Abstract
1,25-dihydroxy1,25(OH)2D3 [1,25(OH)2D3] is the biologically active form of Vitamin D and is immunoregulatory. 1,25(OH)2D3 binds the Vitamin D Receptor (VDR) complex present in many immune populations and can illicit transcriptional responses that vary amongst different immune subsets. The effects of 1,25(OH)2D3 on mature and developing human natural killer (NK) cells are not well characterized. Here we studied the influence of 1,25(OH)2D3 using an established NK cell differentiation system. Briefly, UCB CD34+ cells were isolated and cultured in conditions optimal for natural killer (NK) cell differentiation and varying concentrations of 1,25(OH)2D3 were administered. At physiological concentrations (10 nM),1,25(OH)2D3 impaired NK cell development. Moreover, the NK cells that did develop under the influence of 1,25(OH)2D3 showed a significant reduction in function (cytotoxicity and cytokine production). Conversely,1,25(OH)2D3 strongly induced hematopoietic stem cells to differentiate along a myeloid pathway, giving rise to CD14+ cells. Mechanistically, 1,25(OH)2D3 drives hematopoietic progenitor cells to rapidly upregulate monocyte genes (i.e. C/EBPα and CD14). There were no effects of 1,25(OH)2D3 on mature NK cytotoxicity or cytokine production. Collectively, these studies provide novel data showing the negative regulatory effect of 1,25(OH)2D3 on NK cell development.
Introduction
25(OH)D, is fat-soluble hormone which, when converted to its active from, 1,25(OH)2D, regulates calcium metabolism and skeletal health by stimulating gastrointestinal calcium absorption, thereby promoting bone mineralization. Evidence for the role of vitamin D intake on health first came from studies on rickets (1). Vitamin D deficiency is also associated with the development of cardiovascular diseases, cancer and autoimmune disorders (2). Extensive media coverage of the potential health benefits of vitamin D supplementation have translated into steady increases in vitamin D intake by the public. Accordingly, sales of vitamin D supplements in the United States have increased from $75 million in 2006 to $550 million in 2010, suggesting that large numbers of individuals are using these supplements 15. Given the increased usage, research is needed to better understand the benefits, as well as the risks, of vitamin D supplementation. These issues could be considered a matter of both consumer protection and public health.
There has been increasing recognition that the active form of vitamin D [1,25(OH)2D3],impacts the immune system. For instance, 1,25(OH)2D3 has potent anti-proliferative activity on T-cells after mitogen activation through the upregulation of inhibitory ligand receptors such as CTLA-4 1, 3. Inhibition of proliferation in lymphoid and myeloid leukemia cell lines is also seen at the level of cell cycle regulation, as 1,25(OH)2D3 upregulates p21 and p27 proteins and down regulates CDK2/4, cyclin D1 and cyclin A (3–5). In addition to inhibiting proliferation,1,25(OH)2D3 also activates pro-apoptotic pathways by down-regulating BCL2, thereby sensitizing lymphocytes to apoptosis (6, 7). Vitamin D has also been shown to skew T cells to a less inflammatory state. For instance, 1,25(OH)2D3 decreases T cell IFN-γ production, and increases IL-4 production (8). Both the generation and immune suppressive capacity of Foxp3+CD4 regulatory T cells are increased by 1,25(OH)2D3 (5), (9). More recent studies also show that 1,25(OH)2D3 prevents T cells from producing the inflammatory cytokine IL-17 (10, 11). In line this these results, other groups have documented that 1,25(OH)2D3 negatively modulates development of Th17 T cells (6). Physiologically relevant doses of 1,25(OH)2D3 also inhibit the production of IL-17, IL-21 and IL-22 in Th17-skewed T cells, suggesting that major transcription changes are driven by the vitamin D receptor (VDR) transcription factor complex.
Natural killer (NK) cells are innate immune effector cells that play a crucial role in both tumor and viral surveillance (12). Unlike T or B cells which express a single germline rearranged antigen receptor, NK cells clonally display a diverse repertoire of both activating and inhibitory receptors that recognize aberrant cells that have lost MHC class I expression or acquired stress receptors that trigger NK cell activation(13). NK cells are the first lymphocyte population to recover after allogeneic transplantation (allo-HCT), potentially linking these cells to the early graft vs. leukemia reactions that occur after allo-HCT. Using heavy water labeling, prior studies show that human NK cells disappear from the peripheral circulation relatively rapidly (6.9%/day; ½ life of <10 days). Thus, unlike T or B cells, which are believed to be long lived, NK cells need to be replenished constantly by hematopoietic stem cells (HSCs) (14). The effects of 1,25(OH)2D3 on mature NK cell proliferation and modulation of functional activity have been previously explored. Contrary to the above studies on T cells,the literature regarding the impact of 1,25(OH)2D3 on NK cells have been varied and ranges from augmentation of NK cell proliferation and cytotoxicity, to an inhibition in these activities.(15–17) The extent to which 1,25(OH)2D3 affects NK cell development from CD34+ UCB stem cells remains understudied.
In both mice and humans, NK cells have been shown to undergo a series of developmental intermediates that can be identified on the basis of cell surface receptors.(18, 19) These intermediates vary anatomically and functionally. For instance, in humans, the earliest NK progenitors (NKp) are stage II NK cells and are identified by the following surface phenotype (CD34+CD117highCD45RAlow). These cells likely emerge from the bone marrow and traffic to secondary lymphoid tissues (SLTs), such as the lymph nodes, where they subsequently differentiate to stage III NKp (CD56+/−CD117highCD94−) under the influence of instructive cytokines, including IL-15. Stage III progenitors lack NK functionality (i.e. the ability to produce INF-γ or kill tumor targets). Through an undefined process stage III NK cells develop into stage IV NK cells (CD56brightCD117highCD94+) where they acquire the ability to produce IFN-γ in response to cytokine stimulation (IL-12 and IL-18) and have attenuated cytotoxicity. Following this, stage IV NK cells are released from the SLTs to further differentiate into mature, stage V NK cells (CD56dimCD16+CD94+/−KIR+/−) which have potent cytotoxicity and cytokine production. We have previously reported that umbilical cord blood (UCB) CD34+ progenitors cultured with cytokines (IL-15, IL-3, IL-7, Flt 3 ligand and stem cell factor (SCF)) and a fetal liver stromal cell line can differentiate into functional stage IV and V human NK cells over the course of 28 days. This model closely mirrors the developmental stages described in primary human SLT(19, 20).
To study the role of vitamin D on NK cell development, physiologically relevant concentrations of 1,25(OH)2D3 (10 nm) was added to the NK differentiation cultures. In conditions that contained 1,25(OH)2D3 there was a significant reduction in overall cell expansion and a marked reduction of NK differentiation. Of the cells that did differentiate into NK cells, they were immature and had reduced function. The reduction in NK cell differentiation was further explained by an increase in the number of CD14+ monocytes differentiating from CD34+ stem cells, despite conditions that are optimal for NK cell differentiation. Stem cells cultured in 1,25(OH)2D3 rapidly upregulated monocyte associated genes, including C/EBP-α and CD14 within 72 hours. Interestingly, there was no impact of 1,25(OH)2D3 on short-term (7 day) cultures of mature, peripheral blood NK cell that were tested for cytotoxicity or IFN-g production. Collectively, these results show that 1,25(OH)2D3 favors monocyte development, at the cost of NK cells.
MATERIAL/METHODS
Isolation of CD34+ cells from umbilical cord blood (UCB) and CD56+ cells from peripheral blood (PB)
After ficoll separation, CD34+ progenitor cells were isolated from UCB using magnetic bead selection (Miltenyi Biotech, Auburn, CA). Selected cells were routinely >90% pure. CD3-CD56+ cells were isolated from human peripheral blood using the Rosette separation method according to the manufactures speciation’s (Stem Cells, Vancouver, Canada). Cells were tested for purity and were 85–90% pure.
Culture of the stromal cell line EL08.1D2
The embryonic liver cell line EL08.1D2 was cultured on gelatinized plates at 32°C in 40.5% α–minimum essential medium (α-MEM; Invitrogen, Carlsbad, CA), 50% myelocult (M5300; Stem Cell Technologies, Vancouver, BC, Canada), 7.5% fetal bovine serum (FBS), with β-mercaptoethanol (50 μM/L), glutamax (2 mM), penicillin (100 U/mL)/streptomycin (100 U/mL), and hydrocortisone (10–6 M). Prior to co-culture with progenitor cells, EL08.1D2 cells were irradiated (3000 rads).
NK-cell differentiation cultures
CD34+ selected cells (500 per well) were plated in a 24-well plate on an irradiated confluent monolayer of EL08.1D2 cells in Ham F12 plus Dulbecco modified Eagle medium (DMEM; 1:2 ratio) with 20% male, human AB− sera (Sera Care Life Sciences, Oceanside, CA), ethanolamine (50 μM), ascorbic acid (20 mg/L), 5 μg/L sodium selenite (Na2SeO3), β-mercaptoethanol (24 μM), and penicillin (100 U/mL)/streptomycin (100 U/mL). At the start of cultures, IL-3 (5 ng/mL), IL-7 (20 ng/mL), IL-15 (10 ng/mL), SCF (20 ng/mL), and FLT-3L (10 ng/mL) were added along with equal volumes of either a vehicle control (100% ethanol) or 1,25(OH)2D3 (Sigma Aldrich). Cultures were refreshed by demi-depletion (50% volume change) supplemented with the previously mentioned cytokines except IL-3. The number of cells was determined in each condition on days 14, 21, 28.
FACS staining and monoclonal antibodies
The following antibodies were used: CD34 (PercpCy5.5 or PE, clone 581), CD56 (PercpCy5.5 or PeCy7, clone B159), CD94 (FITC, clone HP-3D9), CD117 (PeCy7, clone 104D2 or PercpCy5.5, clone YB5.B8), CD161 (FITC,clone DX12), CD14 (ApcCy-7, clone MφP9), CD158a (FITC clone, HP3E4), CD158b (FITC, clone CH-2), NKB1 (FITC, clone DX9) all from BD Biosciences, San Jose, CA. Additional antibodies included NKG2D (PE, clone 149810), NKp30 (PE, clone 210845), NKp44 (PE, clone 253415), and NKp46 (PE, clone 195314) all obtained from R&D Systems, Minneapolis, MN. Intracellular staining for IFN-γ (PE clone 45.B3) was performed using cytofix/cytoperm (BD Biosciences). IFN-γ staining was performed after 16 hours of stimulation with IL-12 (10 ng/mL) and IL-18 (100 ng/mL). Brefeldin A was added for the last 4 hours. Data were analyzed using Flowjo Version 7.6. FACS sorting was performed on either a FACS Vantage or FACS Aria (BD Biosciences).
51Cr release assay
The immortalized erythroleukemia cell line K562 was used as an NK target for cytotoxicity and were labeled with 51Cr (Dupont-NEN, Boston, MA) by incubating 1 × 106 cells in 11.1 MBq (300 μCi) 51Cr for 1 hour at 37°C, 5% CO2. The cells were washed with phosphate-buffered saline (PBS), resuspended in RPMI with 10% FBS, and plated in 96-well plates at 1 × 104 cells/well in triplicate. Effector cells were added at specified ratios (from 5:1 to 1.25:1) and incubated for 4 hours at 37°C, 5% CO2. Supernatants were collected and counted (Wizard 1470; Perkin-Elmer, Shelton, CT). Specific 51Cr lysis was calculated using the equation: % specific lysis = 100 × (test release–spontaneous release)/(maximal release–spontaneous release).
RNAseq Analysis
UCB CD34+ HSC’s were freshly isolated from three different donors and used for NK cell differentiation (as described above) with either 1,25(OH)2D3 (10 nM) or vehicle control (100% ethanol). After 72 hours the cells were harvested, and RNA was isolated using an RNeasy mini kit (Qiagen). Samples were assessed by RNAseq analysis (llumina’s HiSeq 2000)at the Minnesota Biomedical Genomics Center, Minneapolis MN. Data was analyzed for differences in fold change of gene expression using the Partex Genomics Suit version 6.6 software.
Statistical Analysis
Difference between the various conditions was determined using performed using Graphpad Prism Software Version 5, (graph pad prism version 5)using either T-test or ANOVA where appropriate.
RESULTS
1,25(OH)2D3 Suppresses Overall Cell Expansion, Percentage of CD56+ cells and Absolute Number of NK cells
To investigate the influence of vitamin D on NK cell differentiation and maturation, physiological concentrations of 1,25(OH)2D3 (10 nM) were added to NK cell differentiation cultures. Briefly, CD34+ cells were cultured on a stromal line (EL08.1D2) and in the presences of IL-3, SCF, FLT-3L, IL-7 and IL-15. As we have previously demonstrated, these conditions are optimal for NK cell differentiation and give rise to ~2–3000× NK cell expansion from a single stem cell over the course of 21–28 days (21–24). As shown in Figure 1A, 1,25(OH)2D3 significantly decreased the number of mononuclear cells compared to the vehicle controls (p=0.01). Examining the cultures on days 14, 21 and 28 showed that a significantly lower percentage of NK cells were present in 1,25(OH)2D3 containing cultures compared to the vehicle control (Figure 1B, p<0.01). Combining the above information showed that the total number of NK cells that developed in the presence of 1,25(OH)2D3 were significantly less than vehicle controls (Figure 1C, p<0.01). Thus, under conditions that are optimal for NK cell differentiation (20, 22–24),1,25(OH)2D3 markedly inhibits NK development.
Figure 1. Vitamin D Reduces The Number of CD34-derived NK cells.
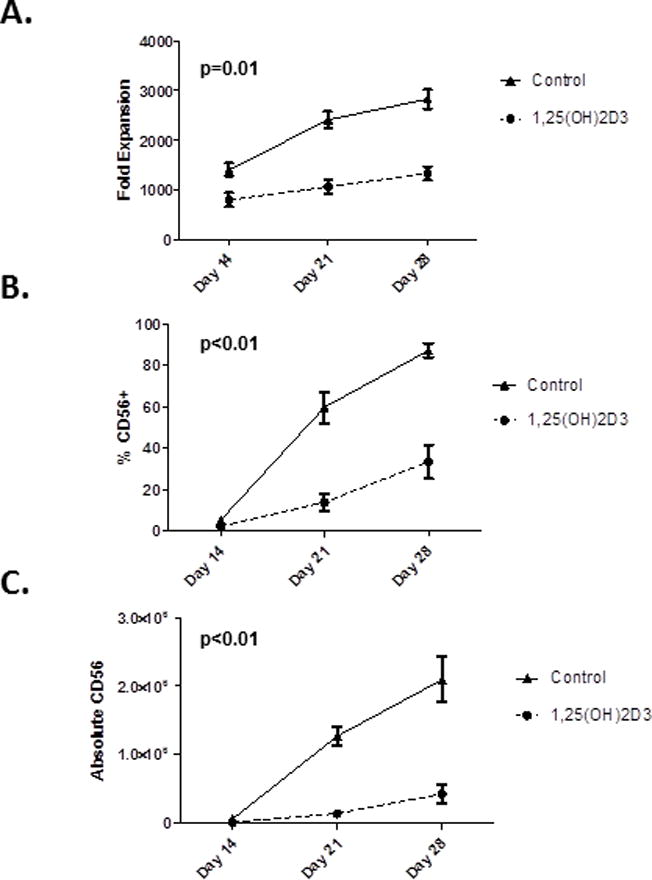
A)Fold expansion of MNCs following culture of CD34+ HSCs in the presence of 1,25(OH)2D3 or vehicle control. On days 14, 21 and 28 cells were enumerated using trypan blue staining. B) The percentage of CD56+ cells as determined by FACS on day 14, 21 and 28 of culture. C) The absolute numbers of NK cells on days 14, 21 and 28 of culture with vehicle or 1,25(OH)2D3. Shown are the average results of 10 donors (p<0.01).
Effect of Supplemental 1,25(OH)2D3 on The Function of Stem Cell Derived NK cells
While significantly fewer NK cells develop in the presence of 1,25(OH)2D3, the function of these cells are unknown. On day 21 and 28 of differentiation, 51Cr release assays were performed to compare the cytotoxic capacity of NK cells that differentiated in the presence of 1,25(OH)2D3 to the vehicle controls. NK cells were purified from cultures and used to kill the prototypic NK target, K562 cells. As shown in Figure 2A, purified HSC-derived NK cells that developed in the presence of 1,25(OH)2D3 showed a reduction in cytotoxicity compared to the vehicle control group (p<0.05, average of n=8 donors). We also investigated the potential changes in the ability of NK cells to produce INF-γfollowing stimulation with monokines (IL-12 and IL-18). As shown in Figure 2B, the percentage of INF-γ-producing NK cells was decreased in NK cells that developed in the presence of 1,25(OH)2D3 (p=0.02, average of n=4 donors). These results could be explained by either by slower NK differentiation (see below), impaired function or both. To address the effect of 1,25(OH)2D3 on function,mature NK cells (stage IV-V) were purified from differentiation cultures on the basis of CD94 expression (which marks stage IV and V NK cells). Purified CD94+ NK cells that were differentiated in the presence of 1,25(OH)2D3 were less cytotoxic then controls (Figure 2C, p=0.02, n=4). Collectively, the above studies show that HSC-derived NK cells differentiated in the presence of 1,25(OH)2D3 show a marked attenuation of both cytotoxicity and cytokine production.
Figure 2. Reduced Function of HSC-derived NK cells Developed in the Presence of 1,25(OH)2D3.
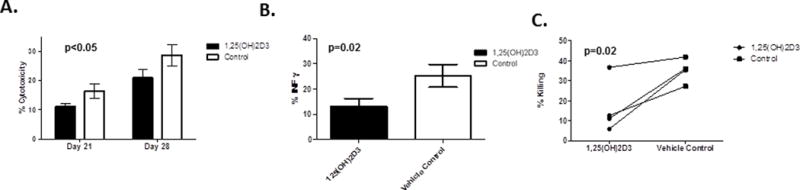
A) On day 21 and 28 NK cells were isolated from cultures using CD56+ selection and used for 51Cr release assay. Results are shown as the average +/− SD of 8 individual donors (n=8, * = p<0.05). B) Purified NK cells were stimulated for 16 hours with IL12 (10ng/ml) and IL18 (100 ng/ml) and cells were assessed IFN-γ production using intracellular cytokine staining (n=4, p=0.03). C) Function of purified stage IV and V NK cells differentiated with 1,25(OH)2D3 or vehicle control. Cells were cultured as described and then purified based on CD94 expression and used in a 51Cr release assay. Shown are the individual results of 4 separate CD34+ donors used to generate NK cells (p=0.02).
Effect of 1,25(OH)2D3 on NK Differentiation
The developmental stage of NK cells can be determined using CD117 and CD948,13. Specifically, stage III NK progenitors lack cytotoxicity and cytokine production, and are marked by a CD56+CD117highCD94− phenotype. During differentiation, stage III NK progenitors down modulate CD117 and acquire the CD94/NKG2A inhibitory receptor while progressing to the next developmental stage (stage IV, CD56+ CD117int/lowCD94+).13 At day 28, the majority of cells in the control groups are stage IV, but when 1,25(OH)2D3 is present, more stage III NK progenitors are noted (Figure 3). Thus, 1,25(OH)2D3 also delays NK maturation.
Figure 3. Comparative phenotypes of developing NK cultures in the presence of 1,25(OH)2D3.
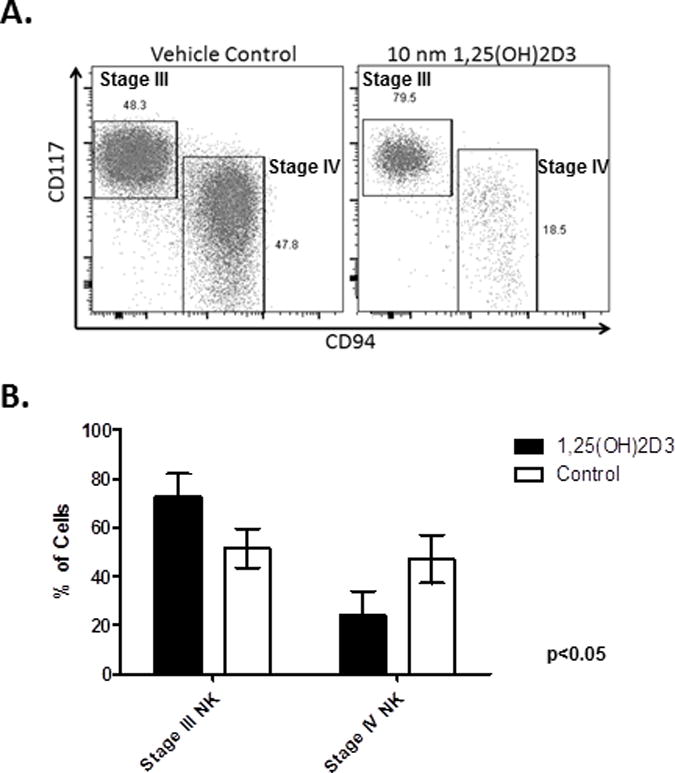
A) FACS plots showing the distribution of stage III and IV NK progenitors in the presence of 1,25(OH)2D3 or vehicle control at Day 21 B) The mean (+/−SD) percentage of stage III and stage IV at Day 21 (n=4/group, p<0.05).
1,25(OH)2D3 Drives Monocyte Differentiation at the Expense of NK cells
In the presence of 1,25(OH)2D3 the percentage and absolute numbers of CD56+ cells were significantly less than in controls (Figure 1). Implicit with a lower percentage of NK cells is a higher proportion of other cells in 1,25(OH)2D3 containing cultures. Cultures were tested at days 14, 21 and 28 to determine the identity of these cells and demonstrated significantly elevated percentages of monocytes in the 1,25(OH)2D3-treated samples compared to controls (p<0.01) (Figure 4A), which was consistent across a series of donors (n =4) (Figure 4B). There was also a significant elevation in the absolute number of monocytes generated in the presence of 1,25(OH)2D3 (p=0.04, Figure 4C). This information, coupled with a lower fraction of NK cells, suggest that 1,25(OH)2D3 induces monocyte differentiation at the cost of NK development.
Figure 4. Monocytes develop at the cost of NK development in the presence of 1,25(OH)2D3.
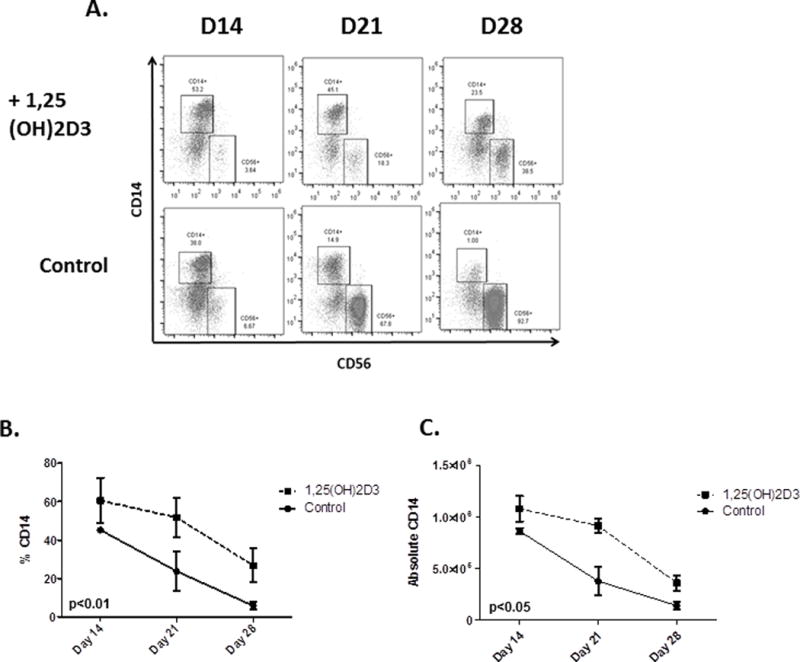
A) Results from a representative donor showing an increase in the percentage monocytes and a decrease in the percentage of CD56+ cells when HSCs were cultured in the presence of 1,25(OH)2D3. B and C) Cumulative data showing the percentage (p<0.01) and absolute numbers on monocytes in cultures containing 1,25(OH)2D3 (p<or vehicle (n=4, p<0.05).
1,25(OH)2D3 Acts on Early Progenitors To Inhibit NK Development
The above studies show that 1,25(OH)2D3 inhibits NK cell development, but it was unclear where in development this was acting. To investigate this,1,25(OH)2D3 was added at varying times of culture (day 0, 4, 7, 12 and 16). The total number of cells were dramatically reduced when 1,25(OH)2D3 was added at early time points (Days 0, 4 and 7) (Figure 5A), however, at later times (days 12 and 16), there was no impact on total cell number. Similarly, the percentage of NK cells were decreased when 1,25(OH)2D3 was added at the early time points (Days 0, 4 and 7), but not when it was added at later times (Days 12 and 16, Figure 5B). Collectively, these results show that 1,25(OH)2D3 acts upon early progenitors, significantly impairing NK development.
Figure 5. 1,25(OH)2D3 Acts Early in NK cell Differentiation to Impair NK Development.
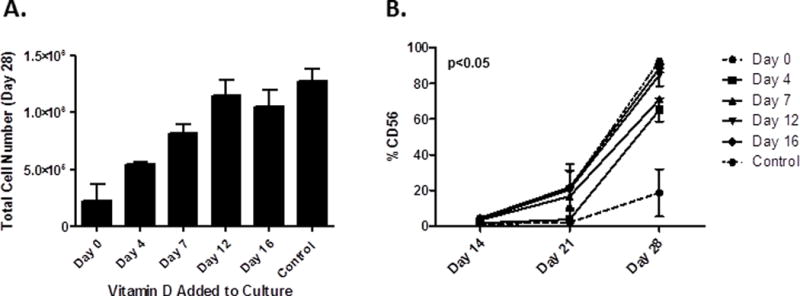
CD34+ cells were purified and place in NK differentiation cultures. At varying times (Day 0, 4, 7, 12, or 16) either 1,25(OH)2D3 (10 nM) or vehicle was added to cultures. At D28 cultures were assessed for: A) the total cell numbers (MNCs) and B) percent NK cells, (n=2, p≤0.05 for Days 0, 4, 7 compared to control).
Impact of 1,25(OH)2D3 on Gene Expression in Early Progenitors In NK Differentiation Cultures
Based on the above data showing that monocytes develop in the presence of 1,25(OH)2D3 (Figure 4) and that this occurs early in development (Figure 5), we hypothesized that 1,25(OH)2D3 initiates a genetic program in early progenitors that favors monocyte development. Given that we could detect CD14+ cells as early as day 7 after the start of cultures (not shown), we analyzed the changes in gene expression using an RNASeq Array Analysis at day 3 of culture in either vehicle or 1,25(OH)2D3 to identify genes that were differentially expressed in early progenitors. As shown in Table 1, relatively few genes were significantly altered in the presence of 1,25(OH)2D3 (defined as >1.5 fold over control). Interestingly, CD14 was the only gene to change >2.5 fold, reinforcing the notion 1,25(OH)2D3 drives developing progenitors to differentiate toward the monocyte lineage. Other monocyte-associated genes that were unregulated over the first 72 hours of culture with 1,25(OH)2D3 include Seglec12 (CD33 family member), CEBP/α and CEBP/δ.
Table 1. Changes in gene expression progenitors cultured in NK differentiation cultures in 1,25(OH)2D3.
Purified CD34+ cells were cultured on EL08.1D2 cells as described in the methods in the presence of IL-3, IL-7, IL-15, SCF and FLT3L with 1,25(OH)2D3 or vehicle for 72 hours and cells were collected for RNA isolation and RNA-seq analysis.
| Symbol | Fold Change |
|---|---|
| CD14 | +5.43 |
| CD38 | +2.12 |
| CD24 | +2.11 |
| UPF2 | +1.95 |
| OXCT2 | +1.86 |
| SIGLEC12 | +1.82 |
| HPGD | +1.79 |
| IL8 | +1.77 |
| DOK1 | +1.7 |
| OSM | +1.62 |
| CEBP/α | +1.58 |
| CEBP/δ | +1.53 |
| Granzyme B | −1.81 |
| MX1 | −1.78 |
| NFIX | −1.75 |
| TAP1 | −1.65 |
| Galectin-1 | −1.62 |
| Vimentin | −1.55 |
1,25(OH)2D3 Has Minimal Impact on The Expansion and Cytotoxicity of Human Peripheral blood NK cells
We next set out to test the effects of 1,25(OH)2D3 on NK cells isolated from peripheral blood (PB). NK cells were purified by negative selection and cultured for 7 days with media supplemented with IL-15 (10 ng/ml) with either 1,25(OH)2D3 or vehicle control. Unlike the effects of 1,25(OH)2D3 on HSC-derived NK cells, there was no significant changes in either NK cell expansion (Figure 6A, p=0.06, n=4), cytokine production (Figure 6B, p=ns, n=4) or cytoxicity (Figure 6C, p=ns, n=8).
Figure 6. Peripheral Blood NK Expansion and Cytotoxicity with 1,25(OH)2D3.
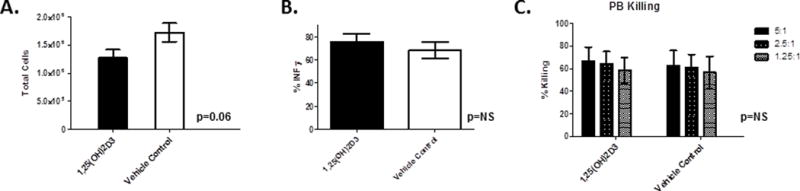
Cells were isolated from healthy donors, purified using CD3−CD56+ magnetic beads and cultured for 7days with 10nm 1,25(OH)2D3 and analyzed for A) total cell expansion (n=4, p=0.06) and B) IFN-γ production in response to IL-12/IL-18 stimulation (n=4, p=ns), and C) cytotoxicity to K562 cells (n=4, ns).
DISCUSSION
In this study, we assessed the impact of 1,25(OH)2D3 on the development of NK cells using a validated NK cell differentiation model(22). This optimized system is highly robust, yielding ~2,0x00–3000 fold expansion of functional NK cells from a single stem cell(20, 22). Here, we show that physiologic concentrations of 1,25(OH)2D3 inhibits the overall cell expansion and causes the developing progenitors to shift away from differentiating into the NK lineage. While significantly reduced in number, the NK cells that do differentiate in the presence of 1,25(OH)2D3 are less mature and show a reduction in NK function, with less IFN-γ production and cytotoxicity. Interestingly, we found no differences in the expression of NK activating receptors, including NKG2D, NKp30 and NKp46 (not shown), suggesting the role of 1,25(OH)2D3 in attenuating function lies at a deeper level than recognition and activation of surface receptor-ligand interactions. Surprisingly, despite conditions that have been optimized for NK cell development, the addition of 1,25(OH)2D3 led to rapid commitment of hematopoietic progenitor cells to the myeloid lineage with significant monocyte development.
Lineage specification during hematopoiesis is a complex process where stem and progenitor cells integrate external signals from cytokine receptors and surface proteins. Triggering of these receptors feed into signaling cascades that activate transcription factors to drive lineage restricted genetic programs, specifying developmental fates. A variety of transcription factors have been shown to be critical for NK cell differentiation, including E4BP4, ID family transcription factors, EOMES and T-bet (reviewed in (25)). By altering the time that 1,25(OH)2D3 was added to the cultures, we observed dramatically different developmental fates of stem cells that were otherwise treated identically. Adding 1,25(OH)2D3 at the start of the culture strongly inhibited NK development, while delaying the introduction of 1,25(OH)2D3 by just 3 or 7 days diminished both the percentage and absolute numbers of NK cells that differentiated. In sharp contrast, adding 1,25(OH)2D3 at later times (≥12 days) had no major impact on NK cell development. These results, coupled with the rapid appearance of CD14+ cells (within 7 days of the start of cultures)when 1,25(OH)2D3 was present, suggests that 1,25(OH)2D3 acts early in differentiation to skew progenitors away from the NK lineage. RNA-seq analysis on HSCs cultured in NK differentiation conditions with and without 1,25(OH)2D3 showed that a strikingly small number genes were altered by 1,25(OH)2D3. However, some of these have clearly been implicated in myeloid specification, including C/EBP-α and C/EBP-δ, which form homo- or heterodimers and drive myeloid differentiation(26, 27). Interesting, vitamin D responsive elements have been identified in the proximal regions of C/EBP family genes and exogenous administration of 1,25(OH)2D3 has previously been shown to increase the expression of one of the family members, C/EBP-β(28). The C/EBP family of transcription factors are especially important in monocytic development, supporting our findings(29). In our studies there was no decrease in the expression of transcription factors involved in NK differentiation, perhaps suggesting that genes such as C/EBP-α and C/EBP-δdominate over NK associated transcription factors to drive monocyte differentiation. In the presence of IL-3, IL-6 and SCF or GM-CSF, stem cells with ectopic expression of C/EBP-α show monocyte development(29); conditions similar to ours. The gene that was most strongly upregulated by 1,25(OH)2D3 in our studies was CD14, again, consistent with existing literature showing that C/EBP-α and C/EBP-δ bind to the CD14 promoter to transactivate gene expression(30). Interestingly, other investigators have examined the impact of 1,25(OH)2D3 on the ability of monocytes to acquire functions of dendritic cells (after with GM-CSF and IL-4 and inflammatory stimuli) and show that the antigen presentation properties were markedly impaired (31), perhaps suggesting that the monocytes generated under these conditions would have tolerogenic properties.
Prior experiments testing the influence of 1,25(OH)2D3 on mature (PB) NK function are varied and seemingly contradictory. Here we show for the first time that NK cells that develop from progenitors in the presence of 1,25(OH)2D3 show reduced differentiation and function. In contrast, we found that adult NK cells cultured with IL-15 were not affected by exogenous 1,25(OH)2D3 and showed no difference in expansion, cytokine production or cytotoxicity. These findings are different from those previously published including prior studies showing an inhibitory effect of 1,25(OH)2D3 on mature NK function(8, 32). However, caution is needed in interpreting these older experiments since they were performed using mixed cultures of peripheral blood lymphocytes following mitogen stimulation(33). The described inhibitory effect of 1,25(OH)2D3 might be explained by the now known negative regulatory action of 1,25(OH)2D3 on IL-2 production by CD4+ T cells, especially since the investigators showed that exogenous IL-2 could overcome the inhibition(8, 32). Conversely, other investigators have shown that short term culture (24 hours) with 1,25(OH)2D3 activated NK function (16, 34). Vitamin D supplementation in patients undergoing chronic renal dialysis (35, 36) or with rickets (37) also showed improvements in NK function, suggesting that chronic deficiency may also negatively regulate NK function. NK cells are also known to exist in the uterus where they lack cytotoxicity but play a central role in embryo implantation (reviewed in (38)). It is not clear how NK cells acquire their unique properties in the uterus, but it is interesting to note that vitamin D is produced by trophoblasts(39, 40) and that vitamin D deficiency is a risk factor for recurrent fetal loss after in vitro fertilization and embryo implantation (39, 41). Considering a dynamic cross talk between NK cells and trophoblasts perhaps suggests that 1,25(OH)2D3 in utero participates in the unique functions of this NK subset.
Here using an in vitro NK cell differentiation system we show that 1,25(OH)2D3 has a negative regulatory effect on NK cell development from CD34+ progenitors, which is consistent with the literature showing an antiproliferative effect of this steroid hormone on numerous cell types. As well, the NK cells that do differentiate in the presence of 1,25(OH)2D3 have attenuated inflammatory capacity, with reduced IFN-γ and cytotoxicity. Despite conditions that have been optimized for NK cell development, the addition of 1,25(OH)2D3 favors monocyte development. Collectively, these studies show that 1,25(OH)2D3 negatively influences NK differentiation from hematopoietic progenitors. An important caveat with this data is that both the activation of 25(OH)D3 to 1,25(OH)2D3 and the subsequent inactivation of 1,25(OH)2D3 are tightly regulated enzymatic processes which are difficult to model in vitro. As well, the local concentrations of 1,25(OH)2D3 in the bone marrow or secondary lymphoid tissues (where NK cell development occurs) is not known. Future studies are needed to address whether vitamin D supplementation impacts NK cell number or function in experimental animals or human populations.
Acknowledgments
This work was supported by RO1 AI100879 (MRV), Children’s Cancer Research Fund (MRV), P01 CA65493 (JSM), P01 111412 (MRV and JSM) and R01 HL55417 (JSM), ASBMT New Investigators Award (YOA and RB), and PO1067493 (JSM). Running Title: Effect of 1,25(OH)2D3 on NK development
References
- 1.Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:974–979. doi: 10.1359/jbmr.080420. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15:1555–1563. doi: 10.1038/sj.onc.1201329. [DOI] [PubMed] [Google Scholar]
- 4.Park WH, Seol JG, Kim ES, Jung CW, Lee CC, Binderup L, Koeffler HP, Kim BK, Lee YY. Cell cycle arrest induced by the vitamin D(3) analog EB1089 in NCI-H929 myeloma cells is associated with induction of the cyclin-dependent kinase inhibitor p27. Experimental cell research. 2000;254:279–286. doi: 10.1006/excr.1999.4735. [DOI] [PubMed] [Google Scholar]
- 5.Morales-Tirado V, Wichlan DG, Leimig TE, Street SE, Kasow KA, Riberdy JM. 1alpha,25-dihydroxyvitamin D3 (vitamin D3) catalyzes suppressive activity on human natural regulatory T cells, uniquely modulates cell cycle progression, and augments FOXP3. Clin Immunol. 2011;138:212–221. doi: 10.1016/j.clim.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, Nishimura T. 1alpha,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunology letters. 2010;134:7–16. doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. The Journal of biological chemistry. 2003;278:46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 8.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 9.Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. 2010;120:86–95. doi: 10.1016/j.jsbmb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, van Leeuwen JP, Lubberts E. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–142. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, Nishimura T. 1 alpha,25-Dihydroxyvitamin D(3) and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 2010 doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, Griffin GE, Taylor GP, Tough DF, Beverley PC, Macallan DC. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. The Journal of clinical investigation. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravid A, Koren R, Maron L, Liberman UA. 1,25(OH)2D3 increases cytotoxicity and exocytosis in lymphokine-activated killer cells. Mol Cell Endocrinol. 1993;96:133–139. doi: 10.1016/0303-7207(93)90103-q. [DOI] [PubMed] [Google Scholar]
- 17.Balogh G, de Boland AR, Boland R, Barja P. Effect of 1,25(OH)(2)-vitamin D(3) on the activation of natural killer cells: role of protein kinase C and extracellular calcium. Experimental and molecular pathology. 1999;67:63–74. doi: 10.1006/exmp.1999.2264. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nature immunology. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 19.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzywacz B, Kataria N, Sikora M, Oostendorp RA, Dzierzak EA, Blazar BR, Miller JS, Verneris MR. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzywacz B, K N, Sikora M, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- 23.Grzywacz B, Kataria N, Blazar BR, Miller JS, Verneris MR. Natural killer-cell differentiation by myeloid progenitors. Blood. 2011;117:3548–3558. doi: 10.1182/blood-2010-04-281394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullar V, Oostendorp R, Panoskaltsis-Mortari A, Yun G, Lutz CT, Wagner JE, Miller JS. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Experimental hematology. 2008;36:598–608. doi: 10.1016/j.exphem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luevano M, Madrigal A, Saudemont A. Transcription factors involved in the regulation of natural killer cell development and function: an update. Frontiers in immunology. 2012;3:319. doi: 10.3389/fimmu.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heath V, Suh HC, Holman M, Renn K, Gooya JM, Parkin S, Klarmann KD, Ortiz M, Johnson P, Keller J. C/EBPalpha deficiency results in hyperproliferation of hematopoietic progenitor cells and disrupts macrophage development in vitro and in vivo. Blood. 2004;104:1639–1647. doi: 10.1182/blood-2003-11-3963. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS, Lekstrom-Himes JA, Akashi K, Tenen DG. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004;21:853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Dhawan P, Peng X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, Centrella M, McCarthy TL, Christakos S. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Molecular and cellular biology. 2005;25:472–487. doi: 10.1128/MCB.25.1.472-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, D’Costa J, Civin CI, Friedman AD. C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108:1223–1229. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Z, Hetherington CJ, Zhang DE. CCAAT/enhancer-binding protein activates the CD14 promoter and mediates transforming growth factor beta signaling in monocyte development. The Journal of biological chemistry. 1999;274:23242–23248. doi: 10.1074/jbc.274.33.23242. [DOI] [PubMed] [Google Scholar]
- 31.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 32.Leung KH. Inhibition of human natural killer cell and lymphokine-activated killer cell cytotoxicity and differentiation by vitamin D3. Scand J Immunol. 1989;30:199–208. doi: 10.1111/j.1365-3083.1989.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 33.Merino F, Alvarez-Mon M, de la Hera A, Ales JE, Bonilla F, Durantez A. Regulation of natural killer cytotoxicity by 1,25-dihydroxyvitamin D3. Cell Immunol. 1989;118:328–336. doi: 10.1016/0008-8749(89)90381-x. [DOI] [PubMed] [Google Scholar]
- 34.Ota K, Dambaeva S, Han AR, Beaman K, Gilman-Sachs A, Kwak-Kim J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum Reprod. 2013 doi: 10.1093/humrep/det424. [DOI] [PubMed] [Google Scholar]
- 35.Quesada JM, Solana R, Martin A, Santamaria M, Serrano I, Martinez ME, Aljama P, Pena J. The effect of calcitriol on natural killer cell activity in hemodialyzed patients. Journal of steroid biochemistry. 1989;34:423–425. doi: 10.1016/0022-4731(89)90120-9. [DOI] [PubMed] [Google Scholar]
- 36.Quesada JM, Serrano I, Borrego F, Martin A, Pena J, Solana R. Calcitriol effect on natural killer cells from hemodialyzed and normal subjects. Calcified tissue international. 1995;56:113–117. doi: 10.1007/BF00296341. [DOI] [PubMed] [Google Scholar]
- 37.Kitajima I, Maruyama I, Matsubara H, Osame M, Igata A. Immune dysfunction in hypophosphatemic vitamin D-resistant rickets: immunoregulatory reaction of 1 alpha(OH) vitamin D3. Clinical immunology and immunopathology. 1989;53:24–31. doi: 10.1016/0090-1229(89)90097-4. [DOI] [PubMed] [Google Scholar]
- 38.Le Bouteiller P. Human Decidual NK Cells: Unique and Tightly Regulated Effector Functions in Healthy and Pathogen-Infected Pregnancies. Frontiers in immunology. 2013;4:404. doi: 10.3389/fimmu.2013.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod. 2012;27:3321–3327. doi: 10.1093/humrep/des280. [DOI] [PubMed] [Google Scholar]
- 40.Rubin LP, Yeung B, Vouros P, Vilner LM, Reddy GS. Evidence for human placental synthesis of 24,25-dihydroxyvitamin D3 and 23,25-dihydroxyvitamin D3. Pediatric research. 1993;34:98–104. doi: 10.1203/00006450-199307000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertility and sterility. 2010;94:1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


