Abstract
While acute effects of toxic radiation doses on intestine are well established, we are yet to acquire a complete spectrum of sub-lethal radiation-induced chronic intestinal perturbations at the molecular level. We investigated persistent effects of a radiation dose (2 Gy) commonly used as a daily fraction in radiotherapy on oxidants and anti-oxidants, and autophagy pathways, which are interlinked processes affecting intestinal homeostasis. Six to eight weeks old C57BL/6J mice (n=10) were exposed to 2 Gy γ-ray. Mice were euthanized two or twelve months after radiation, intestine surgically removed, and flushed using sterile PBS. Parts of the intestine from jejunal-ilial region were fixed, frozen, or used for intestinal epithelial cell (IEC) isolation. While oxidant levels and mitochondrial status were assessed in isolated IEC, autophagy and oxidative stress related signaling pathways were probed in frozen and fixed samples using PCR-based expression arrays and immunoprobing. Radiation exposure caused significant alterations in the expression level of 26 autophagy and 17 oxidative stress related genes. Immunoblot results showed decreased Beclin1 and LC3-II and increased p62, PI3K/Akt, and mTOR. Flow cytometry data showed increased oxidant production and compromised mitochondrial integrity in irradiated samples. Immunoprobing of intestinal sections showed increased 8-oxo-dG and nuclear PCNA, and decreased autophagosome marker LC3-II in IEC after irradiation. We show that sub-lethal radiation could persistently downregulate anti-oxidants and autophagy signaling, and upregulate oxidant production and proliferative signaling. Radiation-induced promotion of oxidative stress and downregulation of autophagy could work in tandem to alter intestinal functions and have implications for post-radiation chronic gastrointestinal diseases.
Keywords: Radiation, autophagy, oxidative stress, intestine, mitochondria
1. Introduction
Autophagy is a complex catabolic process involved in removing and recycling damaged or unwanted cellular constituents in autophagolysosomal vesicles to sustain energy supply during nutritional stress. The mammalian target of rapamycin (mTOR), a member of the phosphatidylinositol kinase-related kinase (PIKK) family and a nutrient sensor, is a major regulator of autophagy [1]. Formation of the autophagosome, and its subsequent fusion with lysosomal vesicle to form the autophagolysosome, is essential for autophagy and at least thirty autophagy-related (Atg) genes have been identified and demonstrated to be involved in different sub-types of autophagy [2,3]. However, the core autophagy pathway requires seventeen genes and in mammalian cells the process is initiated when the mammalian homolog of yeast Atg1 complex comprising ULK1, ULK2, Atg13, Atg101 and FIP200 is activated due to mTOR inhibition [1-3]. Major proteins involved in the elongation of the autophagosome membrane are Vps34/PI3PIII complex, Vps15, and Beclin1. Subsequently, the Atg12-Atg5-Atg16L protein complex joins the process and usher in maturation of the autophagosome [1-4]. Elongation and maturation of autophagosomes also involve mammalian orthologs of yeast Atg8 such as LC3, Gabarapl1 and Gabarapl2. Conversely, Atg7, Atg4, and Atg3 are involved in activation of these proteins to generate their mature forms. For LC3, it is initially converted to LC3-I or LC3A and finally to LC3-II or LC3B and similar to LC3, the GABARAP members are finally converted to their active forms of GABARAPL1-II and GABARAPL2-II. The activated LC3 and GABARAP family of proteins on the autophagosome allow binding of adaptor proteins such as p62 and Nbr-1, which in turn recruits ubiquitinated proteins for autophagolysosomal degradation [1-4]. Importantly, the autophagy pathway through its influence on cell death and proliferation, oxidative stress and inflammation, and on immune responses is also involved in the maintenance of cellular homeostasis in intestine, and dysregulation of autophagy has been linked to altered intestinal functionality and development of diseases such as inflammatory bowel diseases and cancer [1-5].
Maintenance of gastrointestinal (GI) homeostasis is essential for health and radiation exposures have been shown to affect cellular functionality in the intestine, a rapidly proliferating tissue. Radiation effects on intestine are dependent on radiation dose, and duration of exposure. Radiation exposure to intestine has been reported to cause a myriad of short- and long-term ailments due to perturbation of intestinal cell functions [6-8]. While higher doses of radiation exposure invariably leads to cell death-associated normal tissue complications, lower sub-lethal doses of radiation exposure cause most of the alterations at the molecular level and cells survive with damage leading to long-term heath risks [9]. Furthermore, epidemiological studies in atomic bomb survivors and nuclear workers, most of whom were exposed to sub-lethal doses of <1 Gy, have demonstrated that radiation is a long-term risk factor for non-cancer diseases as well as solid cancers [10-13]. Furthermore, radiation exposure has been intimately linked to increased reactive oxygen species (ROS) production and persistent oxidative stress in cells, and oxidative stress has been reported to activate the pro-growth PI3K/Akt pathway, which in turn is known to activate mTOR [14,15]. On the other hand oxidative stress and autophagy are in a co-dependent but inverse relationship where decreased autophagic activity increases oxidative stress and increased oxidative stress in turn is known to downregulate the autophagy pathway [16-18]. Notably, most of the radiation exposure studies on autophagy are in relation to cancer therapy, and effects of a sub-lethal radiation dose on autophagy has not been explored [19-24]. Here we demonstrated that radiation exposure led to persistently increased oxidant production and decreased anti-oxidant gene expression leading to oxidative stress and activation of proliferative PI3K/Akt and mTOR signaling. When considered along with our results showing radiation-induced downregulation of autophagy pathway associated factors at the mRNA as well as at the protein level, our observations have implications for radiation-induced persistent alterations of intestinal functions.
2. Methods and materials
2.1. Mice
Six to eight weeks old female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed at Georgetown University's research animal facility, which is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited facility. Mice were housed in autoclaved cages and bedding materials in a separate room with 12 h dark and light cycle maintained at 22 °C in 50% humidity. All animals were provided certified rodent diet with filtered water ad libitum and CO2 asphyxiation was used for euthanasia as per animal care facility guideline. Post-irradiation, any mouse showing signs of declining health assessed using approved criteria such as hunched posture, ruffled fur, diarrhea, and reduced activity was euthanized by CO2 asphyxiation and was excluded from the specific study group. All animal procedures used in the study were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee and we followed Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council, and U.S. National Academy of Sciences for our research.
2.2 Irradiation
We used a 137Cs source to expose the mice (n = 10 per group) to a sub-lethal whole-body γ radiation dose of 2 Gy (dose rate 0.7 Gy/min). For this study, the radiation dose of 2 Gy was chosen due to the fact that it is a common daily fraction used in radiotherapy to deliver planned total radiation dose to cancer patients. Additionally, we have used the 2 Gy dose to correlate the current and earlier [25] radiation related persistent effect studies in intestinal cells to radiation-induced colorectal carcinogenesis studies in mouse models [26]. Post-irradiation mice were returned to their home cages and monitored regularly. Irradiation experiments were repeated three times and mice were euthanized two months after each exposure. In all experiments the control mice were sham-irradiated.
2.3 Tissue harvesting and intestinal epithelial cell (IEC) isolation
Mice were euthanized as per approved protocol and small intestine was surgically removed two or twelve months after radiation exposure. For our previous radiation-induced persistent effect studies in different tissues [25,27-29] we choose two and twelve months time points and the same time points were used in the current study. Intestinal lumen was flushed with phosphate buffered saline (PBS) at room temperature, and the jejunal-ileal region was used for intestinal epithelial cell (IEC) isolation, tissue fixation, or freezing. IEC was isolated, characterized, and viability assessed according to a protocol described previously [25]. Isolated cells were used for flow cytometry experiments and samples from six mice in each group were processed in triplicate for each experiment. For immunohistochemistry and immunoflurescence, 3 cm of intestinal tissue was fixed in 10% buffered formalin, paraffin embedded, and 4 µm sections were made. Tissues were also snap-frozen in liquid nitrogen and stored at -80 °C either for RNA isolation or for immunoblot analysis.
2.4. RNA isolation and quantitative real time PCR (qRT-PCR) using PCR array
For RNA extraction, we used pooled samples of five mice from each experimental group. Total RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA) and RNeasy column according to the manufacturer's instructions (Qiagen, Germantown, MD) from flash frozen intestine. RNA concentration and quality was determined using a Bioanalyzer (Agilent Technologies, Palo Alto, CA) and stored in aliquots at -80 °C for further analysis. Mouse autophagy (PAMM-084Z) and oxidative stress (PAMM-065Z) RT2 Profiler PCR Array was obtained from SA Biosciences (Frederick, MD) and intestinal RNA samples were assayed by qRT-PCR according to manufacturer's instructions. Briefly, RT2 First Strand Kit (SA Biosciences) was used to reverse-transcribe RNA into cDNA and RT2 real time SYBR green PCR master mix was used for qRT-PCR in an iCycler (Bio-Rad, Hercules, CA) following a protocol provided by the manufacturer (SA Biosciences). The PCR array probes expression of 84 genes in each of the autophagy and oxidative stress arrays and relative changes in gene expression were calculated using β-actin as an endogenous control following the comparative Ct (AACt) method using web-based tools provided by the manufacturer (SA Biosciences). Results were expressed relative to sham-irradiated control samples.
2.5. Intracellular ROS measurements
Intracellular ROS level was measured in IEC as per protocol described previously using the fluorescent probe 2'-7'-dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen, Carlsbad, CA) [25,30].
2.6. Mitochondrial superoxide assay
Mitochondrial superoxide level was measured in IEC using a mitochondrial superoxide specific fluorescent probe, MitoSOX red (Invitrogen), as per manufacturer's instructions and described previously [25].
2.7. Mitochondrial membrane potential (MMP) analysis
Changes in MMP were measured in IEC using Rhodamine 123 as per protocol described previously [25,31].
2.8. Immunohistochemistry in intestinal sections
Staining for 8-oxo-dG was performed using a primary antibody from Trevigen (Gaithersburg, MD; dilution-1:200) according to a protocol described previously [25]. For proliferating cell nuclear antigen (PCNA) staining, sections were depraffinized, antigen retrieved in pH 6.0 citrate buffer (Dako, Carpinteria, CA), and endogenous peroxidase quenched. Sections were incubated in blocking buffer (0.1% bovine serum albumin in PBS) before exposing to PCNA antibody (sc7907; Santa Cruz Biotechnology, Dallas, TX; dilution-1:100). SuperPictureTM 3rd Gen IHC detection kit (87-9673; Invitrogen) was used for signal detection and color development. All the IHC slides were mounted and visualized under a bright field microscope and images were captured at microscopic magnification (8-oxo-dG at 40X and PCNA at 20X microscopic magnification). For immunofluorescence of LC3-II, following the blocking step described for PCNA, tissue sections were incubated overnight with anti-LC3-II antibody (PA1-33197, Thermo Scientific, Pittsburgh, PA; dilution 1:100) at 4 °C. After necessary washing steps sections were incubated with AlexaFluor488-conjugated goat-anti-rabbit antibody (A-1 1034, Life Technologies, Grand Island, NY) for 1 h in dark at room temperature. Samples were washed and mounted using DAPI containing VECTASHIELD mounting medium (H-1200, Vector Laboratories, Burlingame, CA). Sections were visualized and images captured at 20X magnification using an Olympus BX61 DSU fluorescent microscope and images were analyzed using SlideBook v5.0 software (Intelligent Imaging Innovations, Inc, Denver, CO). To determine specificity of the staining, appropriate controls were run in parallel with the experimental sections.
2.9. Immunoblot analysis
Intestinal tissues from five mice were pooled and subjected to immunoblot analysis according to a protocol described previously [28] and repeated in three sets of experimental samples. Briefly, tissues were homogenized in ice-cold extraction buffer (0.5% sodium deoxycholate, 0.5% NP-40, 10 mM EDTA in phosphate-buffered saline containing protease inhibitor cocktail obtained from Sigma-Aldrich, St. Louis, MO). Supernatants from the homogenates were collected by centrifugation, proteins were resolved by SDS-PAGE, transferred onto polyvinylidene fluoride membrane, and incubated with appropriate primary antibody for LC3-II (PA1-33197, Thermo Scientific, Pittsburgh, PA; dilution-1:1000), Beclin 1 (sc11427, Santa Cruz Biotechnology; dilution-1:400), p62 (5114s, Cell Signaling Technology, Danvers, MA; dilution-1:500), p85 (4292s, Cell Signaling Technology; dilution-1:500), mTOR (PA5-17780, Thermo Scientific; dilution-1:1000), phospho-mTOR (2971s, Cell Signaling Technology; dilution-1:500), Akt (sc8312; Santa Cruz Biotechnology; dilution-1:400,), and phospho-Akt (9277s; Cell Signaling Technology; dilution-1:500,), β-actin (sc4778, Santa Cruz Biotechnology; dilution-1:2500). Immunoblot membranes were developed with horseraddish peroxidase conjugated secondary antibody and enhanced chemiluminescence detection system. Images were captured on photographic films and scanned, and representative results are displayed. Densitometric quantification of the immunoblots was performed by normalizing to β-actin band intensity using ImageJ v1.46r (National Institutes of Health, Bethesda, MD).
2.10. Data analysis and statistics
Immunohistochemistry images were analyzed using color deconvolution and/or Image-based Tool for Counting Nuclei (ITCN) plug-ins of ImageJ v1.46r software by two observers blinded to treatment groups as per protocol described earlier [25,32,33]. We used five random image frames from each section for analysis and mean data from six mice in each group are presented graphically and a representative image from one animal of each group is shown in the results. We used WinMDI v2.9 to analyze flow cytometry data and average percent change of mean fluorescence from triplicate samples of six mice are presented in bar graphs. A representative histogram comparing sham-irradiated (black) to irradiated (red) samples is shown in the results. Two independent viewer blinded to the experimental groups counted LC3-II staining by observation in 24 random image frames (4 frames per section; n= 6 per group) captured at 20X magnification and results are expressed as average number of positive cells per 20X field and a representative image (20X magnification) from one animal of each group of one experiment is shown in the results. Statistical significance between the two groups was determined using a two-tailed paired student's t test and p<0.05 was taken as statistically significant. Error bars represent ± standard error of the mean (SEM).
3. Results
3.1. Increased oxidant production and compromised mitochondrial status accompanied decreased anti-oxidant gene expression after radiation exposure
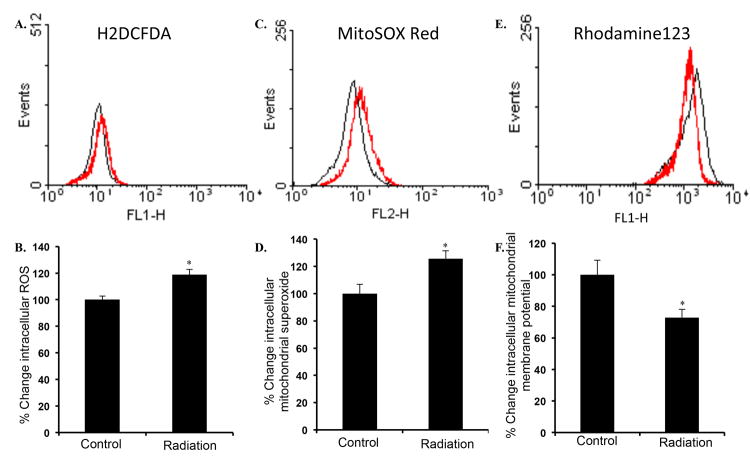
Earlier we reported that 2 Gy irradiation of mice (same strain, gender, and age) used in this study was associated with oxidative stress in intestinal cells even twelve months after exposure [25]. In the current study, significantly increased level of ROS was observed in intestinal epithelial cells two months after exposure to 2 Gy γ radiation relative to controls (Figure 1A and B; p<0.007). Concurrently, we also observed increased mitochondrial superoxide (Figure 1C and D; p<0.001) and decreased mitochondrial membrane potential (Figure 1E and F; p<0.005) two months after radiation exposure relative to controls. Quantitative PCR analysis of oxidative stress related gene expression showed significant (determined using a fold change cutoff of 1.25 and a p-value of <0.05) alterations of 17 genes (Table 1). While expression of only one anti-oxidant gene (Gpx2) was upregulated, expression of a total of 14 anti-oxidant genes were downregulated (Gpx1, Gpx3, Gpx8, Prdx1, Prdx2, Prdx3, Prdx4, Prdx6, Sod1, Sod2, Sod3, Txnrd3, Cat, and Gstk1) in intestine two months after radiation exposure. In contrast, expression of two oxidant production related genes was perturbed. While expression of Nox4 was downregulated, expression of Nos2 was significantly increased in irradiated samples (Table 1).
Figure 1.
Increased oxidative stress in intestinal epithelial cells after radiation exposure. A) Flow cytometry histogram showing intracellular reactive oxygen species (ROS) measurement in IEC of control (black) and irradiated (red) mice. B) Quantification of ROS presented as percent change in mean fluorescence relative to control. C) Flow cytometry histogram showing mitochondrial superoxide measurement in IEC of control (black) and irradiated (red) mice. D) Quantification of mitochondrial superoxide presented as percent change in mean fluorescence relative to control. E) Flow cytometry histogram showing mitochondrial membrane potential measurement in control (black) and irradiated (red) cells. F) Quantification of mitochondrial membrane potential presented as percent change in mean fluorescence relative to control. *denotes p<0.05.
Table 1. Expression of oxidant and anti-oxidant related genes in mouse intestine two months after radiation exposure.
| Gene symbol | Gene name | Gene function | Fold change ±SD |
|---|---|---|---|
| Gpx1 | Glutathione peroxidase 1 | Detoxify hydrogen peroxide. | ↓4.94±0.15 |
| Gpx2 | Glutathione peroxidase 2 | Detoxify hydrogen peroxide. Gastrointestinal specific isoform. | ↑1.25±0.06 |
| Gpx3 | Glutathione peroxidase 3 | Detoxify hydrogen peroxide. Extracellular or plasma isoform. | ↓2.38±0.25 |
| Gpx8 | Glutathione peroxidase 8 | Detoxify hydrogen peroxide. | ↓3.38±0.48 |
| Prdx1 | Peroxiredoxin 1 | Removes hydrogen peroxide and alkyl hydroperoxides. | ↓3.16±0.23 |
| Prdx2 | Peroxiredoxin 2 | Removes hydrogen peroxide and alkyl hydroperoxides. | ↓6.02±0.16 |
| Prdx3 | Peroxiredoxin 3 | Removes hydrogen peroxide and alkyl hydroperoxides. Present in mitochondria. | ↓6.39±0.14 |
| Prdx4 | Peroxiredoxin 4 | Removes hydrogen peroxide and alkyl hydroperoxides. Present in cytoplasm. | ↓3.67±0.26 |
| Prdx6 | Peroxiredoxin 6 | Removes hydrogen peroxide and alkyl hydroperoxides. | ↓1.60±0.20 |
| Sod1 | superoxide dismutase 1 | Neutralizes superoxide radicals. Present in cytoplasm. | ↓8.93±0.12 |
| Sod2 | superoxide dismutase 2 | Neutralizes superoxide radicals. Mitochondria specific enzyme. | ↓5.15±0.29 |
| Sod3 | superoxide dismutase 3, extracellular | Neutralizes superoxide radicals | ↓1.86±0.41 |
| Txnrd3 | thioredoxin reductase 3 | Reduces thioredoxin | ↓4.26±0.34 |
| Cat | Catalase | Converts hydrogen peroxide to water and oxygen | ↓6.63±0.14 |
| Gstk1 | glutathione S-transferase kappa 1 | Removes hydrophobic toxic compounds by conjugating them to glutathione | ↓3.53±0.22 |
| Nos2 | nitric oxide synthase 2, inducible | Increased nitric oxide production | ↑1.27±0.3 |
| Nox4 | NADPH oxidase 4 | Functions as a catalytic subunit of the NADPH oxidase enzyme complex and present in non-phagocytic cells. | ↓2.18±0.21 |
3.2. Ionizing radiation downregulated autophagy-associated gene expression in intestine
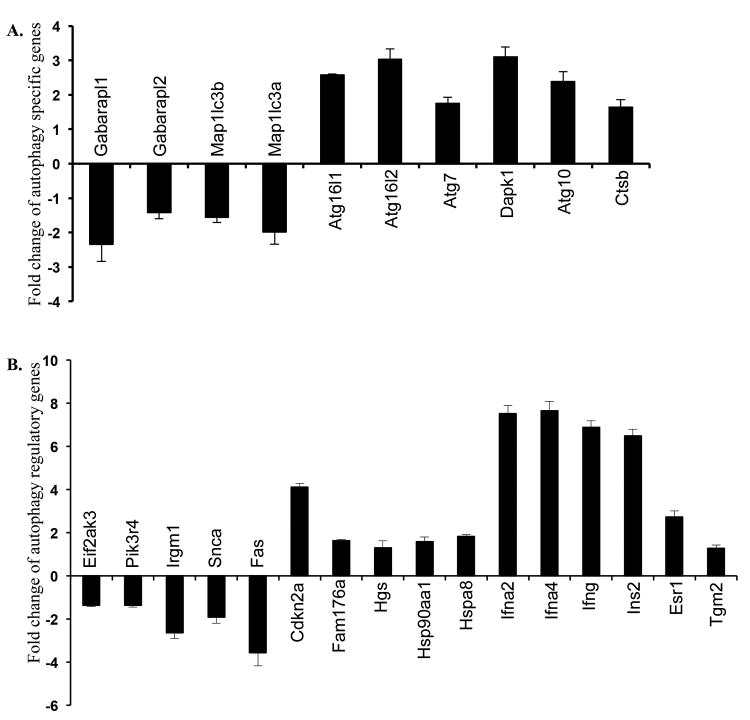
PCR array results showed significant (determined using a fold change cutoff of 1.25 and a p-value of <0.05) perturbation of 10 autophagy pathway specific (Figure 2A) as well as 16 autophagy regulatory genes (Figure 2B) in two-month post-irradiation samples. Among the autophagy specific genes, Gabarapl1, Gabarapl2, Map1lc3a, and Map1lc3b involved in elongation and closer of autophagosomes were significantly downregulated. On the contrary, expression of six autophagy pathway specific genes (Atg16l1, Atg16l2, Atg7, Dapk1, Atg10, and Ctsb) were significantly upregulated. Additionally, we also observed downregulation of five (Eif2ak3, Pik3r4, Irgm, Snca, and Fas) and upregulation of eleven (Cdkn2a, Fam176a, Hgs, Hsp90aal, Hspa8, Ifna2, Ifna4, Ifng, Ins2, Esrl, and Tgm2) genes, which are known to influence the autophagy pathway.
Figure 2.
Radiation exposure altered expression of genes associated with autophagy in mouse intestine. A) Fold change in the expression of autophagy pathway specific genes. B) Fold change in the expression of autophagy regulatory genes.
3.3. Radiation exposure decreased autophagy pathway specific proteins, and activated pro-growth pathways
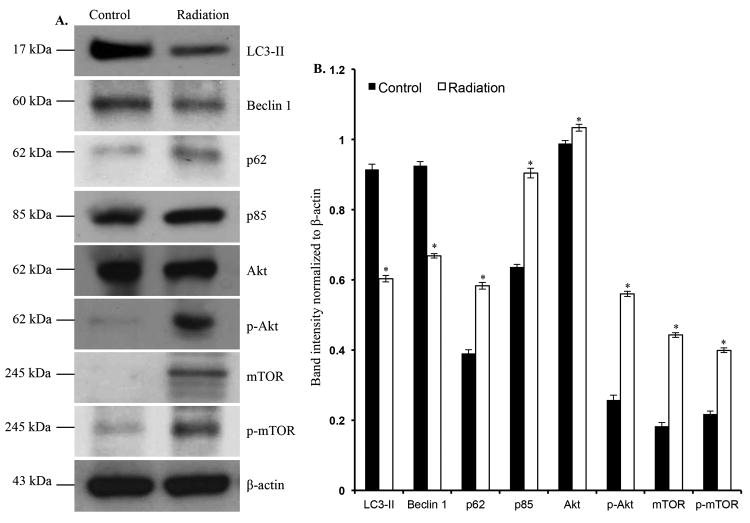
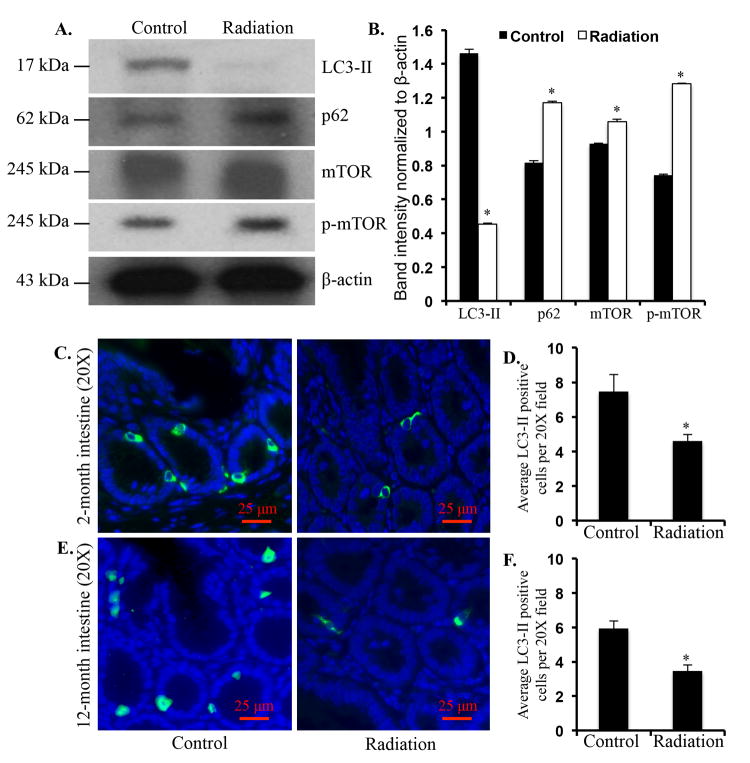
In immunoblot analysis of two-month post-irradiation intestinal samples, levels of autophagosome forming LC3-II (p<0.001) and Beclinl (p<0.003) were decreased (Figure 3A and B). However, p62, an autophagy scaffolding protein was significantly (p<0.004) increased in irradiated samples (Figure 3A and B). Additionally, we also observed significantly increased p85a (p<0.0005), a regulatory subunit of PI3K, in the irradiated samples (Figure 3A and B). Levels of Akt (p<0.01), phospho-Akt (p<0.004) and its downstream target mTOR (p<0.002) and its active form phospho-mTOR (p<0.0001) were significantly increased in intestine two months after radiation exposure (Figure 3A and B). Significantly decreased LC3-II (p<0.0004), and increased p62 (p<0.0003), mTOR (p<0.01), and phospho-mTOR (p<0.00003) was also observed in twelve-month post-irradiation samples (Figure 4A and B). We immunostained intestinal sections for LC3-II, a protein known to be associated with autophagosome membrane, and observed decreased staining in both the two- (Figure 4C and D) and twelve-month (Figure 4E and F) post-irradiation samples relative to sham-irradiated controls.
Figure 3.
Decreased autophagy increased proliferative markers two months after radiation exposure. A) Immunoblots showing alteration in autophagy and proliferative signaling pathway molecules. B) Quantification of immunoblot band intensities normalized to β-actin band intensity. *denotes p<0.05.
Figure 4.
Decreased autophagy increased proliferative markers twelve months after radiation exposure. A) Immunoblots showing alteration in autophagy and proliferative signaling pathway molecules. B) Quantification of immunoblot band intensities normalized to β-actin band intensity. C) Representative images of intestinal sections from control and two-month post-irradiation mice showing LC3-II immunofluorescence staining. D) Quantification of control and two-month post-irradiation LC3-II staining presented as pixel units per 20X field. E) Representative images of intestinal sections from control and twelve-month post-irradiation mice showing LC3-II immunofluorescence staining. F) Quantification of control and twelve-month post-irradiation LC3-II staining presented as pixel units per 20X field. *denotes p<0.05.
3.4. Radiation caused increased oxidative DNA damage and cell proliferation in intestine
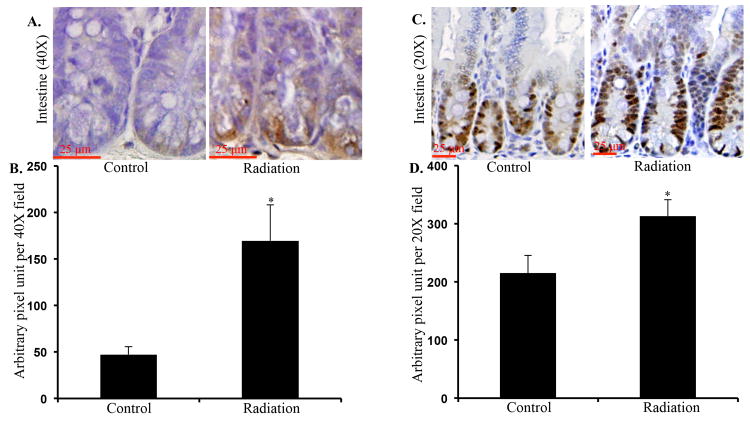
Cellular consequence of increased oxidative stress was assessed using 8-oxo-dG staining. We show that two months after irradiation there was significantly greater 8-oxo-dG staining in the intestinal crypts relative to sham-irradiated control (Figure 5A and B; p<0.01). Furthermore, we also observed significantly increased number of intestinal epithelial cells stained positive for PCNA in irradiated samples relative to controls (Figure 5C and D; p<0.04).
Figure 5.
Greater oxidative DNA damage and higher cell proliferation was observed in irradiated mice. A) Representative images of intestinal sections from control and irradiated mice showing 8-oxo-dG staining. B) Quantification of 8-oxo-dG staining presented as pixel units per 40X field. C) Representative images of intestinal sections from control and irradiated mice showing PCNA staining. B) Quantification of PCNA staining presented as pixel units per 20X field. *denotes p<0.05.
4. Discussion
Exposure to ionizing radiation, still a major modality for cancer treatment, has been demonstrated to have long-term side effects in exposed normal tissues including intestine. Effective use of radiation is playing its role in increasing cancer therapy success rate and consequently, there is increased number of cancer survivors who could potentially develop chronic enteropathies or even a second cancer in the colorectal region especially after pelvic irradiation [34]. Although the dose (2 Gy) used in the current study is one fraction of a total radiation dose delivered in planned fractionated radiotherapies, it has allowed us to discern underlying molecular events that we believe could play a role in the long-term sequela of radiation exposures in normal intestine and is not reported previously. We show that radiation exposure caused decreased expression of autophagy and anti-oxidant genes. Additionally, increased intracellular ROS production was associated with decreased levels of key autophagy proteins and activation of proliferative pathways.
Autophagy, which is a self-cannibalistic process and provides energy during stress, is widely studied for its roles in normal development and homeostasis as well as in pathological states such as inflammatory bowel disease, neurodegeneration, and cancer [3]. However, autophagy in relation to radiation has mostly been studied for radiosensitization of cancer cells [19-24]. Notably, these studies, which commonly involved short-term cell culture system dissecting molecular pathways involved in radiation-induced autophagy activation and cancer cell survival, aimed to develop strategies to inhibit autophagy and thus sensitize cancer cell to therapy [19-24,35]. While autophagy activation provides an alternate energy source to cell for survival and may contribute to promotion and progression of established cancers, its downregulation in normal cells has been implicated in functional alterations and cancer initiation [24]. The autophagosome is formed de novo and is a complex process involving multiple steps of vesicle induction, nucleation, elongation, and finally closure [2]. The Map1lc3a and Map1lc3b along with Map1lc3c are the three isoforms of Map1lc3 (microtubule-associated protein light chain 3), which is the yeast Atg8 ortholog. The Map1lc3 or commonly termed LC3 undergoes extensive post-translational modification including protease cleavage by Atg4 enzyme leading to formation of LC3-I and then phospholipidated by Atg7 and Atg3 to LC3-II, which is required for elongation of autophagosome membrane. While LC3-I is cytosolic, LC3-II is associated with autophagosome membrane and its level denotes number of autophagosome in cell [36]. Furthermore, the Gabarapl1 and Gabarapl2, essential for autophagosome vesicles closure, are also activated by the Atg4 to Gabarapl1-I and Gabarapl2-I and phospholipidated by Atg7 and Atg3 to form autophagosome closure elements Gabarapl1-II and Gabarapl2-II. Radiation exposure associated decreased expression of four important autophagy specific genes (Gabarapl1, Gabarapl2, Map1lc3a, and Map1lc3b) we believe, will reduce overall autophagy activity in intestinal cells. Decreased expressions of Map1lc3 were further supported by decreased LC3-II in immunoblots demonstrating that decreased levels of active form, in spite of increased/unaltered expression of activating proteins, was due to transcriptional downregulation and reduced availability of LC3. Importantly, Beclin1 as well as Pik3r4, which are components of the class III PI3K complex and are involved in the nucleation phase of the autophagy [37], are significantly decreased after radiation exposure. Although expression of a number of genes (Dapk1, Cdkn2a, Fam176a, Hgs, Hsp90aa1, Hspa8, Ifna2, Ifna4, and Ifng) implicated in triggering autophagy in response to different stimuli [38-42] were upregulated, our results show that exposure to a sub-lethal dose of radiation led to long-term downregulation of the core components involved in the three critical autophagy steps of nucleation, elongation, and closure of autophagosomes. Our results further show that radiation-induced perturbation of the autophagy specific factors could act in tandem with downregulated autophagy regulating factors such as Eif2ak3, Irgm1, Snca, and Fas to produce an inhibitory effect on autophagic processes. Indeed, accumulation of p62, which is an adaptor protein facilitating recruitment of ubiquitinated proteins to autophagosome via its interaction with LC3 and Gabarap family of proteins and is degraded during autophagic process [43], further argues in favor of autophagy downregulation after radiation exposure. Considering that Eif2ak3 and Irgm1 respectively are required for endoplasmic reticulum (ER) [44], and bacterial pathogen [45] mediated stress-induced autophagy activation, decreased expression of these factors will cause a faulty autophagic response to ER stress and immune challenges and increased vulnerability of intestinal cells to functional deregulation and pathologic infection. On the contrary, increased level of Snca [46] as well as ligand-mediated activation of Fas [47] is known to activate autophagy and downregulation of both the factors observed in our study further supports long-term downregulatory effects of sub-lethal radiation exposure on autophagy. Although we noted upregulation of a number of genes (Ctsb, Atg10, Ins2, Esr1, and Tgm2) linked to autophagy, they play mostly regulatory roles in autophagosome formation. On the contrary, upregulation of these genes have been reported to promote inflammation, oxidative stress, insulin resistance, and proliferation with adverse cellular consequences [48-53]. Taken together we have shown that although the activators/regulators such as Atg7, Atg10, Atg16l1, and Atg16l2 of key autophagy molecules are upregulated, the key molecules themselves involved in initiation (Beclin 1), elongation (LC3-II, and closure (Gabarapl1 and Gabarapl2) of the autophagosome membrane were downregulated suggesting potential implications for autophagy inhibition. When combined with immunofluorescence evidence of decreased LC3-II widely used as a marker for autophagosome number, our results strongly suggest that exposure to a sub-lethal radiation dose persistently downregulated autophagy with implications for chronic cellular functional deregulation and transformation.
Oxidative stress, which could be due to increased oxidant production, decreased antioxidant activity, or a combination of both, has been linked to radiation as well as autophagy. Radiation is known to impart its damaging effects directly by depositing energy into the traversed biomolecules or indirectly by oxidant production especially ROS. While radiation at higher doses causes cell death in part by producing lethal levels of ROS, at lower doses it causes sub-lethal oxidative stress, DNA damage, and activation of proliferative signaling pathway thus allowing damaged cells to proliferate with increasing transformation potential [25]. Persistent oxidative stress after radiation exposure observed in the current study is consistent with others as well as our earlier studies [25,54] and was due to increased ROS production as a results of compromised mitochondrial function, as well as due to transcriptional downregulation of important antioxidant genes including a number of Gpx and Prdx isoforms. Considering that histological examination of intestinal sections did not show differences in inflammatory cell number between control and irradiated samples and isolated epithelial cells were typified (data not shown) [55,56], our results are suggestive of epithelial cell dysregulation and oxidative stress and is in agreement with our earlier twelve month post-radiation results [25]. Importantly, there was increased Nos2 (inducible nitric oxide synthase or iNos) expression, which could in combination with ROS promote reactive nitrogen species (RNS) generation and more oxidative stress. Apart from its effects on furthering oxidative stress, increased expression of Nos2 via increased NO production has also been reported to adversely affect autophagosome formation [57]. Additionally, oxidative stress is known to downregulate autophagy and reduced autophagy is in turn known to promote cellular stress not only due to increased accumulation of damaged and aggregated proteins but also due to decreased removal of damaged mitochondria (mitophagy) [36]. Taken together, our results of decreased autophagy and increased oxidative stress lead us to propose that radiation exposure is propelling cells into a cycle of persistent dysregulated homeostasis (Figure 6). Our belief of radiation-induced cyclical deregulation is further strengthened by the fact that there is increased PI3K/Akt signaling invariably linked to increased ROS production as well as Akt-mediated activation of mTOR, which is known to downregulate autophagy (Figure 6).
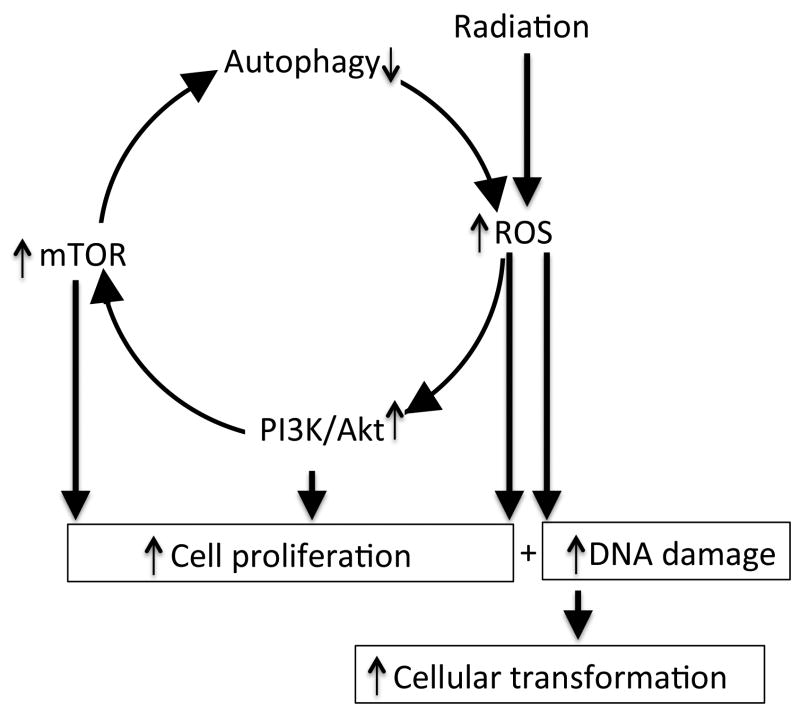
Figure 6.
Schematic overview of radiation-induced persistent effects on autophagy. Radiation caused oxidative stress leading to downregulation of autophagy and downregulated autophagy could in turn further promote oxidative stress. Additionally, activation of proliferative pathways such as PI3K/Akt and mTOR is expected to induce proliferation of cells damaged by oxidative stress leading transformation and functional alterations.
The prevailing linear no-threshold (LNT) model and the seventh Biological Effects of Ionizing Radiation (BEIR VII) report proposed that no radiation dose is safe and that irrespective of dose, radiation exposure has short as well as long-term cellular consequences [58], and fundamental understanding of molecular events will support developing preventive and therapeutic strategies against adverse long-term sequel of radiation exposure. While the current study focused on long-term effects of radiation on small intestine and investigating persistent radiation effects on colonic and other tissues remains a future goal, we believe that our results have relevance to radiation associated chronic GI tract ailments. To our knowledge this is the first report on long-term effects of a sub-lethal dose of radiation on oxidative stress in relation to autophagy, which is considered a major mechanism involved in maintaining intestinal homeostasis. The current study showed that radiation exposure is initiating a chronic chain of events starting with oxidative stress leading to PI3K/Akt and mTOR activation, downregulation of autophagy pathway, and further promotion of oxidative stress and has implications for chronic intestinal pathologies such as inflammatory bowel diseases, chronic enteritis, and cancer (Figure 6).
Acknowledgments
This study is supported in part by NASA Grant# NNX13AD58G and NNX09AU95G. We acknowledge the Histopathology and Tissue Shared Resources supported by Award Number P30CA051008 from the National Cancer Institute.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 4.Le Grand JN, Chakrama FZ, Seguin-Py S, Fraichard A, Delage-Mourroux R, Jouvenot M, Boyer-Guittaut M. GABARAPL1 (GEC1): original or copycat? Autophagy. 2011;7:1098–1107. doi: 10.4161/auto.7.10.15904. [DOI] [PubMed] [Google Scholar]
- 5.Patel KK, Stappenbeck TS. Autophagy and intestinal homeostasis. Annu Rev Physiol. 2013;75:241–262. doi: 10.1146/annurev-physiol-030212-183658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 7.Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359–365. doi: 10.1177/1756283X10371558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiagarajah JR, Gourmelon P, Griffiths NM, Lebrun F, Naftalin RJ, Pedley KC. Radiation induced cytochrome c release causes loss of rat colonic fluid absorption by damage to crypts and pericryptal myofibroblasts. Gut. 2000;47:675–684. doi: 10.1136/gut.47.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert ES, Omohundro E, Buchanan JA, Holter NA. Mortality of workers at the Hanford site: 1945-1986. Health Phys. 1993;64:577–590. doi: 10.1097/00004032-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 12.Ritz B. Radiation exposure and cancer mortality in uranium processing workers. Epidemiology. 1999;10:531–538. [PubMed] [Google Scholar]
- 13.Sont WN, Zielinski JM, Ashmore JP, Jiang H, Krewski D, Fair ME, Band PR, Letourneau EG. First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 2001;153:309–318. doi: 10.1093/aje/153.4.309. [DOI] [PubMed] [Google Scholar]
- 14.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang JH. Activation of the PI3K/Akt pathway by oxidative stress mediates high glucose-induced increase of adipogenic differentiation in primary rat osteoblasts. J Cell Biochem. 2013;114:2595–2602. doi: 10.1002/jcb.24607. [DOI] [PubMed] [Google Scholar]
- 16.Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo C, Li Y, Wang H, Feng Z, Li Y, Long J, Liu J. Mitochondrial accumulation under oxidative stress is due to defects in autophagy. J Cell Biochem. 2013;114:212–219. doi: 10.1002/jcb.24356. [DOI] [PubMed] [Google Scholar]
- 19.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–1494. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang W, Qin Z, Liang Z. The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochim Biophys Sin (Shanghai) 2009;41:341–351. doi: 10.1093/abbs/gmp028. [DOI] [PubMed] [Google Scholar]
- 21.Liang B, Kong D, Liu Y, Liang N, He M, Ma S, Liu X. Autophagy inhibition plays the synergetic killing roles with radiation in the multi-drug resistant SKVCR ovarian cancer cells. Radiat Oncol. 2012;7:213. doi: 10.1186/1748-717X-7-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YS, Song HX, Lu Y, Li X, Chen T, Zhang Y, Xue JX, Liu H, Kan B, Yang G, Fu T. Autophagy inhibition contributes to radiation sensitization of esophageal squamous carcinoma cells. Dis Esophagus. 2011;24:437–443. doi: 10.1111/j.1442-2050.2010.01156.x. [DOI] [PubMed] [Google Scholar]
- 23.He G, Wang Y, Pang X, Zhang B. Inhibition of autophagy induced by TSA sensitizes colon cancer cell to radiation. Tumour Biol. 2014;35:1003–1011. doi: 10.1007/s13277-013-1134-z. [DOI] [PubMed] [Google Scholar]
- 24.Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee CW, Chan FK, Yu J, Sung JJ. The autophagic paradox in cancer therapy. Oncogene. 2012;31:939–953. doi: 10.1038/onc.2011.295. [DOI] [PubMed] [Google Scholar]
- 25.Datta K, Suman S, Kallakury BV, Fornace AJJ. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS One. 2012;7:e42224. doi: 10.1371/journal.pone.0042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta K, Suman S, Kallakury BV, Fornace AJJ. Heavy ion radiation exposure triggered higher intestinal tumor frequency and greater beta-catenin activation than gamma radiation in APC(Min/+) mice. PLoS One. 2013;8:e59295. doi: 10.1371/journal.pone.0059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta K, Hyduke DR, Suman S, Moon BH, Johnson MD, Fornace AJJ. Exposure to ionizing radiation induced persistent gene expression changes in mouse mammary gland. Radiat Oncol. 2012;7:205. doi: 10.1186/1748-717X-7-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suman S, Johnson MD, Fornace AJJ, Datta K. Exposure to ionizing radiation causes long-term increase in serum estradiol and activation of PI3K-Akt signaling pathway in mouse mammary gland. Int J Radiat Oncol Biol Phys. 2012;84:500–507. doi: 10.1016/j.ijrobp.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suman S, Rodriguez OC, Winters TA, Fornace AJJ, Albanese C, Datta K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging (Albany NY) 2013;5:607–622. doi: 10.18632/aging.100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallmyr A, Fan J, Datta K, Kim KT, Grosu D, Shapiro P, Small D, Rassool F. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111:3173–3182. doi: 10.1182/blood-2007-05-092510. [DOI] [PubMed] [Google Scholar]
- 31.Huigsloot M, Tijdens IB, Mulder GJ, van de Water B. Differential regulation of doxorubicin-induced mitochondrial dysfunction and apoptosis by Bcl-2 in mammary adenocarcinoma (MTLn3) cells. J Biol Chem. 2002;277:35869–35879. doi: 10.1074/jbc.M200378200. [DOI] [PubMed] [Google Scholar]
- 32.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21:489–497. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP. Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol. 2007;20:1307–1315. doi: 10.1038/modpathol.3800972. [DOI] [PubMed] [Google Scholar]
- 34.Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5(1):15–29. doi: 10.1177/2040622313510730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang N, Jia L, Liu Y, Liang B, Kong D, Yan M, Ma S, Liu X. ATM pathway is essential for ionizing radiation-induced autophagy. Cell Signal. 2013;25:2530–2539. doi: 10.1016/j.cellsig.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Renna M, Schaffner C, Winslow AR, Menzies FM, Peden AA, Floto RA, Rubinsztein DC. Autophagic substrate clearance requires activity of the syntaxin-5 SNARE complex. J Cell Sci. 2011;124:469–482. doi: 10.1242/jcs.076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning Y, Riggins RB, Mulla JE, Chung H, Zwart A, Clarke R. IFNgamma restores breast cancer sensitivity to fulvestrant by regulating STAT1, IFN regulatory factor 1, NF-kappaB, BCL2 family members, and signaling to caspase-dependent apoptosis. Mol Cancer Ther. 2010;9:1274–1285. doi: 10.1158/1535-7163.MCT-09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, Cannizzaro A, Colbert RA, Alessandro R, Triolo G. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalvakolanu DV, Gade P. IFNG and autophagy: a critical role for the ER-stress mediator ATF6 in controlling bacterial infections. Autophagy. 2012;8:1673–1674. doi: 10.4161/auto.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budina-Kolomets A, Hontz RD, Pimkina J, Murphy ME. A conserved domain in exon 2 coding for the human and murine ARF tumor suppressor protein is required for autophagy induction. Autophagy. 2013;9 doi: 10.4161/auto.25831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie H, Hu J, Pan H, Lou Y, Lv P, Chen Y. Adenovirus vector-mediated FAM176A overexpression induces cell death in human H1299 non-small cell lung cancer cells. BMB Rep. 2014;47:104–109. doi: 10.5483/BMBRep.2014.47.2.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Kang R, Huang H, Xi X, Wang B, Wang J, Zhao Z. Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression. Autophagy. 2014;10 doi: 10.4161/auto.27954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He S, Wang C, Dong H, Xia F, Zhou H, Jiang X, Pei C, Ren H, Li H, Li R, Xu H. Immune-related GTPase M (IRGM1) regulates neuronal autophagy in a mouse model of stroke. Autophagy. 2012;8:1621–1627. doi: 10.4161/auto.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plotegher N, Civiero L. Neuronal autophagy, alpha-synuclein clearance, and LRRK2 regulation: a lost equilibrium in parkinsonian brain. J Neurosci. 2012;32:14851–14853. doi: 10.1523/JNEUROSCI.3588-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Wu Y, Cheng Y, Zhao Z, Tashiro S, Onodera S, Ikejima T. Fas-mediated autophagy requires JNK activation in HeLa cells. Biochem Biophys Res Commun. 2008;377:1205–1210. doi: 10.1016/j.bbrc.2008.10.151. [DOI] [PubMed] [Google Scholar]
- 48.Li S, Du L, Zhang L, Hu Y, Xia W, Wu J, Zhu J, Chen L, Zhu F, Li C, Yang S. Cathepsin B contributes to autophagy-related 7 (Atg7)-induced nod-like receptor 3 (NLRP3)-dependent proinflammatory response and aggravates lipotoxicity in rat insulinoma cell line. J Biol Chem. 2013;288:30094–30104. doi: 10.1074/jbc.M113.494286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 50.Codogno P, Meijer AJ. Autophagy: a potential link between obesity and insulin resistance. Cell Metab. 2010;11:449–451. doi: 10.1016/j.cmet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Luciani A, Villella VR, Esposito S, Gavina M, Russo I, Silano M, Guido S, Pettoello-Mantovani M, Carnuccio R, Scholte B, De Matteis A, Maiuri MC, Raia V, Luini A, Kroemer G, Maiuri L. Targeting autophagy as a novel strategy for facilitating the therapeutic action of potentiators on DeltaF508 cystic fibrosis transmembrane conductance regulator. Autophagy. 2012;8:1657–1672. doi: 10.4161/auto.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke R, Shajahan AN, Riggins RB, Cho Y, Crawford A, Xuan J, Wang Y, Zwart A, Nehra R, Liu MC. Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114:8–20. doi: 10.1016/j.jsbmb.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo YK, Kim SC, Park IJ, Park SJ, Jin DH, Hong SW, Cho DH, Kim JC. Increased expression of ATG10 in colorectal cancer is associated with lymphovascular invasion and lymph node metastasis. PLoS One. 2012;7:e52705. doi: 10.1371/journal.pone.0052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim GJ, Fiskum GM, Morgan WF. A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res. 2006;66:10377–10383. doi: 10.1158/0008-5472.CAN-05-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bissonnette R, Lee MJ, Wang E. The differentiation process of intestinal epithelial cells is associated with the appearance of statin, a non-proliferation-specific nuclear protein. J Cell Sci. 1990;95:247–254. doi: 10.1242/jcs.95.2.247. [DOI] [PubMed] [Google Scholar]
- 56.Macartney KK, Baumgart DC, Carding SR, Brubaker JO, Offit PA. Primary murine small intestinal epithelial cells, maintained in long-term culture, are susceptible to rotavirus infection. J Virol. 2000;74:5597–5603. doi: 10.1128/jvi.74.12.5597-5603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarkar S, Korolchuk VI, Renna M, Imarisio S, Fleming A, Williams A, Garcia-Arencibia M, Rose C, Luo S, Underwood BR, Kroemer G, O'Kane CJ, Rubinsztein DC. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43:19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davidson ST. Any dose is too high. Environ Health Perspect. 2005;113:A735. doi: 10.1289/ehp.113-a735a. [DOI] [PMC free article] [PubMed] [Google Scholar]