Abstract
TGFβ1 activity depends on a complex signaling cascade that controls expression of several genes. Among others, TGFβ1 regulates expression of matrix metalloproteinases (MMPs) through activation of Smads. Here, we demonstrate for the first time that the αvβ6 integrin interacts with TGFβ receptor II (TβRII) through the β6 cytoplasmic domain and promotes Smad3 activation in prostate cancer cells. Another related αv integrin, αvβ5, as well as the αvβ6/3 integrin, which contains a chimeric form of β6 with a β3 cytoplasmic domain, do not associate with TβRII and fail to show similar responses. We provide evidence that αvβ6 is required for upregulation of MMP2 by TGFβ1 through a Smad3-mediated transcriptional program in prostate cancer cells. The functional relevance of these results is underscored by the finding that αvβ6 modulates cell migration in a MMP2-dependent manner on an αvβ6 specific ligand, latency associated peptide (LAP)-TGFβ. Overall, these mechanistic studies establish that expression of a single integrin, αvβ6, is sufficient to promote activation of Smad3, regulation of MMP2 levels, and consequent catalytic activity, as well as cell migration. Our study describes a new TGFβ1/αvβ6/MMP2 signaling pathway that, given TGFβ1 pro-metastatic activity, may have profound implications for prostate cancer therapy.
Keywords: Integrins, prostate cancer, SMAD3, TGFβ1 receptor, MMP2
INTRODUCTION
The interactions between extracellular matrix (ECM) proteins and integrins, transmembrane receptors which comprise an α-subunit and a β-subunit [1, 2], support cancer cell functions including cell adhesion, proliferation, gene expression and modulation of migratory/invasive phenotypes [3–5]. Among others, the αvβ6 integrin is largely expressed in a wide variety of cancer types [6–10]. Its function is likely to be crucial for cancer progression [10–12], including prostate cancer (PrCa) where this molecule is largely undetectable in normal tissues, but abundantly expressed in metastatic tumors as evaluated in a mouse model of PrCa [13].
TGFβ1 has both tumor suppressor and tumor promoter activities [14, 15]. However, tumor cells often acquire a resistance to the growth-suppressive activities of TGFβ1 during tumor progression and hence, TGFβ1 shows growth stimulatory effects on these cells [16]. There are three types of TGFβ receptors (TβR)s: type I (TβRI), type II (TβRII) and type III (TβRIII) receptors. Type I and type II receptors comprise a single transmembrane segment and a cytoplasmic segment with a Ser/Thr kinase domain and form a heterodimeric complex upon ligand binding to initiate intracellular signaling [17]. Phosphorylation of TβRII at Ser213 is required for TGFβ1-mediated downstream signaling [18]. TβRIII, known as betaglycan, does not have an intrinsic signaling function, and has been shown to increase TGFβ1 binding to the signaling receptor TβRII [19].
As downstream effectors of TGFβ1 signaling, Smad molecules [20], whose genes have been reported to be mutated in various cancers [21], are often exploited in malignancy. While Smads 1, 5 and 8 are downstream of bone morphogenetic protein, rather than of TGFβ1, and Smad6 is an inhibitor of this growth factor [17, 22], Smads 2, 3 and 4 are downstream of TGFβ1. Phosphorylated Smad2/3 form dimers or trimers with Smad4, translocate to the nucleus and interact with both DNA-binding cofactors as well as co-activators or co-repressors, to modulate transcription of TGFβ-target genes [20]. Previous studies reported that Smad2 and Smad3 expression are highly relevant to PrCa progression as upregulation of these molecules correlates with higher Gleason scores in patients. Higher nuclear p-Smad2 and Smad4 are found in PrCa Gleason grade 5 as compared to PrCa with Gleason grade 3 or 4, whereas the levels of Smad7, which prevents phosphorylation of Smad2 or Smad3, are not changed with different Gleason grades [23]. Overexpression of Smad3 has been shown to correlate with Gleason score in human PrCa, and may contribute to disease progression in humans [24]. Both nuclear and cytoplasmic overexpression of Smad4 protein correlates with tumor grade, stage and DNA ploidy in PrCa patients [25], although nuclear Smad4 is decreased in high Gleason score cancer [26] and Smad4 downregulation enhances the metastatic potential of PrCa cells [27].
Critical downstream effectors of TGFβ1 in cancer progression are integrins [28] and matrix metalloproteinases (MMPs) [29, 30]. Among others, TGFβ1 upregulates αvβ6 [28] and MMP2 protein expression [31], MMP2 mRNA stability [32] as well as secretion [31], and this response has been functionally implicated in osteolytic lesions in murine models of PrCa metastasis [33, 34]. Another study by Miralles et al. shows that TGFβ1 is a key activator of MMP2 in the pancreatic islets [35]. A different study by Sehgal et al. shows that TGFβ1 induces secreted protein activity for MMP2 although it has no effect on new MMP2 RNA synthesis [36]. Specifically, Ahmed et al. report expression of αvβ6 in ovarian cancer tissues and show that αvβ6 causes enhanced levels of two pro-MMPs, MMP2 and 9, in several cancer cell lines [7, 37]. Smad 2, 3 and Smad4 are involved in regulating MMP2 [38] and other MMPs [39, 40]. In addition, there are reports showing that MMP2 and MMP9 are increased in response to integrin expression [7, 37, 41, 42] and that TGFβ in cooperation with α3β1 promotes MMP9 mRNA stability in normal keratinocytes [42]. Although clinical data have implicated MMP2 and MMP9 as independent predictors of PrCa metastasis [43] and associated MMP2 with reduced disease-free survival [44], a potential role of integrins in their regulation by TGFβ1 has not been investigated.
Our data show that the αvβ6 integrin interacts with TβRII and promotes TGFβ1-induced phosphorylation of Smad3. This causes upregulation of MMP2 and as a result, αvβ6 –expressing cells show increased cell migration in a MMP2-dependent manner.
MATERIALS AND METHODS
Reagents and Antibodies
BSA was from Sigma-Aldrich, type I Collagen (type I Col) was from Invitrogen, TGFβ1 and LAP-TGFβ1 were from R&D Systems. Specific inhibitor of Smad3 (SIS3) was from Santa Cruz Biotechnology [45]. SIS3 was dissolved in DMSO. We used the following rabbit antibodies (Abs) against: TβRII, ERK1/2, fibronectin (FN), focal adhesion kinase (FAK) and AKT from Santa Cruz Biotechnology; phospho-Smad3 (ser423/425) from Cell Signaling Technology; Smad3 from Zymed; β3 cytoplasmic domain [46]; β5 cytoplasmic domain and membrane type-1 matrix metalloproteinase (MT1-MMP) from Millipore; and MMP2 from Cell Signaling Technology. This Ab against MMP2 is specific for MMP2 and reacts with pro- and active human MMP2.
C19 goat Ab against β6 cytoplasmic domain and goat IgG was from Santa Cruz Biotechnology.
We used the following mouse (m)Abs against: Smad2 from Invitrogen, tissue inhibitor of metalloproteinases2 (TIMP2) from Abeam, β6 from Chemicon, 10D5, for FACS and immunoprecipitation (IP) and 2A1 for immunoblotting (IB) [12, 47]. In addition, the following mouse (m)Abs against: αv, L230; β1, TS2/16; β3, AP3, all from Life Technologies; β1, C-18 and Smad4 from Santa Cruz Biotechnology were used.
Non-immune mouse and rabbit IgG (from Sigma-Aldrich) were used as negative controls.
Cells and Culture Conditions
PC3 cells were from ATCC. Two PC3 sublines: PC3-high and PC3-zero, previously designated PC3-H and PC3-L respectively [48], were used: PC3-high (PC3-H) which are positive for β6 expression and PC3-zero (PC3-L) which are negative for β6 expression; these cells were cultured in RPMI medium containing 10% FBS, 2 mmol/L glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin. Another prostate cancer cell line, RWPE (from ATCC), was maintained in Defined Keratinocyte Serum Free Medium (K-SFM) (from Invitrogen) containing Defined K-SFM growth supplement. Cells were starved in serum-free culture medium for 24 h before being incubated with TGFβ1 (10, 20, 40 ng/ml) [49] for 48 h. After TGFβ1 stimulation, cells were analyzed by IB, zymography or quantitative real-time PCR (qRT-PCR). BPH1 cells were provided by Dr. Simon W. Hayward (Vanderbilt University, TN) and authenticated as previously described [50]. These cells were maintained in RPMI supplemented with 5% FBS, 2 mmol/L glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin and the serum-free culture medium was used as a control for MMP activity by zymography [51].
Viral Constructs and Cell Transfection
PC3-zero cells were stably transfected using human β6 integrin cDNA subcloned in pBABE retroviral vector as described before [52]. The β6 integrin cDNA was generously provided by Dr. Ian Hart, Cancer Research UK Clinical Centre [53]. Human β5 integrin cDNA in pBluescript ATCC (NO. 95496) was subcloned into pBABE retroviral vector by EcoRI sites. The pBABE-β5 construct was confirmed by DNA sequencing. PC3-zero cells were stably transfected using human β6 or β5 integrin cDNA in pBABE retroviral vector, selected and maintained in culture medium containing 10% FBS and 0.5 μg/ml puromycin.
PC3-zero cells expressing chimeric β6/β3 or β3/β6 integrin were generated in our laboratory by subcloning in pcDNA-3 (pcDNA) mammalian expression vector (Invitrogen). Detailed construct information is included in the section of construction of integrin chimeras. PC3-zero cells expressing pcDNA vector were also generated. All these transfectants were selected and maintained in culture medium containing 10% FBS and 100 μg/ml G418. αvβ6 -PC3-zero cells expressing either a Ctrl.-shRNA or the MMP2-shRNA were generated in our laboratory as previously published [52].
All lentiviral constructs were obtained from Open Biosystems. PC3-high cells were stably transfected with lentivirus pLKO.1 β5-shRNA (Number RHS3979-9624928) or lentivirus pLKO.1 β6-shRNA (Number RHS3979-9624888). Stable transfectants of PC3-high and PC3-zero cells were screened using puromycin and maintained in culture medium containing 10% FBS and 0.5 μg/ml puromycin. Two different clones for each stable transfectants were used for each experiment.
Cells were transiently transfected using oligofectamine with 100 nM of the following siRNAs against: Smad2 (Dharmacon) [5′-GUCCCAUGAAAAGACUUAA(TT)-3′], Smad3 (Dharmacon) [5′-AAUGGUGCGAGAAGGCGGUCAdTdT-3′], Smad4 (Dharmacon) [5′-GUGUGCAGUUGGAAUGUAAUU-3′(UU overhang)], β1C (Dharmacon) [5′-CCUCUGACUUCCAGAUUCC-3′], a mixture of two different siRNAs against TβRII (Dharmacon) [5′-CAACAACGGUGCAGUCAAG-3′ and 5′-GACGAGAACAUAACACUAG-3′], β6 integrin (siRNA duplex, D2, IDT Inc.) [(sense) 5′-ACCACGGGAACGGCUCUUUCCAGTG-3′ and (antisense) 5′-CACUGGAAAGAGCCGUUCCCGUGGUGA-3′]. After two rounds of transfection with siRNA, cells were serum starved for 24 h and stimulated with 20 ng/ml TGFβ1 for 48 h. Cells were then analyzed for β6 integrin protein levels and MMP2 protein or mRNA levels.
Construction of Integrin Chimeras
Two chimeric integrins were expressed in PC3-zero cells: one referred to as β3/β6, contains the β3 extracellular and transmembrane domains (residues 1 – 741 [54]) and a β6 cytoplasmic tail (corresponding to residues 731 to 788 of β6); another, called β6/β3, consists of β6 extracellular and transmembrane domains (residues 1 – 730 [55]) and a β3 cytoplasmic tail (corresponding to residues 742 to 788 of β3). The amino acids spanning the putative transmembrane domain/cytoplasmic domain transitions are identical: IWKLL, corresponding to residues 740 - 744 in β3 and 729 - 733 in β6. The putative transmembrane domain ends at the residue W 741 in β3, 730 in β6, and the cytoplasmic domain starts at the residue K 742 in β3, 731 in β6. Therefore the sequences surrounding the junctions in the newly generated constructs are: β3/β6 integrin chimera …AALLIWKLLVSFH… and β6/β3 integrin chimera … VLLCIWKLLITIH….
β3/β6 integrin chimera: A recombinant cDNA encoding the β3/β6 chimera was constructed in our laboratory by directional ligation of cDNA and PCR-generated fragments. Identical sequences of β3 and β6 at the transmembrane domain - cytoplasmic domain interface allowed for the generation of cohesive termini between β3 and β6 fragments at this juncture. This was effected by incorporating a restriction enzyme site (Bsa I) that cuts at a distance away from its recognition sequence (GGTCTCN′NNNN′) into oligos used in PCR; the cohesive termini are generated upon digestion with BsaI. Two PCR products, one containing the transmembrane domain of β3 integrin (fragment 1) and one containing the cytoplasmic domain of β6 (fragment 2), were generated by using the following oligos and templates: fragment 1. (β3 forward, nucleotide (nt) 2001-2022) GTGACGAGATTGAGTCAGTGAA and (β3 reverse, nt 2218-2239, containing the Bsa-restriction enzyme site) GGTCTCCCAGATGAGCAGGGCGGCAAGG using β3 cDNA in pRc/CMV as template; fragment 2. (β6 forward nt 2410-2431, containing the Bsa-restriction enzyme site) GGTCTCATCTGGAAGCTACTGGTGTCA and (SP6 in vector) ATTTAGGTGACACTATAG using β6 in pcDNA-3 as template. The following gel-purified (QIAEX II) fragments (cDNAs or fragments) were assembled in a ligation reaction with pcDNA-3 digested with EcoRI and XbaI to create a recombinant cDNA encoding the β3/β6 chimera: β3 cDNA digested with EcoRI and AflII + fragment 1 digested with AfllI and BsaI + fragment 2 digested with BsaI and XbaI.
β6/β3 integrin chimera: Similarly, a recombinant cDNA encoding the β6/β3 chimera was constructed in our laboratory by directional ligation of cDNA and PCR-generated fragments. The two PCR products, one containing the transmembrane domain of β6 integrin (fragment 3) and one containing the cytoplasmic domain of β3 (fragment 4), were generated by using the following oligos and templates: fragment 3. (β6 forward nt 2060-2081) CCAACCTGTGAACGATGTCCTA and (β6 reverse nt 2395-2416, containing the Bsa-restriction enzyme site) GGTCTCCCAGATGCACAGTAGGACAACC using β6 in pcDNA-3 as template; fragment 4. (β3 forward nt 2231-2253, containing the Bsa-restriction enzyme site) GGTCTCATCTGGAAACTCCTCATCAC and (SP6 in vector) ATTTAGGTGACACTATAG using β3 cDNA in pRc/CMV as template. The following gel-purified (QIAEX II) fragments were assembled in a ligation reaction with pcDNA-3 digested with EcoRI and XbaI to generate a recombinant cDNA encoding the β6/β3 chimera: β6 cDNA digested with EcoRI and BstEII + fragment 3 digested with BstEII and BsaI + fragment 4 digested with BsaI and XbaI. The absence of mutations in all PCR-amplified fragments was verified by sequencing.
Flow Cytometric analysis
FACS analysis was performed to determine integrin expression using mAbs: TS2/16 to β1 or 10D5 to β6-Non-immune mouse IgG was used as negative control.
Immunoblotting and Immunoprecipitation
PC3 or RWPE cells were resting or stimulated with 20 ng/ml TGFβ1 for 48 h. Cell lysates were prepared, separated by SDS-PAGE and analyzed by IB as described before [56].
IP experiments were performed as described before [56] with the following modification: cells were lysed in the following lysis buffer [20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 1% NP-40, 1 mM Na3VO4, 1 mM PMSF, 10 μg/ml Aprotinin, 10 μg/ml Leupeptin, 4 μg/ml Pepstatin and 1 μM Calpain inhibitor] and pre-clearing was performed by two consecutive incubations with protein G-Sepharose at 4°C for 45 min. Binding to the specific Abs was achieved by incubation at 4°C overnight, followed by incubation with protein G-Sepharose for 2 h at 4°C. After five washes with lysis buffer, immunocomplexes were eluted and separated under reducing or non-reducing condition (reducing for β3, β5 or TβRII and non-reducing for β6). The immunocomplexes were separated by SDS-PAGE and analyzed by IB. In order to detect the association between TβRII and β6, either β6 or TβRII was immunoprecipitated from the cell lysates using 10D5 (this mAb reacts with mouse, rat or human β6) to β6 and sc-400 (designated as 1, a rabbit polyclonal affinity purified Ab raised against a peptide mapping within a cytoplasmic domain of TβRII of human origin; reacts with mouse, rat or human TβRII) or sc-1700 (designated as 2, a rabbit polyclonal Ab raised against amino acids 1-567 representing full length TβRII of human origin; reacts with mouse, rat or human TβRII) to TβRII. To perform the IP experiment with other integrin subunits, β3 or β5 was immunoprecipitated from the cell lysate using mAb AP3 to β3 or a rabbit Ab against the cytoplasmic domain of β5.
Gelatin Zymography
Serum-free culture media collected from PC3-high, PC3-zero cells and their stable transfectants, were concentrated using Centricon filters (Millipore, MA) by centrifuging at 3000 rpm for 30 min at 4°C. Gel loading buffer [0.5 M Tris–HCl, pH 6.8, 10% SDS, 50% glycerol and 0.1% bromophenol blue] was added to 7 μg (PC3-high) or 10 μg (PC3-zero) of concentrated culture supernatant. Proteins were loaded and electrophoresis was performed using SDS-PAGE (8%) containing 0.1% gelatin (Sigma-Aldrich) at 12 mA for 2 h at room temperature. After electrophoresis, the gels were incubated in 2.5% Triton X-100 solution for 1 h at room temperature and then in substrate buffer containing 50 mM Tris–HCl, pH 7.4, 10 mM CaCl2 for 24 h at 37°C. The gels were stained with Coomassie brilliant blue, and destained in methanol: acetic acid: water (50: 10: 40) [57].
Quantitative Real-Time PCR analysis
qRT-PCR was performed as described before using Parental, shβ5- and shβ6-PC3-high cells [58]. Before RNA isolation and PCR analysis, cells were treated as follows: Parental, shβ5- and shβ6-PC3-high were stimulated with TGFβ1 or resting for 48 h. PC3-high cells were transiently transfected with β1C, Smad2, Smad3, Smad4 siRNA or not transfected before being stimulated with or without TGFβ1 for 48 h. PC3-high and αvβ6-PC3-zero cells were pre-treated with 3, 10 or 30 μM SIS3 for 1 hr and then stimulated with TGFβ1 or unstimulated. The oligo sequences used are as follows: MMP2 (Forward) 5′-GCAACCCAGATGTGGCCAAC-3′, (Reverse) 5′-CGCTCCAGACTTGGAAGGCA-3′; and MMP9 (Forward) 5′-ATAGACTACTACAGGCT-3′, (Reverse) 5′-TAGCACGGATAGACCA-3′; GAPDH (Forward) 5′-GGGAAGGTGAAGGTCGGAGT-3′, (Reverse) 5′-GTTCTCAGCCTTGACGGTGC-3′.
Statistical analysis
Statistical significance between datasets was calculated using t-test and all graphs were generated using Microsoft Excel. The error bars were calculated and represented in terms of mean ± SD. A two-sided P-value of less than 0.05 was considered statistically significant.
Migration assay
Transwell chambers (12 μm pore diameter, Costar) were coated with BSA (1%), type I Collagen (50 μg/ml) or LAP-TGFβ1 (10 μg/ml) overnight at 4°C and migration assay were performed as described previously [59]. Briefly, cells were stimulated with TGFβ1 for 48 hr; after detachment and trypsin inactivation, the cells were seeded (300,000 cells/well) on transwell chambers at 37°C for 6 h in presence or absence of TGFβ1. After fixation with 3.7% paraformaldehyde (PFA), cells attached on both layers of the porous filter were stained with DAPI (1 μg/ml) and pictures of nuclei were acquired by fluorescence microscopy (Olympus IX71 or Nikon Eclipse TS-100 inverted microscopes equipped with fluorescence unit). Then, cells on the top layer were removed using a cotton swab, and pictures of nuclei from cells migrated to the bottom layer were acquired. Five and twenty random fields were acquired for quantification of attached and migrated cells, respectively. Cell Profiler [60] software (www.cellprofiler.org) was then used for quantification of nuclei number, using 10–30 pixel units as range for discrimination between single nuclei and potential aggregates. The ratio between number of cells migrated onto the bottom layer and total (top + bottom) number of cells attached on the filter was calculated for each group of transfectants. Chi-Square test was used for statistical analysis.
RESULTS
αvβ6 is Required for TGFβ1 Upregulation of MMP2
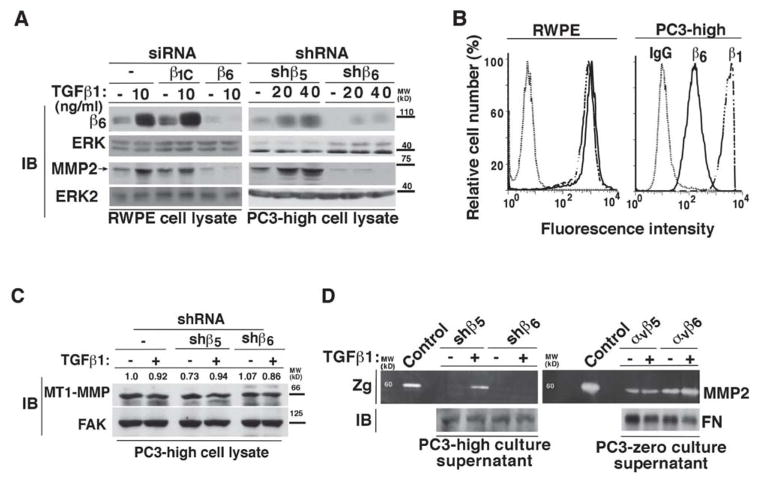
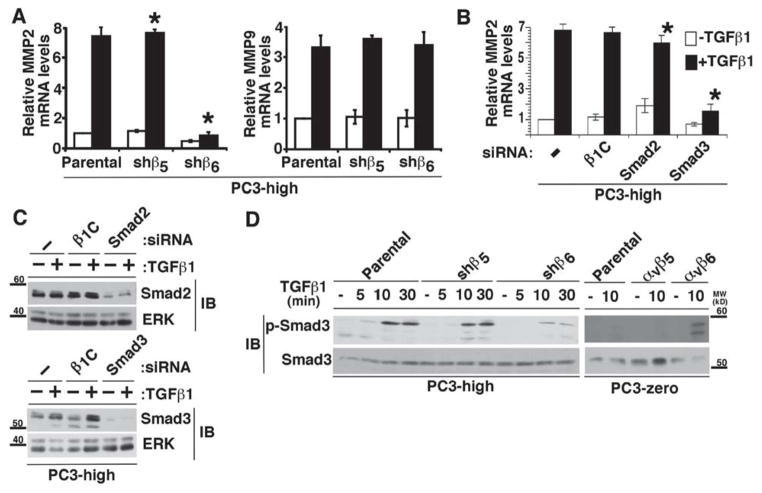
Given the known ability of TGFβ1 to upregulate αvβ6 [28] and MMP2 [31, 32], we first asked whether αvβ6 is a downstream effector of TGFβ1 which mediates MMP2 induction (Figure 1 and Figure 2). For this study, we used two PrCa PC3 cell sublines, PC3-high (αvβ6+) or PC3-zero (αvβ6−) (described in the Material and Methods section) and another PrCa cell line, RWPE, which expresses high levels of αvβ6. In RWPE cells, TGFβ1 induces the expression of β6 and MMP2 (Figure 1A, left panels). However, TGFβ1 stimulation fails to significantly increase β6 and consequently MMP2 expression in β6-silenced RWPE cells as compared to non-transfected or cells transfected with a non silencing (β1C) siRNA (Figure 1A, left panels). Similarly, in RWPE cells, PC3-high cells stably transfected with β5-shRNA (shβ5) show increased levels of β6 upon TGFβ1 stimulation. Densitometric analysis shows a 6.4 or 9.3 fold increase of β6 expression in shβ5-PC3-high cells upon 20 or 40 ng/ml TGFβ1 stimulation respectively, as compared to the unstimulated shβ5-PC3-high cells (Figure 1A, right panels). However, downregulation of β6 by shRNA (shβ6) in PC3-high fails to induce MMP2 expression upon TGFβ1 stimulation (Figure 1A, right panels). Our FACS analysis confirms high levels of surface expression of αvβ6 integrin in RWPE and PC3-high cells (Figure 1B). This αvβ6-dependent TGFβ1 regulation is specific for MMP2 as another MMP family member, MT1-MMP, known to be upregulated by TGFβ1 in several cell types [61–63], is not affected upon abrogation of β6 in PC3-high cells in presence or absence of TGFβ1 (Figure 1C). Densitometric analysis confirms that no significant changes occur in MT1-MMP expression upon these treatments. In parallel, we observed that TGFβ1 induces MMP2 activity in shβ5-PC3-high cells as evaluated in culture supernatants by gelatin zymography, but it fails to induce MMP2 activity in shβ6-PC3-high cells (Figure 1D). Similarly, MMP2 activity is induced by TGFβ1 in αvβ6 expressing PC3-zero transfectants, but not in αvβ5 expressing PC3-zero cells (Figure 1D).
Figure 1. αvβ6 is required for TGFβ1 induction of MMP2.
(A) RWPE cells (left panels) transiently transfected with 100 nM β1C siRNA, β6 siRNA or non- transfected, were serum starved for 24 h, before being stimulated with 10 ng/ml TGFβ1 for 48 h. Cell lysates were analyzed by IB and probed with 2A1 Ab to β6 or an Ab to MMP2. ERK was used as a loading control. PC3-high cells (right panels) stably transfected with β5-shRNA (shβ5) or β6-shRNA (shβ6) were serum starved for 24 h; then stimulated with 20 or 40 ng/ml TGFβ1 for 48 h. Cell lysates were analyzed by 10% SDS-PAGE, under non-reducing conditions and probed with 2A1 Ab to β6, or under reducing conditions and probed with an Ab to MMP2. ERK was used as a loading control. (B) FACS analysis of β6 (solid line) in RWPE and PC3-high cells is shown. β1 (broken line) was used as positive control for each cell line. Dotted-lines represent staining with an isotype negative control Ab. (C) PC3-high cells, stably transfected with β5- shRNA (shβ5) or β6-shRNA (shβ6) were serum starved for 24 h; then stimulated with 20 ng/ml TGFβ1 for 48 h. Cell lysates were analyzed by 10% SDS-PAGE, under reducing conditions and probed with MT1-MMP Ab. FAK was used as a loading control. (D) MMP2 activity in culture supernatants of shβ5- or shβ6-PC3-high cells (left panels) and αvβ5- or αvβ6-PC3-zero (right panels) cells was analyzed by gelatin zymography (Zg). Cells were either stimulated by TGFβ1 (20 ng/ml) or left resting for 48 h. Serum-free culture medium from BPH1 cells was used as a control for MMP activity. Fibronectin (FN) protein levels, analyzed by IB, were used as a loading control.
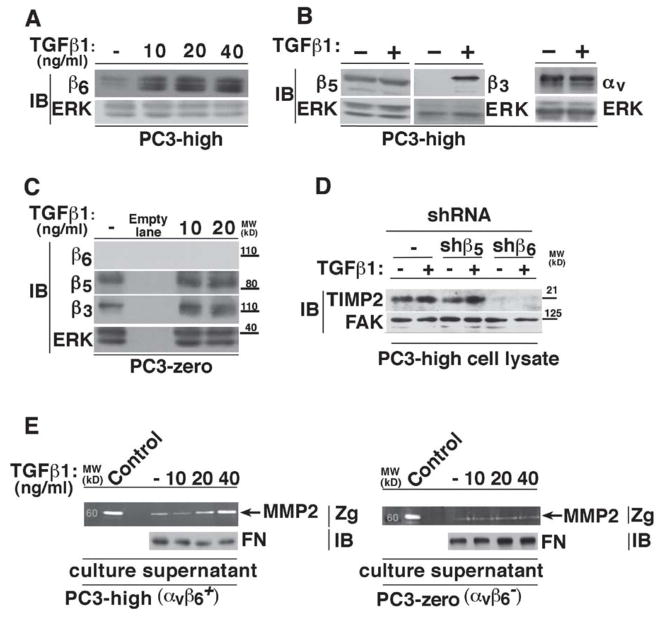
Figure 2. TGFβ1 upregulates MMP2 in αvβ6+ - cells.
(A-B) PC3-high cells were serum starved for 24 h; then stimulated with increasing concentrations of TGFβ1 for 48 h. PC3-high cell lysates were analyzed by IB and probed with 2A1 Ab to β6 (10% SDS-PAGE under non-reducing conditions) (A) or with an Ab to β5 (left panel; 10% SDS-PAGE under reducing conditions), to β3 (middle panel; 10% SDS-PAGE under reducing conditions) or to αv (right panel; 7.5% SDS-PAGE under non-reducing conditions) (B). ERK was used as a loading control. (C) Cell lysates from 10, 20 ng/ml TGFβ1 (48 h) stimulated or unstimulated PC3-zero cells were analyzed by 10% SDS-PAGE and probed with 2A1 Ab to β6 (under non-reducing conditions), an Ab to β5 or to β3 (under reducing conditions). ERK was used as loading control. (D) PC3-high cells stably transfected with β5-shRNA (shβ5) or β6-shRNA (shβ6) were serum starved for 24 h; then stimulated with 20 ng/ml TGFβ1 for 48 h. Cell lysates were analyzed by 10% SDS-PAGE, under reducing conditions and probed with TIMP2 Ab. FAK was used as a loading control. (E) PC3-high (αvβ6+; left panel) and PC3-zero (αvβ6-; right panel) cells were serum starved for 24 h; then stimulated with 10, 20, 40 ng/ml TGFβ1 for 48 h or unstimulated (-). MMP2 activity in serum-free culture supernatants was analyzed by gelatin zymography (Zg). Serum-free culture medium from BPH1 cells was used as a control for MMP activity. Fibronectin (FN) protein levels, analyzed by IB, were used as loading controls.
As expected [28], we observed that TGFβ1 stimulation of PC3-high cells specifically increases β6 and β3 protein levels without affecting αv or β5 integrin subunits (Figure 2A and 2B). However, the cells that lack αvβ6 expression (PC3-zero), do not show a similar response as TGFβ1 stimulation of PC3-zero cells specifically increases β3, but not β5 or β6 (Figure 2C). Since TGFβ1 is known to upregulate TIMP2 in gastric cancer [64], we investigated whether αvβ6 is required for TIMP2 regulation by TGFβ1 in PrCa. We observe that TIMP2 is increased upon TGFβ1 treatment in PC3-high cells and that abrogation of β6 by shRNA (shβ6) in these cells drastically downregulates the expression of TIMP2 under basal and induced conditions (Figure 2D). Furthermore, our data also show that TGFβ1 promotes MMP2 expression and consequently activity only in αvβ6 –expressing PC3-high cells (Figure 2E and not shown), but fails to show a similar response in αvβ6− PC3-zero cells (Figure 2E and not shown). Altogether, these data show that αvβ6 is required for TGFβ1-mediated increase in MMP2 expression and, consequently, activity.
αvβ6 Integrin Associates with TβRII
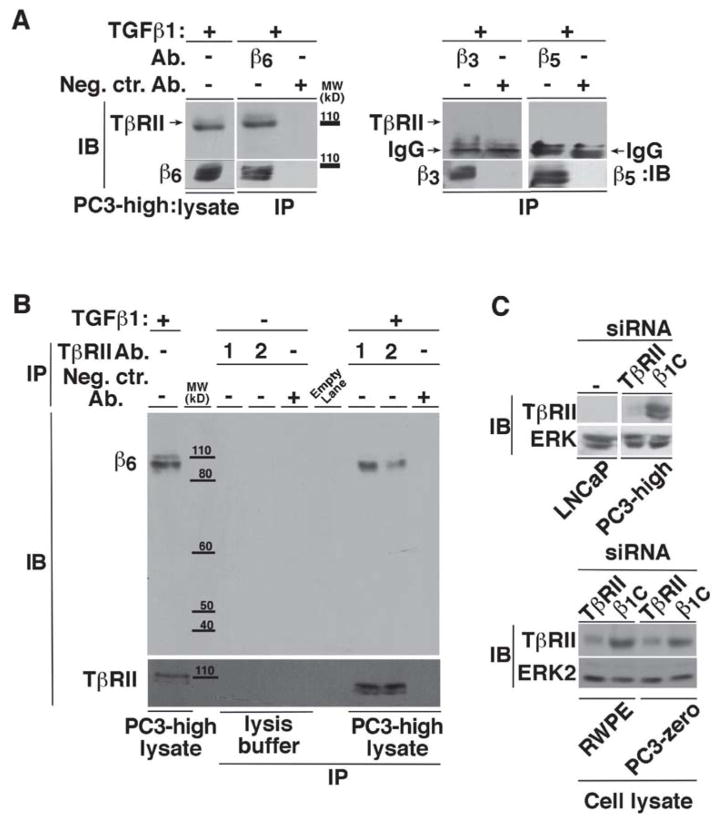
Given previous studies that have demonstrated that TβRII associates with integrins [65], we tested whether TβRII associates with αvβ6. TβRII co-immunoprecipitates with αvβ6 but not with other integrins such as αvβ3 or αvβ5 in PC3-high cells (Figure 3A); a reverse co-IP experiment was also performed to confirm this association (Figure 3B). Additional immunoblotting analysis to confirm the specificity of the Ab to TβRII was also performed using a mixture of two different TβRII siRNAs in PC3-high, PC3-zero and RWPE cells (Figure 3C).
Figure 3. αvβ6 specifically interacts with TβRII.
(A) αvβ6, αvβ3 or αvβ5 integrins were immunoprecipitated from 20 ng/ml TGFβ1 stimulated (48 h) PC3-high cell lysates and the immunoprecipitates were analyzed by 10% SDS-PAGE (under non-reducing conditions, left panels; or under reducing conditions, right panels) in order to detect TβRII. Mouse or rabbit IgG was used as a negative control (Neg.Ctr.) Ab. β6 (left panel), integrin expression was analyzed in the immunoprecipitates by IB using 2A1 Ab to β6. In the right panels an Ab to β3 or β5 was used. αvβ6 and TβRII expression was also detected in the cell lysate (left panel). (B) TβRII was immunoprecipitated from TGFβ1 stimulated PC3-high cell lysates using two different Abs against TβRII (1 and 2) and analyzed by 10% SDS-PAGE, under non-reducing conditions followed by IB to detect β6. (C) PC3-high (top panels), RWPE and PC3-zero (bottom panels) cells were transiently transfected with β1C or TβRII siRNA and the cell lysates were analyzed by 10% SDS-PAGE under non-reducing conditions and probed with rabbit Ab against TβRII. LNCaP cell lysate was used as a negative control. ERK was used as a loading control.
Association of αvβ6 Integrin with TβRII Induces MMP2 Expression
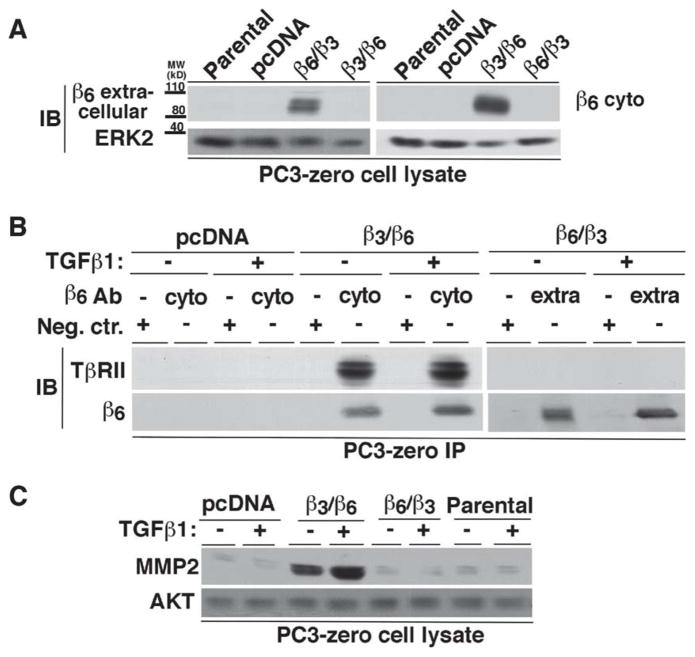
To confirm whether the association of αvβ6 with TβRII causes MMP2 expression, we transfected either a cDNA chimeric variant of the β6 and β3 integrin subunits containing the β3 extracellular domain and β6 cytoplasmic domain (β3/β6) or a chimeric form containing the β6 extracellular domain and β3 cytoplasmic domain (β6/β3) in PC3-zero cells (Figure 4A). IP using an Ab specific for the β6 cytoplasmic domain shows association of TβRII with β3/β6 in PC3-zero cells, either in the absence or presence of TGFβ1 (Figure 4B, left panels). However, PC3-zero cells expressing β6/β3 do not show any association with TβRII when immunoprecipitated using an Ab directed against the extracellular domain of β6 (Figure 4B, right panels). We next stimulated β3/β6-PC3-zero and β6/β3-PC3-zero cells with TGFβ1 and analyzed MMP2 expression. The results show MMP2 induction only in β3/β6-PC3-zero cells as compared to Parental, control cells transfected with a vector alone (pcDNA) or β6/β3-PC3-zero cells (Figure 4C) in the absence of TGFβ1. In addition, TGFβ1 stimulation to β6/β3-PC3-zero cells further increases MMP2 expression, but it fails to show a similar response in Parental, pcDNA or β6/β3-PC3-zero cells (Figure 4C). These results confirm that TβRII associates with the cytoplasmic domain of β6 and the interaction between β6 cytoplasmic domain and TβRII promotes TGFβ1-mediated induction of MMP2.
Figure 4. Association of TβRII with β6 through the β6 cytoplasmic domain induces MMP2.
(A) PC3-zero cells were transfected as follows: pcDNA, β6 extracellular and β3 cytoplasmic (β6/β3) or β3 extracellular and β6 cytoplasmic (β3/β6) chimera. Non-transfected PC3-zero cells are designated as Parental. Expression of either extracellular (left panel) or cytoplasmic (right panel) domain of β6 in Parental and PC3-zero transfectants was analyzed by IB. 2A1 Ab (10% SDS-PAGE under non-reducing conditions; left panel) was used to detect β6 extracellular domain or C-19 Ab (10% SDS-PAGE under reducing conditions; right panel) to detect β6 cytoplasmic domain. ERK was used as a loading control. (B) PC3-zero cells, transfected with pcDNA, β6/β3 integrin chimera or β3/β6 integrin chimera, were stimulated with 20 ng/ml TGFβ1 (48 h) or unstimulated. αvβ6 integrin was immunoprecipitated from lysates using either C-19 (which recognizes β6 cytoplasmic domain (cyto); left panel) or 10D5 (specific for β6 extracellular domain (extra); right panel) Ab. Goat IgG (left panel) or mouse IgG (right panel) was used as a negative control (Neg.Ctr.) Ab. The immunoprecipitates were analyzed by IB in order to detect TβRII. β6 integrin expression was also analyzed in the immunoprecipitates by using C-19 (10% SDS-PAGE under reducing conditions; left panel) or 2A1 (10% SDS-PAGE under non-reducing conditions; right panel) as described above. (C) PC3-zero cells were transfected with pcDNA, β6/β3 integrin chimera, β3/β6 integrin chimera or were non-transfected (Parental). Cells were serum starved for 24 h before being incubated with 20 ng/ml TGFβ1 for 48 h. Cell lysates were analyzed by 10% SDS-PAGE under reducing conditions and probed with an Ab to MMP2. AKT was used as a loading control.
Smad3 Mediates MMP2 Regulation by TGFβ1 in αvβ6–Expressing Cells
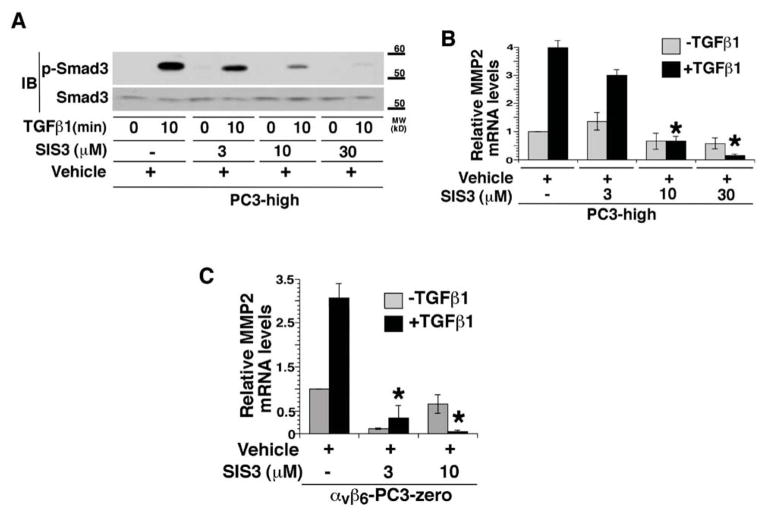
We performed a qRT-PCR analysis to investigate whether a TGFβ1 induction of MMP2 in Parental and shβ5-PC3-high cells occurs at the mRNA levels. ShRNA mediated silencing of β6 in PC3-high cells results in reduced MMP2 mRNA levels. On the other hand, MMP9 mRNA levels do not show any change upon β6 downregulation in PC3-high cells (Figure 5A). Studies by different groups have shown that MMP2 is a Smad3 target gene [39], and since Smad3 is known to promote PrCa progression [24], we investigated the role of Smad3 in inducing MMP2 in TGFβ1-stimulated PC3-high cells. Silencing of Smad3 (Figure 5B and 5C) and Smad4 (data not shown) in PC3-high cells reduces TGFβ1-induced MMP2 expression; however, downregulation of Smad2 by siRNA or transfection of a non-relevant siRNA does not show a similar effect (Figure 5B and 5C). In addition, the differences in MMP9 transcript levels in the presence or absence of Smad3 in PC3-high cells are not statistically significant (P>0.05; data not shown). When we tested the activation of Smad3 by αvβ6 in the presence of TGFβ1 for 5, 10 or 30 min, we observe that TGFβ1 induces robust phosphorylation of Smad3 in Parental and shβ5-PC3-high, but only a minor increase in shβ6-PC3-high cells (Figure 5D, left panel). In support of this result, we confirm that exogenous expression of αvβ6 in PC3-zero cells sustains phosphorylation of Smad3 (Figure 5D, right panel). Furthermore, to evaluate whether activation of Smad3 is required for TGFβ1 induction of MMP2, we pre-treated PC3-high (Figure 6A and 6B) and αvβ6 expressing PC3-zero (Figure 6C) cells with different concentrations of SIS3, which specifically blocks the phosphorylation of Smad3 [45]. TGFβ1 stimulation of these cells results into decreased phosphorylation of Smad3 as compared to vehicle treated cells (Figure 6A). As a consequence, TGFβ1 induction of MMP2 mRNA is reduced in SIS3 treated cells as compared to vehicle treated cells (Figure 6B and 6C). These data show that activation of Smad3, mediated by αvβ6, selectively regulates TGFβ1-induced MMP2 levels.
Figure 5. αvβ6 supports TGFβ1-induced Smad3 phosphorylation.
(A) MMP2 (left) and MMP9 (right) mRNA levels were analyzed by qRT-PCR in Parental, shβ5- and shβ6-PC3-high cells. These cells were serum starved for 24 h, followed by incubation with or without 20 ng/ml TGFβ1 for 48 h. MMP2 and MMP9 mRNA expression levels were normalized to GAPDH. *, P=0.003. (B–C) PC3-high cells were transiently transfected with the following siRNAs: 100 nM β1C, Smad2, Smad3 and starved for 24 h in serum-free medium before being incubated with 20 ng/ml TGFβ1 for 48 h. Cells were analyzed for MMP2 mRNA levels using total RNA by qRT-PCR; expression levels were normalized to GAPDH (B). *, P=0.014. Cells were also lysed (C) and analyzed by 10% SDS-PAGE under reducing conditions, followed by IB using an Ab to Smad2 or Smad3. ERK was used as loading control. (D) shβ5-, shβ6- and Parental PC3-high cells (left panels) were serum starved for 24 h, incubated with 20 ng/ml TGFβ1 for 0, 5, 10 or 30 min. Similarly, αvβ5 or αvβ6-transfected and Parental PC3-zero cells (right panels) were also serum starved for 24 h before being incubated with 20 ng/ml TGFβ1 for 0 or 10 min. Proteins in cell lysates were separated by 10% SDS-PAGE under reducing conditions and probed with Abs to phospho- Smad3 or Smad3.
Figure 6. Smad3 phosphorylation is required for TGFβ1 induction of MMP2 mRNA.
(A–B) PC3- high cells were serum starved for 24 h, pre-treated with 3, 10, 30 μM SIS3 or same volume of DMSO (vehicle) for 1 h before being incubated with 20 ng/ml TGFβ1 for 0 or 10 min. Cell lysates were analyzed by 10% SDS-PAGE under reducing conditions and probed with phospho-Smad3 Ab. Smad3 was used as loading control (A). MMP2 mRNA levels were analyzed by qRT-PCR (B). MMP2 mRNA expression levels were normalized to GAPDH. *, P=0.00078. **, P=0.00079. (C) αvβ6 stably transfected PC3-zero cells (αvβ6-PC3-zero) were pre-treated with 10 μM SIS3 or the same volume of DMSO (vehicle) for 1 h and incubated with 20 ng/ml TGFβ1 for 0 and 10 min. MMP2 mRNA levels were analyzed by qRT-PCR (C). MMP2 mRNA expression levels were normalized to GAPDH. *, P=0.0087. **, P=0.0079.
MMP2 mediates PrCa cell migration supported by αvβ6 integrin
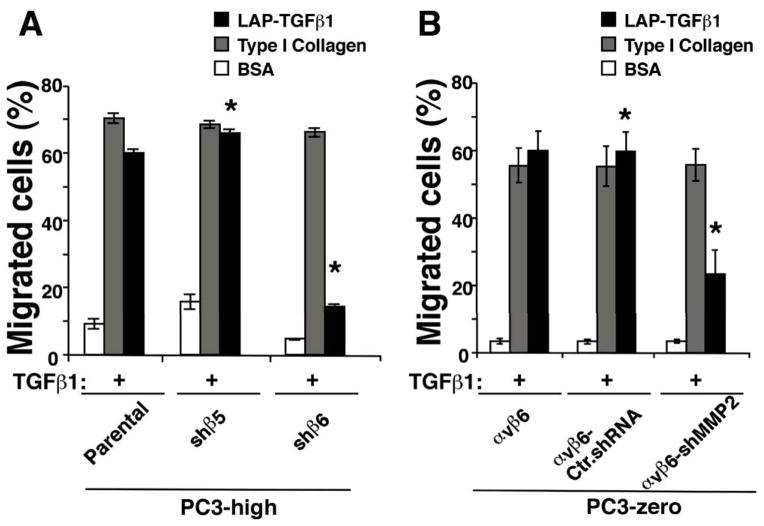
Given our previous observations showing that MMP2 mediates αvβ6 downstream signaling in bone metastasis in vivo [52], here we investigated the contribution of αvβ6-dependent MMP2 on cell migration upon TGFβ1 stimulation of PrCa cells on an αvβ6 specific ligand LAP-TGFβ1[66]. TGFβ1 stimulation of Parental or shβ5-PC3-high cells enhances migration on LAP-TGFβ1, whereas TGFβ1 stimulation of shβ6-PC3-high cells has a minimal effect on cell migration on this ligand. On the other hand, migration of Parental, shβ6-PC3-high cells and shβ5-PC3-high cells on type I collagen is comparable (Figure 7A). On the basis of these results, we investigated whether downregulation of MMP2 in αvβ6 expressing cells contributes to this phenotype. We observe that TGFβ1 stimulation of αvβ6-PC3-zero or αvβ6-Ctr.shRNA-PC3-zero enhances migration on LAP-TGFβ1, whereas TGFβ1 stimulation of αvβ6-shMMP2-PC3-zero cells has a reduced effect on cell migration on this ligand. On the other hand, αvβ6-PC3-zero, αvβ6-Ctr.shRNA-PC3-zero and αvβ6-shMMP2-PC3-zero cells migrate equally well on type I Collagen (Figure 7B). Overall, our data indicate that MMP2 promotes TGFβ1-dependent PrCa cell migration in αvβ6-expressing PC3 cells.
Figure 7. MMP2 promotes cell migration in αvβ6-expressing cells.
Migration assays were performed using TGFβ1 pre-stimulated cells seeded on BSA, type I Collagen or LAP-TGFβ1-coated transwell chambers. Cells were allowed to migrate on different matrix ligands for 6 hr in the presence of TGFβ1. The differences in cell migration between shβ5- and shβ6-PC3-high cells (A) as well as between αvβ6-Ctr.shRNA-PC3-zero and αvβ6-shMMP2-PC3-zero (B) on LAP-TGFβ1 are statistically significant. *, P=0.004 and **, P=0.002.
αvβ6 Association with TβRII Increases MMP2 Levels in a Smad3-dependent Manner
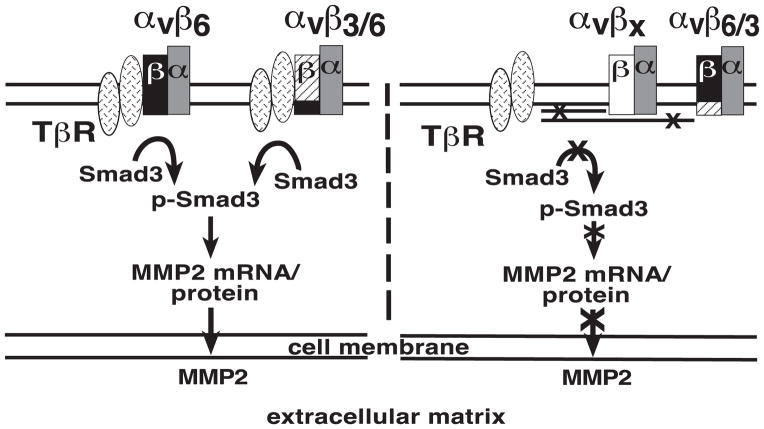
Our working model shows that the αvβ6 integrin interacts with TβRII and promotes phosphorylation of Smad3. As a result, tumor cells produce MMP2, which is released in the ECM. On the other hand, either αvβ6/3 or other αv- integrins (αvβx) fail to associate with TβR and to phosphorylate Smad3, thus preventing TGFβ1 ability to induce MMP2 (Figure 8).
Figure 8. αvβ6 increases MMP2 levels through Smad3 activation.
Our model shows that the αvβ6 integrin, through its cytoplasmic domain, interacts with TβR, initiates the Smad3-mediated downstream signaling cascade. As a consequence, tumor cells produce MMP2, which is released in the extracellular matrix (left). On the other hand, other related αv-containing integrins, αvβx, as well as the αvβ6/3 integrin, which contains a chimeric form of β6 with a β3 cytoplasmic domain, fail to show similar responses due to the lack of association with TβRII (right).
DISCUSSION
In this study, we show that the αvβ6 integrin is required for TGFβ1 signaling. We demonstrate that αvβ6 associates with TβRII and is required for TGFβ1-stimulated upregulation of MMP2, through Smad3 activation, and consequent MMP2-dependent cell migration.
The ability of αvβ6 to associate with TβRII and consequently activate the TGFβ1 pathway is novel. Although a direct association between αvβ6 and TβRII is not confirmed by our findings, specific molecular requirements appear to be necessary. We present evidence that the β6 cytoplasmic domain mediates this association and provides a high degree of specificity to the system since it cannot be replaced by the β3 cytoplasmic domain. The functional implication of this interaction mediated by the β6 cytoplasmic domain is that MMP2 upregulation is observed only if this domain is expressed and does not require ligand binding to αvβ6. Specifically, increased MMP2 levels are evident as shown by using a chimeric integrin in our assays, only if an association of an integrin containing the β6 cytoplasmic domain and TβRII occurs. Thus, minute changes in cellular integrin repertoires, such as downregulation of αvβ6 or expression of αvβ3 or αvβ5, which fail to cause the described signaling cascade, may cause pathological events where TGFβ1 signaling becomes aberrant. In this context, it is worth stressing that this mechanism appears to occur in the absence of the specific cytokine-ligand; αvβ6-TβRII interaction is observed in either presence or absence of TGFβ1, as previously described for TβRII interaction with α5β1 or αvβ5 in normal cells [67, 68]. In contrast, TGFβ1 stimulation is needed for the association of αvβ3 integrin with TβRII in human normal lung fibroblasts [65].
αvβ6 activates Smads whereas an αvβ3-TβRII-mediated pathway activates p38 MAPK, thus suggesting that the difference in the status of TβRII upon association with two different integrins which share the same α partner, αv, may cause significant variations in the downstream signaling [69].
In our study, the relevance of αvβ6-TβRII association lies on the effect of TGFβ1 on MMP2 production and consequent MMP2-dependent cell migration. Our results highlight a specific function of αvβ6 integrin in upregulating MMPs, and for the first time the requirement of integrins in the TGFβ1/MMP pathway. Other studies have shown that in vitro integrins upregulate MMPs [7, 70] with apparent discrepancies attributed to differences in cancer cell types, and that stimulation of TGFβ1 induces secretion and activation of MMP2 [71, 72] as well as increased half-life of MMP2 mRNA [32]. However, a selective upregulation of MMP2 mediated by integrins upon TGFβ1 stimulation had not been previously shown. Noteworthy is the evidence that ex vivo primary cultures of breast cancer cells produce mature form of MMP9 when expressing activated αvβ3 integrin [73]. We conclude that the increased levels of MMP2 facilitate cell migration regulated by αvβ6 expressing cells and are likely to recapitulate previous effects observed in vivo where cancer cells were shown to cause osteolytic lesions [52] or metastasize to a higher extent when expressing αvβ6 [11]. We speculate that this pathway may be shared by other integrins, such as αvβ3, whose interaction with TβRII results in enhanced EMT, invasion [69] and proliferation [65] in a TGFβ1-dependent manner. Overall, these studies and our analysis suggest that MMP regulation by integrins is likely to be relevant to human cancer progression to a metastatic phenotype.
Our results show a direct correlation between MMP2 and TIMP2 expression which appears to be regulated by αvβ6 upon TGFβ1 stimulation. These data are in agreement with a previous study that showed MMP2 and TIMP2 co-expression in prostate adenocarcinoma [74], although increased TIMP2 expression is usually associated with decreased tumor growth, invasiveness and metastasis in PrCa [75]. A direct or inverse correlation between MMP2 and TIMP2 expression appears to be organ-site specific; indeed, the correlation has been shown to be direct and to predict poor prognosis in studies related to renal cell carcinomas and bladder cancer [76, 77], but to be inverse in endometrial carcinoma [78]. It should be stressed that MMP2 is known to be activated on the cell surface by forming a complex with TIMP2, which functions as inhibitor of MMPs but is also required for pro-MMP2 activation [79], and with MT1-MMP. We speculate that this migration promoting activity occurs through specific induction of MMP2 and TIMP2 without any change in MT1-MMP levels, as seen in Figure 1C. Moreover, MMP2 enzymatic activity is known to be controlled by its binding to the cell surface αvβ3 integrin [80]. This previous observation explains why this membrane-bound active MMP2 is detected in whole cell lysates as also described by another study showing its accumulation in the intracellular vesicles of endothelial cells [81].
The relevance of our results is also shown by the signaling pathway activated by αvβ6, which requires activation of Smad3 for the observed increase in MMP2 levels as evaluated using SIS3, an inhibitor of Smad3 phosphorylation [45]. Smad3 has been shown to be overexpressed in human PrCa, and may contribute to disease progression in humans [24]. It remains to be established whether another Smad, Smad4, which also contributes to TGFβ1 induction of MMP2 (data not shown), is also regulated by αvβ6. This signaling cascade appears consistent with a previously described pathway whereby a different αvβ3 integrin elevates levels of phosphorylated Smad5 in a Runx2-dependent manner in PrCa cells [82]. Overall, Gupta et al.’s study and our analysis allow us to conclude that differential integrin expression regulates specific downstream TGFβ signaling. In conclusion, in our model (Figure 8), we propose that expression of αvβ6 integrin by associating with TβRII promotes Smad3-mediated downstream signaling pathway and consequent upregulation of MMP2 and MMP2-dependent cell migration in response to TGFβ1.
Acknowledgments
We thank Drs. M. Trerotola and B. Zerlanko in Dr. Languino’s laboratory for constructive discussion. We also thank Drs. L.W. Chung for providing PC3-high cells; I. Hart for pBABE-β6 integrin construct; S.W. Hayward for providing the BPH cell line.
FUNDING
This work was supported by the following grants: NIH-R01 CA89720, CA109874 and PO1 CA140043-Project 2 (L.R. Languino). This project is also funded, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.); the Department specifically disclaims responsibility for any analyses, interpretations or conclusions. C. F. was supported in part by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship. Research in this publication includes work carried out using the Sidney Kimmel Cancer Center Cancer Genomics and Flow Cytometry Shared Resources, which is supported in part by NCI Cancer Center support grant P30CA056036. Shelia Violette is an employee and shareholder of Biogen, Cambridge, MA.
Footnotes
The abbreviations used are: ECM, Extracellular matrix; PrCa, Prostate cancer; TβR, TGFβ receptor; MMP, Matrix metalloproteinase; LAP, Latency associated peptide; type I Col, type I Collagen; SIS3, Specific inhibitor of Smad3; IB, Immunoblotting; IP, Immunoprecipitation; qRT-PCR, Quantitative Real Time PCR; Fn, Fibronectin; Zg, Zymography; TIMP2, Tissue Inhibitor of Metalloproteinase2; MT1-MMP, Membrane type-1 matrix metalloproteinase.
The other authors do not have any conflict of interests.
AUTHORS’ CONTRIBUTION
Conception and design: Anindita Dutta, Jing Li and L.R. Languino.
Performed experiments: Anindita Dutta, Jing Li, Carmine Fedele, Aejaz Sayeed and Thomas Manes (who generated chimeric integrins).
Acquisition of data: Anindita Dutta, Jing Li, Carmine Fedele, Aejaz Sayeed.
Analysis and interpretation of data: Anindita Dutta, Jing Li, Carmine Fedele, Aejaz Sayeed, Amrita Singh.
Writing, review, and/or revision of the manuscript: Anindita Dutta, Jing Li, Carmine Fedele, Aejaz Sayeed, Amrita Singh.
Administrative, technical, or material support: Shelia M. Violette for having provided antibodies to αvβ6 integrin.
Study supervision: LR. Languino.
References
- 1.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 2.Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, Otey CA, Zhau HE, Chung LW. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001;12:99–107. [PubMed] [Google Scholar]
- 3.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin J, Vinogradova O, Plow EF. Integrin bidirectional signaling: a molecular view. PLoS Biol. 2004;2:0726–0729. doi: 10.1371/journal.pbio.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay A, Raghavan S. Defining the role of integrin αvβ6 in cancer. Curr Drug Targets. 2009;10:645–652. doi: 10.2174/138945009788680374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok S, Baker MS. Overexpression of αvβ6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis. 2002;23:237–244. doi: 10.1093/carcin/23.2.237. [DOI] [PubMed] [Google Scholar]
- 8.Hecht JL, Dolinski BM, Gardner HA, Violette SM, Weinreb PH. Overexpression of the αvβ6 Integrin in Endometrial Cancer. Appl Immunohistochem Mol Morphol. 2008;16:543–547. doi: 10.1097/PAI.0b013e31816bc5ee. [DOI] [PubMed] [Google Scholar]
- 9.Azare J, Leslie K, Al-Ahmadie H, Gerald W, Weinreb PH, Violette SM, Bromberg J. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin αvβ6. Mol Cell Biol. 2007;27:4444–4453. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, Ellis IO, Green AR, Haider S, Brouilette K, Saha A, Vallath S, Bowen R, Chelala C, Eccles D, Tapper WJ, Thompson AM, Quinlan P, Jordan L, Gillett C, Brentnall A, Violette S, Weinreb PH, Kendrew J, Barry ST, Hart IR, Jones JL, Marshall JF. Therapeutic targeting of integrin alphavbeta6 in breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS, Chen R, Niu J. Integrin αvβ6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879–887. doi: 10.1111/j.1349-7006.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Aarsen LA, Leone DR, Ho S, Dolinski BM, McCoon PE, LePage DJ, Kelly R, Heaney G, Rayhorn P, Reid C, Simon KJ, Horan GS, Tao N, Gardner HA, Skelly MM, Gown AM, Thomas GJ, Weinreb PH, Fawell SE, Violette SM. Antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res. 2008;68:561–570. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- 13.Garlick DS, Li J, Sansoucy B, Wang T, Griffith L, FitzGerald TJ, Butterfield J, Charbonneau B, Violette SM, Weinreb PH, Ratliff TL, Liao C, Roy-Burman P, Vietri M, Lian J, Stein G, Altieri DC, Languino LR. αvβ6 integrin expression is induced in the POET and PTENpc−/− mouse models of prostatic inflammation and prostatic adenocarcinoma Am. J Transl Res. 2012;4:165–174. [PMC free article] [PubMed] [Google Scholar]
- 14.Akhurst RJ, Derynck R. TGF-β signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 15.Wakefield LM, Roberts AB. TGF-β signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 16.Padua D, Massague J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 17.Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 18.Luo K, Lodish HF. Positive and negative regulation of type II TGF-β receptor signal transduction by autophosphorylation on multiple serine residues. EMBO J. 1997;16:1970–1981. doi: 10.1093/emboj/16.8.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-β receptor system. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 20.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 21.Riggins GJ, Kinzler KW, Vogelstein B, Thiagalingam S. Frequency of Smad gene mutations in human cancers. Cancer Res. 1997;57:2578–2580. [PubMed] [Google Scholar]
- 22.Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- 23.Perttu MC, Martikainen PM, Huhtala HS, Blauer M, Tammela TL, Tuohimaa PJ, Syvala H. Altered levels of Smad2 and Smad4 are associated with human prostate carcinogenesis. Prostate Cancer Prostatic Dis. 2006;9:185–189. doi: 10.1038/sj.pcan.4500871. [DOI] [PubMed] [Google Scholar]
- 24.Lu S, Lee J, Revelo M, Wang X, Dong Z. Smad3 is overexpressed in advanced human prostate cancer and necessary for progressive growth of prostate cancer cells in nude mice. Clin Cancer Res. 2007;13:5692–5702. doi: 10.1158/1078-0432.CCR-07-1078. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr, Ross JS. Smad4 protein expression correlates with grade, stage, and DNA ploidy in prostatic adenocarcinomas. Hum Pathol. 2005;36:1204–1209. doi: 10.1016/j.humpath.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Horvath LG, Henshall SM, Kench JG, Turner JJ, Golovsky D, Brenner PC, O’Neill GF, Kooner R, Strieker PD, Grygiel JJ, Sutherland RL. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate. 2004;59:234–242. doi: 10.1002/pros.10361. [DOI] [PubMed] [Google Scholar]
- 27.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, Perry SR, Labrot ES, Wu X, Lis R, Hoshida Y, Hiller D, Hu B, Jiang S, Zheng H, Stegh AH, Scott KL, Signoretti S, Bardeesy N, Wang YA, Hill DE, Golub TR, Stampfer MJ, Wong WH, Loda M, Mucci L, Chin L, DePinho RA. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A, De Luca M. Transforming growth factor-β1 modulates β1 and β5 integrin receptors and induces the de novo expression of the αvβ6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol. 1995;129:853–865. doi: 10.1083/jcb.129.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokeshwar BL. MMP inhibition in prostate cancer. Ann NY Acad Sci. 1999;878:271–289. doi: 10.1111/j.1749-6632.1999.tb07690.x. [DOI] [PubMed] [Google Scholar]
- 30.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 31.Kwak HJ, Park MJ, Cho H, Park CM, Moon SI, Lee HC, Park IC, Kim MS, Rhee CH, Hong SI. Transforming growth factor-β 1 induces tissue inhibitor of metalloproteinase-1 expression via activation of extracellular signal-regulated kinase and Spl in human fibrosarcoma cells. Mol Cancer Res. 2006;4:209–220. doi: 10.1158/1541-7786.MCR-05-0140. [DOI] [PubMed] [Google Scholar]
- 32.Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by growth factor-β 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064–114071. [PubMed] [Google Scholar]
- 33.Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R, Cher ML. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94:17–25. doi: 10.1093/jnci/94.1.17. [DOI] [PubMed] [Google Scholar]
- 34.Deng X, He G, Levine A, Cao Y, Mullins C. Adenovirus-mediated expression of TIMP-1 and TIMP-2 in bone inhibits osteolytic degradation by human prostate cancer. Int J Cancer. 2008;122:209–218. doi: 10.1002/ijc.23053. [DOI] [PubMed] [Google Scholar]
- 35.Miralles F, Battelino T, Czernichow P, Scharfmann R. TGF-β plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol. 1998;143:827–836. doi: 10.1083/jcb.143.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sehgal I, Thompson TC. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-β1 in human prostate cancer cell lines. Mol Biol Cell. 1999;10:407–416. doi: 10.1091/mbc.10.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas GJ, Lewis MP, Hart IR, Marshall JF, Speight PM. αvβ6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer. 2001;92:641–650. doi: 10.1002/1097-0215(20010601)92:5<641::aid-ijc1243>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Huang X, Huang S, Zhang F, Han X, Miao L, Liu Z, Fan Z, Ji G. Lentiviral-mediated Smad4 RNAi promotes SMMC-7721 cell migration by regulation of MMP-2, VEGF and MAPK signaling. Mol Med Report. 2010;3:295–299. doi: 10.3892/mmr_00000254. [DOI] [PubMed] [Google Scholar]
- 39.Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fandrich F. Differential roles of Smad2 and Smad3 in the regulation of TGF-β 1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: control by Rac1. Mol Cancer. 2011;10:67. doi: 10.1186/1476-4598-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiercinska E, Naber HP, Pardali E, van der Pluijm G, van Dam H, ten Dijke P. The TGF-β/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res Treat. 2011;128:657–666. doi: 10.1007/s10549-010-1147-x. [DOI] [PubMed] [Google Scholar]
- 41.Lamar JM, Iyer V, DiPersio CM. Integrin α3β1 potentiates TGFβ-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol. 2008;128:575–586. doi: 10.1038/sj.jid.5701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer V, Pumiglia K, DiPersio CM. α3β1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci. 2005;118:1185–1195. doi: 10.1242/jcs.01708. [DOI] [PubMed] [Google Scholar]
- 43.Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998;58:1395–1399. [PubMed] [Google Scholar]
- 44.Trudel D, Fradet Y, Meyer F, Harel F, Tetu B. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 2003;63:8511–8515. [PubMed] [Google Scholar]
- 45.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 46.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 47.Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, Sheppard D, Violette SM. Function-blocking integrin αvβ6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- 48.Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–821. doi: 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheppard D, Cohen DS, Wang A, Busk M. Transforming growth factor β differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992;267:17409–17414. [PubMed] [Google Scholar]
- 50.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips JL, Hayward SW, Wang Y, Vasselli J, Pavlovich C, Padilla-Nash H, Pezullo JR, Ghadimi BM, Grossfeld GD, Rivera A, Linehan WM, Cunha GR, Ried T. The consequences of chromosomal aneuploidy on gene expression profiles in a cell line model for prostate carcinogenesis. Cancer Res. 2001;61:8143–8149. [PubMed] [Google Scholar]
- 52.Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T, Zerlanko BJ, FitzGerald TJ, Jiang Z, Birbe R, Wixted J, Violette SM, Stein JL, Stein GS, Lian JB, Languino LR. The αvβ6 integrin promotes an osteolytic program through upregulation of MMP2. Cancer Res. 2014;74:1598–1608. doi: 10.1158/0008-5472.CAN-13-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan MR, Thomas GJ, Russell A, Hart IR, Marshall JF. The integrin cytoplasmic-tail motif EKQKVDLSTDC is sufficient to promote tumor cell invasion mediated by matrix metalloproteinase (MMP)-2 or MMP-9. J Biol Chem. 2004;279:26533–26539. doi: 10.1074/jbc.M401736200. [DOI] [PubMed] [Google Scholar]
- 54.Zimrin AB, Eisman R, Vilaire G, Schwartz E, Bennett JS, Poncz M. Structure of platelet glycoprotein IIIa. A common subunit for two different membrane receptors. J Clin Invest. 1988;81:1470–1475. doi: 10.1172/JCI113478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheppard D, Rozzo C, Starr L, Quaranta V, Erie DJ, Pytela R. Complete amino acid sequence of a novel integrin β subunit (β6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem. 1990;265:11502–11507. [PubMed] [Google Scholar]
- 56.Goel HL, Fornaro M, Moro L, Teider N, Rhim JS, King M, Languino LR. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 58.Sayeed A, Alam N, Trerotola M, Languino LR. Insulin-like growth factor 1 stimulation of androgen receptor activity requires β1A integrins. J Cell Physiol. 2012;227:751–758. doi: 10.1002/jcp.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A, Languino LR. Trop-2 promotes cancer metastasis by modulating β1 integrin functions. Cancer Res. 2013;73:3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuo YC, Su CH, Liu CY, Chen TH, Chen CP, Wang HS. Transforming growth factor-beta induces CD44 cleavage that promotes migration of MDA-MB-435s cells through the up-regulation of membrane type 1-matrix metalloproteinase. Int J Cancer. 2009;124:2568–2576. doi: 10.1002/ijc.24263. [DOI] [PubMed] [Google Scholar]
- 62.Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ, Munshi HG. Collagen regulation of let-7 in pancreatic cancer involves TGF-beta1-mediated membrane type 1-matrix metalloproteinase expression. Oncogene. 2011;30:1002–1008. doi: 10.1038/onc.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quan J, Elhousiny M, Johnson NW, Gao J. Transforming growth factor-beta1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclasts. Clin Exp Metastasis. 2013;30:659–670. doi: 10.1007/s10585-013-9570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson E, Komuro A, Iwata C, Hagiwara A, Fuse Y, Watanabe A, Morishita Y, Aburatani H, Funa K, Kano MR, Miyazono K. Exogenous introduction of tissue inhibitor of metalloproteinase 2 reduces accelerated growth of TGF-beta-disrupted diffuse-type gastric carcinoma. Cancer Sci. 2010;101:2398–2403. doi: 10.1111/j.1349-7006.2010.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. αvβ3 Integrin interacts with the transforming growth factor β (TGFβ) type II receptor to potentiate the proliferative effects of TGFβ1 in living human lung fibroblasts. J Biol Chem. 2004;279:37726–37733. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- 66.Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 67.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galliher AJ, Schiemann WP. β3 integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGFβ1 type II receptor and regulates TGFβ1 stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 70.Niu J, Gu X, Turton J, Meldrum C, Howard EW, Agrez M. Integrin-mediated signalling of gelatinase B secretion in colon cancer cells. Biochem Biophys Res Commun. 1998;249:287–291. doi: 10.1006/bbrc.1998.9128. [DOI] [PubMed] [Google Scholar]
- 71.Huang X, Chen S, Xu L, Liu Y, Deb DK, Platanias LC, Bergan RC. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65:3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 72.Binker MG, Binker-Cosen AA, Gaisano HY, de Cosen RH, Cosen-Binker LI. TGF-β1 increases invasiveness of SW1990 cells through Rac1/ROS/NF-kappaB/IL-6/MMP-2. Biochem Biophys Res Commun. 2011;405:140–145. doi: 10.1016/j.bbrc.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin αvβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross JS, Kaur P, Sheehan CE, Fisher HA, Kaufman RA, Jr, Kallakury BV. Prognostic significance of matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod Pathol. 2003;16:198–205. doi: 10.1097/01.MP.0000056984.62360.6C. [DOI] [PubMed] [Google Scholar]
- 75.Rabbani SA, Harakidas P, Guo Y, Steinman D, Davidsen SK, Morgan DW. Synthetic inhibitor of matrix metalloproteases decreases tumor growth and metastases in a syngeneic model of rat prostate cancer in vivo. Int J Cancer. 2000;87:276–282. doi: 10.1002/1097-0215(20000715)87:2<276::aid-ijc20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 76.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–3119. [PubMed] [Google Scholar]
- 77.Kanayama H, Yokota K, Kurokawa Y, Murakami Y, Nishitani M, Kagawa S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer. 1998;82:1359–1366. [PubMed] [Google Scholar]
- 78.Honkavuori-Toivola M, Santala M, Soini Y, Turpeenniemi-Hujanen T, Talvensaari-Mattila A. Combination of strong MMP-2 and weak TIMP-2 immunostainings is a significant prognostic factor in endometrial carcinoma. Dis Markers. 2013;35:261–266. doi: 10.1155/2013/416870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta A, Cao W, Chellaiah MA. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-KB ligand signaling axis. Mol Cancer. 2012;11:66. doi: 10.1186/1476-4598-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]