Abstract
Postpartum mammary gland involution has been identified as tumor-promotional and is proposed to contribute to the increased rates of metastasis and poor survival observed in postpartum breast cancer patients. In rodent models, the involuting mammary gland microenvironment is sufficient to induce enhanced tumor cell growth, local invasion, and metastasis. Postpartum involution shares many attributes with wound healing, including upregulation of genes involved in immune responsiveness and infiltration of tissue by immune cells. In rodent models, treatment with non-steroidal anti-inflammatory drugs (NSAIDs) ameliorates the tumor-promotional effects of involution, consistent with the immune milieu of the involuting gland contributing to tumor promotion. Currently, immunotherapy is being investigated as a means of breast cancer treatment with the purpose of identifying ways to enhance anti-tumor immune responses. Here we review evidence for postpartum mammary gland involution being a uniquely defined ‘hot-spot’ of pro-tumorigenic immune cell infiltration, and propose that immunotherapy should be explored for prevention and treatment of breast cancers that arise in this environment.
Keywords: Macrophages, Immunotherapy, Microenvironment, Chemoprevention
Introduction
In the breast, epithelial cells are the source of milk and the target of oncogenic transformation; thus, understandably, the field of mammary gland biology is epithelial cell-centric. However, in the last decade, stromal-epithelial interactions, including immune cell interactions, have been recognized as key to physiologic and pathologic breast development. In all organs, including breast, the percent of tissue composed of immune cells is high. Data obtained from immunohistochemical analyses and genetic models that permit lineage tracing estimate a minimum of 10–30 % of cells within “epithelial” organs as being of immune origin [1, and references therein]. Immunohistochemical analyses for epithelial and immune cell lineage markers demonstrate that this is also true for human breast tissue (Fig. 1). Historically, the role of immune cells in the mammary gland was thought to be restricted to immune-surveillance, particularly during lactation and postpartum involution due to increased risk for mastitis. More recently, a paradigm shift has occurred and immune cells are being studied as obligate partners in normal tissue development. The mammary gland field has led in this area, in large part due to the pioneering macrophage work from the laboratory of Jeffery Pollard [2]. One advantage of the mammary gland as a model to study the roles of immune cells in normal development is that the majority of mammary development occurs postnatally, over the course of weeks in rodents, which is in contrast to fetal organ development that occurs within hours and days. Roles for immune cells in mammary gland development have been identified in pubertal duct elongation [3, 4], estrous cycle regulation [5, 6], gland expansion during pregnancy [7], cell death during weaning-induced involution [8], and adipocyte repopulation after weaning [9]. In this review, our objective is to explore immune cells in the postpartum involuting mammary gland as potential targets for the prevention and treatment of postpartum breast cancers.
Fig 1.
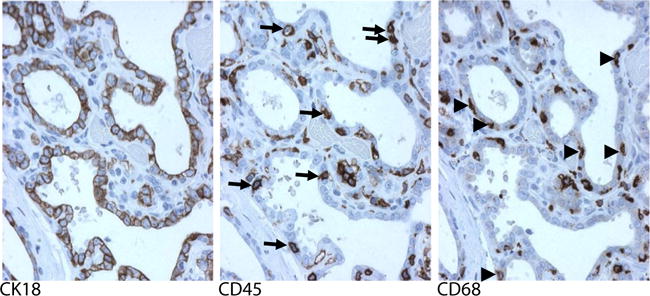
Serial sections of human involuting breast tissue demonstrate immune cells in very close proximity to breast epithelium. Representative immunohistochemical staining (brown) for the epithelial cell marker cytokeratin 18 (CK18), the general leukocyte marker CD45, and the macrophage marker CD68 are shown. CD45 and CD68 positive cells within the breast epithelium are indicated with arrows and arrowheads, respectively. Modified with permission from O’Brien et al. [39]
Postpartum Breast Cancer
Following pregnancy, women experience a transient increase in breast cancer risk that peaks approximately 5 to 6 years postpartum and may persist for up to 30 years [10–14]. Over time, the increased risk following pregnancy diminishes, such that a crossover in risk occurs and women who have had their first birth below age 35, have a lower breast cancer risk than age-matched women who have never given birth [10, 12, 15–17]. The phenomenon of a transient increase in breast cancer risk postpartum followed by protection was first described by Janerich and Hoff in the early 1980s and is referred to as the “dual effect” of pregnancy [17]. Breast cancer diagnosis in the postpartum period has been identified as an independent risk factor for poor outcomes [12, 18]. Stensheim et al. reported 11 year survival rates of 33 % for breast cancer cases diagnosed within 7 months postpartum, compared to 69 % for non-pregnancy-associated cases [19]. Surprisingly, this same study found that survival rates in cases diagnosed during pregnancy were not significantly different from nulliparous cases [19]. These data are further supported by subsequent studies from Johansson et al. reporting similar results for 10 year survival rates [20]. More recently, Callihan et al. reported a breast cancer diagnosis within 5 years of a recent pregnancy independently associated with a 2.8-fold increased risk for metastasis and a 2.7-fold increase in mortality as compared to nulliparous cases [21]. This study was unique in the large number of cases with known reproductive histories (n=619), and thus indicates that postpartum breast cancer carries a significantly worse prognosis when diagnosed within 5 years postpartum, an assertion also supported by earlier, albeit lower powered studies [22–24]. One potential mediator underlying the poor prognosis of breast cancer diagnoses following pregnancy is postpartum mammary gland involution [12, 25].
Postpartum Mammary Gland Involution
In virgin rodents, the mammary gland consists of a rudimentary, epithelial ductal network embedded within a stroma comprised of adipocytes, extracellular matrix, fibroblasts, immune cells, blood vessels, and lymphatics. Upon pregnancy, the epithelium extensively proliferates to meet the demand of lactation (Fig. 2). Recent lobular analysis of human breast tissue has revealed a >10-fold increase in epithelial content in the lactating breast [26]. Following lactation, or pregnancy if lactation does not occur, the mammary gland undergoes the process of postpartum involution to return to a state morphologically resembling the relatively simple ductal network of the pre-pregnant gland (Fig. 2). Though involution has been predominantly characterized in rodent models, support for postpartum involution similarly eliminating lactationally-competent lobules in women is demonstrated by the observation that the epithelial content in the breast following pregnancy becomes indistinguishable from that of nulliparous women within 18 months postpartum [26].
Fig 2.
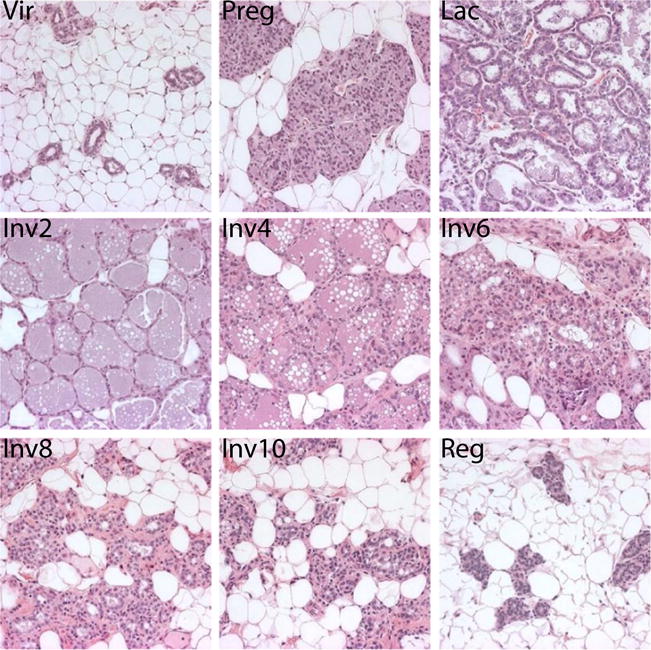
Epithelial regression and adipocyte repopulation during postpartum involution in the rat mammary gland. Hematoxylin and eosin staining of rat mammary tissue generated as described [39]. Vir = virgin, Preg = pregnancy day 18, Lac = lactation day 10, Inv2–10 = 2–10 days post-wean, Reg = Regressed (4 weeks post-wean)
In rodents, postpartum involution is characterized by programmed death of the majority of alveolar epithelial cells, extracellular matrix remodeling, leukocyte infiltration, and adipocyte repopulation [27–30]. The involution process has been described as occurring in two phases—a reversible cell-death phase with maintenance of the lobuloalveolar structures, followed by a non-reversible, tissue-remodeling phase with additional cell death and lobuloalveolar loss [27]. Through teat-sealing experiments, the initial phase of involution has been found to be locally regulated by milk stasis and in mice, lasts approximately 48 h following cessation of lactation [31, 32]. Recently, it has been reported that cell death during the first phase of involution is lysosomally-mediated through signal transducer and activator of transcription 3 (STAT3) and activation of cathepsins B and L [33]. Somewhat surprisingly, in a separate study, macrophages were found to be essential for execution of cell death during involution, as distended milk-filled lumens and STAT3 activation were not sufficient to induce cell death following macrophage depletion [8]. How macrophages mediate epithelial cell death during early involution remains to be determined. The non-reversible tissue remodeling phase of involution is characterized histologically by loss of lobuloalveolar structures and adipocyte repopulation [27]. This phase is regulated by changes in systemic hormones and associated with downregulation of protease inhibitors and upregulation of proteases, including matrix metalloproteinases (MMPs) 2, 3, 9, and 11, interleukin-1β converting enzyme (ICE), and urokinase-type plasminogen activator (uPA) [28, 31, 34–37]. The complexity of weaning-induced mammary gland involution and its regulation are also evident from transcriptional activity analyses. In one murine study, nine temporal patterns of expression profiles were identified, each with distinct gene ontology pathways [29]. However, one unifying theme when comparing histological, immunological, biochemical and RNA expression profiling data across involution is evidence for tissue remodeling reminiscent of wound healing.
Similarities Between Physiologic and Pathology-Associated Tissue Remodeling
Physiologic tissue remodeling during postpartum involution shares multiple attributes with wound healing, a process known to be tumor promotional [28, 29, 38–43]. These attributes include expression of the inflammatory mediator cyclooxygenase-2 (COX-2), elevated protease activity as described above, release of bioactive extracellular matrix fragments, deposition of fibrillar collagen and significant leukocytic infiltration [29, 38–42, 44]. Based on the similarities between wound healing and postpartum involution, the involution-hypothesis was proposed to account for the increased metastasis and poor prognosis of breast cancers diagnosed following pregnancy [25]. In support of this hypothesis, orthotopic injection of human tumor cells into mammary glands of immunocompromised mice on involution day one leads to increased tumor growth, local invasion, and metastatic seeding as compared to tumor cells injected into mammary glands of virgin mice [18, 45]. Here we review data implicating the immune microenvironment in promoting mammary tumors in the postpartum period [46].
Immune Milieu of the Postpartum Involuting Mammary Gland
During postpartum involution, increased expression of immune-related genes and leukocyte infiltration is observed in the absence of overt inflammatory insult [47]. Provocative evidence for immune cell involvement in postpartum involution was provided by microarray analyses demonstrating upregulation of waves of immune-related genes throughout the involution period in weaning-induced murine models. Within the first 12 h post-weaning, upregulation of acute-phase response genes were observed, such as STAT3, lipopolysaccharide-binding protein (LBP), cluster of differentiation (CD) 14, and inflammatory mediators, including interleukin (IL)-1α, IL-1β, and IL-13 [29, 38]. In addition, the neutrophil chemoattractant chemokine (C-X-C motif) ligand (CXCL) 1 and the neutrophilic-granulocyte marker leucine-rich α2-glycoprotein (LRG) were also upregulated during early involution, peaking 2 days post-wean [29, 38]. These neutrophil-associated gene expression data were corroborated by histological analysis demonstrating neutrophil influx with early involution [29]. This initial wave of pro-inflammatory gene expression and neutrophil infiltrate was followed by increased expression of monocyte and macrophage chemoattractants including chemokine (C-C motif) ligand (CCL) 6, CCL7, CCL8, and CXCL14, followed by upregulation of macrophage-specific antigens themselves, including colony stimulating factor-1 receptor (CSF-1R), CD68, and low density lipoprotein-related protein (LRP1), all of which peaked at 72–96 h post-wean [29, 38]. Consistent with upregulation of macrophage chemoattractants and antigens, macrophage infiltration into the involuting gland has been reported in numerous studies in mice and rats, with peak macrophage influx occurring mid- to late-involution [29, 39, 48]. Furthermore, increased numbers of eosinophils, mast cells, and plasma cells have been described in the involuting mammary glands of rodents [29, 49, 50].
In women, extensive characterization of immune cell infiltration during postpartum breast involution has been hindered by lack of tissue; however, data to date are consistent with involvement of immune cells during involution in women as observed in rodents. Immunohistochemical staining for the general leukocyte antigen CD45 is markedly increased in involuting breast lobules (Fig. 3), and is significantly increased in breast tissue in the first 12 months postpartum [26, 39]. Within this 12 month postpartum window, macrophages are also significantly increased, as determined by positivity for CD68, a marker highly expressed on macrophages (Fig. 3) [26, 39]. In addition, we observe increased presence of CD4-, CD8-, and CD19-positive cells, consistent with the presence of effector T and B cells within involuting lobules of the postpartum breast (Fig. 3).
Fig 3.
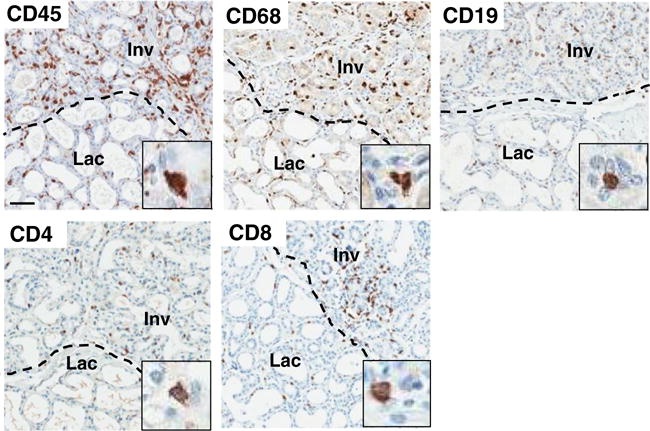
Postpartum involuting lobules have increased immune cell infiltrate.
Immunohistochemical staining for the general leukocyte marker CD45, the macrophage marker CD68, the B cell marker CD19, and the T cell markers CD4 and CD8 in actively involuting (Inv) and lactating (Lac) human breast tissue lobules. Scale bar = 100 μM; inset = representative positively-stained cell at 6× magnification of the larger image
Of the immune cells present in the involuting rodent mammary gland, macrophages are the most well-characterized. During maturation, monocytes mature into a spectrum of macrophage phenotypes in response to the surrounding cytokine milieu. The two ends of this spectrum include the classically-activated and alternatively-activated macrophages, also variably referred to as M1 or M2, respectively [51–53]. Classically-activated macrophages are promoted by tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), IL-1β, and lipopolysaccharide (LPS), and can be identified by inducible nitric oxide synthase (iNOS), IL-1, and IL-12 expression. These macrophages have pro-inflammatory and antigen presentation properties, thus likely function in immunosurveillance. In contrast, IL-4, IL-10 and IL-13 promote alternatively activated macrophages, which can be identified by expression of arginase-1 (arg-1), mannose receptor, FIZZ1, Ym1, IL-1RA, and IL-10 [51–53]. Alternatively-activated macrophages function in immunosuppression, wound repair and tissue remodeling [51, 54]. In the context of cancer, classically-activated macrophages are thought to function predominantly in tumor cell elimination, while alternatively-activated macrophages have tumor-promotional attributes and share many properties with tumor-associated macrophages [52, 55]. Importantly, macrophages in the involuting mammary gland have been characterized as having an alternatively-activated phenotype based on arg-1 and mannose receptor expression [39]. Known inducers of alternative macrophages, IL-4, IL-10, and IL-13, are also increased in the involuting gland [39, 56], data consistent with promotion of alternative activation.
It is anticipated that elucidating how the immune microenvironment of the involuting gland is established and maintained will reveal immunotherapeutic targets for postpartum breast cancer. However, regulation of immune cell infiltration, differentiation and activation in the postpartum involuting mammary gland remains largely unexplored. Possible mediators of the immune milieu attributes include COX-2 expression by mammary epithelial cells, generation and clearance of apoptotic cells, extracellular matrix remodeling, adipocyte repopulation, and changes in systemic hormones.
COX-2 Expression by Mammary Epithelial Cells
In the mammary gland, normal mammary epithelial cells are the dominant cell type expressing the inflammatory mediator COX-2, and furthermore, epithelial cell expression of COX-2 increases during involution [44]. In mammary tumor models, COX-2 overexpression is sufficient to induce tumorigenesis and is associated with tumor cell migration and invasion [57–62]. COX-2 is an enzyme involved in formation of prostaglandins from arachidonic acid. Prostaglandin E2 (PGE2) is considered the dominant prostaglandin secreted in cancer and has many pro-tumorigenic properties, acting on tumor cells themselves, as well as on the tumor microenvironment. In addition, COX-2 expression and synthesis of PGE2 contribute to a tumor-promotional immune microenvironment. PGE2 promotes tumor-associated macrophages by inhibiting proinflammatory cytokines, such as IL-12, required for classical macrophage activation [63, 64]. Furthermore, PGE2 has been implicated in recruitment and promotion of suppressive myeloid cells and regulatory T cells, both of which inhibit anti-tumor immunity [65]. In a murine model of postpartum breast cancer, COX-2 inhibition reduced tumor promotion during postpartum involution [62]. Moreover, NSAID treatment during postpartum involution reduced mammary PGE metabolite levels [66]. Altogether, these data implicate a role for mammary epithelial cell-derived COX-2 in establishing a protumorigenic immune milieu during involution.
Generation and Clearance of Apoptotic Cells
The dominant feature of postpartum involution is death and removal of the lactationally-competent mammary epithelium. While macrophages from the involuting gland are capable of engulfing apoptotic cells, temporal morphometric analyses and gene knockout of the apoptotic cell receptor MerTK identifies mammary epithelial cells as the primary phagocyte [48, 67]. Insights from previous studies in other non-involution systems have demonstrated that apoptotic cell clearance contributes to local immune suppression required for maintenance of tissue homeostasis and prevention of autoimmunity. Upon binding to apoptotic cells, professional phagocytes, such as macrophages, have been shown to promote a locally suppressive environment through production of TGF-β, PGE2, and IL-10 [68–70]. Consistent with mammary epithelial cells similarly promoting local immune suppression during involution, mammary epithelial cells secrete TGF-β and, in vitro, are able to suppress proinflammation cytokine production upon engulfment of apoptotic cells [71]. Furthermore, STAT3 expression by mammary epithelial cells contributes to the immune milieu in the involuting gland, and is implicated in poor prognosis in breast cancer [49, 72]. In addition to contributing to an immunosuppressive environment, phagocytic mammary epithelial cells may also directly contribute to tumor promotion by functioning like tumor-associated macrophages, as suggested by elevated production of the pro-angiogenic factor vascular endothelial growth factor (VEGF) [73, 74].
While studies characterizing the immune suppressive environment of apoptotic cell clearance have primarily focused on the role of phagocytes in this clearance, the apoptotic cells themselves also contribute directly to the surrounding immune milieu. Apoptotic cells promote recruitment of macrophages and other phagocytes to areas of cell death by releasing cellular constituents such as lysophosphatidylcholine (LPC), adenosine triphosphate (ATP), and uridine-5′-triphosphate (UTP) which bind to specific receptors on phagocytic cells [75–77]. Following leukocyte recruitment, apoptotic cells induce immune suppressive gene responses in local leukocytes by releasing adenosine monophosphate (AMP), transforming growth factor-beta (TGF-β), and PGE2 [78, 79]. Furthermore, PGE2 from apoptotic cells also suppresses local T cell function [80, 81]. Given the vast amount of apoptosis occurring in the involuting mammary gland, it is anticipated that the apoptotic cells themselves, as well as the phagocytic mammary epithelial cells responsible for their clearance, contribute to an immune suppressive milieu which facilitates tumor progression [46].
Extracellular Matrix
The tissue-remodeling phase of postpartum mammary gland involution results in numerous changes in extracellular matrix (ECM) abundance, organization, and proteolysis, and ECM from involuting rat mammary glands has demonstrated protumor activity. Culturing of MDA-MB-231 human breast tumor or D2.OR murine mammary tumor cells on ECM isolated from involuting rat mammary glands leads to disruption of cell-cell adhesion junctions, loss of apical-basal polarity and induction of front-back polarity, resulting in formation of more invasive cells as compared to cells cultured on matrix isolated from nulliparous rat mammary glands [28, 66]. Furthermore, in orthotopic xenograft models, tumor cells coinjected with involution-mammary ECM metastasize at high rates [28]. These data indicate that involution-mammary ECM is sufficient to enhance tumor growth and metastasis in breast cancer models, with increased abundance of collagen and tenascin-C, radial alignment of collagen, and proteolysis of collagen, fibronectin and laminin all implicated as mediators [28, 39, 42, 62, 66, 82]. While these ECM changes likely have direct pro-tumorigenic effects on tumor cells themselves, the role of ECM proteins in influencing immune complexity of the involuting mammary gland cannot be discounted. ECM has roles in immune cell recruitment, activation, and function [83, 84]. Of potential relevance to the protumorigenic environment in the postpartum involuting mammary gland, some collagen and laminin fragments are chemotactic for neutrophils and macrophages [39, 85, 86]. Collagen has also been found to regulate tumor cytotoxicity by macrophages [85, 87]. Treatment of lung alveolar macrophages with native and synthetic collagen peptides enhance cytotoxicity by macrophages against both normal and transformed cells [85]. Conversely, culturing macrophages on collagen fiber-coated plates impaired their ability to kill target cells [87]. Tenascin-C is another involution-associated ECM protein that may modify leukocyte function during involution, as tenascin-C has been found to inhibit T cell activation in multiple models [88–92]. Interestingly, in rodent models, the tumor-promotional properties of ECM isolated from involuting glands can be ameliorated by NSAID treatment during involution [66]. Specifically, mammary gland collagen and tenascin-C deposition are reduced by NSAID treatment [66, 62], raising the hypothesis that epithelial cell-derived COX-2 activity during involution promotes changes in the ECM, which in turn alter leukocyte function.
In addition to the ability of ECM proteins to directly influence recruitment of immune cells and their subsequent activation, ECM can also serve as a reservoir for cytokines. Many ECM molecules have glycosaminoglycan side chains that interact with and sequester cytokines (as reviewed by [93]), including fibroblast growth factor (FGF), IFNγ, TNFα, IL-8, CCL12, IL-4, and TGF-β [94–96]. Upon ECM cleavage and remodeling during mammary gland involution [40, 42, 97], cytokines may be released from ECM, permitting them to act upon local immune cells. For example, upon protease activation (thrombospondin, MMP2, MMP9), engagement of integrin binding (αvβ6), or mechanical stress upon the ECM [98], active TGF-β fragments are released from ECM stores [98–100]. TGF-β inhibits T cell cytotoxicity [101], macrophage effector function [102], neutrophil activation [103], and NK cell activity [104]. In addition, TGF-β can augment immunosuppression by down-regulating major histocompatibility complex (MHC) expression and enhancing Treg function [105, 106]. IL-4 levels may also be regulated by the ECM during involution as IL-4 also binds glycosaminoglycan side chains [96]; thus, ECM remodeling and cleavage during postpartum involution may release IL-4 into the local environment, and notably, IL-4 levels are increased in the mammary gland during involution [39].
Another mechanism by which ECM proteolysis appears to modulate immune cell function is through release of bioactive fragments (matrikines) that have different activities than the intact molecule. The anti-angiogenic factors endostatin, a 20kD C-terminal fragment of collagen XVIII, and tumstatin, a fragment of collagen IV α3 chain, serve as classic examples of matrikines [107, 108]. It has been found that specific ECM matrikines alter immune cell chemotaxis, phagocytosis, differentiation, activation, and cytokine production. Fibronectin fragments, which are generated during mammary gland involution [28], can enhance phagocytosis and oxidative burst in monocytes and macrophages [109–111]. In addition, fibronectin matrikines can increase macrophage production of the protease MMP9, and the pro-inflammatory cytokines IL-1, IL-6, and TNFα [109, 112, 113]. Laminin fragments, which are also generated during mammary gland involution [28], can function as matrikines by attracting macrophages and enhancing expression of the proteases MMP9 and MMP14, as well as TNFα [114, 115].
Adipocyte Repopulation
In rodent models, the adipocyte content of the mammary gland changes dramatically across the pregnancy-lactation-involution cycle. In the nulliparous mouse mammary gland, adipocytes occupy approximately 97 % of the tissue volume; however, the adipocyte content of the lactating gland is only ~10 % [116]. During involution, adipocyte re-population occurs such that adipocyte content in the fully regressed gland is comparable to that of pre-pregnancy [116]. While the relationship between adipocytes and immune cells during postpartum involution is yet to be investigated, the role of adipocytes in obesity-associated inflammation indicates that adipocytes are worth considering as potential contributors to the immune profile of the involuting gland. During involution, adipocytes may have roles in both macrophage attraction and activation; adipocytes can make CCL2 (also known as monocyte chemoattractant protein 1 (MCP-1)), which is upregulated during postpartum involution, though the cell type responsible for CCL2 expression is unknown [39, 117, 118]. In addition, adipocytes influence macrophage activation through production of leptin [119, 120]. Leptin has been demonstrated to promote a combination of alternative and classical activation phenotypes in macrophages, inducing mannose receptor expression as well as the pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IL-1RA [119]. Leptin can also serve as a chemoattractant for monocytes and macrophages [121]. Importantly, leptin expression is increased in mammary adipocytes and ductal epithelium throughout involution [122]. Cumulatively, these data support a role for adipocyte regulation of macrophage activation during postpartum involution.
Hormones
In a model of postpartum breast cancer, treatment with the aromatase inhibitor letrozole led to reduced tumor growth of estrogen receptor negative tumors, indicating estrogen may act on stromal cells to contribute to tumor promotion [45]. Co-injection of tumor cells and bone marrow isolated from estrogen-treated NOD/SCID mice into nulliparous hosts was sufficient to promote tumor growth, providing support for estrogen acting on cells of hematopoietic origin [45]. Additional support for a potential role for estrogen in modulating the immune profile of the mammary gland comes from studies demonstrating changes in macrophage number and phenotype in the mammary gland across the estrous cycle and with estradiol and progesterone treatment [5, 6]. Further, in a wound-healing model, estradiol and progesterone were found to promote an alternative phenotype in macrophages [123]. However, it should be noted that estradiol can also promote a pro-inflammatory phenotype in macrophages [124]. Mast cell number in the mammary gland has also been found to fluctuate throughout the estrous cycle and during involution in rats [50]. Furthermore, in response to estrogen and progesterone, mast cells upregulate chemokine receptors, maturation markers, and degranulate [125, 126]. While changes in T and B cells have not been reported in the mammary gland during the estrous cycle, there is considerable evidence indicating these cells can also be directly regulated by estrogen [127–132] and as such, should be considered as potential targets of hormone regulation in the postpartum mammary gland.
Immune Mediators of Breast Cancer Promotion
The immune environment of the involuting mammary gland is anticipated to contribute to poor outcomes in postpartum breast cancer. In breast cancer patients, increased macrophage infiltration into primary tumors correlates with tumor cell proliferation and significant decreases in relapse-free and overall survival [133–137]. In addition, high levels of the macrophage chemoattractant and growth factor colony stimulating factor 1 (CSF-1) and the chemoattractant CCL2 associate with breast cancer metastasis and poorer outcomes [138, 139]. Notably, CCL2 significantly increases in the mammary gland during involution [39]. Direct evidence supporting a role for macrophages in breast cancer progression comes from murine studies. For example, in the MMTV-PyMT mammary carcinoma model, macrophage depletion through CSF-1 deletion slows tumor progression and significantly decreases metastasis, while overexpression of CSF-1 increases macrophage infiltration in primary tumors and elevates rates of metastasis [140]. Subsequently, it was reported that CD4+ T cells indirectly regulate metastasis in this model by inducing a pro-tumorigenic phenotype in CD11b(+)Gr1(−)F4/80(+) macrophages via IL-4 secretion [141]. Moreover, therapeutic depletion of macrophages through delivery of CSF-1 receptor (CSF-1R) antagonists, in combination with taxol-based chemotherapy, significantly improves outcomes for mice harboring mammary carcinomas by reducing metastasis through CD8+ T cell-dependent mechanisms [142].
In addition to macrophages, there is convincing evidence that myeloid suppressive cells (MSCs) and regulatory T cells (Tregs) play important roles in breast cancer promotion. In breast cancer patients, elevated numbers of MSCs correlate with increased clinical stage and metastasis, and are also associated with a poorer response to chemotherapy [143, 144]. Similarly, increased numbers of Tregs within the tumor and peripheral blood correlates with disease progression and worse outcomes for breast cancer patients [145–147]. MSCs and Tregs contribute to tumor progression predominantly by suppressing anti-tumor immunity in both innate and adaptive immune cells. One example of adaptive immune suppression by MSCs is suppression of T-cell activation and proliferation, occurring in part through increased MSC production of arginase and iNOS that depletes local L-arginine levels [148]. L-arginine is required for expression of the T cell receptor associated ζ chain, a receptor essential for T cell activation and subsequent proliferation; thus, L-arginine depletion by MSCs (and macrophages) contributes directly to T cell suppression [142, 148, 149].
Potential Immunotherapeutic Targets During Postpartum Involution
The influx of leukocytes into the postpartum involuting mammary gland, and the known roles for immune cells in breast cancer progression, raises the question of whether immunotherapy during normal mammary involution could block incidence or progression of postpartum breast cancer. The goal of immunotherapy is to activate a patient’s own immune system to elicit an anti-tumor response that detects and eliminates cancer cells. Cancer immunotherapies include vaccines, adoptive cell transfer, and targeting tumor-associated macrophages and immune checkpoint pathways (Reviewed in [150]). Here, we discuss the potential of targeting involution macrophages and immune checkpoints, as well as the use of NSAIDs, in postpartum breast cancer.
Involution macrophages have been proposed to contribute to the poor prognosis of postpartum breast cancer [25], and as such represent one target for immunotherapy directed at blocking the tumor-promotional attributes of the involuting gland (Fig. 4). Targeting tumor-associated macrophage recruitment and activation has had therapeutic success in pre-clinical mammary cancer models. For example, reducing macrophage recruitment into murine mammary tumors by blocking CSF-1 or CSF-1R decreases tumor growth and metastasis and increases sensitivity to chemotherapy [142, 151, 152]. These data indicate that targeting CSF-1/CSF-1R may be beneficial in postpartum breast cancer as well. However, CSF-1R is not expressed on all macrophage populations in the involuting mammary gland [39], indicating that anti-CSF-1/CSF-1R treatment may need to be combined with additional therapies when targeting involution. Notably, levels of the macrophage chemoattractant CCL2 greatly increase in the involuting gland and precede the macrophage influx [39], identifying CCL2 as an additional target with immunotherapeutic potential. Blocking CCL2 levels in models of non-small cell lung cancer was found to effectively reduce local immunosuppression and enhance development of vaccine-mediated anti-tumor immunity [153]. Another way to target involution macrophages in postpartum breast cancer would be to “re-educate” macrophages away from a tumor-promotional, alternatively-activated phenotype toward classical activation with increased anti-tumor attributes. In murine mammary carcinoma models, inducing classical activation in tumor-associated macrophages with GM-CSF treatment or by targeting STAT3 expression has proven successful at reducing tumor growth, angiogenesis, and metastasis [154, 155]. Candidate cytokines to target for macrophage “reeducation” in the involution gland include IL-4, IL-13, IL-10, and TGF-β (Fig. 4) [39, 56, 156, 157]. Given the role of resident macrophages in the execution of cell death during postpartum involution [8], successful immunotherapies targeting involution macrophages will be those which tip the balance of the immune microenvironment toward one of anti-tumor immunity, while allowing involution to progress unabated.
Fig 4.
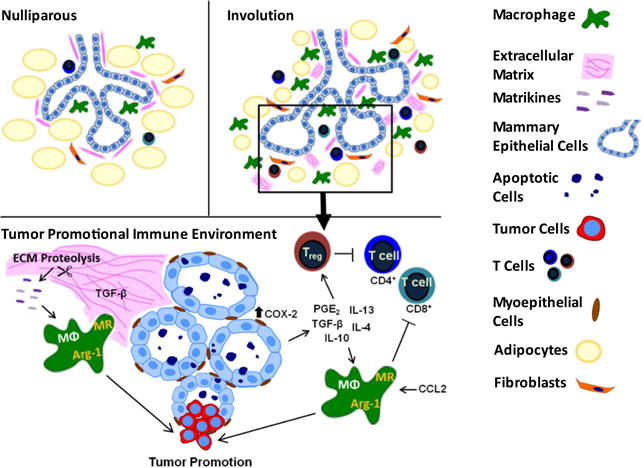
Macrophages as orchestrators of a tumor-promotional immune environment during postpartum mammary involution. Alternatively activated macrophages, characterized by mannose receptor and arg-1 expression, increase in the mammary gland during involution. Involution macrophages are anticipated to contribute to tumor promotion directly through the production of growth factors, such as epidermal growth factor (EGF) [176], and indirectly by suppressing anti-tumor immunity. Targeting macrophage recruitment and activation may be one way to alleviate macrophage-induced tumor promotion during postpartum involution. Involution macrophage recruitment and activation are anticipated to be promoted by ECM components, cytokines, growth factors, and prostaglandins, all of which represent potential immunotherapeutic targets directed toward involution macrophages
In addition to promoting anti-tumor immunity through macrophages, targeting immune checkpoints is another way to relieve immunosuppression in the tumor microenvironment. Immune checkpoints are negative regulators of the immune system that modulate the extent of immune responses and function in maintaining self-tolerance [158]. Antibodies targeting the immune checkpoint molecules cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PD-L1) are finding success in the clinic. Ipilimumab, a monocolonal antibody against CTLA-4, has been FDA approved since 2011 for metastatic melanoma and has been successfully combined with other immunotherapies [159]. Immune checkpoint modulation is currently being evaluated in breast cancer, with early studies showing promise [160].
As an alternative approach to targeting specific cell types or molecules, targeting the immune environment with general anti-inflammatory agents may also be effective in the involuting mammary gland. NSAID treatment limited to the window of involution has previously been identified as a potential strategy for prevention and treatment of postpartum breast cancer through ECM- and tumor cell-mediated mechanisms [62, 66]. Effects on the immune cells were not evaluated in these studies, as they utilized orthotopic xenograft models in immunocompromised mice. However, given the function of NSAIDs as anti-inflammatory agents, it is likely that the immune microenvironment is also affected and warrants further investigation.
Limitations and Future Questions
In rodents, characterization of the immunologic programs activated during postpartum involution has utilized forced-weaning models to synchronize involution programs throughout the entire gland. While there are multiple advantages of these models, including the ability to perform molecular analyses under controlled conditions, it is important to acknowledge potential limitations as the field of postpartum breast cancer research progresses. In most circumstances, forced-wean models do not fully recapitulate the onset of involution in women, which more frequently occurs through a gradual-weaning process. Recently it has been proposed that abrupt weaning, as used in the postpartum breast cancer models described here, may be associated with an increased risk of developing breast cancer, while gradual weaning may protect against breast cancer [161]. This hypothesis has yet to be tested; however, epidemiologic data are consistent with gradual involution contributing to tumor promotion, as postpartum women, regardless of lactation history, are at increased risk for early onset breast cancer with poor prognosis [21]. Characterization of involution in women may provide some insight, as gradual involution is associated with lobule by lobule regression with hallmarks of a tumor-promotional environment associated with involuting lobules, but not adjacent lactational lobules [26, 39]. Since local interactions between tumor cells and the microenvironment are shown to be sufficient to promote metastasis [162], it is anticipated that tumor cells present within involuting lobules may be promoted in this environment independent of whether the neighboring lobules are lactating or involuting.
An additional question to address moving forward is the role of lactation. Multiple meta-analyses using both case–control and large cohort studies support a role for lactation in reducing overall breast cancer risk [18, 163]. Briefly, these meta-analyses revealed modest reduction in breast cancer risk with any lactation [164], reduction in risk with prolonged lactation [165], and/or no correlation between lactation and reduction in breast cancer risk [166]. The results of these studies may be confounded by the difficulties associated with obtaining accurate lactation history. In addition, breast cancer is a complex disease with significant differences in onset and/or severity based on tumor biologic subtype, as well as patient race, age, body mass index (BMI), age at first birth, time since last child birth, and menopausal status [21]. Thus, more refined analyses are necessary for additional insight into the role of lactation in breast cancer risk as well as disease prognosis. Several recent studies have taken such an approach.
In a case–control study of Tanzanian pre-menopausal women, prolonged lactation was associated with modest risk reduction (OR 0.98, 95 % CI 0.97–0.99) [167]. Furthermore, in the Carolina Breast Cancer Study, a cohort of primarily African American women, and the Black Women’s Health Study, lack of breastfeeding was associated with significant increases in risk for basal-like and hormone receptor negative breast cancers, respectively [168, 169]. An additional study, from a cohort of pre-menopausal primarily white women, revealed that risk for triple negative tumors was similarly increased in women who did not breastfeed [170]. In addition, Stuebe et al. revealed that breastfeeding was protective in premenopausal women with a family history that included a first-degree relative with breast cancer [171]. In contrast, some studies have reported that breast cancers diagnosed during lactation exhibit aggressive phenotypes and poor survival rates [19, 172]. However, in these studies the lactation group was defined as less than 2 years or less than 6 months postpartum, respectively, and no data were presented to indicate whether the women were lactating at the time of diagnosis. Thus, the data are likely confounded by inclusion of women who are post-lactational and at higher risk for more aggressive tumors and poor survival rates [21]. Recently, several studies have more specifically examined the role that breastfeeding may play in promoting more aggressive disease phenoytpes. In one study, breastfeeding for more than 12 months was associated with increased risk for triple negative tumors compared to luminal A tumors in women of Mexican descent [173]. Furthermore, a study of Swedish women revealed that excessive milk production during breastfeeding and breastfeeding for >12 months was associated with a two-fold increased risk for early breast cancer events, defined as new, local, regional, or distant recurrence in primary breast cancer patients [174]. While these studies are in contrast to data from a transgenic rodent model of continuous lactation, which revealed that the lactogenic microenvironment protected against mammary tumor growth and lung metastasis [175], more recent data support a role for mammary adipose stromal cells obtained from lactating mammary glands in breast tumor promotion [116]. Cumulatively, these studies highlight the need for additional animal models to address the role of lactation and involution in mammary tumor promotion. Furthermore, longitudinal prospective studies on the effects of lactation and weaning on breast cancer risk with women grouped by race, age at diagnosis, BMI, parity status, menopause status, and tumor biologic subtype may shed light on the roles for lactation and involution in breast cancer risk.
Conclusion
The increased rate of metastasis and poor prognosis of postpartum breast cancer are anticipated to be due, in part, to the pro-tumorigenic immune milieu of the involuting mammary gland. While exposure to gestational hormones and lactation may contribute to risk and poor prognosis of breast cancers diagnosed in the postpartum period, therapies targeted to the postpartum window have clear benefits. For example, strategies targeting pregnant or lactating women have the undesirable consequence of cross-targeting the developing fetus or infant. However, the postpartum involution window is unencumbered by these potential problems. The dramatic upregulation of immune-associated genes and influx of immune cells into the involuting gland indicate that immunotherapeutic strategies may be particularly effective. Future work should be directed toward investigating the efficacy of immunotherapies directed toward the window of postpartum mammary involution as preventive and therapeutic agents for postpartum breast cancers.
Abbreviations
- ATP
adenosine triphosphate
- arg-1
arginase 1
- AMP
adenosine monophosphate
- BMI
body mass index
- CCL
chemokine (C-C motif) ligand
- CD
cluster of differentiation
- CK
cytokeratin
- COX-2
cyclooxygenase-2
- CSF-1
colony stimulating factor-1
- CSF-1R
colony stimulating factor-1 receptor
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- CXCL
chemoattractant chemokine (C-X-C motif) ligand
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- ICE
interleukin-1β converting enzyme
- IFNγ
interferon gamma
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- Inv
Involution
- Lac
lactation
- LBP
lipopolysaccharide binding protein
- LRP1
low density lipoprotein-related protein 1
- LPC
lysophosphatidylcholine
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein 12
- MHC
major histocompatibility complex
- MMPs
matrix metalloproteinases
- MMTV
mouse mammary tumor virus
- MSC
myeloid suppressor cell
- NK
natural killer
- NOD
non-obese diabetic
- NSAIDs
non-steroidal anti-inflammatory drugs
- PD-L1
programmed death ligand 1
- PD-1
programmed cell death protein 1
- PGE2
prostaglandin E2
- Preg
Pregnant
- PyMT
polyoma virus middle T antigen
- Reg
Regressed
- SCID
severe combined immunodeficiency
- STAT3
signal transducer and activator of transcription 3
- TGF-β
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
- Treg
regulatory T cell
- uPA
urokinase-type plasminogen activator
- UTP
uridine-5′-triphosphate
- VEGF
vascular endothelial growth factor
- Vir
Virgin
Contributor Information
Jaime Fornetti, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA; Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA; Program in Reproductive Sciences, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA.
Holly A. Martinson, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA; Cancer Biology Program, University of Colorado Anschutz Medical Campus, 12801 E 17th Ave, Aurora, CO 80045, USA.
Courtney B. Betts, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA; Cell Biology, Stem cells, and Development, 12801 E 17th Ave, Aurora, CO 80045, USA.
Traci R. Lyons, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA.
Sonali Jindal, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA; Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA.
Qiuchen Guo, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA; Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA; Cancer Biology Program, University of Colorado Anschutz Medical Campus, 12801 E 17th Ave, Aurora, CO 80045, USA.
Lisa M. Coussens, Department of Cell & Developmental Biology, Knight Cancer Institute, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA
Virginia F. Borges, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA.
Pepper Schedin, Email: Schedin@OHSU.edu, Department of Medicine, Division of Medical Oncology, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA; Young Women’s Breast Cancer Translational Program, University of Colorado Cancer Center, University of Colorado Anschutz Medical Campus, 1665 Aurora Court, Aurora, CO 80045, USA; Program in Reproductive Sciences, University of Colorado Anschutz Medical Campus, 12801 East 17th Avenue, Aurora, CO 80045, USA; Cancer Biology Program, University of Colorado Anschutz Medical Campus, 12801 E 17th Ave, Aurora, CO 80045, USA; Cell Biology, Stem cells, and Development, 12801 E 17th Ave, Aurora, CO 80045, USA; Department of Cell & Developmental Biology, Knight Cancer Institute, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA.
References
- 1.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101(3):1155–63. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127(11):2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 4.Van Nguyen A, Pollard JW. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol. 2002;247(1):11–25. doi: 10.1006/dbio.2002.0669. [DOI] [PubMed] [Google Scholar]
- 5.Chua AC, Hodson LJ, Moldenhauer LM, Robertson SA, Ingman WV. Dual roles for macrophages in ovarian cycle-associated development and remodelling of the mammary gland epithelium. Development. 2010;137(24):4229–38. doi: 10.1242/dev.059261. [DOI] [PubMed] [Google Scholar]
- 6.Hodson LJ, Chua AC, Evdokiou A, Robertson SA, Ingman WV. Macrophage phenotype in the mammary gland fluctuates over the course of the estrous cycle and is regulated by ovarian steroid hormones. Biol Reprod. 2013;89(3):6. doi: 10.1095/biolreprod.113.109561. [DOI] [PubMed] [Google Scholar]
- 7.Pollard JW, Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci U S A. 1994;91(20):9312–6. doi: 10.1073/pnas.91.20.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. 2012;139(2):269–75. doi: 10.1242/dev.071696. [DOI] [PubMed] [Google Scholar]
- 9.Lilla JN, Joshi RV, Craik CS, Werb Z. Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. J Biol Chem. 2009;284(20):13792–803. doi: 10.1074/jbc.M900508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13(4):299–305. doi: 10.1023/a:1015287208222. [DOI] [PubMed] [Google Scholar]
- 12.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–91. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 13.Albrektsen G, Heuch I, Hansen S, Kvale G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–75. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chie WC, Hsieh C, Newcomb PA, Longnecker MP, Mittendorf R, Greenberg ER, et al. Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol. 2000;151(7):715–22. doi: 10.1093/oxfordjournals.aje.a010266. [DOI] [PubMed] [Google Scholar]
- 15.Albrektsen G, Heuch I, Kvale G. The short-term and long-term effect of a pregnancy on breast cancer risk: a prospective study of 802,457 parous Norwegian women. Br J Cancer. 1995;72(2):480–4. doi: 10.1038/bjc.1995.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leon DA, Carpenter LM, Broeders MJ, Gunnarskog J, Murphy MF. Breast cancer in Swedish women before age 50: evidence of a dual effect of completed pregnancy. Cancer Causes Control. 1995;6(4):283–91. doi: 10.1007/BF00051403. [DOI] [PubMed] [Google Scholar]
- 17.Janerich DT, Hoff MB. Evidence for a crossover in breast cancer risk factors. Am J Epidemiol. 1982;116(5):737–42. doi: 10.1093/oxfordjournals.aje.a113462. [DOI] [PubMed] [Google Scholar]
- 18.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14(2):87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stensheim H, Moller B, van Dijk T, Fossa SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27(1):45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 20.Johansson AL, Andersson TM, Hsieh CC, Cnattingius S, Lambe M. Increased mortality in women with breast cancer detected during pregnancy and different periods postpartum. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1865–72. doi: 10.1158/1055-9965.EPI-11-0515. [DOI] [PubMed] [Google Scholar]
- 21.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138(2):549–59. doi: 10.1007/s10549-013-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnier P, Romain S, Dilhuydy JM, Bonichon F, Julien JP, Charpin C, et al. Influence of pregnancy on the outcome of breast cancer: a case–control study. Societe Francaise de senologie et de pathologie mammaire study group. Int J Cancer. 1997;72(5):720–7. doi: 10.1002/(SICI)1097-0215(19970904)72:5<720::AID-IJC3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Daling JR, Malone KE, Doody DR, Anderson BO, Porter PL. The relation of reproductive factors to mortality from breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(3):235–41. [PubMed] [Google Scholar]
- 24.Olson SH, Zauber AG, Tang J, Harlap S. Relation of time since last birth and parity to survival of young women with breast cancer. Epidemiology. 1998;9(6):669–71. [PubMed] [Google Scholar]
- 25.O’Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia. 2009;14(2):145–57. doi: 10.1007/s10911-009-9118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindal S, Gao D, Bell P, Albrektsen G, Edgerton S, Ambrosone C, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res. 2014;16(2):R31. doi: 10.1186/bcr3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122(1):181–93. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. 2006;168(2):608–20. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein T, Morris J, Davies C, Weber-Hall S, Duffy M-A, Heath V, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6(2):R75–91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185(1):19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, et al. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci U S A. 1997;94(7):3425–30. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marti A, Feng Z, Altermatt HJ, Jaggi R. Milk accumulation triggers apoptosis of mammary epithelial cells. Eur J Cell Biol. 1997;73(2):158–65. [PubMed] [Google Scholar]
- 33.Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol. 2011;13(3):303–9. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- 34.Dickson SR, Warburton MJ. Enhanced synthesis of gelatinase and stromelysin by myoepithelial cells during involution of the rat mammary gland. J Histochem Cytochem. 1992;40(5):697–703. doi: 10.1177/40.5.1315355. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre O, Wolf C, Limacher JM, Hutin P, Wendling C, LeMeur M, et al. The breast cancer-associated stromelysin-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol. 1992;119(4):997–1002. doi: 10.1083/jcb.119.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- 37.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118(5):1271–82. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarkson R, Wayland M, Lee J, Freeman T, Watson C. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6(2):R92–109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–55. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog. 2004;41(4):207–20. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- 41.Schedin P, O’Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12(1):71–82. doi: 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- 42.Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci. 2000;113(Pt 5):795–806. doi: 10.1242/jcs.113.5.795. [DOI] [PubMed] [Google Scholar]
- 43.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fornetti J, Jindal S, Middleton KA, Borges VF, Schedin P. Physiological COX-2 expression in breast epithelium associates with COX-2 levels in ductal carcinoma in situ and invasive breast cancer in young women. Am J Pathol. 2014;184(4):1219–29. doi: 10.1016/j.ajpath.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor–negative cancers. Cancer Res. 2007;67(5):2062–71. doi: 10.1158/0008-5472.can-06-3895. [DOI] [PubMed] [Google Scholar]
- 46.Fornetti J, Martinson H, Borges V, Schedin P. Emerging targets for the prevention of pregnancy-associated breast cancer. Cell Cycle. 2012;11(4):639–40. doi: 10.4161/cc.11.4.19358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein T, Salomonis N, Nuyten DS, van de Vijver MJ, Gusterson BA. A mouse mammary gland involution mRNA signature identifies biological pathways potentially associated with breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2009;14(2):99–116. doi: 10.1007/s10911-009-9120-1. [DOI] [PubMed] [Google Scholar]
- 48.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during postlactation involution of the mouse mammary gland. Biol Reprod. 2008;78(4):586–94. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- 49.Hughes K, Wickenden JA, Allen JE, Watson CJ. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol. 2012;227(1):106–17. doi: 10.1002/path.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez RA, Lee A, Schedin P, Russell JS, Masso-Welch PA. Alterations in mast cell frequency and relationship to angiogenesis in the rat mammary gland during windows of physiologic tissue remodeling. Dev Dyn. 2012;241(5):890–900. doi: 10.1002/dvdy.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 56.Sohn BH, Moon HB, Kim TY, Kang HS, Bae YS, Lee KK, et al. Interleukin-10 up-regulates tumour-necrosis-factor-alpha-related apoptosis-inducing ligand (TRAIL) gene expression in mammary epithelial cells at the involution stage. Biochem J. 2001;360(Pt 1):31–8. doi: 10.1042/0264-6021:3600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101(2):591–6. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106(9):3372–7. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012;7(9):e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larkins T, Nowell M, Singh S, Sanford G. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6(1):181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu CH, Chang S-H, Narko K, Trifan OC, Wu M-T, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276(21):18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 62.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17(9):1109–15. doi: 10.1038/nm.2416. http://www.nature.com/nm/journal/v17/n9/abs/nm.2416.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 64.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen EP, Smyth EM. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 2011;96(1–4):14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brien J, Hansen K, Barkan D, Green J, Schedin P. Non-steroidal anti-inflammatory drugs target the pro-tumorigenic extracellular matrix of the postpartum mammary gland. Int J Dev Biol. 2011;55(7–9):745–55. doi: 10.1387/ijdb.113379jo. [DOI] [PubMed] [Google Scholar]
- 67.Sandahl M, Hunter DM, Strunk KE, Earp HS, Cook RS. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Dev Biol. 2010;10:122. doi: 10.1186/1471-213X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fadok VA, Bratton DL, Konowal A, Freed OW. Macrophages That Have Ingested Apoptotic Cells In Vitro Inhibit Proinflammatory Cytokine Production Through Autocrine/Paracrine Mechanisms Involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101 doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, et al. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and prep-1. Immunity. 2007;27(6):952–64. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brecht K, Weigert A, Hu J, Popp R, Fisslthaler B, Korff T, et al. Macrophages programmed by apoptotic cells promote angiogenesis via prostaglandin E2. FASEB J. 2011;25(7):2408–17. doi: 10.1096/fj.10-179473. [DOI] [PubMed] [Google Scholar]
- 71.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12(2):107–14. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 72.Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, et al. The role of Stat3 in apoptosis and mammary gland involution. Conditional deletion of Stat3. Adv Exp Med Biol. 2000;480:129–38. doi: 10.1007/0-306-46832-8_16. [DOI] [PubMed] [Google Scholar]
- 73.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18(14):1716–8. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 74.Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223(2):177–94. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- 75.Somersan S. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001;155(4):501–4. doi: 10.1083/jcb.200110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lauber K, Bohn E, Kröber SM, Xiao Y-j, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 77.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamaguchi H, Maruyama T, Urade Y, Nagata S. Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. eLife. 2014 doi: 10.7554/eLife.02172.001. doi: 10.7554/eLife.02172.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta Released by Apoptotic T Cells Contributes to an Immunosuppressive Milieu. Immunity. 2001;14 doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 80.Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3(110):ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316(19):3109–23. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 82.O’Brien JH, Vanderlinden LA, Schedin PJ, Hansen KC. Rat mammary extracellular matrix composition and response to ibuprofen treatment during postpartum involution by differential GeLC-MS/MS analysis. J Proteome Res. 2012;11(10):4894–905. doi: 10.1021/pr3003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morwood SR, Nicholson LB. Modulation of the immune response by extracellular matrix proteins. Arch Immunol Ther Exp (Warsz) 2006;54(6):367–74. doi: 10.1007/s00005-006-0043-x. [DOI] [PubMed] [Google Scholar]
- 84.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;0(0):712–23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 85.Laskin DL, Soltys RA, Berg RA, Riley DJ. Activation of alveolar macrophages by native and synthetic collagen-like polypeptides. Am J Respir Cell Mol Biol. 1994;10(1):58–64. doi: 10.1165/ajrcmb.10.1.8292381. [DOI] [PubMed] [Google Scholar]
- 86.Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, et al. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;283(15):9513–22. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaplan G. In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not. J Exp Med. 1983;157(6):2061–72. doi: 10.1084/jem.157.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hauzenberger D, Olivier P, Gundersen D, Ruegg C. Tenascin-C inhibits beta1 integrin-dependent T lymphocyte adhesion to fibronectin through the binding of its fnIII 1–5 repeats to fibronectin. Eur J Immunol. 1999;29(5):1435–47. doi: 10.1002/(SICI)1521-4141(199905)29:05<1435::AID-IMMU1435>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 89.Hemesath TJ, Marton LS, Stefansson K. Inhibition of T cell activation by the extracellular matrix protein tenascin. J Immunol. 1994;152(11):5199–207. [PubMed] [Google Scholar]
- 90.Hibino S, Kato K, Kudoh S, Yagita H, Okumura K. Tenascin suppresses CD3-mediated T cell activation. Biochem Biophys Res Commun. 1998;250(1):119–24. doi: 10.1006/bbrc.1998.9258. [DOI] [PubMed] [Google Scholar]
- 91.Puente Navazo MD, Valmori D, Ruegg C. The alternatively spliced domain TnFnIII A1A2 of the extracellular matrix protein tenascin-C suppresses activation-induced T lymphocyte proliferation and cytokine production. J Immunol. 2001;167(11):6431–40. doi: 10.4049/jimmunol.167.11.6431. [DOI] [PubMed] [Google Scholar]
- 92.Ruegg CR, Chiquet-Ehrismann R, Alkan SS. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci U S A. 1989;86(19):7437–41. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maller O, Martinson H, Schedin P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. J Mammary Gland Biol Neoplasia. 2010;15(3):301–18. doi: 10.1007/s10911-010-9189-6. [DOI] [PubMed] [Google Scholar]
- 94.Korpos E, Wu C, Sorokin L. Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des. 2009;15(12):1349–57. doi: 10.2174/138161209787846685. [DOI] [PubMed] [Google Scholar]
- 95.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67(2):149–59. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 96.Lortat-Jacob H, Garrone P, Banchereau J, Grimaud JA. Human interleukin 4 is a glycosaminoglycan-binding protein. Cytokine. 1997;9(2):101–5. doi: 10.1006/cyto.1996.0142. [DOI] [PubMed] [Google Scholar]
- 97.Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague–Dawley Rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5(2):211–25. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- 98.Hinz B. Formation and function of the myofibroblast during tissue repair. J Investig Dermatol. 2007;127(3):526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 99.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 100.Flanders KC, Wakefield LM. Transforming growth factor-(beta)s and mammary gland involution; functional roles and implications for cancer progression. J Mammary Gland Biol Neoplasia. 2009;14(2):131–44. doi: 10.1007/s10911-009-9122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174(9):5215–23. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitamura M. Identification of an inhibitor targeting macrophage production of monocyte chemoattractant protein-1 as TGF-beta 1. J Immunol. 1997;159(3):1404–11. [PubMed] [Google Scholar]
- 103.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282(5394):1714–7. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 104.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4 + CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162(8):4567–75. [PubMed] [Google Scholar]
- 106.Wahl SM, Chen W. Transforming growth factor-beta-induced regulatory T cells referee inflammatory and autoimmune diseases. Arthritis Res Ther. 2005;7(2):62–8. doi: 10.1186/ar1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 108.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3(6):589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40(6–7):1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown EJ. The role of extracellular matrix proteins in the control of phagocytosis. J Leukoc Biol. 1986;39(5):579–91. doi: 10.1002/jlb.39.5.579. [DOI] [PubMed] [Google Scholar]
- 111.Yang KD, Augustine NH, Shaio MF, Bohnsack JF, Hill HR. Effects of fibronectin on actin organization and respiratory burst activity in neutrophils, monocytes, and macrophages. J Cell Physiol. 1994;158(2):347–53. doi: 10.1002/jcp.1041580217. [DOI] [PubMed] [Google Scholar]
- 112.Beezhold DH, Personius C. Fibronectin fragments stimulate tumor necrosis factor secretion by human monocytes. J Leukoc Biol. 1992;51(1):59–64. doi: 10.1002/jlb.51.1.59. [DOI] [PubMed] [Google Scholar]
- 113.Marom B, Rahat MA, Lahat N, Weiss-Cerem L, Kinarty A, Bitterman H. Native and fragmented fibronectin oppositely modulate monocyte secretion of MMP-9. J Leukoc Biol. 2007;81(6):1466–76. doi: 10.1189/jlb.0506328. [DOI] [PubMed] [Google Scholar]
- 114.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, et al. A site on laminin alpha 5, AQARSA ASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003;171(1):398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 115.Adair-Kirk TL, Atkinson JJ, Kelley DG, Arch RH, Miner JH, Senior RM. A chemotactic peptide from laminin alpha 5 functions as a regulator of inflammatory immune responses via TNF alpha-mediated signaling. J Immunol. 2005;174(3):1621–9. doi: 10.4049/jimmunol.174.3.1621. [DOI] [PubMed] [Google Scholar]
- 116.McCready J, Arendt LM, Glover E, Iyer V, Briendel JL, Lyle SR, et al. Pregnancy-associated breast cancers are driven by differences in adipose stromal cells present during lactation. Breast Cancer Res. 2014;16(1):R2. doi: 10.1186/bcr3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 118.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K-i, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Acedo SC, Gambero S, Cunha FG, Lorand-Metze I, Gambero A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell Dev Biol Anim. 2013;49(6):473–8. doi: 10.1007/s11626-013-9629-x. [DOI] [PubMed] [Google Scholar]
- 120.Klein-Wieringa IR, Andersen SN, Kwekkeboom JC, Giera M, de Lange-Brokaar BJ, van Osch GJ, et al. Adipocytes modulate the phenotype of human macrophages through secreted lipids. J Immunol. 2013;191(3):1356–63. doi: 10.4049/jimmunol.1203074. [DOI] [PubMed] [Google Scholar]
- 121.Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293(5):C1481–8. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 122.Lin Y, Li Q. Expression and function of leptin and its receptor in mouse mammary gland. Sci China C Life Sci. 2007;50(5):669–75. doi: 10.1007/s11427-007-0077-2. [DOI] [PubMed] [Google Scholar]
- 123.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17(1):42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 124.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185(2):1169–76. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 125.Jensen F, Woudwyk M, Teles A, Woidacki K, Taran F, Costa S, et al. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS One. 2010;5(12):e14409. doi: 10.1371/journal.pone.0014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44(8):1977–85. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, et al. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187(5):2386–93. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 128.Pernis AB. Estrogen and CD4+ T cells. Curr Opin Rheumatol. 2007;19(5):414–20. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- 129.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–64. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 130.Fu Y, Li L, Liu X, Ma C, Zhang J, Jiao Y, et al. Estrogen promotes B cell activation in vitro through down-regulating CD80 molecule expression. Gynecol Endocrinol. 2011;27(8):593–6. doi: 10.3109/09513590.2010.507281. [DOI] [PubMed] [Google Scholar]
- 131.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109(12):1625–33. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176(5):2703–10. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- 133.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14(2):425–31. [PubMed] [Google Scholar]
- 134.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56(20):4625–9. [PubMed] [Google Scholar]
- 135.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82(5):765–70. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 136.Lee AH, Happerfield LC, Bobrow LG, Millis RR. Angiogenesis and inflammation in invasive carcinoma of the breast. J Clin Pathol. 1997;50(8):669–73. doi: 10.1136/jcp.50.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Volodko N, Reiner A, Rudas M, Jakesz R. Tumour-associated macrophages in breast cancer and their prognostic correlations. Breast. 1998;7(2):99–105. doi: 10.1016/S0960-9776(98)90065-0. [DOI] [Google Scholar]
- 138.McDermott RS, Deneux L, Mosseri V, Vedrenne J, Clough K, Fourquet A, et al. Circulating macrophage colony stimulating factor as a marker of tumour progression. Eur Cytokine Netw. 2002;13(1):121–7. [PubMed] [Google Scholar]
- 139.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–9. [PubMed] [Google Scholar]
- 140.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. 2001. pp. 727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.cd-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132(1):215–23. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohara M, Yamaguchi Y, Matsuura K, Murakami S, Arihiro K, Okada M. Possible involvement of regulatory Tcells in tumor onset and progression in primary breast cancer. Cancer Immunol Immunother. 2009;58(3):441–7. doi: 10.1007/s00262-008-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 147.Yan M, Jene N, Byrne D, Millar EK, O’Toole SA, McNeil CM, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13(2):R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277(24):21123–9. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 149.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70(19):7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fend L, Accart N, Kintz J, Cochin S, Reymann C, Le Pogam F, et al. Therapeutic effects of anti-CD115 monoclonal antibody in mouse cancer models through dual inhibition of tumor-associated macrophages and osteoclasts. PLoS One. 2013;8(9):e73310. doi: 10.1371/journal.pone.0073310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncolmmunology. 2013;2(12):e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, et al. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010;70(1):109–18. doi: 10.1158/0008-5472.can-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, et al. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 2009;69(5):2133–40. doi: 10.1158/0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X, Tian W, Cai X, Wang X, Dang W, Tang H, et al. Hydrazinocurcumin Encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS One. 2013;8(6):e65896. doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Faure E, Heisterkamp N, Groffen J, Kaartinen V. Differential expression of TGF-beta isoforms during postlactational mammary gland involution. Cell Tissue Res. 2000;300(1):89–95. doi: 10.1007/s004410000183. [DOI] [PubMed] [Google Scholar]
- 157.Nguyen AV, Pollard JW. Transforming growth factor beta3 induces cell death during the first stage of mammary gland involution. Development. 2000;127(14):3107–18. doi: 10.1242/dev.127.14.3107. [DOI] [PubMed] [Google Scholar]
- 158.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol. 2011;18(3):603–7. doi: 10.1245/s10434-010-1442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12(12):1597–611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Silanikove N. Natural and abrupt involution of the mammary gland affects differently the metabolic and health consequences of weaning. Life Sci. 2014;102(1):10–5. doi: 10.1016/j.lfs.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 162.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, et al. Postpartum remodeling, lactation, and breast cancer risk: summary of a national cancer institute-sponsored workshop. J Natl Cancer Inst. 2013;105(3):166–74. doi: 10.1093/jnci/djs505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bernier MO, Plu-Bureau G, Bossard N, Ayzac L, Thalabard JC. Breastfeeding and risk of breast cancer: a metaanalysis of published studies. Hum Reprod Update. 2000;6(4):374–86. doi: 10.1093/humupd/6.4.374. [DOI] [PubMed] [Google Scholar]
- 165.Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187–95. doi: 10.1016/s0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 166.Michels KB, Willett WC, Rosner BA, Manson JE, Hunter DJ, Colditz GA, et al. Prospective assessment of breastfeeding and breast cancer incidence among 89,887 women. Lancet. 1996;347(8999):431–6. doi: 10.1016/s0140-6736(96)90010-0. [DOI] [PubMed] [Google Scholar]
- 167.Jordan I, Hebestreit A, Swai B, Krawinkel MB. Breast cancer risk among women with long-standing lactation and reproductive parameters at low risk level: a case-control study in Northern Tanzania. Breast Cancer Res Treat. 2013;142(1):133–41. doi: 10.1007/s10549-010-1255-7. [DOI] [PubMed] [Google Scholar]
- 168.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1883–91. doi: 10.1158/1055-9965.EPI-11-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137(2):579–87. doi: 10.1007/s10549-012-2365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stuebe AM, Willett WC, Xue F, Michels KB. Lactation and incidence of premenopausal breast cancer: a longitudinal study. Arch Intern Med. 2009;169(15):1364–71. doi: 10.1001/archinternmed.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ishida T, Yokoe T, Kasumi F, Sakamoto G, Makita M, Tominaga T, et al. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case–control study in Japan. Jpn J Cancer Res. 1992;83(11):1143–9. doi: 10.1111/j.1349-7006.1992.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martinez ME, Wertheim BC, Natarajan L, Schwab R, Bondy M, Daneri-Navarro A, et al. Reproductive factors, heterogeneity, and breast tumor subtypes in women of Mexican descent. Cancer Epidemiol Biomark Prev. 2013;22(10):1853–61. doi: 10.1158/1055-9965.epi-13-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gustbee E, Anesten C, Markkula A, Simonsson M, Rose C, Ingvar C, et al. Excessive milk production during breast-feeding prior to breast cancer diagnosis is associated with increased risk for early events. Springerplus. 2013;2(1):298. doi: 10.1186/2193-1801-2-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sotgia F, Casimiro MC, Bonuccelli G, Liu M, Whitaker-Menezes D, Er O, et al. Loss of caveolin-3 induces a lactogenic microenvironment that is protective against mammary tumor formation. Am J pathol. 2009;174(2):613–29. doi: 10.2353/ajpath.2009.080653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64(19):7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]


