Abstract
Elevated levels of the chemokine, CXCL13, have been observed in the plasma of chronic HIV-1 infected subjects and have been correlated with plasma viremia, which in turn has been linked to progressive dysregulation of humoral responses. In this study we sought to identify mechanisms of CXCL13 induction in response to HIV-1 infection. Plasma levels of CXCL13 in HIV-1 infected ART-naïve subjects correlated with viral load and were higher compared to ART-treated HIV-1 infected and HIV-1-uninfected subjects. To elucidate the relationship between HIV-1 viremia and CXCL13 plasma levels, peripheral blood mononuclear cells from uninfected donors were stimulated with HIV-1 infectious virions, HIV-1 ssRNA, TLR7/8 agonists or IFNα. The cellular sources of CXCL13 were determined by intracellular cytokine staining of cell populations. CXCL13 was produced by monocytes after stimulation with TLR7/8-ligands or HIV-1-derived ssRNA. CXCL13 production by monocytes required TLR7-activation of plasmacytoid dendritic cells and secretion of type I interferon. IFNα alone was sufficient to induce CXCL13-expression in human monocytes. In sum, we identified a novel mechanism of HIV-1-induced CXCL13 secretion; one due to TLR7 induction of type I interferon by pDCs and subsequent IFN stimulation of monocytes. Our findings are relevant in understanding how HIV-1 infection leads to immune dysregulation and provide the opportunity to develop and test potential therapeutic interventions.
Introduction
A characteristic immunological defect of HIV-1 infection is progressive humoral dysregulation (reviewed in (1, 2)), which includes changes in the frequencies of specific B cells subsets (3), results in hypergammaglobulinemia (4), and in the impaired induction of de novo antibody responses to vaccination (5). Although evidence exists that some of the above-mentioned defects are associated with viremia (6–8), the specific mechanisms of B cell dysregulation have yet to be elucidated. Understanding such mechanisms may assist the development of therapeutic interventions to restore B cell functionality in chronic HIV-1 infection.
CXCL13 is a chemokine crucial for the development of secondary lymphoid structures, where it is secreted by follicular dendritic cells in response to lymphotoxin receptor activation (9, 10). CXCL13 is chemotactic for cells expressing the receptor CXCR5 (9, 11), including mature B cells and T follicular helper (Tfh) cells (12–14), and is expressed at high levels in the B cell follicles of lymphoid organs (9). Importantly, CXCL13 facilitates the co-migration of B cells and Tfh cells into B cell follicles and germinal centers, where high-affinity antibody-secreting memory B and plasma cells are generated (15, 16). Conversely, aberrant CXCL13 secretion has been implicated in the pathogenesis of many chronic inflammatory conditions, including various infections and autoimmune disorders associated with dysregulated lymphoid genesis and humoral responses (17–20). Elevated levels of CXCL13 have been observed in the plasma of chronic HIV-1 infected subjects and have been correlated with viremia (21–24). Additionally, it has been shown that CXCL13 plasma levels decline after suppression of virus replication by anti-retroviral therapy (21). Furthermore, increased CXCL13 plasma levels were found to be associated with reduced chemokine receptor CXCR5 expression on B cells, with alterations in the chemotactic potential of B cells, and correlated inversely with the frequency of circulating Tfh cells during chronic HIV-1 infection (22, 24). It appears therefore that CXCL13 is a factor linked with the dysregulation of B cell function during HIV-1 infection. However, it is unclear how HIV-1 infection leads to increased plasma CXCL13 concentrations and how changes in CXCL13 plasma concentration are linked with B cell dysregulations during HIV-1 infection. While increased transcriptional expression of CXCL13 is detected in B cells from HIV-1-infected subjects, secretion of CXCL13 by those B cells is only detectable at low levels and only after stimulation for 6 days with CD40 ligation, CpG, IL-2, and IL-10 (22). Therefore, the reported increases in the CXCL13 concentrations during HIV-1 infection are likely not due to increases in CXCL13 production by B cells. In contrast, in lymph node biopsies from these same subjects, the majority of CXCL13 was found in macrophages and immature dendritic cells (22). Whether these two cell types secrete CXCL13 in the periphery is unknown. It is also not understood whether CXCL13 expression by macrophages and immature dendritic cells is the direct result of their infection by HIV-1, or due to a bystander effect of immune activation that takes place during HIV-1 infection.
In this regard, it is known that HIV-1 derived RNA triggers cytokine secretion directly through activation of toll-like receptors 7 and 8 (TLR7/8) (25, 26), which are predominantly expressed by antigen presenting cells such as monocytes, monocyte-derived dendritic cells and myeloid cells (27). Additionally, monocytes, macrophages, and monocyte-derived dendritic cells have been identified as inducible producers of CXCL13 by TLR2 and TLR4 activation (17, 20, 28–30). TLRs 2, 4, 7 and 8 share overlapping signaling pathways and downstream transcription factors. Therefore increases in CXCL13 plasma concentrations during HIV-1 infection could be due to secretion of this chemokine by any (or all) of these cells through TLR-activated pathways.
Here, we report that CXCL13 is secreted by monocytes in response to stimulation with HIV-1 virions, HIV-1-derived ssRNA and the TLR7/8 ligand, R848. Interestingly, maximal CXCL13 production required pDC-secretion of IFN-I. Therefore our study reveals a novel mechanism of CXCL13 induction in human monocytes via the TLR7 stimulation of pDCs and secretion of IFN-I and subsequent IFN induced production of CXCL13 in monocytes.
Materials and Methods
Study Participants
Fifty HIV-1 infected subjects and ten HIV-1-negative subjects, included in the plasma CXCL13 determination studies, were enrolled in protocols at the Massachusetts General Hospital. Additional, HIV-1-negative subjects were enrolled in a control cohort at Seattle Biomedical Research Institute and HIV-1 infected adult subjects were enrolled in either the Seattle HIV Vaccine Clinic Long-Term Non-Progressor or Natural Progression cohorts at the Fred Hutchinson Cancer Research Center. The latter subjects were chronically HIV-1 infected with a median viral load of 1773 RNA copies/mL (range: 320-9999) and CD4+ T cell count of 483 CD4+ T cells/mm3 (range: 261-1330) at the time of the blood draw and were not receiving anti-retroviral therapy (ART). The relevant institutional review boards approved all human subject protocols, and all subjects provided written informed consent before enrollment. Peripheral blood mononuclear cells (PBMCs) were isolated within 4 hours of venipuncture by density-gradient centrifugation. In vitro stimulation experiments were performed on fresh PBMCs from HIV negative and HIV-1-infected subjects. Plasma samples were aliquoted and frozen at −80°C. Concentrations of CXCL13 in thawed plasma samples were determined by ELISA according to the manufacturers’ instructions (CXCL13 Quantikine ELISA kit; R & D systems).
Intracellular Cytokine Staining
For stimulation with HIV-1 virions, PBMCs were isolated from HIV-negative donors and resuspended at 2x106/mL in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin (cRPMI). HIV-1 YU2 infectious molecular clones (IMC) were added to the PBMCs at 50ng/mL P24 for 18 hours at 37°C and 5% CO2. Brefeldin A (Sigma) was added at a final concentration of 5 μg/mL and incubated for an additional 18hrs. Samples were stained for extracellular markers, fixed, permeabilized and stained for FACS analysis following manufacturer’s recommendations (BD cytofix/cytoperm kit). Samples were surface-stained with the following antibodies to distinguish cell subsets: CD11c (Pe-Cy5), CD14 (FITC), HLA-DR (APC-Cy7), CD3 (Qdot 800), CD19 (Qdot 605), and CD123 (Brilliant Violet). Samples were stained intracellularly for CXCL13 (APC; R & D Systems).
Cell subsets were defined as follows: monocytes (live/dead aqua−, CD3−, CD19−, CD56−, HLA-DR+, CD11c+, CD123−, and CD14+), mDCs (live/dead aqua−, CD3−, CD19−, CD56−, HLA-DR+, CD11c+, CD123−, and CD14−), and pDCs (live/dead aqua-, CD3−, CD19−, CD56−, HLA-DR+, CD11c+, CD123+, and CD14−) and acquired using a Becton Dickinson (BD) LSR II ™ flow cytometer and analyzed using FlowJo (Treestar). Antibodies were from BD unless otherwise stated.
Isolation of Cell Subsets
Plasmacytoid dendritic cells (pDCs) were depleted from human PBMCs using a CD123 Microbead Kit (Miltenyi Biotec). Since CD123 is also expressed on basophils, pDCs were positively selected using CD304 (BDCA-4/Neuropilin-1) magnetic bead isolation (Miltenyi Biotec). Monocytes were isolated from PBMCs using the monocyte negative selection enrichment kit and depleted using the CD14 positive selection kit (Stem Cell Technologies). All PBMCs and cell subsets were cultured in complete RPMI media as described above. Cell purity was consistently greater than 95%, and confirmed by surface staining with mAbs against CD19, CD3, CD14, CD11c and CD123 (BD Biosciences) and analyzed using an LSR II flow cytometer.
In-vitro Stimulation of PBMCs with Toll-like Receptor Ligands
Two million freshly isolated PBMCs or 2 x 105 monocytes incubated with or without 25,000 purified pDCs, were cultured in 1 ml of complete media and treated for 1–4 days with the TLR7/8 agonist R848 (Invivogen) at 1 μg/mL. Increasing concentrations of HIV-1 LTR derived GU-rich ssRNA40/LyoVec (5′GCCCGUCUGUUGUGUGACUC-3′) and negative control RNA, where U nucleotides were replaced with adenosine, ssRNA41/LyoVec (5′GCCCGACAGAAGAGAGACAC-3′) (26) (Invivogen), and recombinant human IFNα2A (PBL Interferon) were added to cells. The soluble IFN receptor, B18R (Affymetrix), was added (at 0.1μg/mL) to cells directly preceding stimulation with the above TLR7/8 agonists in order to neutralize type I IFN in the supernatant. For time course experiments, 500 μL of supernatant were removed and replaced with fresh media at 24 hr intervals. Concentrations of CXCL13 and IFNα in culture supernatants were determined by ELISA according to the manufacturers’ instructions (IFNα kit from PBL Biomedical Laboratories; CXCL13 kits from R & D systems).
Quantification of mRNA by Real-Time PCR
Total RNA was isolated from monocytes and monocyte-depleted PBMCs using the Qiagen RNAeasy Kit (Qiagen) and was reverse transcribed into cDNA using QuantiTect Reverse Transcription Kit. Briefly, genomic DNA was removed by DNAse digestion by incubation with gDNA wipeout buffer. First-strand cDNA synthesis was carried out for 30 min at 42°C in 20 μL solution containing Quantiscript Reverse Transcriptase, Quantiscript RT Buffer, and RT Primer Mix (Qiagen), followed by denaturation for 3 min at 95°C. 4 μL of cDNA templates were used for real-time PCR reactions to quantify CXCL13 (assay number Hs00757930_m1) in a 96-well plate. Each reaction was carried out in 20 μL solution containing 10μL TaqMan reaction mix and 1 μL TaqMan FAM dye–labeled MGB probe using a 7500 Fast Real-Time PCR machine (Applied Biosystems). Fold change of CXCL13 gene expression was calculated by ΔΔCT method using GAPDH (assay number Hs02758991_g1) as an internal control and compared to unstimulated controls.
Statistical Analysis
Data are presented as the medians of a minimum of two independent experiments carried out using PBMCs from different donors unless stated otherwise. Two-tailed Mann-Whitney U tests, Wilcox-paired nonparametric tests and Spearman’s rank correlations were calculated using GraphPad Prism 5.02 software (GraphPad Software). P values of less than 0.05 were considered significant.
Results
HIV-1 Induces CXCL13 Production
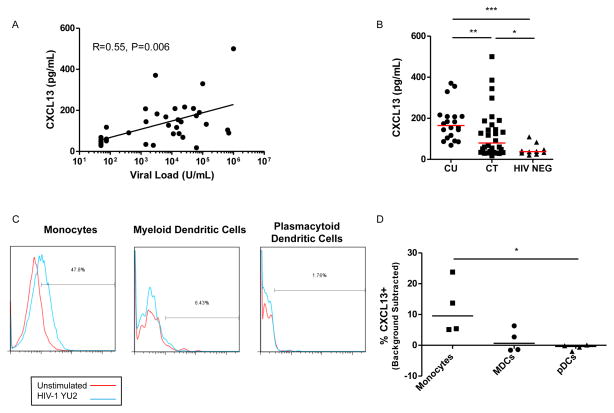
In order to confirm the relationship between HIV-1 viremia and induction of CXCL13, we compared levels of CXCL13 and viral load in the plasma of chronically HIV-1-infected subjects. We found a significant correlation between viral load and CXCL13 plasma concentrations of chronic untreated HIV-1 infected subjects (Figure 1A; t=0.55, p=0.006), in agreement with previous reports (21–23). Similarly, we found that subjects with suppressed viral load by ART had significantly lower levels of plasma CXCL13 (Figure 1B), suggesting that viral replication drives the production of CXCL13 and not the reverse. Overall, the CXCL13 levels following ART in HIV-1 infected subjects were higher than those observed in HIV-1-negative control subjects. To determine whether HIV-1 induces CXCL13-production directly and to define potential mechanism(s) by which viremia contributes to elevated CXCL13 expression, we incubated PBMCs from uninfected donors with HIV-1 replication-competent virions and monitored for increases in CXCL13 by intracellular cytokine staining in different cell subsets. Indeed, we observed that CXCL13 expression was significantly increased in monocytes (Figure 1C and 1D), but not in other cell subsets (such as mDCs, pDCs (Figures 1C and 1D) or T and B cells, data not shown).
Figure 1. CXCL13 plasma levels in chronic HIV-1 infected subjects.
A) A correlation of CXCL13 plasma levels in chronic HIV-1 infected untreated subjects with contemporaneous plasma viremia. Correlation coefficient and significance was calculated using a Spearman’s rank-order correlation for nonparametric data. B) CXCL13 concentrations were measured in the plasma of HIV-1 infected subjects: 20 chronic untreated (CU), and 30 chronic ART-treated (CT), as compared to 10 HIV-1 negative control subjects (HIV-NEG). The horizontal lines are at the median. The stated p-values were calculated using Mann-Whitney test for nonparametric data and considered significant if p<0.05 (*), p<0.01(**), or p<0.0001(***). PBMCs from four HIV negative donors were stimulated with HIV-1 YU2 virions for 36 hours and evaluated by intracellular staining by flow cytometry for CXCL13. C) Histograms depict the expression of CXCL13 in different cell populations after stimulation with HIV-1 virions, for a representative sample. D) Represents a summary of the expression of CXCL13 in monocytes, mDCs and pDCs in PBMCs after stimulation with HIV-1 virions for (n=4) HIV-1 uninfected subjects. Significance was determined by Kruskal-Wallis test, p<0.05 (*).
Monocytes produce CXCL13 through TLR7/8 activation
As mentioned above, HIV-1 derived ssRNA is known to activate TLR7 and 8 pathways (26, 31). Because monocytes produce CXCL13 upon stimulation of the TLR2/4 pathways (17, 29, 30), and because TLRs 2, 4, 7 and 8 share overlapping signaling pathways and downstream transcription factors (27), we hypothesized that HIV-1 infection of monocytes may induce CXCL13 through TLR7 and/or TLR8 activation. To investigate this possibility, we treated PBMCs from HIV-negative subjects with ssRNA derived from the long terminal repeat of HIV-1 (ssRNA40) at different concentrations and measured CXCL13 production after two days stimulation. We observed that CXCL13 was detectable in response to stimulation with HIV-1 ssRNA, in a dose-dependent manner (Figure 2A). In contrast, CXCL13-secretion was not observed with a control ssRNA in which uridines have been replaced with adenosines (ssRNA41) (Figure 2A). However, it has been shown that infection with HIV-1 modulates the immune response to TLR stimulation. Therefore, to determine whether TLR7/8 induction of CXCL13 secretion is relevant in the context of HIV-1-infection, we stimulated PBMCs from untreated HIV-1 infected subjects with HIV-1 derived ssRNA, control ssRNA or the TLR7/8 agonist, R848, (Figure 2B). We also stimulated PBMCs in the presence of the antiretroviral drug, azidothymidine (AZT), to prevent endogenous HIV-1 replication in vitro. Addition of either ssRNA or R848 resulted in significant CXCL13 secretion (p=0.008). The presence of AZT had no discernable effect on CXCL13 secretion; an indication that the observed increase in CXCL13 secretion was primarily due to the addition of exogenous ssRNA.
Figure 2. Monocytes secrete CXCL13 following TLR7/8 stimulation of PBMCs.
A) PBMCs isolated from HIV-negative subjects were stimulated with increasing doses of HIV-1 ssRNA or with a negative control ssRNA. CXCL13 levels in the supernatants were determined after 2 days of stimulation. The lines represent linear regression curves. B) PBMCs isolated from HIV-1-infected subjects were stimulated with ssRNA derived from HIV-1 or control ssRNA (1μg/mL) or the TLR7/8 agonist (R848; 1μg/mL) in the presence or absence of AZT (5μM). CXCL13 levels in the supernatants were determined 2 days post-stimulation. C) PBMCs or isolated monocytes from HIV-negative subjects were stimulated with ssRNA derived from HIV-1 or control ssRNA at indicated concentrations. CXCL13 levels in the supernatants were determined after 2 days post-stimulation. D) PBMCs, monocytes, and monocyte-depleted PBMCs from HIV-1 negative subjects were stimulated with R848. CXCL13 levels in the supernatants were determined by ELISA at the indicated time-points. The horizontal lines are at the median. P-values were calculated using Mann-Whitney test for nonparametric data. P<0.05 (*). E) PBMCs from HIV-negative subjects were stimulated with R848 for 2 days. Monocytes were isolated from the PBMCs, RNA was extracted from both the monocytes and the monocyte-depleted PBMCs and CXCL13 transcripts were measured by real-time PCR. Fold change was calculated by ΔΔCT using GAPDH as internal control and compared to unstimulated controls. Values represent mean of triplicates and SEM. The stated p-values were calculated by paired T test, p<0.05 (*).
To define the role of monocytes in the observed induction of CXCL13 via TLR7/8 activation, we stimulated isolated monocytes from HIV-1-negative subjects with HIV-1 ssRNA or with the TLR7/8 agonist, R848. Interestingly, although TLR7/8 stimulation of purified monocytes resulted in CXCL13 secretion, the amounts of CXCL13 produced were significantly lower than those observed when the corresponding PBMCs were similarly stimulated (Figure 2C). These results were obtained after two days of PBMC or monocyte stimulation with ssRNA, or R848. A time course experiment indicated that a significant induction of CXCL13 production by PBMC was observed as early as 1 day post-stimulation (p=0.03) that peaked at day 3 (Figure 2D). Here, isolated monocytes secreted significantly less CXCL13 than the stimulated PBMCs and the levels of CXCL13 detected in the stimulated monocytes were not significant compared to the unstimulated monocytes. Additionally, CXCL13 secretion was almost completely abrogated in the monocyte-depleted PBMCs under these stimulatory conditions (Figure 2D). The above results, led us to conclude that monocytes seem to be necessary, but not sufficient to promote the secretion of high concentrations of CXCL13 in response to TLR7/8 stimulation in human PBMCs. We envisioned two most likely possibilities to explain these results: (a) monocytes are the major producers of CXCL13, but that additional cell types, depleted when monocytes were enriched, are necessary for optimal CXCL13 production by monocytes and (b) monocytes are not the major producers of CXCL13 but are critical for CXCL13 production by another cell subset in PBMCs. To determine which of these two possibilities was responsible for the above observations, we stimulated PBMCs from HIV-negative donors with R848 for 2 days and subsequently isolated the monocytes. Then, we compared the levels of CXCL13 mRNA expression in the monocytes and monocyte-depleted PBMCs by real-time PCR. We observed significant up-regulation of CXCL13 mRNA expression in the monocytes, but not in the monocyte-depleted PBMCs (Figure 2E). Together, these results confirmed that: (a) monocytes were the primary cells secreting CXCL13 under TLR7/8 stimulation of PBMCs, (b) that TLR7/8 stimulation of monocytes is sufficient for the production of CXCL13 and (c) that an intermediary cell type in PBMCs exists that is required for optimal CXCL13 secretion by monocytes.
Type I interferon is necessary and sufficient for CXCL13 secretion
To identify the intermediate cell subtype necessary for the optimal production of CXCL13 by monocytes upon activation, we performed targeted cell-depletions from PBMCs. We focused on pDCs, since pDCs express TLR7, and are known to secrete significant quantities of cytokines, IFNα in particular, in response to TLR7 stimulation (25, 32–34). A significant decrease in CXCL13 production in PBMC cultures was observed when pDCs were depleted from the PBMCs (Figure 3A). To determine whether pDC-produced IFN-I was involved in the production of CXCL13 by monocytes, we stimulated PBMCs with TLR7/8 agonists after depleting pDCs or in the presence of the soluble IFN-I receptor, B18R, which neutralizes IFN-I. Under these conditions, a significant reduction of CXCL13 production was observed (Figure 3A).
Figure 3. Type I IFN is required for maximal induction of CXCL13 in monocytes.
A) PBMCs and pDC-depleted PBMCs from HIV-1 negative subjects (the same subjects from figure 2D) were stimulated for 4 days with either: media alone, or R848 in the presence or absence of the IFN-I–neutralizing protein (B18R). CXCL13 levels in the supernatants were determined by ELISA at the indicated time-points. The horizontal lines are the medians. P-values were calculated using Mann-Whitney test for nonparametric data. P<0.05 (*). B) PBMCs, pDC-depleted PBMCs, monocyte-depleted PBMCs, and isolated monocytes from HIV-negative subjects were stimulated with the TLR agonists: R848, R837, or the indicated amounts of recombinant IFNα2A and CXCL13 concentrations in the cell supernatants were determined. C) IFNα concentrations were determined and D) compared to the levels of CXCL13 detected in the supernatants of the unstimulated, TLR- or IFN-stimulated cultures of PBMCs or pDC-depleted PBMCs from HIV-negative subjects. Spearman’s rank correlation was used to determine the significance of the correlation. The line is a linear regression curve. E) PBMC-isolated monocytes combined with or without purified autologous pDCs were stimulated for 2 days with R848 and the CXCL13 levels were determined by ELISA. The horizontal lines are at the median. P<0.05 (*).
To determine whether IFNα alone was sufficient to induce CXCL13 production, we stimulated PBMCs, pDC-depleted PBMCs or purified monocytes from HIV-negative subjects with exogenous recombinant IFNα. In all cases, we observed that CXCL13 expression was induced in an IFNα-dose-dependent manner (Figure 3B). IFNα-mediated CXCL13-expression was abrogated when the soluble IFN receptor, B18R, was added to the cell culture during stimulation (Figure 3B). Furthermore, similar levels of CXCL13 were secreted from PBMCs stimulated with the TLR7/8 agonist, R848, or with the TLR7 agonist, R837 (Figure 3B). This suggests that TLR8-activation is most likely dispensable for maximal secretion of CXCL13. To further confirm the role of pDC-secreted IFNα in the stimulation of monocytes and the subsequent secretion of CXCL13 by these cells, we isolated pDCs and monocytes recombined them, stimulated them with a TLR7/8 agonist (R848) and after 2 days we measured CXCL13 in the supernatant (Figure 3E). The recombined pDCs and monocytes recapitulated the level of CXCL13 detected in the PBMC response to TLR7/8 stimulation (Figure 3E and Figure 2B).
Discussion
The chemokine, CXCL13, is the ligand for the receptor, CXCR5, expressed on B and Tfh cells and is critical for follicle development, affinity maturation of B cells, and organization of secondary lymphoid architecture. While follicular dendritic cells have historically been considered the main producers of CXCL13, monocytes, macrophages and myeloid DCs have also been shown to secrete CXCL13 in response to bacterial derived TLR2/4 ligands (17, 19, 20, 28–30). Previous reports demonstrated increases in plasma levels of CXCL13 during HIV-1 infection and of a positive correlation between the plasma CXCL13 concentration and plasma viremia (21–24). In chronic HIV-1 infection, a correlation has been observed between plasma CXCL13 concentrations and changes in the chemotactic potential of B cells (22), and with a loss of circulating Tfh cells (24). However, the mechanistic connections between HIV-1 viremia and CXCL13-production are unknown.
Here, we confirmed the correlation between plasma HIV-1 viremia and CXCL13 plasma concentrations in untreated chronic HIV-1 infection and identified a novel mechanism of HIV-induced CXCL13 secretion. We report that HIV-1 induces potent monocyte production of CXCL13 and that this occurs through TLR7-induced IFN-I secretion from pDCs and subsequent IFN-I stimulation of monocytes. Specifically, we identified HIV-1-derived ssRNA, which is a TLR7/8 agonist, as a potent inducer of CXCL13 secretion from PBMCs. We note that although we established that monocytes were the main producers of CXCL13 in PBMCs, under the stimulatory conditions used here, and that maximum CXCL13 secretion was dependent on pDC-secreted IFN-I, it is possible that low CXCL13 amounts are secreted by other cell types. Additionally, the promoter region of CXCL13 contains the binding site of the transcription factor, ISGF-3, which is downstream of IFN-I, further corroborating the potential induction of CXCL13 secretion by IFN signaling (35). IFNα was sufficient to induce high levels of CXCL13 secretion from isolated monocytes. This observation indicates that direct contact between pDCs and monocytes upon TLR7/8 stimulation of these two cell types is probably not required for secretion of CXCL13 by the monocytes. Future experiments will be necessary to better define to what extent direct monocyte-pDC contributes to the observed CXCL13-secretion. Therefore, the IFN-I-dependent TLR7/8-mediated up-regulation of CXCL13 in monocytes may explain the elevated levels of CXCL13 found in the periphery during chronic HIV-1 infection. Lastly, we were able to confirm that the HIV-1 derived ssRNAs and the TLR7/8 agonist, R848 were still able to induce CXCL13 secretion PBMCs from untreated HIV-1 chronically infected subjects.
Previous studies have already demonstrated that increased CXCL13 during chronic HIV-1 infection is associated with reduced expression of the cognate receptor, CXCR5, on mature B cells, and increased B cell chemotaxis (22). In addition, the fact that CXCL13 expression can be upregulated in peripheral monocytes by IFN-I activation may indicate a similar mechanism of CXCL13 expression in lymphatic tissues where substantial HIV-1 replication occurs (36). While we did not have access to lymph node samples from HIV-infected subjects, in previous studies of lymph node biopsies from HIV-1-infected subjects, the majority of CXCL13+ cells were detected in the T-dependent zone and co-localized with CD68+ monocytes or macrophages and CD1a+ immature myeloid dendritic cells (22). pDCs have been shown to accumulate in the lymph nodes (LN) of HIV-infected subjects and to secrete IFNα (37, 38). Likewise, IFN-I-inducible genes have been reported as being increased in the LN of SIV-infected macaques and HIV-1-infected humans (37, 39–41) and several studies have addressed the potential contribution of TLR7-mediated activation of plasmacytoid dendritic cells (pDCs) and induction of IFN-I to chronic immune activation, immune cell dysfunction, disruption of lymphoid architecture, and reduced de novo antibody responses (31, 34, 35, 42–45). Furthermore, the accumulation of Tfh cells and memory B cells, which express CXCR5 and migrate in response to CXCL13, has been described in the LNs of chronically HIV-1-infected humans and some SIV-infected macaques (41, 46–48). The accumulation of Tfh cells in LNs during chronic HIV-1 infection has also been associated with skewing of the B cell compartment and hypergammaglobulinemia (46, 49). Taken together our results suggest that HIV-1-induced IFN-I-dependent CXCL13 secretion may contribute to the observed disruption of the lymphoid architecture and dysregulation of humoral responses during untreated chronic HIV-1-infection by increasing migration and accumulation of mature B and Tfh cells in lymphoid organs.
Acknowledgments
Funding: These studies were supported by NIH grants AI081625 (LS) and AI080289 (MJM).
References
- 1.Amu S, Ruffin N, Rethi B, Chiodi F. Impairment of B-cell functions during HIV-1 infection. Aids. 2013 doi: 10.1097/QAD.0b013e328361a427. [DOI] [PubMed] [Google Scholar]
- 2.Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunological reviews. 2013;254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
- 3.Ho J, Moir S, Malaspina A, Howell ML, Wang W, DiPoto AC, O’Shea MA, Roby GA, Kwan R, Mican JM, Chun TW, Fauci AS. Two overrepresented B cell populations in HIV-infected individuals undergo apoptosis by different mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19436–19441. doi: 10.1073/pnas.0609515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sonnerborg A, Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 5.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, Levandowski RA, Mican JM, Fauci AS. Compromised B cell responses to influenza vaccination in HIV-infected individuals. The Journal of infectious diseases. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 6.Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O’Shea MA, Roby G, Mican JM, Kottilil S, Chun TW, Proschan MA, Fauci AS. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. The Journal of infectious diseases. 2008;197:572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 7.Sciaranghella G, Tong N, Mahan AE, Suscovich TJ, Alter G. Decoupling activation and exhaustion of B cells in spontaneous controllers of HIV infection. Aids. 2013;27:175–180. doi: 10.1097/QAD.0b013e32835bd1f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pensieroso S, Galli L, Nozza S, Ruffin N, Castagna A, Tambussi G, Hejdeman B, Misciagna D, Riva A, Malnati M, Chiodi F, Scarlatti G. B-cell subset alterations and correlated factors in HIV-1 infection. Aids. 2013 doi: 10.1097/QAD.0b013e32835edc47. [DOI] [PubMed] [Google Scholar]
- 9.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. The Journal of experimental medicine. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. The Journal of experimental medicine. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 12.Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 13.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nature immunology. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 16.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 17.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 18.Wong CK, Wong PT, Tam LS, Li EK, Chen DP, Lam CW. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. Journal of clinical immunology. 2010;30:45–52. doi: 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- 19.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infection and immunity. 2003;71:3572–3577. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermi W, Facchetti F, Riboldi E, Heine H, Scutera S, Stornello S, Ravarino D, Cappello P, Giovarelli M, Badolato R, Zucca M, Gentili F, Chilosi M, Doglioni C, Ponzi AN, Sozzani S, Musso T. Role of dendritic cell-derived CXCL13 in the pathogenesis of Bartonella henselae B-rich granuloma. Blood. 2006;107:454–462. doi: 10.1182/blood-2005-04-1342. [DOI] [PubMed] [Google Scholar]
- 21.Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB, Zack JA, Detels R, Martinez-Maza O. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2005;25:702–706. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 22.Cagigi A, Mowafi F, Phuong Dang LV, Tenner-Racz K, Atlas A, Grutzmeier S, Racz P, Chiodi F, Nilsson A. Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B cells during chronic HIV-1 infection. Blood. 2008;112:4401–4410. doi: 10.1182/blood-2008-02-140426. [DOI] [PubMed] [Google Scholar]
- 23.Regidor DL, Detels R, Breen EC, Widney DP, Jacobson LP, Palella F, Rinaldo CR, Bream JH, Martinez-Maza O. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. Aids. 2011;25:303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, Wloka K, Wheatley A, Narpala S, McDermott A, Roederer M, Haubrich R, Connors M, Ake J, Douek DC, Kim J, Petrovas C, Koup RA. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS pathogens. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 26.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. Journal of immunology. 2004;172:7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 29.Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, Koedel U. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infection and immunity. 2007;75:4351–4356. doi: 10.1128/IAI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-Brouwers J, Pfeilschifter J, Young MF, Schaefer RM, Schaefer L. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. The Journal of clinical investigation. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. Journal of immunology. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 32.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. The Journal of clinical investigation. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cellular immunology. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 34.Simmons RP, Scully EP, Groden EE, Arnold KB, Chang JJ, Lane K, Lifson J, Rosenberg E, Lauffenburger DA, Altfeld M. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. Aids. 2013 doi: 10.1097/01.aids.0000432455.06476.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA, Debernardo R, Rabin RL, Lederman MM, Harding CV. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PloS one. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann C, Lafferty M, Garzino-Demo A, Jung N, Hartmann P, Fatkenheuer G, Wolf JS, van Lunzen J, Romerio F. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PloS one. 2010;5:e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien M, Manches O, Sabado RL, Baranda SJ, Wang Y, Marie I, Rolnitzky L, Markowitz M, Margolis DM, Levy D, Bhardwaj N. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. The Journal of clinical investigation. 2011;121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanghavi SK, Reinhart TA. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. Journal of immunology. 2005;175:5314–5323. doi: 10.4049/jimmunol.175.8.5314. [DOI] [PubMed] [Google Scholar]
- 40.Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 41.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. CD4 T follicular helper cell dynamics during SIV infection. The Journal of clinical investigation. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, van Lunzen J, Altfeld M. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. Journal of virology. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier A, Bagchi A, Sidhu HK, Alter G, Suscovich TJ, Kavanagh DG, Streeck H, Brockman MA, LeGall S, Hellman J, Altfeld M. Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. Aids. 2008;22:655–658. doi: 10.1097/QAD.0b013e3282f4de23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang JJ, Woods M, Lindsay RJ, Doyle EH, Griesbeck M, Chan ES, Robbins GK, Bosch RJ, Altfeld M. Higher Expression of Several Interferon-Stimulated Genes in HIV-1-Infected Females After Adjusting for the Level of Viral Replication. Journal of Infectious Diseases. 2013;208:830–838. doi: 10.1093/infdis/jit262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, Diemand S, Honda K, Kundig TM, Aguzzi A, Speck RF. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113:377–388. doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 46.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. The Journal of clinical investigation. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. The Journal of experimental medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, Wilson K, Suzuki K, Corbeil J, Cooper DA, Kent SJ, Kelleher AD, Zaunders J. Simian Immunodeficiency Virus Infects Follicular Helper CD4 T Cells in Lymphoid Tissues during Pathogenic Infection of Pigtail Macaques. Journal of virology. 2013;87:3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. Journal of immunology. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]