Abstract
Objective
The aim of our study was to observe pelvic organ prolapse (POP) over time, treated and untreated, in a group of highly characterized women being followed subjectively and objectively over 5-7 years following continence surgery.
Study design
We measured baseline prolapse symptoms and anatomic prolapse in subjects enrolled in the Trial of Mid Urethral slings (TOMUS) and E-TOMUS trials, and measured these same parameters annually for five to seven years after the index surgery. Additional information about subsequent treatment for POP was also recorded.
Results
597 women were randomized to one of two mid-urethral sling procedures in the TOMUS trial; concomitant vaginal procedures for POP were allowed at the surgeon’s discretion. Stage 2 POP was present at baseline in 291 of subjects (49%). Symptoms of POP were reported in 67 (25%). Of the asymptomatic women, 34/223 (15%) underwent a concomitant POP repair at the time of index sling surgery. Anatomic progression of prolapse in women with asymptomatic, unoperated stage 2 POP over the next 72 months was infrequent and occurred in only 3/189 subjects (2%); none underwent surgery for POP. Most symptomatic women [47/67 (70%)] underwent a concomitant repair for POP at the index sling surgery. Three of the 47 women who had undergone concomitant repair for symptomatic stage 2 POP underwent repeat POP surgery (2 at 36 months and 1 at 48 months.)
Conclusion
For patient populations similar to the TOMUS and E-TOMUS populations, surgeons may counsel women with asymptomatic stage 2 POP that their prolapse is unlikely to require surgery in the next 5–7 years.
Keywords: asymptomatic cystocele, midurethral sling, stress urinary incontinence, urogynecology
Introduction
Pelvic organ prolapse (POP) is a common finding in women over the age of 60 and likely to become even more common with an aging population. It is estimated that over 40 million women will have POP or another pelvic floor disorder by 2050.1 Symptomatic patients may be offered intervention with pessary or surgery to improve prolapse symptoms. However, not all POP will progress and many patients, particularly with lower stages of POP (Stage 2 and lower), may be safely observed over time.2
A Medicare claims study concluded that patients undergoing surgery for stress urinary incontinence (SUI) may be at fairly high risk for requiring subsequent prolapse surgery within the first year after their SUI procedure.3 This alarming finding suggests that patients with moderate POP (stage 2) should be counseled to consider corrective surgery at the time of SUI surgery to avoid a subsequent additional procedure in the near future. These findings may be explained by study methodology using claims data, may be due to a failure to address potentially asymptomatic POP at the time of SUI surgery, or may be due to the possibility of accelerated progression of POP following SUI surgery.
The trial of mid urethral sling (TOMUS) study and extension trial of the same cohort (E-TOMUS) were carried out to assess efficacy and safety of transobturator and retropubic mid urethral slings (MUS).4i This was a highly characterized group of women with SUI who underwent surgery and agreed to further questioning and exams regarding their outcome and symptom progression for 5-7 years following continence surgery. The current study was a planned secondary analysis which focused on women with stage 2 prolapse at baseline in the TOMUS. The aim of our study to observe POP, symptoms, anatomic progression, and treatment over time in this group of women.
Materials and Methods
This was a planned secondary analysis of uterine and vaginal support after midurethral sling surgery conducted on data from subjects enrolled in the TOMUS trial. The TOMUS trial was a multicenter, randomized equivalence trial comparing the retropubic midurethral sling with the transobturator midurethral sling in women for the treatment of stress urinary incontinence. Study details and the 12- and 24-month post-operative outcomes have been published4,5. Notably, the protocol allowed concomitant procedures for POP, but restricted these procedures to those performed vaginally; additionally, no graft material was permitted in the anterior compartment and the use of synthetic mesh was not permitted at all. Although baseline information was collected as to the bothersomeness and degree of prolapse, the decision as to whether the concomitant procedure for prolapse should be undertaken was an individual decision between the surgeon and the patient.
To gain further insight into the longer-term functional and anatomic outcomes after midurethral slings, the Urinary Incontinence Treatment Network recruited all subjects who had not been surgically retreated for SUI since their TOMUS surgery to an extended follow-up study, E-TOMUS. Consented E-TOMUS participants attended annual in-person study visits for a minimum of 5 years post-surgery to report their continence, any re-treatment for urinary incontinence, any treatment for POP, and any complications. Patients completed a panel of condition specific quality of life and satisfaction questionnaires. Symptoms of pelvic organ prolapse were ascertained on the Urogenital Distress Inventory6 (UDI) questions that asked about seeing or feeling a bulging in the vaginal area, Symptomatic prolapse was defined as a response of “somewhat”, “moderately” or “quite a bit” on either of these prolapse questions of the UDI.
As part of the annual in-person study visits, participants underwent a pelvic exam for prolapse as well as visual or palpable evidence of mesh exposure. The POPQ exam was performed by research staff other than the study surgeon, and anatomic prolapse was categorized by POP-Q ordinal stages. In this system,7 for example, the maximum descent of the anterior vaginal wall (or point Ba) is measured relative to the fixed point of the hymenal ring; a value of minus 2 cm indicates that the maximal descent of the anterior vaginal wall is no more than 2 cm above the hymenal ring, while a value of plus 1 cm indicates the maximal descent of the anterior vaginal wall is no more than 1 cm beyond the hymenal ring. We followed the stage of prolapse over time of each of the following anatomical points: most dependent part of the anterior wall (point Ba), the most dependent part of the posterior wall (point Bp) and the cervix or vaginal cuff (point C). Stage 2 in the POPQ system is defined as the most dependent part of any pelvic organ at one cm above or beyond the hymenal ring; in stage 0-1 the prolapse is above this level, and in stage 3 and 4 the prolapse is beyond this level.
As this was a multi-year extension of a randomized trial initially slated to follow all participants for two years after the index surgery, we allowed some flexibility in follow up. Women who could not attend clinic in person for the annual assessment were surveyed by mail and telephone for new treatments including surgery, and for any new or ongoing symptoms using the same standardized questionnaires.
Statistical methods
Frequency distributions and percentages were used to describe the pattern of prolapse at successive visits for the women enrolled in TOMUS and E-TOMUS. This analysis focuses on women who were had stage II prolapse prior to their TOMUS surgery but whose prolapse was not repaired during surgery. Analyses were performed with the use of SAS statistical software, version 9.2 (SAS Institute). An IRB at each of the 9 clinical sites and the coordinating center approved the study protocol. Written informed consent was obtained from all participants.
Results
Five hundred ninety seven women were randomized to one of two mid-urethral sling procedures in the TOMUS trial, and baseline POP stage for the group is shown in table 1. Stage 2 POP was present at baseline in 291 of subjects (49%); of these 246 (85%) involved the anterior wall and 174 (60%) were limited to the anterior wall. Table 2 demonstrates the relationship of POP stage and symptoms to POP surgery at the time of MUS surgery. For women with stage 2 POP, 67 (25%) reported symptoms while 223 (75%) were asymptomatic. Most symptomatic women [47/67 (70%)] underwent a concomitant repair for POP at the index sling surgery, and 20 (30%) did not. Concomittant surgeries were distributed across all sites, as would be expected in a randomized trial. As reported elsewhere, women in the TOMUS trial who underwent concomitant prolapse surgery had better continence outcomes compared to women who did not undergo concomitant procedures. 8
Table 1.
Frequency of POP Stage at Baseline
| Overall POP Stage | N | % |
|---|---|---|
| Stage 3 at any location | 37 | 6.2 |
| Stage 2 at any location (no stage 3) | 291 | 48.7 |
| Stage 0–1 at all locations | 269 | 45.1 |
| 597 | ||
Table 2.
Symptoms of prolapse at baseline and POP repair at index surgery by POP stage at baseline.
| POP Stage at baseline | Symptoms at baseline | POP Repair | ||
|---|---|---|---|---|
| Yes | No | Total | ||
| Stage 3 | Yes | 19 | 10 | 29 |
| No | 2 | 6 | 8 | |
| Stage 2 | Yes | 47 | 20 | 67 |
| No | 34 | 189 | 223 | |
| Stage 0–1 | Yes | 5 | 18 | 23 |
| No | 6 | 239 | 245 | |
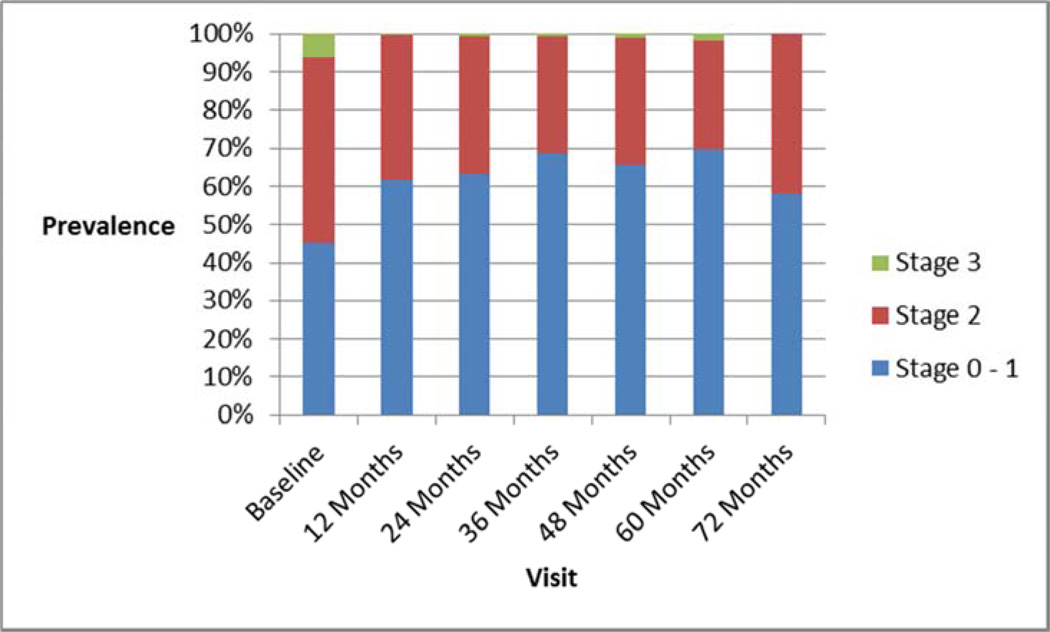
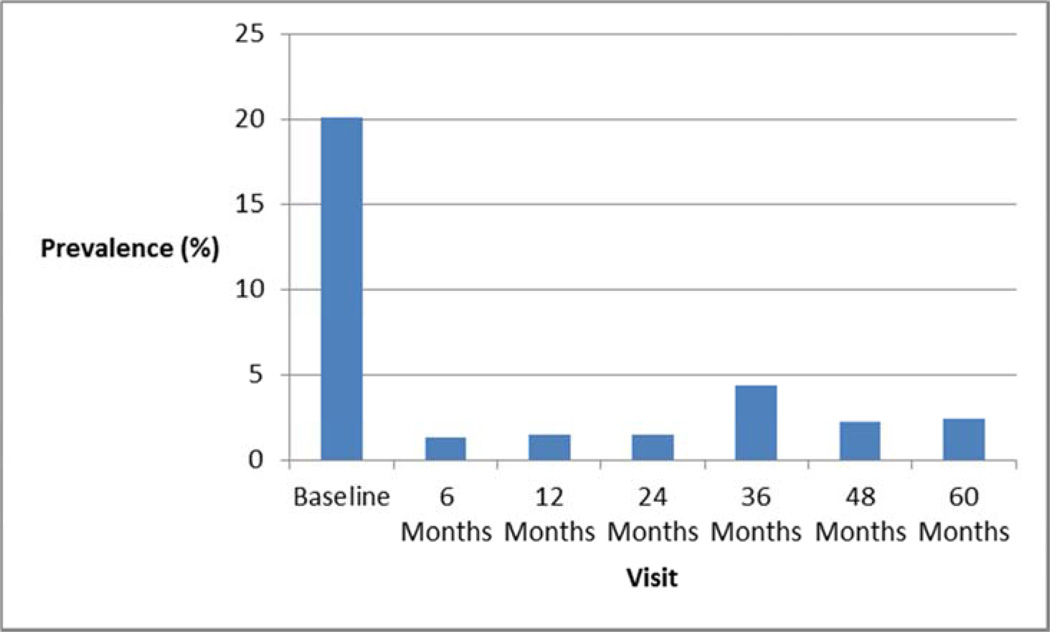
Of the asymptomatic women, 34/223 (15%) underwent a concomitant POP repair at the time of index sling surgery; most [189/223 (85%)] did not. Of the 189 asymptomatic women who were not treated surgically at the index surgery, progression to stage 3 POP occurred in only 3 of 189 (2%), and no one underwent surgery for POP in the next 60-72 months. Figure 1 shows the prevalence of overall POP stage at baseline and each subsequent visit, and figure 2 shows the prevalence of POP symptoms at each of those visits. These demonstrate that progression to stage 3 POP was very uncommon and symptom development was also very uncommon. Three of the 47 women who had undergone concomitant repair for symptomatic stage 2 POP underwent repeat POP surgery (two at 36 months and one at 48 months).
Figure 1.
Prevalence of overall POP Stage at each visit.
Figure 2.
Prevalence of POP symptoms at each visit.
Table 3 demonstrates POPQ ordinal data for the 209 women with overall stage 2 POP at baseline who did not receive prolapse repair at the index continence surgery. Of the 209 included in the analysis, 137 (66%) consented to E-TOMUS . Of these 137 participants, 109 (80%) completed the 5 year patient interview and of those, 57 (42%) underwent the in person POP exam. Eighty-five percent of the women in this group who were examined had point Ba recorded as −1 or 0 at baseline, and this decreased to about 40% after just a midurethral sling. This suggests that the MUS alone stabilizes the anterior wall. None of these women underwent surgery for POP by 60 months after index continence surgery. None of the women with prolapse beyond the hymenal ring (leading edge at + 1 cm) underwent surgery, even though these women might be viewed as more severe than other stage 2 prolapse (e.g. leading edge 0 or −1 cm)
Table 3.
Point Ba at each visit for women who were at stage 2 overall at baseline and did not receive prolapse repair (n=209): N(%).
| Point Ba | Baseline | 12 months | 24 months | 36 months | 48 months | 60 months |
|---|---|---|---|---|---|---|
| −3 | 4 (2%) | 40 (22%) | 38 (23%) | 37 (30%) | 27 (25%) | 12 (21%) |
| −2 | 21 (10%) | 66 (36%) | 58 (36%) | 38 (31%) | 35 (33%) | 30 (53%) |
| −1 | 116 (56%) | 49 (26%) | 43 (26%) | 30 (24%) | 27 (25%) | 9 (16%) |
| 0 | 60 (29%) | 26 (14%) | 23 (14%) | 15 (12%) | 16 (15%) | 5 (9%) |
| +1 | 8 (4%) | 4 (2%) | 1 (1%) | 2 (2%) | 2 (2%) | |
| +2 | 1 (1%) | |||||
| +3 | 1 (2%) | |||||
| Missing | 24 | 46 | 86 | 102 | 152 |
Comments
In this cohort of well characterized women undergoing SUI surgery, we found that unoperated stage 2 POP was unlikely to progress over the ensuing 5-7 years and very unlikely to require surgery. Similarly, treated stage 2 POP was unlikely to require additional surgery over time.
Many women with stress urinary incontinence have concomitant pelvic organ prolapse, and those women with significant POP symptoms commonly undergo concomitant POP and SUI repair. However, there is uncertainty regarding the need for concomitant surgical repair for those women with stage 2 POP, particularly those who are asymptomatic. Patients and surgeons alike will benefit from an evidence-based understanding of the critical components necessary for successful surgical outcomes. Avoiding unnecessary concomitant POP surgery is likely to reduce surgical morbidity and cost.
The findings from this analysis suggest that the often-quoted surgical advice to “repair all prolapse defects” may cause overtreatment in some patients undergoing midurethral sling procedures. Overall, women with stage 2 POP who did not undergo repair had similar outcomes to those who received the additional repairs. This allows surgeons to more confidently counsel patients regarding the necessity of surgically addressing stage 2 POP. Our findings are in contrast to studies using national databases3 which have demonstrated that a significant number of women undergoing surgery for continence require additional POP surgery within 12 months. It was difficult to estimate the extent of baseline POP in that study, making it difficult to know how similar the patient populations were between the two studies.
The evolution in surgical techniques, such as the midurethral sling, may favorably alter anatomy in ways not previously considered. Although the distal anterior wall was supported by more traditional continence repairs like the Burch colposuspension and autologous fascial sling, it was initially thought that the midurethral sling did not contribute such support for POP. In this analysis we found that the midurethral sling seems to stabilize the anterior vaginal wall. Thus, it seems less necessary to add a concomitant anterior prolapse surgery specific for stage 2 anterior prolapse if the patient is scheduled to undergo midurethral sling for symptomatic SUI.
The findings of this study are strengthened by the multi-center, multi-surgeon design and a very common clinical phenotype. These findings are not generalizable to women with higher stage pelvic organ prolapse which often includes significant vaginal apical support loss. Generally, the women in this study had limited anatomical support loss in the anterior and/or posterior vaginal wall. We did not randomize participants to receive or not receive concomitant prolapse repair, thus limiting our ability to control for surgeon or selection bias. In addition, counseling for concomitant prolapse repair was not standardized. Although it would have been interesting to understand how some patients underwent surgery and others did not, this was an observational study and we were not able to make conclusions about subsets other than the largest cohort, women with asymptomatic stage 2 POP who did not undergo repair.
Nonetheless, we can robustly conclude that, for patient populations similar to the TOMUS and e-TOMUS populations, surgeons may counsel women with asymptomatic stage 2 POP that their prolapse is unlikely to progress or require surgery in the next 5-7 years. Said another way, it is not necessary to perform a concomitant anterior prolapse surgery for asymptomatic stage 2 anterior POP when performing a MUS for SUI. The current data suggest that prolapse progression is quite unlikely and further treatment is likely unnecessary for women with stage 2 POP undergoing surgery for stress urinary incontinence.
ACKNOWLEDGMENTS
LS reports research funding from American Medical Systems, Minnetonka Minn and Cook Surgical, Bloomington In, and is a consultant for Johnson and Johnson, New Brunswick, NJ. EV receives research funding from Boston Scientific, Natick, MA.
Supported by cooperative agreements (U01 DK58225, U01DK58229, U01DK58234, U01DK58231, U01DK60379, U01DK60380, U01DK60393, U01DK60395, U01DK60397, and U01KD60401) from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Eunice Kennedy Schriver National Institute of Child Health and Human Development.
Appendix
STEERING COMMITTEE
E. Ann Gormley, Chair (Dartmouth Hitchcock Medical Center, Lebanon, NH); Larry Sirls, MD, Salil Khandwala, MD (William Beaumont Hospital, Royal Oak, MI and Oakwood Hospital, Dearborn, MI; U01 DK58231); Linda Brubaker, MD, Kimberly Kenton, MD (Loyola University Chicago, Stritch School of Medicine, Maywood, IL; U01 DK60379); Holly E. Richter, PhD, MD, L. Keith Lloyd, MD (University of Alabama, Birmingham, AL; U01 DK60380); Michael Albo, MD, Charles Nager, MD (University of California, San Diego, CA; U01 DK60401); Toby C. Chai, MD, Harry W. Johnson, MD (University of Maryland, Baltimore, MD; U01 DK60397); Halina M. Zyczynski, MD, Wendy Leng, MD (University of Pittsburgh, Pittsburgh, PA; U01 DK 58225); Philippe Zimmern, MD, Gary Lemack, MD (University of Texas Southwestern, Dallas, TX; U01 DK60395); Stephen Kraus, MD, Thomas Rozanski, MD (University of Texas Health Sciences Center, San Antonio, TX; U01 DK58234); Peggy Norton, MD, Ingrid Nygaard, MD (University of Utah, Salt Lake City, UT; U01 DK60393); Sharon Tennstedt, PhD, Anne Stoddard, ScD (New England Research Institutes, Watertown, MA; U01 DK58229); Debuene Chang, MD (until 10/2009), John Kusek, PhD (starting 10/2009), Rebekah Rasooly, PhD (National Institute of Diabetes & Digestive & Kidney Diseases).
CO-INVESTIGATORS
Amy Arisco, MD; Jan Baker, APRN; Diane Borello-France, PT, PhD; Kathryn L. Burgio, PhD; Ananias Diokno, MD; MaryPat Fitzgerald, MD; Chiara Ghetti, MD; Patricia S. Goode, MD; Robert L. Holley, MD; Yvonne Hsu, MD; Margie Kahn, MD; Jerry Lowder, MD; Karl Luber, MD; Emily Lukacz, MD; Alayne Markland, DO, MSc; Shawn Menefee, MD; Pamela Moalli, MD; Elizabeth Mueller, MD; Leslie Rickey, MD, MPH; Elizabeth Sagan, MD; Joseph Schaffer, MD; Robert Starr, MD; Gary Sutkin, MD; R. Edward Varner, MD; Emily Whitcomb, MD.
STUDY COORDINATORS
Julie E. Burge, BS; Laura Burr, RN; JoAnn Columbo, BS, CCRC; Tamara Dickinson, RN, CURN, CCCN, BCIA-PMDB; Rosanna Dinh, RN, CCRC; Judy Gruss, RN; Alice Howell, RN, BSN, CCRC; Chaandini Jayachandran, MSc; Kathy Jesse, RN; D. Lynn Kalinoski, PhD; Barbara Leemon, RN; Karen Mislanovich, RN; Elva Kelly Moore, RN; Caren Prather, RN; Jennifer Tabaldo; Tia Thrasher; Mary Tulke, RN; Robin Willingham, RN, BSN; Kimberly Woodson, RN, MPH; Gisselle Zazueta-Damian.
DATA COORDINATING CENTER
Kathleen Cannon, BS; Kimberly J. Dandreo, MSc; Liyuan Huang, MS; Rose Kowalski, MA; Heather Litman, PhD; Marina Mihova, MHA; Anne Stoddard, ScD (Co-PI); Kerry Tanwar, BA; Sharon Tennstedt, PhD (PI); Yan Xu, MS.
DATA SAFETY AND MONITORING BOARD
J. Quentin Clemens MD, (Chair) Northwestern University Medical School, Chicago IL; Paul Abrams MD, Bristol Urological Institute, Bristol UK; Deidre Bland MD, Blue Ridge Medical Associates, Winston Salem NC; Timothy B. Boone, MD, The Methodist Hospital, Baylor College of Medicine, Houston, TX; John Connett PhD, University of Minnesota, Minneapolis MN; Dee Fenner MD, University of Michigan, Ann Arbor MI; William Henderson PhD, University of Colorado, Aurora CO; Sheryl Kelsey PhD, University of Pittsburgh, Pittsburgh PA; Deborah J. Lightner, MD, Mayo Clinic, Rochester, MN; Deborah Myers MD, Brown University School of Medicine, Providence RI; Bassem Wadie MBBCh, MSc, MD, Mansoura Urology and Nephrology Center, Mansoura, Egypt; J. Christian Winters, MD, Louisiana State University Health Sciences Center, New Orleans, LA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The remaining authors report no conflicts of interest.
Presented at the annual meeting of the Society of Gynecologic Surgeons March 26–28, Scottsdale Arizona
References
- 1.Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. women. Obstet & Gynec. 2009;114:1278. doi: 10.1097/AOG.0b013e3181c2ce96. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist AS, Campbell W, Steele H, Brazell H, Foote J, Swift S. Outcomes of observation as therapy for pelvic organ prolapse: a study in the natural history of pelvic organ prolapse. Neurourol and Urodyn. 2013;32:383. doi: 10.1002/nau.22298. [DOI] [PubMed] [Google Scholar]
- 3.Anger JT, Litwin MS, Wang Q, Pashos CL, Rodriguez LV. The effect of concomitant prolapse repair on sling outcomes. J Urol. 2008;180:1003. doi: 10.1016/j.juro.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Albo ME, Litman HJ, Richter HE, Lemack GE, Sirls LT, Chai TC, Norton P, Kraus SR, Zyczynski H, Kenton K, Gormley EA, Kusek JW for the UITN. Treatment success of retropubic and transobturator mid urethral slings at 24 months. J Urol. 2012;199:2281. doi: 10.1016/j.juro.2012.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter HE, Albo ME, Zyczynski HM, Kenton K, Norton PA, Sirls LT, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066. doi: 10.1056/NEJMoa0912658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shumaker SA, et al. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res. 1994;3(5):291. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 7.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 8.Chai TC, Kenton K, Xu Y, Sirls L, Zyczynski H, Wilson TS, et al. Effects of concomitant surgeries during midurethral slings (MUS) on postoperative complications, voiding dysfunction, continence outcomes, and urodynamic variables. Urology. 2012;79(6):1256–1261. doi: 10.1016/j.urology.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]