treat for cervical cancer prevention among HIV-infected women. Aids. 2010;24:2553–61. doi: 10.1097/QAD.0b013e32833e163e. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization International Agency for Research on Cancer. African Population and Health Research Center; Geneva: 2012. [Accessed February 14, 2014]. Prevention of cervical cancer through screening using visual insepction with acetic acid (VIA) and treatment with cryotherapy: A demonstration project in six African countries. Available at http://apps.who.int/iris/bitstream/10665/75250/1/9789241503860_eng.pdf. [Google Scholar]
- 17.World Health Organization. Geneva: 2013. [Accessed February 14, 2014]. Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. Available at http://www.who.int/reproductivehealth/publications/cancers/screening_and_treatment_of_precancerous_lesions/en/index.html. [PubMed] [Google Scholar]
- 18.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. The Journal of infectious diseases. 1998;177:361–7. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 19.Morrison EA, Goldberg GL, Kadish AS, Burk RD. Polymerase chain reaction detection of human papillomavirus: quantitation may improve clinical utility. Journal of clinical microbiology. 1992;30:2539–43. doi: 10.1128/jcm.30.10.2539-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firnhaber C, Mayisela N, Mao L, et al. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PloS one. 2013;8:e53494. doi: 10.1371/journal.pone.0053494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mabeya H, Khozaim K, Liu T, et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid among human immunodeficiency virus-infected women in Western Kenya. Journal of lower genital tract disease. 2012;16:92–7. doi: 10.1097/LGT.0b013e3182320f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firnhaber C, Zungu K, Levin S, et al. Diverse and high prevalence of human papillomavirus associated with a significant high rate of cervical dysplasia in human immunodeficiency virus-infected women in Johannesburg, South Africa. Acta cytologica. 2009;53:10–7. doi: 10.1159/000325079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson J, Lu E, Harris M, Kibwana S, Estep D, Varallo J, Coulibaly TK, Giattas M. Initial Results from a Multi-country Cervical Cancer Screening Program for HIV+ Women. Paper presented at: Conference on Retroviruses and Opportunistic Infections; Feb 27-Mar 2, 2011; Boston, Massachusetts. [Google Scholar]
- 24.Denny L, Boa R, Williamson AL, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstetrics and gynecology. 2008;111:1380–7. doi: 10.1097/AOG.0b013e3181743327. [DOI] [PubMed] [Google Scholar]
- 25.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. British journal of cancer. 2007;96:1480–3. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, et al. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecologic oncology. 2006;103:1017–22. doi: 10.1016/j.ygyno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macleod IJ, O'Donnell B, Moyo S, et al. Prevalence of human papillomavirus genotypes and associated cervical squamous intraepithelial lesions in HIV-infected women in Botswana. Journal of medical virology. 2011;83:1689–95. doi: 10.1002/jmv.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safaeian M, Kiddugavu M, Gravitt PE, et al. Prevalence and risk factors for carcinogenic human papillomavirus infections in rural Rakai, Uganda. Sexually transmitted infections. 2008;84:306–11. doi: 10.1136/sti.2007.027318. [DOI] [PubMed] [Google Scholar]
- 29.Banura C, Franceschi S, Doorn LJ, et al. Infection with human papillomavirus and HIV among young women in Kampala, Uganda. The Journal of infectious diseases. 2008;197:555–62. doi: 10.1086/526792. [DOI] [PubMed] [Google Scholar]
- 30.Motti PG, Dallabetta GA, Daniel RW, et al. Cervical abnormalities, human papillomavirus, and human immunodeficiency virus infections in women in Malawi. The Journal of infectious diseases. 1996;173:714–7. doi: 10.1093/infdis/173.3.714. [DOI] [PubMed] [Google Scholar]
- 31.Bratcher LF, Sahasrabuddhe VV. The impact of antiretroviral therapy on HPV and cervical intraepithelial neoplasia: current evidence and directions for future research. Infectious agents and cancer. 2010;5:8. doi: 10.1186/1750-9378-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. The Journal of infectious diseases. 2010;201:681–90. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paramsothy P, Jamieson DJ, Heilig CM, et al. The effect of highly active antiretroviral therapy on human papillomavirus clearance and cervical cytology. Obstetrics and gynecology. 2009;113:26–31. doi: 10.1097/AOG.0b013e31819225cb. [DOI] [PubMed] [Google Scholar]
- 34.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. The Journal of infectious diseases. 2004;190:37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 35.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. The Journal of infectious diseases. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 36.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. The lancet oncology. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 37.De Vuyst H, Ndirangu G, Moodley M, et al. Prevalence of human papillomavirus in women with invasive cervical carcinoma by HIV status in Kenya and South Africa. International journal of cancer Journal international du cancer. 2012;131:949–55. doi: 10.1002/ijc.26470. [DOI] [PubMed] [Google Scholar]
- 38.Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 39.Van de Velde N, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. Journal of the National Cancer Institute. 2012;104:1712–23. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 40.Alliance G. GAVI funds vaccines to protect girls against cervical cancer. 2013 [Google Scholar]
- 41.Financing HPV vaccination in developing countries. The Lancet. 2011;377:1544. doi: 10.1016/S0140-6736(11)60622-3. [DOI] [PubMed] [Google Scholar]


