Abstract
Objective
To determine if TGF-β3 is a paracrine signal secreted by leiomyoma that inhibits BMP mediated endometrial receptivity and decidualization.
Design
Experimental
Setting
Laboratory
Patients
Women with symptomatic leiomyomas
Interventions
Endometrial stromal cells (ESC) and leiomyoma cells were isolated from surgical specimens. Leiomyoma-conditioned media (LCM) was applied to cultured ESC. TGF-β was blocked by two approaches: TGF-β pan-specific antibody or transfection with a mutant TGF-β receptor type II. Cells were then treated with rhBMP-2 to assess BMP responsiveness.
Main Outcome Measures
Expression of BMP receptor types 1A (BMPR1A), 1B (BMPR1B), 2 (BMPR2), as well as endometrial receptivity mediators HOXA10 and LIF.
Results
ELISA showed elevated TGF-β levels in LCM. LCM treatment of ESC reduced expression of BMPR1B and BMPR2 to approximately 60% of pretreatment levels. Pre-incubation of LCM with TGF-β neutralizing antibody or mutant TGF receptor, but not respective controls, prevented repression of BMP receptors. HOXA10 and LIF expression was repressed in rhBMP-2 treated, LCM exposed ESC. Pre-treatment of LCM with TGF-β antibody or transfection with mutant TGF receptor prevented HOXA10 and LIF repression.
Conclusions
Leiomyoma derived TGF-β was necessary and sufficient to alter endometrial BMP-2 responsiveness. Blockade of TGF-β prevents repression of BMP-2 receptors and restores BMP-2 stimulated expression of HOXA10 and LIF. Blockade of TGF signaling is a potential strategy to improve infertility and pregnancy loss associated with uterine leiomyoma.
Introduction
Endometrial receptivity, critical to embryo implantation, requires coordinated signaling between hormones, growth factors, cytokines, and other signaling molecules. A short “window of implantation” occurs in which the endometrium is able to support blastocyst apposition, adhesion, and invasion. This window begins approximately 4 days after ovulation and continues for 6 days (1–3). Endometrial receptivity is defective when key regulators of implantation, such as HOXA10, HOXA11 and leukemia inhibitory factor (LIF), are altered. The targeted disruption of these genes in mice results in infertility due to failed endometrial receptivity (4–7). HOX genes regulate a number of molecules that function during the window of implantation including: pinopodes, β3 Integrin, Tryptophan dioxygenase, and insulin-like-growth-factor-binding-protein-I (IGFBP-I) (8–11). There are no known human mutations of the HOXA10 or HOXA11 genes; however women affected by conditions known to be associated with implantation defects, including submucosal myomas, have diminished expression of these genes (12–15). Bone morphogenetic protein 2 (BMP-2), a multifunctional growth factor, is also critical to endometrial implantation. Conditional ablation of BMP-2 in the murine endometrium results in failed decidualization and the inability to support embryo implantation (16, 17). BMP-2 regulates expression of HOXA10 and LIF in human endometrial stromal cells, implicating BMP2 in human endometrial receptivity as well (18).
Uterine leiomyomas are the most common benign neoplasms in women of reproductive age, with a lifetime prevalence of 30–70 percent (19, 20). The total economic impact associated with fibroids in the United States (in 2010 dollars) was recently estimated to range between 6 to 34 billion dollars annually (21). Approximately 30 percent of women with leiomyomas are symptomatic, with symptoms including abnormal bleeding, pain, and reproductive dysfunction (impaired implantation, infertility and spontaneous abortion (22, 23). Black women have a 3-fold higher incidence of leiomyomas than white women (24). The presence and severity of symptoms have traditionally been thought to be dependent on the size and location of the myomas (subserosal, intramural or submucosal).
Growth, proliferation and differentiation of myometrial cells are regulated by complex interactions between ovarian steroids and local growth factors (25). Abnormal signaling within these pathways can lead to tumor formation. Leiomyoma tumorigenesis and enlargement are therefore due to signaling errors that cause increased proliferation in response to sex steroids and other growth factors (26). A number of growth factors, including EGF, PDGF, IGF, heparin-binding EGF, TGF-β, TGF-α, VEGF, basic FGF, and acidic FGF are implicated in the development and proliferation of leiomyomas (25, 27, 28). Aberrant regulation of these factors increases extracellular matrix (ECM) components, including collagens, proteoglycans, and fibronectin, in a disorganized fashion (29–33). Recently, TGF-β has been implicated in defective endometrial signaling with adverse effects on embryo implantation (18).
Submucosal leiomyomas, located in the myometrium underlying the endometrium, are known to decrease implantation and clinical pregnancy rates (34–36). It has been hypothesized that anatomic distortion of the endometrial cavity impairs embryo implantation in the endometrium directly overlying the leiomyoma. Leiomyomas are also thought to alter uterine peristalsis, which may interfere with gamete and embryo transport (37). Cavity distortion due to obstruction by large leiomyoma is also thought to hinder sperm migration through the cervix and to the ostiae of the fallopian tubes. However, impaired reproduction is seen in women with small submucosal leiomyomas and intramural leiomyomas that do not alter the endometrial cavity cannot be explained by tubal obstruction (38). These findings suggest that paracrine signaling from leiomyomas to the endometrium may contribute to leiomyoma-related infertility. Expression of HOXA10 and HOXA11, factors known to be necessary for endometrial receptivity, was decreased in the endometrium of women with submucosal myomas (39). Interestingly the decreased expression of these genes was seen globally throughout the endometrial cavity, and not exclusively in the endometrium directly overlying the myomas. This suggests that a diffusible molecule is secreted in women with leiomyomas that can influence the endometrium.
Growth factors, known to be secreted leiomyomas, are plausible mediators of the endometrial effects. Transforming-growth-factor-beta 3 (TGF-β3), a growth factor known to be produced by leiomyomas in far greater amounts than normal myometrium, has detrimental effects on signaling pathways necessary for endometrial receptivity (18, 40). TGF-β3 was shown to decrease the receptivity of human endometrium to BMP-2 by decreasing the expression of BMP receptors (18). HOXA10 and LIF expression did not increase in endometrial cells from women with leiomyomas when treated with recombinant human BMP-2 while similar treatment increased HOXA10 and LIF in cells from women without leiomyomas (18). These findings suggest that TGF-β3, secreted from leiomyomas, impairs the BMP-2 signaling in the endometrium necessary for implantation. Here we sought to determine whether TGF- β blockade in leiomyoma-exposed endometrial stromal cells would restore BMP-2 signaling and restore BMP-2 up-regulation of HOXA10 and LIF expression.
Methods
Patients
Approval was obtained from the Yale School of Medicine Institutional Review board. Leiomyoma samples (N=10) were obtained from 4 patients undergoing abdominal myomectomy, 3 patients undergoing hysteroscopic myomectomy, and 3 patients undergoing hysterectomy. Myometrium samples (N=3) were obtained from hysterectomy specimens from non-pregnant women without fibroids who were not taking hormone containing medications. The mean age of leiomyoma patients was 38.7 (range 32–48 y.o) and the mean age of hysterectomy patients was 44.7 (range 42–48). Control endometrial samples (N=4) were obtained from fertile controls, who were not using hormonal contraception and had no evidence of uterine pathology, undergoing laparoscopic bilateral tubal ligation. The mean age of control patients was 30.5 (range 28–34 y.o.). Written informed consent was obtained prior to specimen collection.
Cell culture
Primary cell culture was performed for leiomyoma, myometrium, and endometrial stromal cells. Endometrial samples were dissected and minced into 2 mm pieces in Hank’s balanced salt solution (HBSS) containing 1% penicillin, 1% streptomycin, 1% amphotericin B, 5% collagenase B, and 0.5% DNAse I. They were incubated at 37° C for 1 hour and vortexed. Epithelial cells were removed by filtration with a 70 µm filter (Millipore, Billerica, MA). Dispersed endometrial stromal cells (ESC) were then centrifuged and the supernatant was discarded. Cell pellets were then re-suspended in DMEM containing 1% penicillin, 1% streptomycin, 1% Amphotericin B, and 10% fetal bovine serum. Cells were grown to confluence in 75 ml flasks (BD Biosciences, San Jose, CA). Cultures were maintained at 37° C in a humidified atmosphere of 5% CO2 in air. Cells were used in experiments between passages 1 and 3.
Leiomyoma and myometrial tissue were dissected from surgical specimens in Hank’s balanced salt solution (HBSS) containing 1% penicillin, 1% streptomycin, 1% amphotericin B, 5% collagenase B, and 0.5% DNAse I. Minced tissue was placed in Petri dishes and incubated at 37° C for 18–20 hours. Cells were then dispersed by vortexing and centrifuged. After discarding the supernatant, cell pellets were re-suspended in DMEM containing 1% penicillin, 1% streptomycin, 1% Amphotericin B, and 10% fetal bovine serum (FBS). Leiomyoma and myometrial cells were grown to confluence in 75 ml flasks (BD Biosciences, San Jose, CA). Cultures were maintained at 37° C in a humidified atmosphere of 5% CO2 in air. Conditioned media from leiomyoma cells (leiomyoma conditioned media, or LCM) and myometrial cells (myometrium-conditioned media, or MCM) was obtained 24 hours after fresh media supplementation and was used in experiments between passages 1 and 3.
Leiomyoma-conditioned media (LCM) and myometrium-conditioned media (MCM) treatment of ESC and TGF-β blockade
The effect of LCM on ESC cultured was assessed by exposing endometrial cells to LCM. ESC derived from control endometrium (patients without fibroids), were plated in 12 well culture dishes and grown to 60–70% confluence. Culture medium was then removed and unattached cells were removed by washing with PBS. LCM was then added and culture continued for 24 hours. Myometrium-conditioned media (MCM) was added to a separate cohort of cells. Independent experiments using cells derived from 8 fibroid patients were used to treat endometrial cells from 4 control patients. Each independent experiment was done in triplicate. The effect of antibody neutralization of TGF-β was assessed by pre-treating LCM with antibody prior to ESC treatment. LCM was incubated with 200µg/ml of pan-specific TGF-β antibody (R&D Systems, Minneapolis, MN) or non-specific control antibody (Polyclonal Rabbit IgG, R&D Systems, Minneapolis, MN) for 2 hours prior to LCM treatment of ESC. BMP receptors 1A (BMPR1A), 1B (BMPR1B), and 2 (BMPR2) were quantified using q-RT PCR after 24 hours of LCM treatment. BMP-2 stimulated expression of HOXA10 and LIF was quantified by q-RT PCR in additional LCM exposed ESC that were treated with recombinant human BMP-2 (rhBMP-2, R&D Systems, Minneapolis, MN) for 24 hours.
To utilize a receptor specific method of TGF-β blockade, ESC were transfected with pCMV5B plasmid vectors containing mutant (K227R) human TGF-β receptor type II cDNA (purchased from Addgene, Cambridge, MA after generous donation to Addgene by J. Wrana and J. Massague). This mutant receptor is able to bind TGF-β ligand, but lacks kinase activity due to the K227R mutation that results in inability to phosphorylate R-SMAD (41). Primary ESC were grown to 60–70% confluence and transfected with pCMV5B-mhTGFβR-II(K227R) or empty pCMV5B plasmid (control) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Transfected ESC were treated with LCM for 24 hours. Following LCM exposure, cells were treated with rhBMP-2 to assess BMP-2 responsiveness.. Independent experiments using cells derived from 8 fibroid patients were used to treat endometrial cells from 4 control patients. Each independent experiment was done in triplicate. BMP-2 stimulated expression of HOXA10 and LIF mRNA expression was then quantified using q-RT PCR.
Quantitative Real Time PCR (qRT-PCR)
Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. 500 ng of total RNA was reverse transcribed in 20 µl of reaction mixture using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Primers were obtained from the W.M. Keck Oligonucleotide Synthesis Facility (New Haven, CT). qRT-PCR reactions were prepared using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). The conditions for PCR were optimized analyzing the melting curves of the products acquired. Amplification efficiency was assessed after varying primer annealing temperatures, primer concentrations, template concentrations and cycle number. All products were analyzed in the linear phase of amplification. Each PCR reaction consisted of the following: 1 µl of cDNA template, 1 µl of forward primer (1 uM), 1µl of reverse primer (1 µM), 9.5 µl of nuclease-free H20, and 12.5 µl of iQ SYBR Green Supermix. The Bio-Rad iCycler iQ system (Bio-Rad, Hercules, CA) was used to quantify fluorescence of PCR products during amplification. Annealing temperatures were optimized for individual primers. Threshold cycle (Ct) and melting curve data were collected for analysis. Relative gene expression was determined by analyzing data using the 2−ΔΔCT method to adjust for expression of β-Actin (42). All experiments were conducted in triplicate. Samples without cDNA template were used as negative controls.
TGF-β3 ELISA
ELISA was used to quantify TGF-β3 levels in conditioned cell culture media from primary endometrial stromal cells and leiomyoma cells. Culture media, consisting of DMEM containing 1% penicillin, 1% streptomycin, 1% Amphotericin B, and 10% FBS was also analyzed. TGF-β ELISA was performed using a commercially available kit per the manufacturer’s protocol (R&D Systems, Minneapolis, MN). Briefly, the microplate was coated with capture antibody overnight. Aliquots of conditioned culture media were obtained from cell culture flasks 24 hours after addition of fresh media, when cells were at 60–70% confluence. Latent TGF-β3 in samples was activated to the immunoreactive form by addition of 1N HCl, followed by 1.2N NaOH/0.5M HEPES prior to assay. After blocking to prevent non-specific binding, diluted samples and standards (rhTGF-β3, R&D Systems, Minneapolis, MN) were plated, followed by detection antibody, Streptavidin-HRP, substrate solution, and a stop solution of 2N H2SO4. Color intensity was quantified by spectrophotometry at 450 nm using an iMark microplate absorbance reader (BioRad, Hercules, CA. A standard curve was generated and the TGF-β3 concentration of each sample was calculated by regression analysis. Each sample was assayed in duplicate. Mean TGF-β3 concentration was calculated for un-conditioned media, ESC-conditioned media, and Leiomyoma cell conditioned media (LCM).
Statistical Analysis
Statistical comparisons of endometrial gene expression and conditioned cell culture media TGF-β3 levels were made using the student’s t-test with P <0.05 considered significant.
Results
TGF-β3 protein concentration in cell culture supernatant
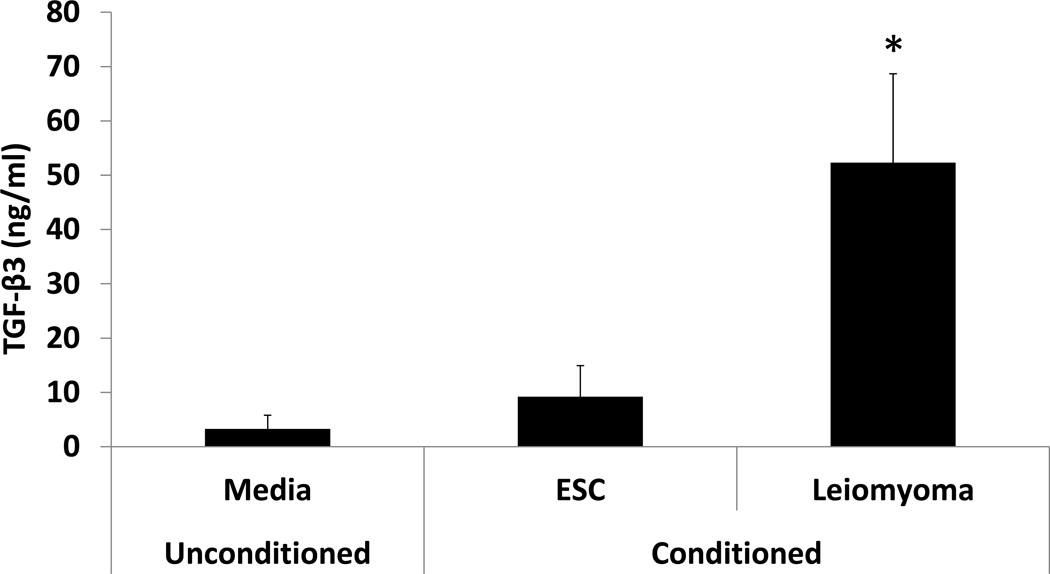
In order to confirm that TGF-β3 is secreted in large amounts by leiomyoma cells, ELISA was used to quantify mean TGF-β3 concentrations in cell culture media after 24 hours of exposure to primary endometrial stromal cells or leiomyoma cells grown to 60–70% confluence. Mean TGF-β3 concentrations were more than 5-fold greater in leiomyoma-conditioned media (LCM) compared to conditioned media from ESC (52 ng/ml vs. 9.2 ng/ml, p<0.05). TGF- β3 was also present in the cell culture media preparation, consisting of DMEM containing 1% penicillin, 1% streptomycin, 1% Amphotericin B, and 10% FBS, at a concentration of 3.29 ng/ml, confirming that contributions of TGF-β3 from FBS were negligible. (Fig 1)
Figure 1. ELISA quantification of TGF-β3 in ESC-conditioned and Leiomyoma-Conditioned media.
TGF-β3 protein concentration was quantified by ELISA in conditioned media from primary ESC and leiomyoma cells grown at 60–70% confluence and cultured for 24 hours. Mean TGF-β3 protein concentration was significantly higher in leiomyoma-conditioned media compared to ESC-conditioned media (52 ng/ml vs. 9.2 ng/ml, p<0.05). TGF-β3 protein was present in low concentration (3.2 ng/ml) in standard media (DMEM containing 1% penicillin, 1% streptomycin, 1% Amphotericin B, and 10% fetal bovine serum).
Expression of BMP receptors in leiomyoma-exposed endometrial stromal cells
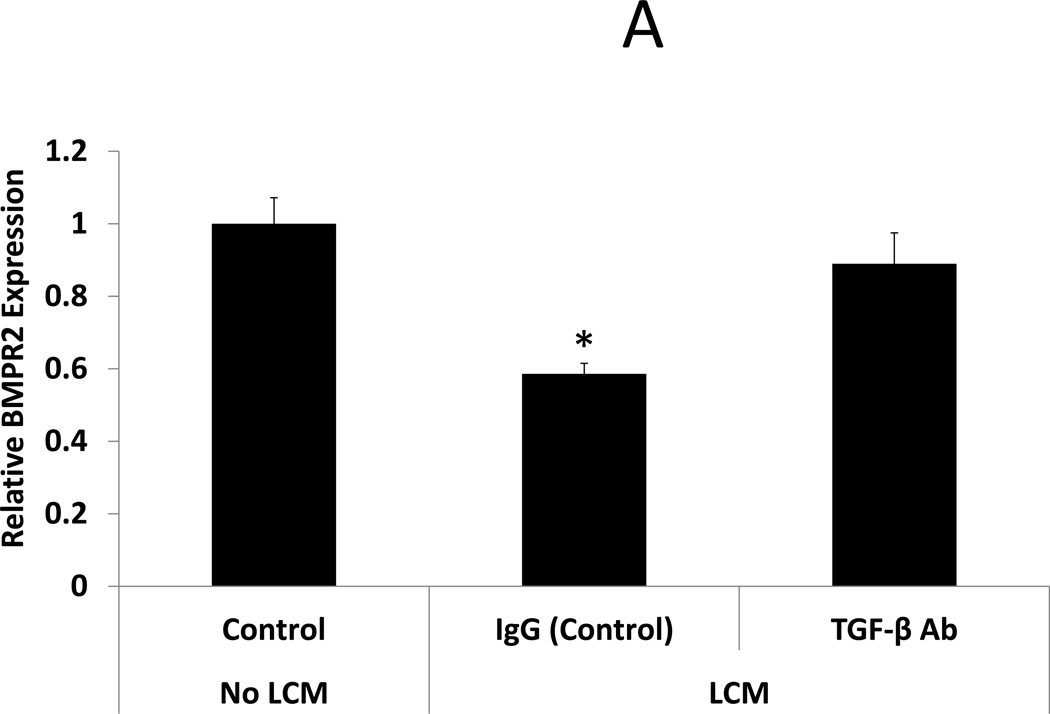
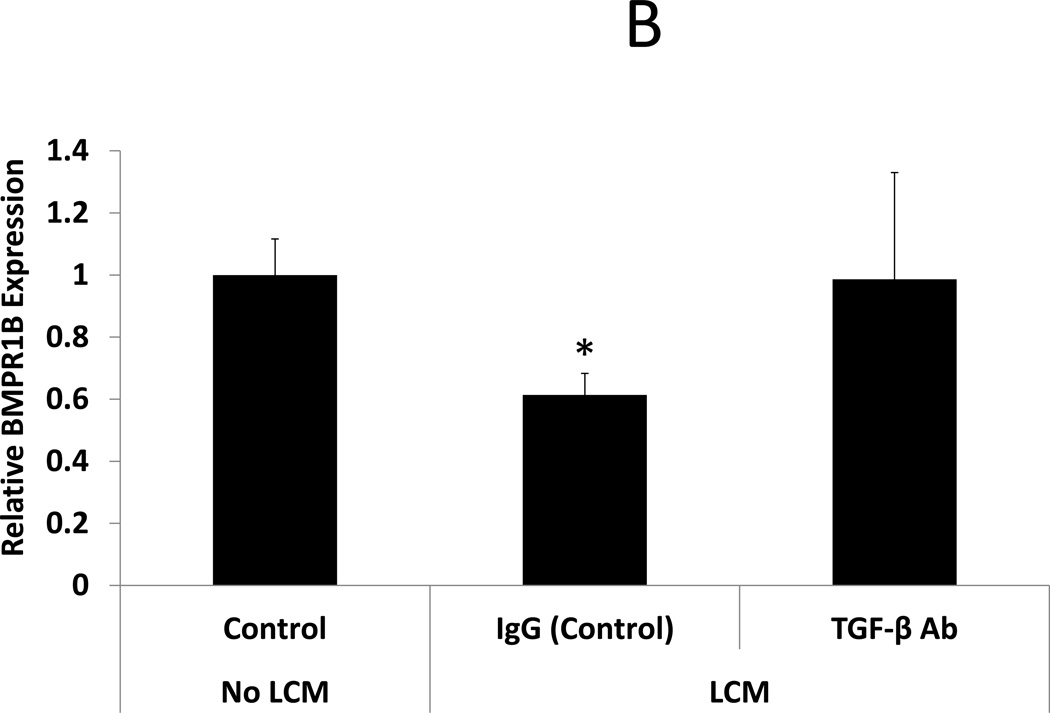
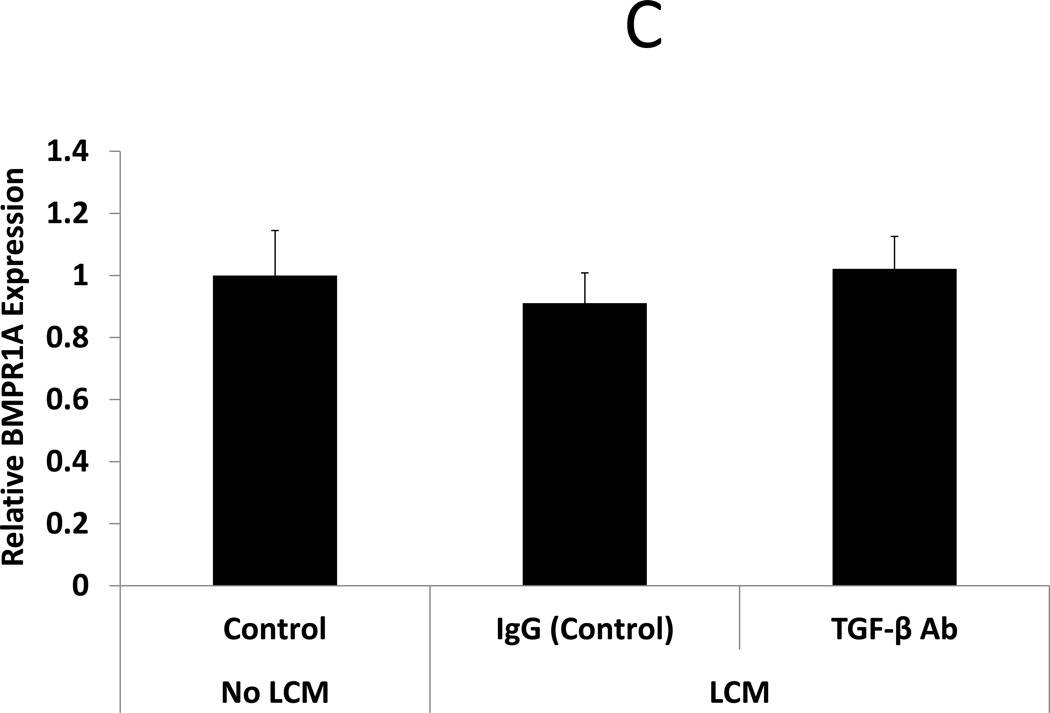
After confirmation that TGF-β3 levels were indeed elevated in LCM, we sought to expand on data from Sinclair et al, showing that TGF-β3 reduces expression of BMP receptors in endometrial stromal cells (18), here using LCM containing TGF- β3. BMP receptor mRNA was quantified by qRT-PCR in ESC exposed to LCM, with and without pre-incubation with TGF-β neutralizing antibody, for 24 hours. LCM treatment of ESC decreased expression of BMPR1B and 2 when compared to control ESC that did not receive LCM treatment (0.61 and 0.59 fold, respectively, p<0.05). BMPR1A was not significantly reduced in LCM treated ESC. Incubation of LCM with TGF-β antibody, prior to ESC treatment, prevented repression of BMPR1B and BMPR2. These data demonstrate that neutralization of TGF- β in LCM is sufficient to prevent repression of BMP receptors in LCM treated ESC. (Fig 2 A–C)
Figure 2. Expression of BMP receptors in LCM exposed ESC.
A: BMP Receptor 2. BMPR2 expression was reduced in ESC exposed to LCM, compared to control non-LCM exposed ESC (0.59 fold, p<0.05). Pre-incubation of LCM with TGF-β neutralizing antibody, prior to ESC exposure, prevented repression of BMPR2 expression.
B: BMP Receptor 1B. BMPR1B expression was reduced in ESC exposed to LCM, compared to control non-LCM exposed ESC (0.61 fold, p<0.05). Pre-incubation of LCM with TGF-β neutralizing antibody, prior to ESC exposure, prevented repression of BMPR1B expression.
C: BMP Receptor 1A. BMPR1A expression was not significantly changed in LCM exposed ESC, compared to control non-LCM exposed ESC.
* denotes statistical significance with p<0.05
BMP-2 stimulated HOXA10 and LIF expression in LCM treated ESC: Effect of TGF-β Antibody
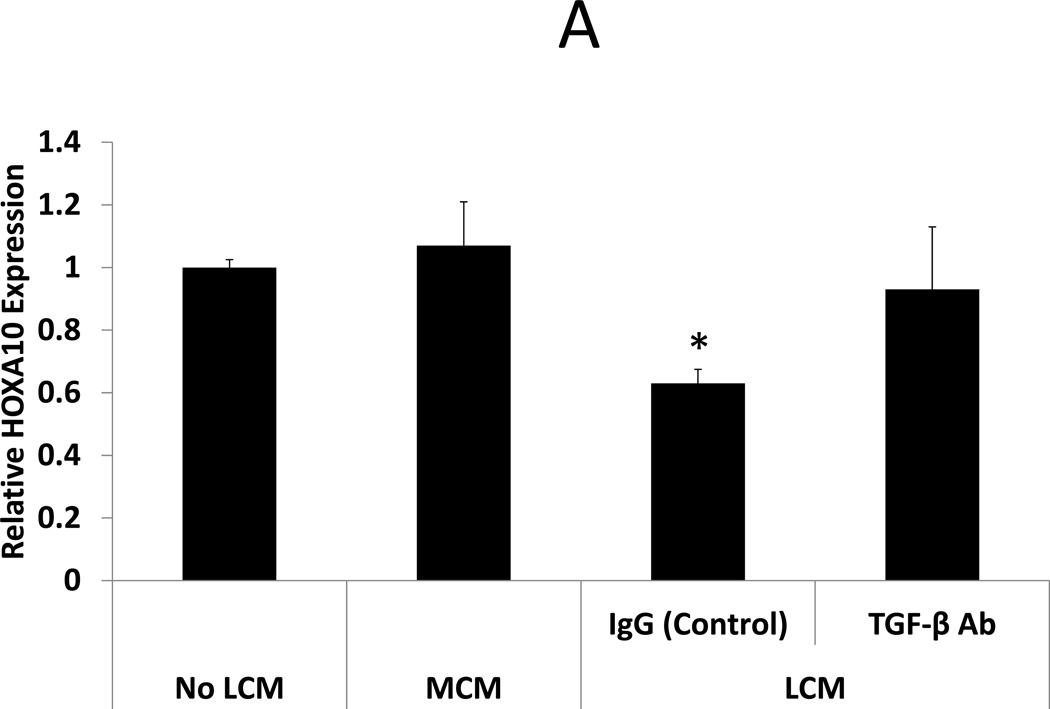
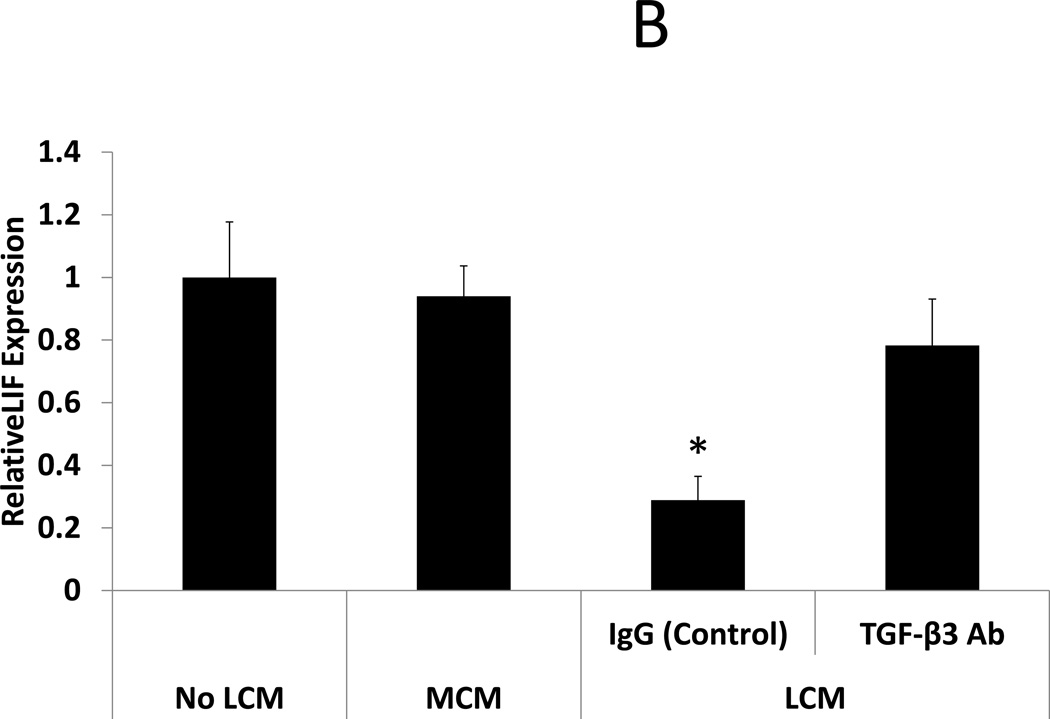
Neutralization of TGF-β3 in LCM is sufficient to restore BMP receptor expression and BMP-2 responsiveness in LCM exposed ESC. BMP-2 responsiveness was assessed by examining HOXA10 and LIF expression in ESC following treatment with rhBMP-2. rhBMP-2 stimulated HOXA10 and LIF expression was compared in 3 groups of ESC: ESC exposed to myometrium-conditioned media (MCM), ESC exposed to leiomyoma-conditioned media (LCM), or non-exposed (control) ESC. Exposure to MCM did not change rhBMP-2 stimulated HOXA10 or LIF expression in MCM-exposed ESC compared to non-exposed control ESC (expression 1.07 and 0.94 fold, respectively, NS). rhBMP-2 stimulated HOXA10 and LIF expression was repressed in LCM-exposed compared to control (expression 0.63 and 0.29 fold, respectively, p<0.05). Pre-incubation of LCM with TGF-β antibody, prior to ESC treatment, prevented repression of BMP-2 stimulated HOXA10 and LIF expression. These data demonstrate that neutralization of TGF- β in LCM is sufficient to restore BMP-2 responsiveness in LCM treated ESC. (Fig 3)
Figure 3. BMP-2 stimulated HOXA10 and LIF expression in LCM treated ESC: Effect of TGF-β Antibody.
BMP-2 responsiveness was assessed in LCM exposed ESC by treatment with rhBMP-2. HOXA10 and LIF expression were quantified by qRT-PCR after 24 hours of rhBMP-2 treatment. A: BMP-2 stimulated HOXA10 expression. BMP-2 stimulated HOXA10 expression was unchanged in MCM exposed ESC, compared to non-exposed control ESC (1.07 fold, NS). BMP-2 stimulated HOXA10 expression was repressed in ESC exposed to LCM, compared to non LCM exposed control ESC (0.63 fold, p<0.05). Incubation of LCM with TGF-β neutralizing antibody, prior to ESC exposure, prevented repression of BMP-2 stimulated HOXA10 expression. B: BMP-2 stimulated LIF expression. BMP-2 stimulated LIF expression was unchanged in MCM exposed ESC, compared to non-exposed control ESC (0.94 fold, NS). BMP-2 stimulated LIF expression was repressed in LCM exposed ESC, compared to non LCM exposed control ESC (0.29 fold, p<0.05). Incubation of LCM with TGF-β neutralizing antibody, prior to ESC exposure, prevented repression of BMP-2 stimulated LIF expression.
* denotes statistical significance with p<0.05
BMP-2 stimulated HOXA10 and LIF expression in LCM treated ESC: Effect of transfection with mutant TGF-β receptor
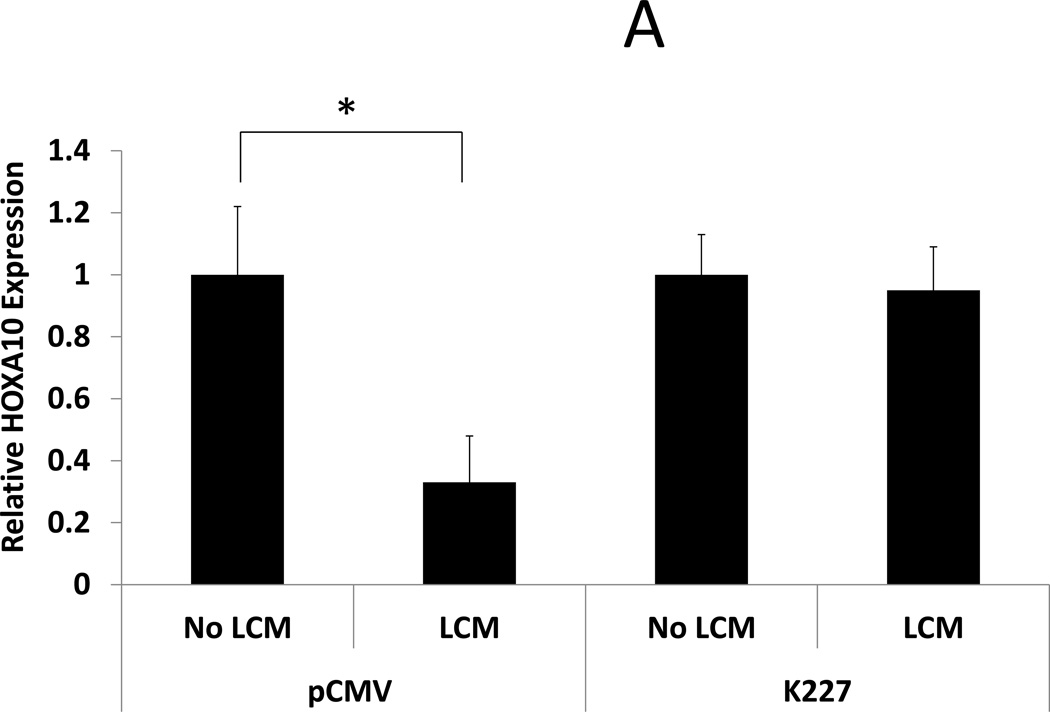
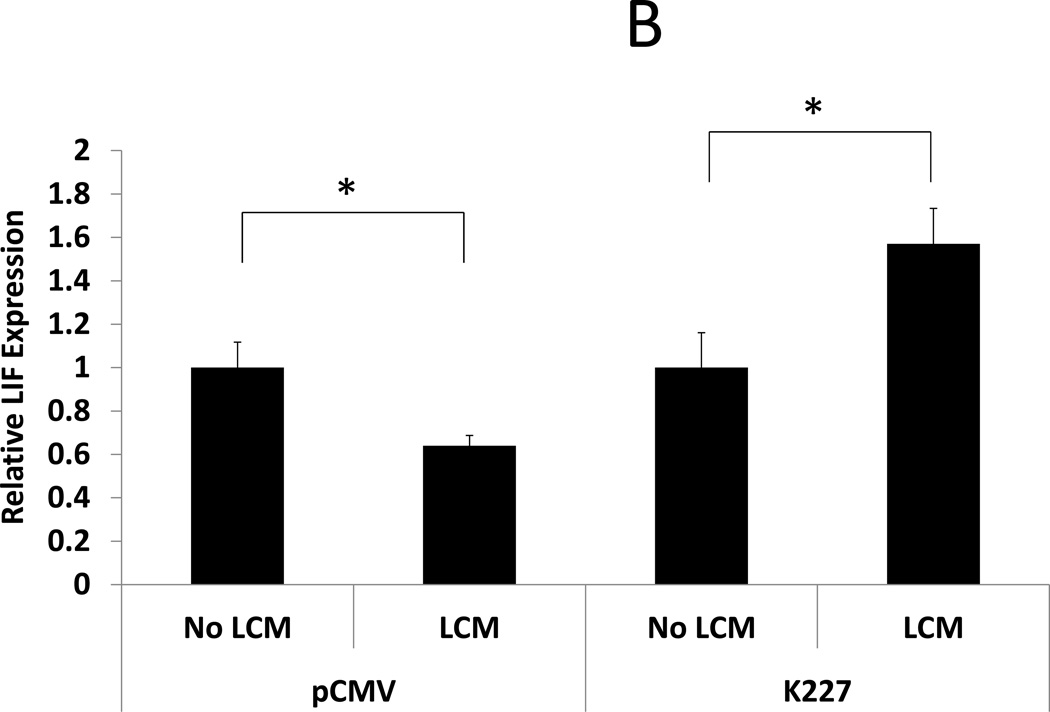
ESC were transfected with a mutant TGF-β type II receptor that allows ligand binding but prevents phosphorylation of SMAD. BMP-2 responsiveness was assessed by examining BMP-2 stimulated HOXA10 and LIF expression in transfected ESC after LCM exposure. LCM exposed control ESC (transfected with empty vector) had reduced HOXA10 and LIF expression after rhBMP-2 treatment compared to control ESC without LCM exposure (0.33 and 0.64 fold, respectively, P<0.05). LCM exposure did not have any effect on rhBMP-2 stimulated HOXA10 and LIF expression in ESC that had been transfected with K227 mutant TGF-β type II receptor (expression 0.95 fold and 1.65 fold, compared to control, NS). These data show that transfection of ESC with a dysfunctional TGF-β type II receptor prior to LCM exposure prevents alterations in BMP-2 signaling and prevents repression of rhBMP-2 stimulated HOXA10 and LIF expression. (Fig 4)
Figure 4. BMP-2 stimulated HOXA10 and LIF expression in LCM treated ESC: Effect of transfection with mutant TGF-β receptor.
BMP-2 stimulated expression of HOXA10 and LIF was examined in LCM exposed ESC that had been transfected with mutant TGF-β Type II receptor or vector control. A: BMP-2 stimulated HOXA10 expression. BMP-2 stimulated HOXA10 expression was repressed in LCM exposed ESC that had been transfected with vector control plasmid, compared to non LCM exposed controls (0.33 fold, p<0.05). BMP-2 stimulated HOXA10 expression was not significantly changed, compared to non LCM exposed control ESC, when ESC had been transfected with mutant TGF-β Type II receptor prior to LCM exposure. B: BMP-2 stimulated LIF expression. BMP-2 stimulated LIF expression was repressed LCM exposed ESC that had been transfected with vector control plasmid, compared to non LCM exposed control ESC (0.64 fold, p<0.05). BMP-2 stimulated LIF expression was significantly increased, compared to non LCM exposed control ESC, when ESC had been transfected with mutant TGF-β Type II receptor prior to LCM exposure (1.57 fold, p<0.05).
* denotes statistical significance with p<0.05
Discussion
Here we have shown that TGF-β3 levels are elevated in leiomyoma-conditioned media and that exposure of endometrial cells to this conditioned media alters BMP-2 responsiveness. TGF exposure leads to repression of BMP receptor types 1B and 2 in ESC leading to the lack of response to BMP. Neutralization of TGF-β was sufficient to prevent reduction in BMP receptor expression and restore endometrial BMP responsiveness. HOXA10 and LIF expression are regulated by BMP-2 (18) and required for endometrial receptivity. Blockade of TGF- β restored BMP-2 mediated up-regulation of HOXA10 and LIF expression. These findings implicate leiomyoma derived TGF-β as a molecular mediator of leiomyoma-associated endometrial receptivity.
Our laboratory has previously demonstrated reductions in HOXA10 expression globally throughout the endometrium (not limited to the region immediately overlying submucosal leiomyomas), suggesting that a diffusible signal mediates the implantation defect seen in women with submucosal leiomyomas (39). Here we demonstrate that TGF-β acts as this signaling molecule to alter BMP-2 signaling in the endometrium and contributes to infertility associated with leiomyoma.
BMP2 mediates Progesterone induced stromal decidualization in both the mouse and the human (16, 43). Decidualization is critical for implantation and the establishment the early pregnancy. Conditional ablation of Bmp2 in the uterus leads to infertility in female mice. Initial embryo attachment is normal in the Bmp2 deficient mice, however the uterine stroma is unable to undergo decidualization and allow continued pregnancy.
TGF-βs are differentially expressed in the endometrium and are influenced by estradiol and progesterone (44, 45). Progesterone and TGF-β act together to repress the expression of MMP’s during the secretory phase prior to the initiation of menstruation (46, 47). Stimulation of TGF-β expression by progesterone has been reported in some studies (46, 48, 49). However, a more recent study demonstrates that progesterone directly inhibits endometrial expression of TGF-β2 and TGF-β3 (45). The discrepancies in regulation of TGF-β by progesterone in these studies have been hypothesized to exist due to a biphasic effect of progesterone or a differential effect that varies with progesterone concentration (45, 50). While the exact interactions between TGF-β and progesterone in the endometrium are yet to be completely defined, our data suggest that TGF-β secreted from leiomyomas functions within endometrial cells to alter BMP-2 signaling.
In the presence of leiomyoma, TGF- β prevents a normal endometrial BMP response and likely hinders decidualization. The failure of decidualization and therefore embryo development after attachment may also contribute to the increased rate of pregnancy loss in women with leiomyoma. Taken together, these findings implicate TGF-β as a paracrine mediator of leiomyoma-related infertility and pregnancy loss. Pharmacotherapy aimed to reduce TGF-β levels or inhibit TGF-β signaling in the endometrium may be a means to improve endometrial receptivity in women with leiomyomas.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant R01 HD076422.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergh PA, Navot D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992;58:537–542. doi: 10.1016/s0015-0282(16)55259-5. [DOI] [PubMed] [Google Scholar]
- 2.Strowitzki T, Germeyer A, Popovici R, von Wolff M. The human endometrium as a fertility-determining factor. Hum Reprod Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- 3.Navot D, Laufer N, Kopolovic J, Rabinowitz R, Birkenfeld A, Lewin A, et al. Artificially induced endometrial cycles and establishment of pregnancies in the absence of ovaries. N Engl J Med. 1986;314:806–811. doi: 10.1056/NEJM198603273141302. [DOI] [PubMed] [Google Scholar]
- 4.Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- 6.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 7.Stewart C, Kaspar P, Brunet L, Bhatt H, Gadi I, Köntgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 8.Bagot CN, Kliman HJ, Taylor HS. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn. 2001;222:538–544. doi: 10.1002/dvdy.1209. [DOI] [PubMed] [Google Scholar]
- 9.Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–579. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- 10.Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, et al. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod. 2003;68:24–30. doi: 10.1095/biolreprod.102.009316. [DOI] [PubMed] [Google Scholar]
- 11.Doherty L, Kwon H, Taylor H. Regulation of Tryptophan 2,3-Dioxygenase by HOXA10 Enhances Embryo Viability Through Serotonin Signaling. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00439.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- 14.Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:238–243. doi: 10.1210/jc.2002-021072. [DOI] [PubMed] [Google Scholar]
- 15.Daftary GS, Kayisli U, Seli E, Bukulmez O, Arici A, Taylor HS. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil Steril. 2007;87:367–372. doi: 10.1016/j.fertnstert.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Lee K, Jeong J, Wang J, Ma L, Martin J, Tsai S, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paria B, Ma W, Tan J, Raja S, Das S, Dey S, et al. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinclair DC, Mastroyannis A, Taylor HS. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-β3. J Clin Endocrinol Metab. 2011;96:412–421. doi: 10.1210/jc.2010-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezzati M, Norian J, Segars J. Management of uterine fibroids in the patient pursuing assisted reproductive technologies. Womens Health (Lond Engl) 2009;5:413–421. doi: 10.2217/whe.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payson M, Leppert P, Segars J. Epidemiology of myomas. Obstet Gynecol Clin North Am. 2006;33:1–11. doi: 10.1016/j.ogc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1–211.e9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 23.Olive DL, Pritts EA. Fibroids and reproduction. Semin Reprod Med. 2010;28:218–227. doi: 10.1055/s-0030-1251478. [DOI] [PubMed] [Google Scholar]
- 24.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 25.Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28:180–203. doi: 10.1055/s-0030-1251476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, et al. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Islam MS, Protic O, Stortoni P, Grechi G, Lamanna P, Petraglia F, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril. 2013 doi: 10.1016/j.fertnstert.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 29.Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- 30.Joseph D, Malik M, Nurudeen S, Catherino W. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 31.Norian J, Malik M, Parker C, Joseph D, Leppert P, Segars J, et al. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–474.e11. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leppert P, Catherino W, Segars J. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez H, Sefrioui O, Virelizier C, Gervaise A, Gomel V, Frydman R. Hysteroscopic resection of submucosal myomas in patients with infertility. Hum Reprod. 2001;16:1489–1492. doi: 10.1093/humrep/16.7.1489. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg M, Sivan E, Sharabi Z, Bider D, Rabinovici J, Seidman DS. Outcome of hysteroscopic resection of submucous myomas for infertility. Fertil Steril. 1995;64:714–716. doi: 10.1016/s0015-0282(16)57844-3. [DOI] [PubMed] [Google Scholar]
- 36.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Yoshino O, Hayashi T, Osuga Y, Orisaka M, Asada H, Okuda S, et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Hum Reprod. 2010;25:2475–2479. doi: 10.1093/humrep/deq222. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Melo NR, Abdelmassih R. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril. 2004;81:582–587. doi: 10.1016/j.fertnstert.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Rackow B, Taylor H. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 41.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358:155–165. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132:217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- 45.Gaide Chevronnay HP, Cornet PB, Delvaux D, Lemoine P, Courtoy PJ, Henriet P, et al. Opposite regulation of transforming growth factors-beta2 and -beta3 expression in the human endometrium. Endocrinology. 2008;149:1015–1025. doi: 10.1210/en.2007-0849. [DOI] [PubMed] [Google Scholar]
- 46.Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, et al. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci U S A. 1995;92:7362–7366. doi: 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruner KL, Eisenberg E, Gorstein F, Osteen KG. Progesterone and transforming growth factor-beta coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids. 1999;64:648–653. doi: 10.1016/s0039-128x(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 48.Casslén B, Sandberg T, Gustavsson B, Willén R, Nilbert M. Transforming growth factor beta1 in the human endometrium. Cyclic variation, increased expression by estradiol and progesterone, and regulation of plasminogen activators and plasminogen activator inhibitor-1. Biol Reprod. 1998;58:1343–1350. doi: 10.1095/biolreprod58.6.1343. [DOI] [PubMed] [Google Scholar]
- 49.Arici A, MacDonald PC, Casey ML. Modulation of the levels of transforming growth factor beta messenger ribonucleic acids in human endometrial stromal cells. Biol Reprod. 1996;54:463–469. doi: 10.1095/biolreprod54.2.463. [DOI] [PubMed] [Google Scholar]
- 50.Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. Role of TGF-betas in normal human endometrium and endometriosis. Hum Reprod. 2010;25:101–109. doi: 10.1093/humrep/dep382. [DOI] [PubMed] [Google Scholar]