Abstract
Mycobacterium tuberculosis (MTB) causes both acute and chronic infections in humans characterized by tolerance to antibiotics and reactivation to cause secondary tuberculosis. These characteristics have led to renewed interested in the in vitro pellicle, or biofilm mode of growth, where bacteria grow to produce a thick aggregate at the air-liquid interface and exhibit increased phenotypic resistance to antibiotics. We infected guinea pigs with the virulent H37Rv strain of MTB for 60 days at which point we collected blood. To identify antigenic proteins, membrane protein extracts of MTB H37Ra pellicles and shaken cultures grown for 3, 5, or 7 weeks were probed with the infected animals’ sera after the proteins were separated by two-dimensional gel electrophoresis (2DGE). Antigenic proteins were then identified using MALDI-TOF/TOF mass spectrometry peptide mass fingerprinting. Antigenic pellicle proteins were compared across the three timepoints to identify those that were produced consistently during pellicle growth. They were also compared to those membrane proteins identified from harvested shaken cultures to determine pellicle-specific versus universally-expressed proteins. Using this technique we identified 44 distinct antigenic proteins, nine of which were pellicle-specific. The sequence of antigenic pellicle-specific proteins was checked for sequence conservation across 15 sequenced MTB clinical isolates, three other members of the MTB complex, as well as Mycobacterium avium and Mycobacterium smegmatis. The antigenic pellicle-specific protein Rv0097 was found to have very high sequence conservation within the MTB complex but not with related mycobacteria while FabG4 was highly conserved in all mycobacteria analyzed. These conserved pellicle-specific proteins represent targets for the development of future diagnostic tests and vaccines.
INTRODUCTION
Mycobacterium tuberculosis (MTB) is the causative agent of tuberculosis (TB) which kills 1.4 million people and infects 8.7 million per year (2012). MTB is an acid-fast bacilli that primarily infects the lungs but can cause disseminated infections in the brain, liver, spleen, spine, and other tissues (Tuli, 2007, Palanisamy, et al., 2008, Skerry, et al., 2013). Antibiotic treatment necessary for cure of TB lasts 4–6 months and multi-drug resistant MTB strains are increasing in prevalence (Hopewell, et al., 2006, Pichugin, et al., 2009, 2012). The only vaccine currently used to protect against TB, the bacillus Calmette-Guérin (BCG) vaccine, was first administered in 1921 and has a roughly 80% efficacy at preventing childhood disseminated TB infections such as TB meningitis but is not effective at preventing adult pulmonary TB (Behr, 2002, Liu, et al., 2009). The widespread use of the BCG vaccine in TB endemic countries further complicates TB diagnosis by making the common screening test, the purified protein derivative (PPD or Mantoux Test) useless because of the cross-reactivity to PPD generated by the vaccine (Jin, et al., 2010). The global prevalence of infection and death associated with TB, plus the poor coverage of the current BCG vaccine, requires that we identify new MTB antigens for use in vaccines as well as diagnostic tests for TB in BCG vaccinated individuals.
With the advent of rapid genome sequencing, in silico antigen screens have been used to predict MTB antigens for new vaccines and diagnostics (Cole, 2002, Chaitra, et al., 2005, Ewer, et al., 2006, Zvi, et al., 2008, Wang, et al., 2010, Tang, et al., 2011, Sundaramurthi, et al., 2012). Many of these screens have focused on the prediction of CD4 and CD8 T-cell epitopes in an effort to discover antigens capable of stimulating a robust cell-mediated immune response (Wang, et al., 2010, Tang, et al., 2011, Sundaramurthi, et al., 2012). Inevitably these studies either incorporate existing published data demonstrating expression of the protein either in vitro or in vivo or require additional testing to verify actual protein expression.
In contrast to in silico screens, two-dimensional gel electrophoresis (2DGE) is a classic proteomic technique that allows for resolution of individual expressed proteins by separation based on each protein’s isoelectric point and molecular weight (Kenrick & Margolis, 1970). 2DGE has been used to map mycobacterial proteins and to demonstrate differences between MTB and BCG. The technique has been applied to MTB in conjunction with mass spectrometry to demonstrate differential protein responses to a variety of stressors such as heat, reactive oxygen/nitrogen species, or hypoxia. Much of the 2DGE proteomics studies on MTB have focused on culture filtrates in order to identify secreted virulence factors or diagnostic targets (Nagai, et al., 1991, Naito, et al., 1998, Malen, et al., 2008). One study by Florczyk et al. examined the difference between the proteome of shaken and standing cultures of BCG Pasteur demonstrating a group of five standing-culture specific proteins (Florczyk, et al., 2001). By Western blotting two-dimensional gels using sera from infected hosts, it is further possible to interrogate the humoral immune response to individual proteins (Pheiffer, et al., 2005, Zhang, et al., 2012).
Recent work using the in vitro pellicle model of static MTB growth in minimal media has led to serious consideration of a potential role for biofilm in tuberculosis. Biofilms are bacterial aggregates that grow at phase interfaces (air/liquid in the case of the MTB pellicle), elaborate a matrix of some type, and gain resistance to killing by stressors such as antibiotics or the host immune system. Bacteria grown in the MTB pellicle have dramatically increased survival following treatment with antibiotics compared to the frequently-used shaken culture of MTB grown in rich media and supplemented with detergents (Tween-80) to prevent MTB’s natural clumping phenotype. Furthermore, this biofilm phenotype can be abolished by specific genetic mutations that restore killing by antibiotics to wild-type levels. Lastly these pellicle-grown bacteria produce specific mycolic acids in high abundance (Ojha, et al., 2008, Sambandan, et al., 2013). Furthermore, MTB produces pilli that are critical to biofilm formation(Ramsugit, et al., 2013).
The question of the role of MTB pellicle/biofilm in human disease is still under active investigation. Tuberculosis has all the hallmarks of a biofilm infection, which has been defined as a disease where the bacteria grow on a surface or tissue, are evident as aggregates or microcolonies in a matrix, the infection is localized but has the ability to disseminate to tissues, and the disease is very difficult to treat with antibiotics (Parsek & Singh, 2003). Although capable of existing in an intracellular environment during infection, M. bovis has also been shown to grow at the air-tissue interface in vivo. The bacterial aggregates produced on the membranes lining tuberculosis lung cavities in cavitary TB are similar to the in vitro pellicle mode of growth (Laennec, 1821). Furthermore, TB is well known for its ability to lay dormant in foci in the lungs, reactivate, and spread to new sites in the lung or other tissues (Gupta, et al., 2012). Lastly, treatment of TB with antibiotics is a prolonged affair; bacteria die off at a rapid rate during the first two weeks, but after that a relatively resistant group of bacteria persist for months of remaining treatment (Wallis, et al., 1999, Jindani, et al., 2003, Sirgel, et al., 2005). These factors together fulfill the criteria for a biofilm infection.
This bioifilm mode of growth is important for MTB during infection and especially during cavitary TB. In this stage of the disease large numbers of infectious bacteria are produced and dispersed in aerosol form (Osler, 1892), so MTB biofilms may play a key role in the transmission of TB. When grown as a pellicle, MTB produces greater amounts of the virulence factor trehalose dimycolate (TDM, cord factor), which is thought to prevent healing of lung cavities (Hunter, et al., 2006). Thus, MTB in the pellicle form mimics an extracellular stage of MTB infection that has been largely neglected and is critical for transmission (Grosset, 2003) (Orme, 2013).
For diagnostic purposes, identifying new antigens that stimulate a humoral response allows the production of new assays for MTB infection using techniques such as enzyme-linked immunosorbant assay (ELISA), indirect fluorescent antibody assay (IFA), or lateral-flow assay. However, in terms of protecting the host against MTB the humoral response may play a supporting role to cell-mediated immunity.
Evidence supporting a role for the humoral response in TB has been found in a number of different mouse model experiments. MTB infection of B-cell knockout mice had 3–8 fold increased counts of CFU compared to wild-type; this increase was reversed after adoptive transfer of wild-type B-cells (Vordermeier, et al., 1996). Passive immunization with serum from mice inoculated with MTB extracts delivered in liposomes has a significant protective effect in a SCID mouse model (Guirado, et al., 2006). Additionally, treatment with monoclonal antibodies against the MTB protein TBA61 reduces bacterial loads in mice (Lopez, et al., 2009). Monoclonal IgG3 against arabinomannan has also improved survival in an endotracheally-infected mouse model and monocloncal IgG1 against the same molecule gave protection in a BALB/c mouse model (Teitelbaum, et al., 1998, Hamasur, et al., 2004). Mechanisms suggested for how humoral responses could help protect against MTB include promoting CMI by allowing antibody-dependent cell-mediate cytotoxicity and by boosting the uptake and processing of antigens for T-cell activation (Moore, et al., 2003). More recently, it was found that humoral protection was specifically dependent upon the glycosylation state of IgG in a murine model of pulmonary TB (Olivares, et al., 2013).
Previously, our group used classic immunoproteomic techniques to show Staphylococcus aureus in vitro biofilms stably express a distinct set of proteins (Brady, et al., 2006). A group of these biofilm proteins were used to create a multi-subunit vaccine that prevented the development of S. aureus osteomyelitis in a rabbit model (Brady, et al., 2011). Antibodies against these same antigens were also used as a novel method of immunodetection of their cognate proteins in vitro S. aureus biofilms (Brady, et al., 2007).
Using the guinea pig model of aerosol infection and in vitro pellicle model of MTB growth we identified a pool of proteins that stimulate a humoral immune response during pulmonary infection. Using 2DGE, Western Blotting, and protein identification by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Time-of-Flight (MALDI-TOF/TOF) mass spectrometry we determined which genes were expressed throughout all three pellicle timepoints. We then separated the data into proteins expressed only in the three pellicle timepoints (pellicle-specific proteins) and those expressed in the three pellicle timepoints and the control shaken culture (referred to as universally expressed proteins). We performed additional bioinformatics studies on both pools of proteins to better gauge their usefulness for vaccine and diagnostic purposes.
MATERIALS AND METHODS
Reagents and Growth Media
All reagents and bacterial growth media were obtained from Thermo Fisher Scientific, Waltham, Massachusetts unless noted otherwise.
Strains
The H37Rv strain of M. tuberculosis (ATCC #25618) was obtained from the Trudeau Institute, Saranac Lake, New York. The H37Ra strain of M. tuberculosis (ATCC #25177) was obtained from the American Type Culture Catalog, Manassas, Virginia.
Bacterial Cultures
Mycobacterium tuberculosis H37Ra was acquired from the American Type Culture Catalog (ATCC Strain 25177). H37Ra was propagated in liquid 7H9 media with Oleic-Albumin-Dextrose-Catalase media enrichment (OADC) plus 0.05% Tween-80 and on solid 7H10 OADC agar. MTB pellicles were grown as previously described (Ojha, et al., 2008), briefly 25 ml of Sauton’s Media in a 250 ml polystyrene bottle was inoculated with 100 µl of a mid-log phase MTB culture grown in 7H9 OADC with 0.05% Tween-80. Biofilms were grown for three, five, and seven weeks in a 37°C incubator. Control shaken cultures were inoculated with 100 µl of the same culture used to inoculate the biofilm cultures into 25 ml of Sauton’s media with no Tween-80 added, shaken for two weeks at 180 rpm at 37°C.
Fluorescence In-Situ Hybridization
MTB pellicle was collected, placed on Gold Plus slides and heat fixed on a hotplate for 10 minutes. Slides were then immersed in 95% ethanol for 10 minutes. MTB smears were treated with a mild acid hydrolysis followed by dual mutanolysin (5,000 U/ml) and lysozyme (10 mg/ml) treatment. The MTB smears were then treated with hybridization solution (50% v/v Formamide, 10% wt/v Dextran Sulfate, 0.1% wt/v Sodium Pyrophosphate, 0.2% wt/v Polyvinylpyrrolidone, 0.2% wt/v Ficoll 400, 0.1% v/v Triton X-100, 0.005 M Disodium EDTA, 0.01 M NaCl) containing 1 nm/ml of the relevant FISH probe and/or 1 µg/ml DAPI for 90 minutes. They were then treat with a wash solution (0.005 M Tris, 0.015 M NaCl, 0.1% v/v Triton X-100) for 30 minutes. The slides were air-dried and mounted using VectaShield mounting solution (Vector Labs, Burlingame, California).
Bacterial Membrane Protein Isolation
H37Ra pellicle biofilms were collected at three, five, and seven week timepoints and shaken cultures after two weeks (late-log stage based on growth curves) by the addition of 20% Tween-80 to a final concentration of 0.05% Tween-80, 10% sodium azide to a final concentration of 0.02%, and 25× concentrated Roche complete, EDTA-free protease inhibitor to a final concentration of 1×. Each MTB sample was centrifuged at 4000 ×g for 15 minutes at 4°C. The pellet was washed twice in PBS with 0.05% Tween-80 and 1× Roche complete, EDTA-free protease inhibitors and resuspended in Mycobacterial Lysis Buffer (50 mM Tris, pH 7.4, 10 mM MgCl2, 0.02% w/v sodium azide) with 1× complete, EDTA-free protease inhibitor. Bacteria were then lysed using 0.1 mm zirconia beads (BioSpec Products Bartlesville, Oklahoma) and a Fastprep FP120 bead beater (Thermo Fisher Scientific, Waltham, Massachusetts) then centrifuged at 15,000 ×g at 4°C for 10 min and the supernatant was removed. The supernatant was centrifuged again at 23,000 ×g at 4°C for 30 minutes, then ultracentrifuged at 150,000 ×g for 1 hour at 4°C to recover the membrane vesicles. Vesicles were resuspended in nanopure water and protein concentration was established using the modified Lowry method.
Two-Dimensional Gel Electrophoresis
Perform as previously described (Brady, et al., 2006). Briefly, an aliquot of 100 µg of membrane protein for each biofilm and shaken culture timepoint was precipitated using the Perfect Focus protein cleanup kit (G-Biosciences, St. Louis, Missouri) and resolubilized in 250 µl of Urea-Thiourea-CHAPS buffer which was used to rehydrate pH 3–10 linear immobilized pH gradient gel strips (GE Healthcare, Pittsburgh, PA).The rehydrated strips were subjected to isoelectric focusing then loaded onto a Criterion precast 12.5% polyacrylamide gel (Biorad Life Science, Hercules, California) covered with an agarose sealing gel, and run at 120 V for and 1.5 h. Proteins were visualized by terminal staining with Sypro Ruby protein stain (Biorad Life Science, Hercules, California) and imaged under UV light. Each timepoint sample was subjected to the process in triplicate.
Experimental infection with M. tuberculosis
Female outbred Hartley guinea pigs [~500 g] were purchased from Charles River Laboratories (North Wilmington, MA) and held in a Biosafety Level III animal laboratory. Culture stocks of M. tuberculosis strain H37Rv collected at mid-log phase of growth in Proskauer-Beck liquid medium containing 0.05% Tween-80 and frozen at −80°C. Thawed aliquots were diluted to 1 × 106 CFU/ml in double-distilled sterile water and delivered by low-dose aerosol infection using a Madison chamber aerosol generation device calibrated to deliver approximately 20 bacilli to each animal.
2D Western Blots
Two-Dimensional gels were run as described and then equilibrated in semi-dry transfer buffer (48mM Tris, 39 mM Glycine, 0.0375% SDS, 20% methanol) for 10 min. Immobilon P membranes (From where) were activated in 100% methanol for 5–10 s, equilibrated in ultrafiltered distilled water for 2 min, and equilibrated in semi-dry transfer buffer for 5–10 min. Semi-dry transfer was conducted using the Bio-Rad Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell as per manufacturer’s instructions (Biorad Life Science, Hercules, California) for 30 min at 20 V. Following transfer the gel was removed and stained with Sypro Ruby while the membrane was incubated in 5% milk Tris-Buffered Saline plus 0.05% Tween-20 (TBS-T) overnight at 4°C. The blot was then incubated with a 1:10 dilution of day 60 TB-infected guinea pig serum again overnight at 4°C. The blot was then washed twice and the secondary antibody, a horseradish peroxidase labeled anti-Guinea Pig IgG (Ref # 61–4620, Life Technologies, Grand Island, New York) was used at a 1:2000 dilution in 5% milk TBST buffer and incubated 1 hour at room-temperature. The blot was then washed and visualized using Super-Signal West Pico-Chemiluminescent Substrate according to manufacturer’s instructions (Thermo Fisher Scientific, Waltham, Massachusetts).
Mass Spectrometry
Gel spots of interest were excised and equilibrated in 100 µl of 50 mM ammonium bicarbonate buffer (pH 8.0, 25mM), washed in 100 µl of water, and incubated twice in 100 µl of acetonitrile for 5 min. Acentonitrile was removed and the samples were then placed in a speedvac for 45 min to remove any excess solvent. 20 mg Trypsin (Promega Corp, Fitchburg, Wisconsin) was dissolved in 2 ml of 25 mM, pH 8.0 ammonium bicarbonate and 10 µl were added to each gel spot and incubated at 37°C for 6 h. After digestion, 1 µl of sample solution was spotted directly onto a MALDI target plate and allowed to dry. 1 µl of alpha-cyano-4-hydroxycinnamic acid (Aldrich Chemical Co.) matrix solution (50:50 acetonitrile/water at 5 mg/ml) was then applied on the sample spot and allowed to dry.
MALDI TOF/TOF mass spectrometry was used to analyze the samples and determine protein identification. Data were acquired with an AB Sciex TOF/TOF™ 5800. AB Sciex software package included TOF/TOF Series Explorer software (v. 4.1.0) with Oracle Database Schema Version (v. 4.0.0), Data Version (4.0.5) to acquire both MS and MS/MS spectral data. The instrument was operated in positive ion reflectron mode, mass range was 850 – 3000 Da, and the focus mass was set at 1700 Da. For MS data, 800 laser shots were acquired and averaged from each sample spot. Automatic external calibration was performed using a peptide mixture with reference masses 904.468, 1296.685, 1570.677, and 2465.199. Mass spectrometry peak filtering included the following parameters: minimum S/N filter = 5, mass exclusion list tolerance = 0.5 Da, and mass exclusion list for common trypsin and keratin-containing compounds.
Following MALDI MS analysis, MALDI MS/MS was performed on the top 10 most abundant ions from each sample spot. A 1kV positive ion MS/MS method was used to acquire data under post-source decay (PSD) conditions. The instrument precursor average selection window was +/− 3 Da. For MS/MS data, 1000 laser shots were acquired and averaged from each sample spot. Automatic external calibration was performed using reference fragment masses 175.120, 684.347, 813.389, 1056.475, and 1441.634 (from precursor mass 1570.670). For MS/MS peak filtering, the minimum S/N filter = 5.
AB Sciex Protein Pilot™ software (v. 4.0.8085) was used in conjunction with MASCOT to search the respective protein database using both MS and MS/MS spectral data for protein identification. Protein match probabilities were determined using expectation values and/or MASCOT protein scores. For protein identification, the M. tuberculosis taxonomy was searched in the NCBI protein database. Other parameters included the following: selecting the enzyme as trypsin; maximum missed cleavages = 2; fixed modifications included carbamidomethyl (C) for 2-D gel analyses only; variable modifications included oxidation (M); precursor tolerance was set at 0.5 Da; MS/MS fragment tolerance was set at 0.5 Da; mass = monoisotopic; and peptide charges were only considered as +1. The significance of a protein match, based on both the peptide mass fingerprint (PMF) in the first MS and the MS/MS data from several precursor ions, is based on expectation values; each protein match is accompanied by an expectation value. The expectation value is the number of matches with equal or better scores that are expected to occur by chance alone. The default significance threshold is p<0.05, so an expectation value of 0.05 is considered to be on this threshold. We used a more stringent threshold of 10−3 for protein identification.
Bioinformatics
Protein functional information was obtained from TubercuList (http://tuberculist.epfl.ch/). Information on homology was obtained through the use of BLAST or by accessing structural information at the Protein Data Bank (PDB). Additional information was obtained from primary literature. Gene sequences obtained from NCBI and analyzed for conservation using tBLASTn to determine protein identity percentages across 15 sequenced MTB strains (http://blast.ncbi.nlm.nih.gov/). We predicted the proteins’ secondary structure using Phyre2 (Kelley & Sternberg, 2009) the results are viewable in Table S2.
RESULTS
MTB in vitro pellicle membrane proteins are antigenic
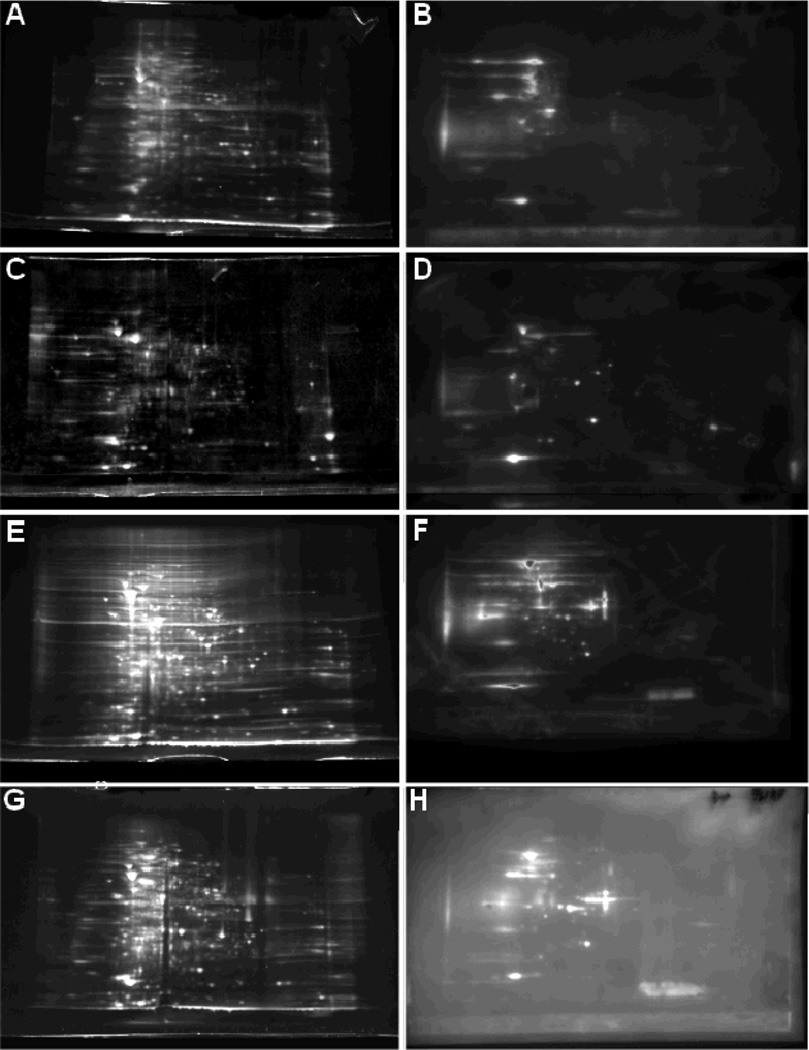
MTB H37Ra pellicles from 3, 5, or 7 weeks in vitro culture was compared to a control shaken culture of H37Ra grown for 2 weeks. Pellicles grown in minimal media for 5 weeks demonstrated robust aggregation macroscopically (Fig. 1A) and microscopically as examined by confocal microscopy following FISH staining of 16S rRNA (Fig. 1B). Bacteria were mechanically lysed and total membrane protein was obtained by differential centrifugation. Protein samples were assayed and 100 µg of sample was precipitated to remove impurities. Protein in each sample was separated by 2DGE in triplicate and transferred to membranes (Fig. 2a, b, c). Western blotting of the membranes of the separated and transferred 3, 5, and 7 week pellicle protein samples identified a number of antigenic proteins. 35, 37, and 29 distinct antigenic proteins spots from 3, 5, and 7-week pellicle two-dimensional gels respectively, were analyzed by MALDI-TOF/TOF mass spectrometry to determine protein identities. This yielded a list of 44 distinct antigenic proteins expressed over the three timepoints, which were compared to two-dimensional gels of 2-week shaken culture H37Ra to determine the temporary expression pattern of the proteins (table 1). Of the 44 identified proteins 43 had previously been showed to fractionate into the total membrane fraction of MTB (Lew, et al., 2011).
Figure 1.
(A) 5 week-old Mycobacterium tuberculosis pellicle grown statically in Sauton’s Minimal Media as described in Ojha et al 2008 and (B) a Fluorescence In-Situ Hybridization stain of the pellicle for MTB 16s rRNA (100× magnification).
Figure 2.
Two-dimensional gel electrophoresis protein-stained gels of (A) 2 week shaken culture, (C) 3 week pellicle, (E) 5 week pellicle, and (G) 7 week pellicle membrane proteins (Sypro Ruby Stain). Two-dimensional Western Blots of (B) 2 week shaken culture, (D), 3 week pellicle, (F) 5 week pellicle, and (H) 7 week pellicle membrane proteins probed with 60-day MTB infected Guinea Pig serum and visualized with horseradish-peroxidase labeled secondary antibody.
Table I. Comprehensive list of antigenic proteins identified grouped by expression patterns (Pellicle Specific, Universal, or Indeterminate).
Protein expression was established for each timepoint by either mass-spectrometry (MS) or Sypro-Ruby (SR) protein stain of two-dimensional gels. Mass spectrometry identities were determined with an expectation value threshold of 10−3 or less, Sypro-Ruby spot identities were representative off at least 3 replicate 2D gels for each timepoint/sample. Protein additional protein information including isoelectric point, molecular weight, and functional information obtained from Tuberculist (tuberculist.epfl.ch).
| Abrv | Name | Rv# | pI | MW (kDa) |
2W Shaken |
3W Pel |
5W Pel |
7W Pel |
Function |
|---|---|---|---|---|---|---|---|---|---|
| Biofilm Specific Proteins | |||||||||
| Rv3237c | Conserved protein | Rv3237c | 4.7 | 17.2 | − | + | + | + | Unknown |
| CeoB | Complementation of E. coli OxyR Mutant B | Rv2691 | 6.3 | 24.2 | − | + | + | + | Trk type potassium transport |
| FabG4 | 3-ketoacyl-acyl carrier protein reductase | Rv0242c | 6.4 | 46.8 | − | + | + | + | Fatty acid biosynthesis |
| FhaA | Conserved protein with FHA domain | Rv0020c | 4.7 | 56.9 | − | + | + | + | Signal transduction |
| Rv0097 | Possible oxidoreductase | Rv0097 | 6.6 | 32.6 | − | + | + | + | Unknown |
| Rv1996 | Universal stress protein family protein | Rv1996 | 7.0 | 33.9 | − | + | + | + | Unknown |
| Rv2216 | Conserved protein | Rv2216 | 7.6 | 31.7 | − | + | + | + | Unknown |
| Rv2296 | Probable haloalkane dehalogenase | Rv2296 | 7.0 | 33.4 | − | + | + | + | Probably haloalkane degradation |
| Rv3519 | Unknown | Rv3519 | 10.0 | 26.0 | − | + | + | + | Unknown |
| Universally Expressed Proteins | |||||||||
| 35kd_ag | 35 kDa Antigen | Rv2744c | 5.8 | 29.3 | + | + | + | + | Unknown |
| AtpD | ATP synthase beta chain | Rv1310 | 4.6 | 53.1 | + | + | + | + | ATP synthesis |
| Eno | Enolase | Rv1023 | 4.3 | 44.9 | + | + | + | + | Glycolysis |
| FadD2 | fatty-acid-CoA synthase | Rv0270 | 6.8 | 59.9 | + | + | + | + | Lipid degradation |
| FixB | Probable electron transfer flavoprotein | Rv3028c | 4.4 | 31.7 | + | + | + | + | Electron acceptor for the respiratory chain. |
| Frr | Ribosome recycling factor | Rv2882c | 5.8 | 20.8 | + | + | + | + | Release of ribosome from mRNA following termination of translation |
| GlnA1 | Glutamine synthetase A1 | Rv2220 | 4.8 | 53.5 | + | + | + | + | glutamine biosynthesis |
| GlnA2 | Glutamine synthetase A2 | Rv2222c | 5.2 | 49.6 | + | + | + | + | glutamine biosynthesis |
| GroEl2 | Heat Shock Protein 65 | Rv0440 | 4.6 | 56.7 | + | + | + | + | Prevention of protein misfolding/promotion of proper assembly of unforlded proteins. |
| HbhA | Heparin binding hemagglutinin | Rv0475 | 9.8 | 21.5 | + | + | + | + | Adhesin involved in extrapulmoary dissemination |
| Mkl | Possible ribonucleotide-transport ABC transporter | Rv0655 | 5.9 | 39.4 | + | + | + | + | Ribonucleotide membrane transport |
| MoxR1 | Probable transcriptional regulatory protein | Rv1479 | 6.4 | 40.8 | + | + | + | + | Transcription |
| NusA | Probable N utilization substance protein A | Rv2841c | 6.9 | 37.6 | + | + | + | + | Possibly involved in termination and antitermination of transcription |
| PepC | Probable aminopeptidase | Rv0800 | 6.5 | 46.0 | + | + | + | + | possibly hydrolyzes peptides and/or proteins |
| PrcA | Proteasome alpha subunit | Rv2109c | 5.2 | 26.8 | + | + | + | + | Protein degradation |
| PyrB | Probable aspartate carbamoyltransferase | Rv1380 | 7.1 | 33.8 | + | + | + | + | pyrimidine biosynthesis |
| RplE | 50S ribosomal protein L5 | Rv0716 | 10.8 | 21.0 | + | + | + | + | Protein translation |
| RplI | 50S ribosomal protein L9 | Rv0056 | 9.7 | 16.2 | + | + | + | + | Protein translation |
| RplL | 50S ribosomal protein L7/L12 | Rv0652 | 4.3 | 13.4 | + | + | + | + | Protein translation |
| RplM | 50S ribosomal protein L13 | Rv3443c | 10.6 | 16.3 | + | + | + | + | Protein translation |
| RplR | 50S ribosomal protein L18 | Rv0720 | 12.1 | 13.2 | + | + | + | + | Protein translation |
| RpoA | DNA-directed RNA polymerase alpha chain | Rv3457c | 4.4 | 37.7 | + | + | + | + | DNA-dependent RNA polymerase |
| RpsE | 30S ribosomal protein S5 | Rv0721 | 10.8 | 22.9 | + | + | + | + | Protein translation |
| Rv1245c | Probable short-chain type dehydrogenase/reductase | Rv1245c | 8.0 | 29.2 | + | + | + | + | Unknown |
| Rv1771 | L-gulono-1,4-lactone dehydrogenase | Rv1771 | 7.6 | 48.0 | + | + | + | + | Possibly vitamin C synthesis |
| SodA | Superoxide dismutase | Rv3846 | 6.4 | 23.0 | + | + | + | + | Peroxidase |
| Ssb | Single-strand binding protein | Rv0054 | 4.8 | 17.3 | + | + | + | + | DNA replication, recombination, and repair |
| Tb31.7 | Universal stress protein family protein | Rv2623 | 5.6 | 31.7 | + | + | + | + | Unknown |
| Tuf | Probable iron-regulated elongation factor TU | Rv0685 | 5.1 | 43.6 | + | + | + | + | Facilitates tRNA binding to ribosomes. |
| Wag31 | DivIVA family protein | Rv2145c | 4.5 | 28.3 | + | + | + | + | Unknown |
| Indeterminate Proteins | |||||||||
| AtpG | ATP synthase gamma chain | Rv1309 | 5.1 | 33.9 | + | ? | + | + | ATP synthesis |
| BfrB | Bacterioferritin B | Rv3841 | 4.5 | 20.4 | ? | + | + | − | Iron Storage |
| Fum | Probable fumarase | Rv1098c | 5.2 | 50.1 | + | ? | + | + | Catalyzes the reversible hydration of fumarate to L-malate |
| Rv0148 | Probable short-chain type dehydrogenase/reductase | Rv0148 | 5.1 | 29.8 | ? | ? | + | ? | Unknown |
| Rv2629 | Conserved protein | Rv2629 | 5.0 | 40.8 | + | ? | + | + | Unknown |
A subset of antigenic proteins is specific to pellicle
In order to identify the subset of antigenic proteins that were specifically produced in the three pellicle timepoints but not during planktonic shaken growth, we performed comparisons of gels at all timepoints to identify proteins that were expressed during all three pellicle timepoints but not in the shaken control culture as previously described(Brady, et al., 2006). We identified 9 proteins that were pellicle-specific in their expression, 30 that were universally expressed, and 5 that did not fall into either class (Table 1). The universally expressed protein class contained a number of housekeeping genes including the glycolysis protein enolase, six ribosomal proteins, and the chaperone GroEl2. Also expressed at all timepoints was the adhesin heparin-binding hemaglutinin A (HbhA) and the unknown function proteins TB31.7 and 35kD antigen.
Pellicle specific antigenic proteins are highly conserved
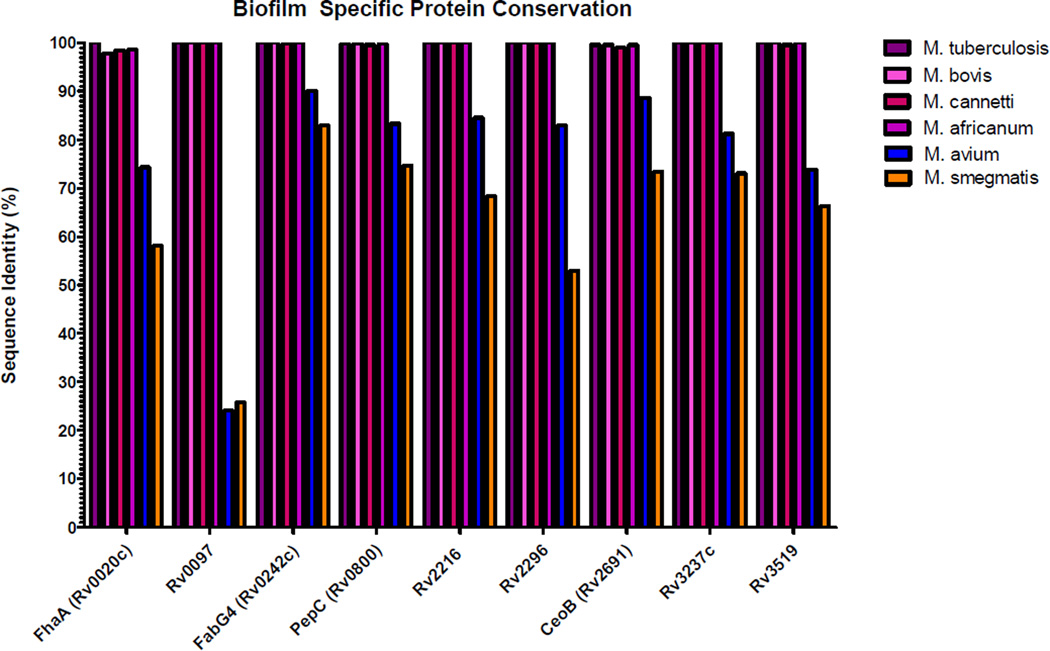
The nine pellicle-specific proteins primary amino acid sequence was compared to 15 sequenced strains of MTB (see Table S2) as well as Mycobacterium bovis AF2122197, Mycobacterium africanum GMO41182, Mycobacterium canetti CIPT 140070008, Mycobacterium smegmatis mc2 155, and Mycobacterium avium 104 using the tBLASTn which uses the Basic Local Alignment Search Tool (BLAST) to compare a protein sequence to a translated genome(Camacho, et al., 2009). The percent identity (Fig. 3) demonstrates the extreme conservation of the protein sequences among the members of the Mycobacterium tuberculosis complex (MTB, M. africanum, M. bovis, M. canetti) and the lower degree of conservation with the related pathogen M. avium or the nonpathogenic M. smegmatis. Rv0097 in particular show high average conservation in the MTB complex (98%) but relatively low sequence conservation in both M. avium (25%) and M. smegmatis (26%). FabG4 (Rv0242c) displayed a high average degree of conservation among the MTB complex (99%) and with M. avium (90%) and M. smegmatis (83%).
Figure 3.
Pellicle Specific protein conservation determined by tBLASTn of protein sequences against translated genomes. M. tuberculosis bar represents median conservation of the protein among 15 sequenced MTB strains.
DISCUSSION
A large number of published studies have attempted to identify new TB diagnostic biomarkers in an effort to detect and combat the large worldwide burden of TB. This work represents a novel approach by utilizing the host antibody response to detect those antigens unique to the pellicle mode of growth that are expressed in vivo. By combining this model of extracellular MTB growth with proven techniques of 2DGE and Western Blotting along with MALDI-TOF/TOF protein identification, we were able to identify 44 antigenic proteins expressed in this model. Out of the 44 antigenic proteins we identified nine that were specifically expressed in the pellicle mode of growth compared to the control shaken culture. The identification of humoral antigens for development as diagnostic biomarkers is advantageous since it allows for a variety of rapid diagnostic tests to be performed on easily isolated serum.
While none of the pellicle-specific proteins identified have well-characterized functions, several existing studies in combination with bioinformatics data shed some light on possible functions for these proteins in MTB pathogenesis. The fork-head domain containing protein FhaA (Rv0020c) has been structurally characterized and is highly similar by BLAST to proteins in Mycobacterium leprae, Rhodococcus spp. and close mycobacterial relatives Amycolicicoccus (Fig. S2) (Roumestand, et al., 2011). Current models of this protein’s function describe it as a signal transducer that, when activated by a sensor of peptidoglycan, mediates the repression of more peptidoglycan synthesis (Gee, et al., 2012). This repression would be expected in the nutrient-deprived pellicle and granuloma since macromolecular synthesis is inhibited.
Another pellicle-specific protein, Rv0097 was expressed preferentially in the three pellicle time-point compared to shaking culture and was highly (98%) conserved within the MTB complex. However, only 25% amino acid sequence similarity was found when compared to M. avium and M. smegmatis. Disruption of Rv0097 in M. bovis (Mb0100) has been shown to abolish production of phthiocerol (PDIM), a lipid virulence factor that protects MTB against nitric oxide-dependent killing in macrophages (Rousseau, et al., 2004, Hotter, et al., 2005, Astarie-Dequeker, et al., 2009). Considering the large amounts of nitric oxide produced by granuloma macrophages in mycobacterial infections, increased expression of Rv0097 might allow for more PDIM production to protect bacteria from this host response (Ehlers, et al., 1999). This protein seems to be conserved among other virulent mycobacteria including M. marinum, M. ulcerans, and M. leprae but also shares considerable identity with tuarine dioxygenases in a large number of bacteria species.
CeoB (Rv2691) is a TrkA homolog and the trk potassium uptake system is a medium-affinity system for potassium uptake (Cholo, et al., 2006). CeoB was noted for its ability to complement the E. coli oxyR mutant, which is susceptible to the antitubercular drug isoniazid unlike wild type E. coli. When CeoB and its homolog oxyR were transformed into the mutant, it regained its natural resistance (Chen & Bishai, 1998). The most common mutations leading to isoniazid residence in clinical isolates of MTB are those of the enzyme KatG, which activates the isoniazid pro-drug (Zhang, et al., 1992, Cade, et al., 2010). Other gene mutations have been identified including kasA, ndh, ahpC, and inhA (Rindi, et al., 2005). Besides low metabolic rates, it is possible that increased expression of CeoB could be responsible for part of the phenotypic resistance to isoniazid displayed by MTB biofilms (Ojha, et al., 2008). CeoB is present in many Mycobacteria species as well as related species Rhodococcus and Pseudonocardia.
Rv3519 contains homology with known acetoacetate decarboxylase proteins, which produce CO2 and acetone from acetoacetate(May, et al., 2013). Acetoacetate is produced in the human liver from acetyl-CoA and secreted into the blood under conditions of starvation or diabetes, metabolized in the brain to CO2 and acetone, both of which are excreted by the pulmonary route as gas(Miekisch, et al., 2001). Acetone is utilized by M. smegmatis as both a carbon source and an energy source, suggesting MTB could utilize Rv3519 to obtain acetone from acetoacetate (Lukins & Foster, 1963). Diabetes is a known risk factor for TB (Dooley & Chaisson, 2009). One possible mechanism for this could be that acetone excreted into the lungs in diabetic patients provides an additional carbon source for MTB. Furthermore, an acetone responsive transcriptional activator, mimR has been found in M. smegmatis (Kotani, et al., 2007, Furuya, et al., 2011). Given acetone’s high permeability across biological membranes there is a possibility that it could act as the, currently uncharacterized, diffusible signaling molecule hypothesized to play a critical role in mycobacterial biofilm development (Ojha, et al., 2008). Acetoacetate decarboxylases are not unique to mycobacteria and are found in both gram-positive and negative bacteria.
FabG4 (Rv0242c) is one of five FabG proteins in the MTB genome. As part of the critical fatty acid synthetase II system (FASII) the protein InhA is transcribed with one FabG protein which uses NADPH to reduce β-oxoacyl-ACP to β-hydroxyacyl-ACP (Dutta, et al., 2013). FabG4 in particular has been shown to be expressed in several minimal media models of growth (Beste, et al., 2009, Sharma, et al., 2010). FabG proteins are common components of fatty-acid synthesis pathways and are not unique to mycobacteria.
PepC (Rv0800) has been annotated as a probable aminopeptidase but no work has been done about its specific cellular function (Ribeiro-Guimaraes & Pessolani, 2007). Aminopeptidases are common proteins in both animal and plant cells and cleave proteins from the amino terminus. PepC production during long-term pellicle growth could reflect a need to break down or cannibalize mycobacterial proteins. Studies in E. coli have demonstrated that certain bacterial proteases are induced strongly in late stationary phase and that mutants for the clpP and clpX genes had reduced viability after stationary phase demonstrating that a subset of bacterial proteases are critical to surviving low-nutrient conditions (Weichart, et al., 2003).
Rv2216 is predicted to be a nucleoside diphosphate sugar epimerase, a class of enzymes that convert sugars (linked to nucleoside diphosphates such as UDP) between different epimers. These enzymes play a crucial role in metabolism and have been shown to be essential virulence factors required for colonization or virulence. They are also critical to the formation of important carbohydrate molecules such as the backbone of bacterial lipopolysaccharide (LPS) (Allard, et al., 2001).
For this study we performed all of the proteomics work using the MTB strain H37Ra, a clinically isolated avirulent strain that produced morphologically identical pellicles to previously published H37Rv pellicles. H37Ra is known to exhibit a number of differences from its more virulent related strain H37Rv, which we used in the animal infections to produce serum for our 2D Western Blots. Chief among these differences are altered morphology (Zheng, et al., 2008), apparent loss of TDM production (Gao, et al., 2004), poor survival inside macrophages (Mackaness, et al., 1954) and somewhat decreased ability to prevent phagosome-lysosome fusion (Hart & Armstrong, 1974). Despite this the two strains are genetically highly similar with only 272 significant genetic variations between them over a 4.4 mb genome containing over 4000 protein coding sequences (Zheng, et al., 2008). While the attenuated strain H37Ra would be improper to use in animal models where it is avirulent we found it to be an excellent model strain for in vitro work.
This study represents an original set of results with novel biomarkers through examining the immunoproteome of tuberculosis that can be studied for rapid diagnostic technologies in subsequent research. A recent high-throughput study examining sera from over 500 patients detected 484 antigenic proteins of which 5 were also detected in this study (35kd_ag, GroEl2, HbhA, RplE, and RplL) (Kunnath-Velayudhan, et al., 2010). Utilizing these proteins in a diagnostic capacity will require further testing using human serum to recognize recombinant or native purified pellicle-specific protein to determine whether MTB infection in humans produces sufficient antibody titers to recognize these antigens. Furthermore, testing of latent vs. active tuberculosis patient serum against these proteins may be useful to identify whether any of these antigens would be suitable for diagnosis of latent TB.
Supplementary Material
ACKNOWLEDGEMENTS
This research was This work was supported by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI069568-02 and ARRA supplement AI069568), and the National Institute of Dental and Craniofacial Research (2T32 DE007309). The authors would also like to thank Rebecca Brady, Anil Ojha, Jan Harro, Nate Archer, Megan Prior.
EFERENCES
- Allard ST, Giraud MF, Naismith JH. Epimerases: structure, function and mechanism. Cell Mol Life Sci. 2001;58:1650–1665. doi: 10.1007/PL00000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh FK, Chalut C, Lopez A, Guilhot C. Phthiocerol dimycocerosates of M tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009;5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr MA. BCG--different strains, different vaccines? Lancet Infect Dis. 2002;2:86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- Beste DJ, Espasa M, Bonde B, Kierzek AM, Stewart GR, McFadden J. The genetic requirements for fast and slow growth in mycobacteria. PLoS One. 2009;4:e5349. doi: 10.1371/journal.pone.0005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74:3415–3426. doi: 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, Leid JG, Kofonow J, Costerton JW, Shirtliff ME. Immunoglobulins to surface-associated biofilm immunogens provide a novel means of visualization of methicillin-resistant Staphylococcus aureus biofilms. Appl Environ Microbiol. 2007;73:6612–6619. doi: 10.1128/AEM.00855-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, O'May GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect Immun. 2011;79:1797–1803. doi: 10.1128/IAI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade CE, Dlouhy AC, Medzihradszky KF, Salas-Castillo SP, Ghiladi RA. Isoniazid-resistance conferring mutations in Mycobacterium tuberculosis KatG: catalase, peroxidase, and INH-NADH adduct formation activities. Protein Sci. 2010;19:458–474. doi: 10.1002/pro.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitra MG, Hariharaputran S, Chandra NR, Shaila MS, Nayak R. Defining putative T cell epitopes from PE and PPE families of proteins of Mycobacterium tuberculosis with vaccine potential. Vaccine. 2005;23:1265–1272. doi: 10.1016/j.vaccine.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Chen P, Bishai WR. Novel selection for isoniazid (INH) resistance genes supports a role for NAD+-binding proteins in mycobacterial INH resistance. Infect Immun. 1998;66:5099–5106. doi: 10.1128/iai.66.11.5099-5106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholo MC, Boshoff HI, Steel HC, et al. Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. J Antimicrob Chemother. 2006;57:79–84. doi: 10.1093/jac/dki409. [DOI] [PubMed] [Google Scholar]
- Cole ST. Comparative mycobacterial genomics as a tool for drug target and antigen discovery. Eur Respir J Suppl. 2002;36:78s–86s. doi: 10.1183/09031936.02.00400202. [DOI] [PubMed] [Google Scholar]
- Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Bhattacharyya S, Roychowdhury A, Biswas R, Das AK. Crystal structure of hexanoyl-CoA bound to beta-ketoacyl reductase FabG4 of Mycobacterium tuberculosis. Biochem J. 2013;450:127–139. doi: 10.1042/BJ20121107. [DOI] [PubMed] [Google Scholar]
- Ehlers S, Kutsch S, Benini J, et al. NOS2-derived nitric oxide regulates the size, quantity and quality of granuloma formation in Mycobacterium avium-infected mice without affecting bacterial loads. Immunology. 1999;98:313–323. doi: 10.1046/j.1365-2567.1999.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer K, Cockle P, Gordon S, et al. Antigen mining with iterative genome screens identifies novel diagnostics for the Mycobacterium tuberculosis complex. Clin Vaccine Immunol. 2006;13:90–97. doi: 10.1128/CVI.13.1.90-97.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florczyk MA, McCue LA, Stack RF, Hauer CR, McDonough KA. Identification and characterization of mycobacterial proteins differentially expressed under standing and shaking culture conditions, including Rv2623 from a novel class of putative ATP-binding proteins. Infect Immun. 2001;69:5777–5785. doi: 10.1128/IAI.69.9.5777-5785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T, Hirose S, Semba H, Kino K. Identification of the regulator gene responsible for the acetone-responsive expression of the binuclear iron monooxygenase gene cluster in mycobacteria. J Bacteriol. 2011;193:5817–5823. doi: 10.1128/JB.05525-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Kripke K, Arinc Z, Voskuil M, Small P. Comparative expression studies of a complex phenotype: cord formation in Mycobacterium tuberculosis. Tuberculosis (Edinb) 2004;84:188–196. doi: 10.1016/j.tube.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Gee CL, Papavinasasundaram KG, Blair SR, et al. A phosphorylated pseudokinase complex controls cell wall synthesis in mycobacteria. Sci Signal. 2012;5:ra7. doi: 10.1126/scisignal.2002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob Agents Chemother. 2003;47:833–836. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado E, Amat I, Gil O, et al. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Gupta A, Kaul A, Tsolaki AG, Kishore U, Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology. 2012;217:363–374. doi: 10.1016/j.imbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab') fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PD, Armstrong JA. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974;10:742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006;6:710–725. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- Hotter GS, Wards BJ, Mouat P, et al. Transposon mutagenesis of Mb0100 at the ppe1-nrp locus in Mycobacterium bovis disrupts phthiocerol dimycocerosate (PDIM) and glycosylphenol-PDIM biosynthesis, producing an avirulent strain with vaccine properties at least equal to those of M. bovis BCG. J Bacteriol. 2005;187:2267–2277. doi: 10.1128/JB.187.7.2267-2277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- Jin L, Wang G, Zhao X, et al. Characterization and immune effect of the hepatitis B-BCG combined vaccine for using a needle innoculation. Vaccine. 2010;28:6041–6051. doi: 10.1016/j.vaccine.2010.06.081. [DOI] [PubMed] [Google Scholar]
- Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kenrick KG, Margolis J. Isoelectric focusing and gradient gel electrophoresis: a two-dimensional technique. Anal Biochem. 1970;33:204–207. doi: 10.1016/0003-2697(70)90454-9. [DOI] [PubMed] [Google Scholar]
- Kotani T, Yurimoto H, Kato N, Sakai Y. Novel acetone metabolism in a propane-utilizing bacterium, Gordonia sp. strain TY-5. J Bacteriol. 2007;189:886–893. doi: 10.1128/JB.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnath-Velayudhan S, Salamon H, Wang HY, et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A. 2010;107:14703–14708. doi: 10.1073/pnas.1009080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laennec R. A treatise on diseases of the chest in which they are described according to their anatomical characters, and their diagnosis established on a new principle by means of acoustick instruments. Underwood, London: 1821. [Google Scholar]
- Lew JM, Kapopoulou A, Jones LM, Cole ST. TubercuList--10 years after. Tuberculosis (Edinb) 2011;91:1–7. doi: 10.1016/j.tube.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Liu J, Tran V, Leung AS, Alexander DC, Zhu B. BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccin. 2009;5:70–78. doi: 10.4161/hv.5.2.7210. [DOI] [PubMed] [Google Scholar]
- Lopez Y, Yero D, Falero-Diaz G, et al. Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16kDa protein in a model of progressive pulmonary infection. Int J Med Microbiol. 2009;299:447–452. doi: 10.1016/j.ijmm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Lukins HB, Foster JW. Methyl ketone metabolism in hydrocarbon-utilizing Mycobacteria. J Bacteriol. 1963;85:1074–1087. doi: 10.1128/jb.85.5.1074-1087.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness GB, Smith N, Wells AQ. The growth of intracellular tubercle bacilli in relation to their virulence. Am Rev Tuberc. 1954;69:479–494. doi: 10.1164/art.1954.69.4.479. [DOI] [PubMed] [Google Scholar]
- Malen H, Softeland T, Wiker HG. Antigen analysis of Mycobacterium tuberculosis H37Rv culture filtrate proteins. Scand J Immunol. 2008;67:245–252. doi: 10.1111/j.1365-3083.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- May A, Fischer RJ, Maria Thum S, Schaffer S, Verseck S, Durre P, Bahl H. A modified pathway for the production of acetone in Escherichia coli. Metab Eng. 2013;15:218–225. doi: 10.1016/j.ymben.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Miekisch W, Schubert JK, Vagts DA, Geiger K. Analysis of volatile disease markers in blood. Clin Chem. 2001;47:1053–1060. [PubMed] [Google Scholar]
- Moore T, Ekworomadu CO, Eko FO, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–624. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- Nagai S, Wiker HG, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Izumi S, Yamada T. Two-dimensional electrophoretic analysis of humoral responses to culture filtrate of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. Int J Lepr Other Mycobact Dis. 1998;66:208–213. [PubMed] [Google Scholar]
- Nedeltchev GG, Raghunand TR, Jassal MS, Lun S, Cheng QJ, Bishai WR. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect Immun. 2009;77:598–603. doi: 10.1128/IAI.01132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares N, Marquina B, Mata-Espinoza D, Zatarain-Barron ZL, Pinzón CE, Estrada I, Parada C, Collin M, Rook G, Hernandez-Pando R. The protective effect of immunoglobulin in murine tuberculosis is dependent on IgG glycosylation. Pathog Dis. 2013 Dec;69(3):176–183. doi: 10.1111/2049-632X.12069. [DOI] [PubMed] [Google Scholar]
- Ojha AK, Baughn AD, Sambandan D, et al. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme IM. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis (Edinb) 2013 doi: 10.1016/j.tube.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler W. The Principles and Practices of Medicine. New York: 1892. [Google Scholar]
- Palanisamy GS, Smith EE, Shanley CA, Ordway DJ, Orme IM, Basaraba RJ. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb) 2008;88:295–306. doi: 10.1016/j.tube.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- Pheiffer C, Betts JC, Flynn HR, Lukey PT, van Helden P. Protein expression by a Beijing strain differs from that of another clinical isolate and Mycobacterium tuberculosis H37Rv. Microbiology. 2005;151:1139–1150. doi: 10.1099/mic.0.27518-0. [DOI] [PubMed] [Google Scholar]
- Pichugin AV, Yan BS, Sloutsky A, Kobzik L, Kramnik I. Dominant role of the sst1 locus in pathogenesis of necrotizing lung granulomas during chronic tuberculosis infection and reactivation in genetically resistant hosts. Am J Pathol. 2009;174:2190–2201. doi: 10.2353/ajpath.2009.081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsugit S, Guma S, Pillay B, Jain P, Larsen MH, Danaviah S, Pillay M. Pili contribute to biofilm formation in vitro in Mycobacterium tuberculosis. Antonie Van Leeuwenhoek. 2013;104:725–735. doi: 10.1007/s10482-013-9981-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Guimaraes ML, Pessolani MC. Comparative genomics of mycobacterial proteases. Microb Pathog. 2007;43:173–178. doi: 10.1016/j.micpath.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Rindi L, Bianchi L, Tortoli E, Lari N, Bonanni D, Garzelli C. Mutations responsible for Mycobacterium tuberculosis isoniazid resistance in Italy. Int J Tuberc Lung Dis. 2005;9:94–97. [PubMed] [Google Scholar]
- Roumestand C, Leiba J, Galophe N, et al. Structural insight into the Mycobacterium tuberculosis Rv0020c protein and its interaction with the PknB kinase. Structure. 2011;19:1525–1534. doi: 10.1016/j.str.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rousseau C, Winter N, Pivert E, et al. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol. 2004;6:277–287. doi: 10.1046/j.1462-5822.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- Sambandan D, Dao DN, Weinrick BC, et al. Keto-mycolic acid-dependent pellicle formation confers tolerance to drug-sensitive Mycobacterium tuberculosis. MBio. 2013;4 doi: 10.1128/mBio.00222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Kumar B, Singhal N, Katoch VM, Venkatesan K, Chauhan DS, Bisht D. Streptomycin induced protein expression analysis in Mycobacterium tuberculosis by two-dimensional gel electrophoresis & mass spectrometry. Indian J Med Res. 2010;132:400–408. [PubMed] [Google Scholar]
- Sirgel FA, Fourie PB, Donald PR, et al. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:128–135. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- Skerry C, Pokkali S, Pinn M, Be NA, Harper J, Karakousis PC, Jain SK. Vaccination with recombinant Mycobacterium tuberculosis PknD attenuates bacterial dissemination to the brain in guinea pigs. PLoS One. 2013;8:e66310. doi: 10.1371/journal.pone.0066310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramurthi JC, Brindha S, Shobitha SR, Swathi A, Ramanandan P, Hanna LE. In silico identification of potential antigenic proteins and promiscuous CTL epitopes in Mycobacterium tuberculosis. Infect Genet Evol. 2012;12:1312–1318. doi: 10.1016/j.meegid.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Tang ST, van Meijgaarden KE, Caccamo N, et al. Genome-based in silico identification of new Mycobacterium tuberculosis antigens activating polyfunctional CD8+ T cells in human tuberculosis. J Immunol. 2011;186:1068–1080. doi: 10.4049/jimmunol.1002212. [DOI] [PubMed] [Google Scholar]
- Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli SM. Tuberculosis of the spine: a historical review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312–316. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis RS, Patil S, Cheon SH, et al. Drug tolerance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:2600–2606. doi: 10.1128/aac.43.11.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang H, Wang H. Analysis of predicted CD8+ T cell epitopes from proteins encoded by the specific RD regions of Mycobacterium tuberculosis for vaccine development and specific diagnosis. Mol Biol Rep. 2010;37:1793–1799. doi: 10.1007/s11033-009-9613-4. [DOI] [PubMed] [Google Scholar]
- Weichart D, Querfurth N, Dreger M, Hengge-Aronis R. Global role for ClpP-containing proteases in stationary-phase adaptation of Escherichia coli. J Bacteriol. 2003;185:115–125. doi: 10.1128/JB.185.1.115-125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global tuberculosis report 2012. World Heath Organization; 2012. [Google Scholar]
- Zhang L, Wang Q, Wang W, et al. Identification of putative biomarkers for the serodiagnosis of drug-resistant Mycobacterium tuberculosis. Proteome Sci. 2012;10:12. doi: 10.1186/1477-5956-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lu L, Wang B, et al. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One. 2008;3:e2375. doi: 10.1371/journal.pone.0002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvi A, Ariel N, Fulkerson J, Sadoff JC, Shafferman A. Whole genome identification of Mycobacterium tuberculosis vaccine candidates by comprehensive data mining and bioinformatic analyses. BMC Med Genomics. 2008;1:18. doi: 10.1186/1755-8794-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.