Abstract
Neuropeptide Y (NPY) is present in the superficial laminae of the dorsal horn and inhibits spinal nociceptive processing, but the mechanisms underlying its anti-hyperalgesic actions are unclear. We hypothesized that NPY acts at neuropeptide Y1 receptors in dorsal horn to decrease nociception by inhibiting substance P (SP) release, and that these effects are enhanced by inflammation. To evaluate SP release, we used microdialysis and neurokinin 1 receptor (NK1R) internalization in rat. NPY decreased capsaicin-evoked SP-like immunoreactivity in microdialysate of the dorsal horn. NPY also decreased non-noxious stimulus (paw brush)-evoked NK1R internalization (as well as mechanical hyperalgesia and mechanical and cold allodynia) after intraplantar injection of carrageenan. Similarly, in rat spinal cord slices with dorsal root attached, [Leu31, Pro34]-NPY inhibited dorsal root stimulus-evoked NK1R internalization. In rat dorsal root ganglion neurons, Y1 receptors colocalized extensively with calcitonin gene-related peptide (CGRP). In dorsal horn neurons, Y1 receptors were extensively expressed and this may have masked detection of terminal co-localization with CGRP or SP. To determine whether the pain inhibitory actions of Y1 receptors are enhanced by inflammation, we administered [Leu31, Pro34]-NPY after intraplantar injection of complete Freund's adjuvant (CFA) in rat. We found that [Leu31, Pro34]-NPY reduced paw clamp-induced NK1R internalization in CFA rats but not uninjured controls. To determine the contribution of increased Y1 receptor-G protein coupling, we measured [35S]GTPγS binding simulated by [Leu31, Pro34]-NPY in mouse dorsal horn. CFA inflammation increased the affinity of Y1 receptor G-protein coupling. We conclude that Y1 receptors contribute to the anti-hyperalgesic effects of NPY by mediating inhibition of SP release, and that Y1 receptor signaling in the dorsal horn is enhanced during inflammatory nociception.
Keywords: pain, G-protein, Neurokinin-1 receptor, calcitonin gene-related peptide, isolectin B4, capsaicin
1.0 INTRODUCTION
Neuropeptide Y (NPY) is a 36- amino acid peptide that acts at G-protein-coupled Y receptors to modulate a variety of physiological processes (Hokfelt et al., 1998). NPY is present in superficial laminae of the dorsal horn of the spinal cord, where it is up-regulated during inflammation (Gibson et al., 1984, Ji et al., 1994). The location and function of NPY receptors in the spinal cord is being actively investigated. In particular, the Y1 receptor (Y1) for NPY is found in small dorsal horn interneurons and in dorsal root ganglion (DRG) neurons that contain calcitonin gene-related peptide (CGRP) (Ji et al., 1994, Zhang et al., 1994). Intrathecal administration of NPY inhibited hyperalgesia associated with nerve injury and inflammation and the expression of Fos, a marker of neuronal activity (Taiwo and Taylor, 2002, Mahinda and Taylor, 2004, Intondi et al., 2008, Kuphal et al., 2008). Both of these effects of NPY were blocked by Y1 receptor antagonists. However, the mechanisms by which NPY exerts its anti-hyperalgesic actions are unknown, and their elucidation is the objective of this study.
Substance P (SP) is released into the dorsal horn upon noxious stimulation or electrical stimulation of C-fibers (Yaksh et al., 1980, Allen et al., 1997a, Calcutt et al., 2000, Adelson et al., 2009, Chen and Marvizon, 2009a). SP then binds to neurokinin 1 receptors (NK1Rs) in lamina I and III neurons causing their internalization (Mantyh et al., 1989), which has been extensively used to indirectly estimate SP release (Mantyh et al., 1995, Abbadie et al., 1997b, Allen et al., 1997b, Honore et al., 1999b, Adelson et al., 2009, Chen and Marvizon, 2009a, Zhang et al., 2010b). Selective elimination of the NK1R neurons with the neurotoxin SP-saporin show that they play a key role in inflammatory and neuropathic nociception (Nichols et al., 1999), as was previously indicated by the effect of NK1R antagonists (Radhakrishnan and Henry, 1991, Yamamoto and Yaksh, 1991, Ren et al., 1996). Taken together, these results indicate that SP release and the resulting NK1 receptor activation contribute to persistent pain.
The great majority of SP immunoreactivity in the dorsal horn is in primary afferent terminals, as indicated by colchicine-free immunohistochemical studies (Tuchscherer and Seybold, 1989, Marvizon et al., 2009). Although early studies suggested that substance P is expressed by dorsal horn neurons (Hunt et al., 1981, Katoh et al., 1988, Senba et al., 1988, Ribeiro-da-Silva et al., 1991), all of these used colchicine to arrest axonal transport of neuropeptides and thus increase their immunoreactivity in the neuronal soma. However, colchicine has been shown to alter gene expression in the CNS, in particular the expression of genes encoding neuropeptides (Cortes et al., 1990, Ceccatelli et al., 1991a, Ceccatelli et al., 1991b, Aguado et al., 1999). Therefore, we investigated the possibility that NPY, acting on Y1 receptors, inhibits the release of SP from primary afferent terminals in the dorsal horn. To test this idea, we measured SP release with in vivo microdialysis and with NK1R internalization in spinal cord slices, in naïve rats and in two rat models of inflammatory pain; intraplantar (i.pl) injection of complete Freund's adjuvant (CFA) or carrageenan.
We recently reported that following cutaneous inflammation or nerve injury, NPY receptors exert a tonic, long-lasting inhibitory control of spinal nociceptive processing (Solway et al., 2011). In uninjured animals, the anti-hyperalgesic effects of NPY are less robust, raising the hypothesis that spinal inhibitory signaling of Y1 receptors increases during inflammation. To evaluate this hypothesis, we performed functional G-protein binding assays in dorsal horn neurons of mouse spinal cord slices following i.pl. CFA.
2.0 MATERIALS AND METHODS
2.1 Animals
Animals were male Sprague-Dawley rats housed on a 12:12 h light-dark cycle or C57BL/6 mice housed on a 14:10 h light-dark cycle. Food and water was provided ad libitum in a humidity-controlled room. For the microdialysis studies at Karolinska Institutet, rats (310–350 g) were obtained from B&K Universal AB (Sollentuna, Sweden) and housed at 20°C. For the carrageenan studies at University of Missouri-Kansas City, rats were obtained from Charles Rivers laboratories (Portage) and housed at 21–23°C. For the spinal cord slice and CFA studies at UCLA, rats were obtained from Harlan laboratories (Indianapolis, IND) and housed at 21–23°C. For functional binding studies at the University of Kansas Medical Center, mice (20–30g) were obtained from Charles Rivers Laboratories (Portage, Michigan) and housed at 20–22°C. Experimental drugs were given only once to each animal. All animal use procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental protocols were approved by the regional ethical committee for experiments on laboratory animals in Stockholm, and the Institutional Animal Care and Use Committee (IACUC) at the University of Missouri-Kansas City, the University of Kentucky and the VA Greater Los Angeles Healthcare System.
2.2 NK1 Internalization Assay in Spinal Cord Slices
2.2.1. Media
Artificial cerebrospinal fluid (aCSF) contained (in mM) 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2 and 10 glucose. Sucrose-aCSF was the same medium with 5 mM KCl and 215 mM sucrose instead of NaCl (iso-osmotic replacement). High K+-aCSF was aCSF containing 5 mM KCl. These media were bubbled with 95% O2 / 5% CO2 for a pH of 7.4.
2.2.2.Preparation
Spinal cords were extracted from 3–4 weeks old Sprague-Dawley rats (Harlan, Indianapolis, IND) under isoflurane anesthesia (Halocarbon Laboratories, River Edge, NJ), as described (Lao et al., 2003a, Marvizon et al., 2003a, Song and Marvizon, 2003, Lao and Marvizon, 2005b, Song and Marvizon, 2005, Adelson et al., 2009). A lumbar spinal cord segment (L2–L4) was rapidly extracted, cleaned of dura mater and ventral roots in ice-cold sucrose-aCSF and glued vertically to a block of agar on the stage of the vibratome. Coronal slices (400 μm, 3–4 per rat) with one dorsal root were cut in ice-cold sucrose-aCSF with a vibratome (Integraslice 7550PSDS, Lafayette Instruments, Lafayette, IN) using low advance speed and fast vibration. Fiber continuity between the root and the dorsal funiculus was assessed by examining the dorsal root and the dorsal surface of the slice with a stereo microscope. Slices were left to recover in oxygenated high-K+ aCSF at 35°C for 1 hr, transferred to regular aCSF and used within 3 hr of preparation.
2.2.3.Dorsal root stimulation
The spinal cord slice with attached dorsal root was placed in a custom-made superfusion chamber, where it was stimulated electrically (Adelson et al., 2009). Slices were superfused at 3–6 ml/min with aCSF at 35°C. A bipolar platinum stimulation electrode (0.5 mm wire diameter, 1 mm separation) was located in a compartment separated from the superfusion chamber by a movable partition. The dorsal root was drawn into the electrode compartment through a hole in the partition (sealed with vacuum grease) and placed on top of the platinum wires. The electrode compartment was then emptied of aCSF and filled with mineral oil. Contact between the root and the electrode wires and the thickness of the sheet of aCSF surrounding the root was monitored with a stereomicroscope, and any excess aCSF short-circuiting the electrode was suctioned away. Electrical stimulation was generated by a Master-8 stimulator and Iso-Flex stimulus isolating unit in constant voltage mode (A.M.P. Instruments, Jerusalem, Israel), and consisted of square pulses of 20 V intensity, 0.4 ms duration. Following electrical stimulation, slices were kept in the chamber for 10 min and then fixed in ice-cold fixative (4 % paraformaldehyde, 0.18 % picric acid). Drugs were added to the aCSF beginning 5–10 min before root stimulation, and were superfused continuously thereafter. Drugs were [Leu31,Pro34]NPY (AnaSpec, Inc) and BIBO3304 (a generous gift from Henri Doods, Boehringer Ingelheim). To recognize the side ipsilateral to the stimulus in the histological sections, a small hole was punched in the contralateral ventral horn of the slice. For the SP-induced NK1R internalization experiment, spinal cord slices without dorsal roots were superfused with 1 μM Substance P (Tocris) and 100 nM [Leu31,Pro34]NPY for 10 min, followed by paraformaldehyde fixation.
2.3 Immunohistochemistry
Rats were euthanized with pentobarbital (100 mg/kg, i.p.) and fixed by aortic perfusion of 100 ml phosphate buffer (0.1 M sodium phosphate, pH 7.4) or phosphate buffer saline (PBS), followed by 400–500 ml of ice-cold 4% paraformaldehyde, 0.18% picric acid in phosphate buffer (CFA studies) or 10% formalin in phosphate buffer (other studies). Spinal cord slices were fixed by immersion in ice-cold 4% paraformaldehyde, 0.18% picric acid overnight. A segment of the lumbar spinal cord (L4–L5), slices or DRG were cryoprotected, frozen and sectioned at 25 μm or 40 μm using a cryostat as previously described (Lao et al., 2003b, Marvizon et al., 2003a, Lao and Marvizon, 2005a). Sections were washed four times and then incubated overnight at room temperature with the NK1R antiserum diluted 1:3000 in PBS containing 0.3 % Triton X-100, 0.001 % thimerosal and 5 % normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes, the secondary antibody (1:2000, Alexa Fluor 488 goat anti-rabbit, Molecular Probes-Invitrogen, Eugene, OR) was applied at for 2 hours at room temperature.
For co-labeling for Y1 receptor, CGRP, SP and isolectin-B4 (IB4), sections were blocked with 3 % normal goat serum in PBS containing 0.3 % Triton X-100, and then incubated overnight at room temperature with a mixture of antisera against Y1 receptor (diluted 1:1000, rabbit, from J. Urban), CGRP (diluted 1:1000, guinea pig, from Bachem, Torrance, CA) or SP (diluted 1:1000, goat, Santa Cruz Biotechnology, Santa Cruz, CA). IB4-biotin (diluted 1:100, Sigma) was combined with the antisera in some experiments. After three washes, sections were incubated for 2 hr at room temperature with a mixture of secondary antibodies: Alexa Fluor 568 donkey anti-rabbit (1:1000, Invitrogen), Alexa Fluor 488 donkey anti-guinea pig (1:800, Jackson ImmunoResearch), Alexa Fluor 633 donkey anti-goat (1:1000, Invitrogen). IB4-biotin staining was revealed with streptavidin-Alexa Fluor 488 (1:200, Vector Laboratories, Burlingame, CA). Sections were washed four more times, mounted on glass slides, and coverslips were applied with Prolong Gold (Invitrogen).
2.4 Quantification of NK1R internalization
NK1R internalization provides a selective, reliable and quantifiable indicator of the extent of NK1R activation (Marvizon et al., 2003b). Compared to microdialysis, NK1R internalization has the advantages of determining release in terms of receptor activation, laminar distribution and target neuron morphology (Liu et al., 1997, Trafton et al., 1999, Riley et al., 2001, Adelson et al., 2009) and having greater sensitivity to detect SP release (Marvizon et al., 2003a, Marvizon et al., 2003b).
The amount of NK1R internalization was quantified using standard methods (Mantyh et al., 1995, Abbadie et al., 1997a, Riley et al., 2001) with minor modifications (Marvizon et al., 1997, Marvizon et al., 1999, Lao et al., 2003b, Lao and Marvizon, 2005a, Adelson et al., 2009). NK1R-expressing neurons in lamina I were visually counted while classifying them as with, or without, internalization. We used a Zeiss Axio-Imager A1 (Carl Zeiss, Inc., Thornwood, NY) fluorescence microscope with a 63x objective, or a Nikon E800 microscope with a 40x objective. The criterion for having internalization was the presence in the neuronal soma of ten or more NK1R-expressing endosomes, defined as a small region of bright staining separated from the cell surface. The investigator counting the neurons was blinded to the treatment. Four sections per slice were used, counting all lamina I NK1R-expressing neurons in each section. Results were expressed as the percentage of the NK1R-expressing neurons in lamina I with NK1R internalization.
2.5 Confocal microscopy and image processing
Confocal images were acquired using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Inc., Thornwood, NY). A 10x objective (numerical aperture 0.3) was used to take images of the whole dorsal horn from transversal spinal cord sections. A 20x objective (numerical aperture 0.8) was used to take images from DRG sections. A 63x oil immersion objective (numerical aperture 1.4) was used to take high magnification images from transverse and sagittal spinal cord sections. Laser excitation lines and emission windows for the different fluorophores were: Alexa Fluor 488 - excitation 488 nm (Ar laser), emission 500–540 nm; Alexa Fluor 568 - excitation 561 nm (diode laser), emission 580–630 nm; Alexa Fluor 633 - excitation 633 nm (HeNe laser), emission 650–720 nm. The pinhole was 1.0 Airy unit, and its actual width was set to that value for each objective and fluorophore. Images were acquired as confocal stacks of sections of 1024 × 1024 pixels. The separation between optical sections was determined by the microscope software by applying the Nyquist formula to the objective used; it was 6.66 μm for the 10x objective, 1.02 μm for the 20x objective and 0.43 μm for the 63x objective.
Imaris 6.1.5 (Bitplane AG, Zurich, Switzerland) was used to crop the images in three dimensions and to generate a two-dimension projection picture. This picture was imported into Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA), which was used to assemble the multi-panel figures.
2.6 Quantification of Co-localization
In dorsal horn images, our goal was to determine whether the targets of the antibodies (for example, the Y1 receptor and a neuropeptide) were present in the same small neuronal compartments: presynaptic terminals, axons or dendrites. This was assumed to be the case when the two labels were present in the tissue with enough proximity that they cannot be resolved optically (Hibbs et al., 2006). This type of co-localization was measured using the Co-localization module of the Imaris software, which analyzes two-channel stacks of confocal sections by measuring the intensity of each label in each voxel. In triple-label images, co-localization was measured in all the three possible two-channel combinations. The extent of co-localization of two labels was measured as the Pearson correlation coefficient of the intensity of the labels in the voxels in a confocal stack. Voxels belonging to the background were excluded from the comparison by limiting the measures to voxels with intensities higher than 20 or 30, in the scale of 0–255. The Pearson coefficient indicates the extent to which the intensities of the two labels increase in parallel with each other in the same voxels. It varies between +1 and −1, with positive values indicating a direct correlation, negative values indicating an inverse correlation, and values near 0 indicating no correlation. Based on the results obtained with negative and positive co-localization controls in a previous study (Marvizon et al., 2009), Pearson coefficients below 0.1 were interpreted as no co-localization, and Pearson coefficients above 0.5 as near-maximal co-localization.
In DRG images, our goal was to determine whether the two labels were present in the same neuronal body regardless of their proximity. It was measured by visually counting neuronal profiles with each of the labels and with the two labels together, and then calculating the percentage of double-labeled profiles relative to each of the single-labeled profiles.
2.7 Substance P Microdialysis
The microdialysis probe was inserted into the lumbar dorsal horn at L5–L6 under visual control, as previously described and illustrated (Gustafsson et al., 1999). In brief, under halothane anesthesia, the spinous processes of the lower thoracic vertebral column were fixed to a stereotaxic apparatus with two clamps. After a laminectomy at T13, a hole was pierced through the dura and pia mater at the level of the left lumbar enlargement (spinal cord segment L5–L6), medial to the dorsal root entry zone. Next, using a micromanipulator, the tip of the microdialysis probe (CMA/11, CMA Microdialysis, Sweden; cuprophan dialyzing membrane: molecular cut-off 6 kD, diameter 0.24 mm, length 2 mm) was inserted into the dorsal horn in a rostro-caudal direction at an angle of 45° from the horizontal plane to a depth of 1.5 mm.
Prior to each experiment, tubing and dialysis probes were perfused with 0.5% sodium hypochlorite buffered with sodium bicarbonate for 15 min, followed by 70% ethanol for 15 minutes, deionized water for 15 min, and finally Krebs-Ringer solution (138 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1mM CaCl2, 11mM NaHCO3, 1 mM NaH2PO4) for 30 min. During the microdialysis experiments, probes were continuously perfused (flow rate: 3.5 μl/min) with Krebs-Ringer solution containing 0.2% BSA (Sigma), 0.2% glucose (Sigma) and 0.03% of the peptidase inhibitor, bacitracin (Sigma). Following a washout period of 60 min, five baseline dialysate samples (105 μl) were collected at 30 min intervals. Twenty min later, 50 μl of either saline or NPY (1 μg/μl, Anaspec) were directly applied to the dorsal surface of the spinal cord. Ten min later, a Krebs-Ringer solution containing capsaicin (Sigma) was delivered by reverse microdialysis at a concentration of 25 μM for 30 min, as described previously (Wallin and Schott, 2002, Kjorsvik Bertelsen et al., 2003, Afrah et al., 2004). After cessation of capsaicin perfusion, two more samples were collected. All samples were collected in Eppendorf vials and stored at −20°C prior to radioimmunoassay.
2.8 Substance P Radioimmunoassay
Following collection of the microdialysis samples and storage at −20o C, the samples were analyzed directly by radioimmunoassay (RIA) without any further preparation. The RIA used to quantify SP-like immunoreactivity (SP-LI) has previously been described in detail elsewhere (Brodin et al., 1986). The lower detection limit for SP was 0.6–1.2 pM, and the IC50 value (i.e., the concentration of synthetic SP required to inhibit the binding of radioactivity by 50%) was 4.8 pM.
2.9 Intrathecal Drug Delivery
2.9.1 Intrathecal catheterization
Rats were implanted with chronic intrathecal catheters inserted between the L5 and L6 lumbar vertebrae (Storkson et al., 1996, Chen and Marvizon, 2009b). Catheterization was performed differently in the carrageenan and in the CFA experiments. Rats (2–4 months old) were anesthetized with isoflurane (CFA experiments) or ketamine/xylazine (carrageenan experiments) and placed on a warmed metal platform. The intrathecal catheter was either a PE-5 tube of 20 mm heat-fused to a PE-10 tube of 150 mm (CFA experiments), or a polyurethane 32-G tube of 10 cm of tubing with a steel wire inside (Micor, carrageenan experiments). A 2 cm incision in the lumbar skin and muscle was made to expose the lumbar vertebrae, and a 20 or 23 gauge needle was inserted at a 30° angle between vertebrae L5 and L6 to puncture the dura mater, which was inferred from a flick of the tail or paw and the backflow of spinal fluid. The needle was removed and the catheter was inserted into the subdural space and advanced rostrally 3.5 cm to terminate over lumbar segments L5–L6. The steel wire was removed (when present) and the catheter was secured to the fascia. Catheters were then tunneled subcutaneously, exteriorized at the nape, and secured to the dorsal neck muscles with suture. The catheter was flushed with 10 μl saline and sealed. CFA rats were given an antibiotic (enrofloxacin) and an analgesic (carprofen) twice daily for 3 days after induction of inflammation. Rats were housed separately and used for the experiment 5–7 days after induction. A criterion for immediate euthanasia of the rat was the presence of motor weakness or signs of paresis, but this did not occur in any of the rats. The position of the catheter was examined postmortem. We established as exclusion criteria: 1) loss of the catheter, 2) termination of the catheter inside the spinal cord, or 3) occlusion of the catheter tip.
2.9.2 Intrathecal injection
volume was 5–10 μl of drug followed by a 10 μl saline flush (Zorman et al., 1982, Jensen and Yaksh, 1984, Aimone et al., 1987, Kondo et al., 2005). These volumes predominantly distribute the drug throughout the spinal cord but not the brain (Yaksh and Rudy, 1976, Chen et al., 2007). Solutions were preloaded, in reverse order of administration, into a tube (PE-10), and delivered with a 50 μl Hamilton syringe within 1 min.
2.10 Pain Models
2.10.1 Intraplantar Carrageenan
Carrageenan (1% in 100 μl of saline) was injected subcutaneously under the dorsal skin of the left hind paw of the rat under halothane anesthesia (Fluothane, AstraZeneca, UK, 1.5 % at an airflow of 2 L/min). We evaluated behavioral responses before (baseline) and 2 hr after carrageenan, and then again at 50 min after the intrathecal injection. Rats were anesthetized 3 min later with pentobarbital (50 mg/kg, i.p.) and a light mechanical stimulus (toothbrush) was applied once per second for 2 min to the plantar skin of the inflamed paw (Honore et al., 1999b). Five minutes after the last period of mechanical stimulation (3 hours after carrageenan, and at the peak of NPY anti-hyperalgesia at 60 min (Taiwo and Taylor, 2002)}), rats were deeply anesthetized and perfused with 10% buffered formalin.
2.10.2 Intraplantar CFA
Rats were anesthetized (2–3% isoflurane) and injected subcutaneously with CFA (undiluted, Sigma) under the plantar surface of the left hindpaw. Volumes of 100 μl and 5 μl were used for rats and mice, respectively.
2.10.3 Noxious mechanical stimulation
Rats were anesthetized with isoflurane (2–3%) and a noxious mechanical stimulus (clamping one hindpaw with a hemostat closed to the first notch) was applied for 30 sec (Abbadie et al., 1997b). Ten minutes later, the rats were euthanized with pentobarbital (100 mg/Kg, i.p.) and perfused with paraformaldehyde.
2.11 Behavioral tests of hyperalgesia and allodynia
Animals were acclimated to a stainless steel grid within individual Plexiglas boxes for at least 60 min, and then first tested for mechanical allodynia, then cold allodynia, and then mechanical hyperalgesia. The observer was blinded to treatment by another experimenter.
2.11.1 Mechanical allodynia: von Frey
Mechanical allodynia was assessed using von Frey filaments (Stoelting, Inc, Wooddale, IL). The medial aspect of the plantar surface of each hind paw was mechanically stimulated with an incremental series of 8 monofilaments of logarithmic stiffness. The 50% withdrawal threshold was determined using a modified up-down method of Dixon, as previously described (Chaplan et al., 1994). First, an intermediate von Frey monofilament (number 4.31, exerts 2.0 g of force) was applied perpendicular to the glabrous skin, causing a slight bending. In the case of a positive response (immediate withdraw of the paw) in any of the three areas tested, a filament exerting less force was tested. In the case of a negative response to all three areas of stimulation, a filament exerting a larger force was tested.
2.11.2 Cold allodynia: acetone
A drop of acetone was applied to the plantar surface of the hindpaw using a syringe connected to PE-90 tubing flared at the tip to a diameter of 3.5 mm. Surface tension maintained the volume of the drop at 10–12μl. The duration of time the animal would lift, shake or lick its paw was recorded. Animals were observed for 60 sec following each application of acetone. Three trials, with an interval of at least 1 min between each, were averaged.
2.11.3 Mechanical hyperalgesia: pin prick
Noxious pressure was applied to the lateral surface of the hindpaw using a safety pin to elicit a response. The duration of time the animal lifted, shook or licked its paw was recorded. Animals were observed for 30 sec following each pin application. Three trials, with an interval of at least 1 min between each, were averaged.
2.11.4 Measurement of thermal hyperalgesia
Paw withdrawal latencies were measured using a “Plantar Analgesia Meter” model 390G (IITC Life Sciences, Woodland Hills, CA), consisting of an acrylic enclosure on an elevated warm glass surface (Cheppudira, 2006). Rats were acclimated to the instrument for 30 min for 3 days. The plantar surface of the hind paw was heated from below with a radiant heat source. The intensity of the lamp was set at 30% of maximal power. Cut-off time was 25 s to prevent tissue damage. Paw withdrawal latencies were measured four times at 5 min intervals. Results were calculated as percentage of the maximum possible response (%MPE) (Paronis and Holtzman, 1991):
2.12 [35S]GTPγS Binding Assay
Mice were used for these studies. Spinally-targeted applications of NPY produce a similar pattern of anti-hyperalgesia and neuronal silencing in rats (Taiwo and Taylor, 2002, Intondi et al., 2008) and mice (Naveilhan et al., 2001, Kuphal et al., 2008, Solway et al., 2011). Furthermore, our pilot studies indicated to us that [Leu31,Pro34]-NPY produces similar [35S]GTPγS binding in rats and mice. Therefore, the results obtained here in mice will likely correlate with those obtained in rat. The lumbar enlargement of the mouse spinal cord was dissected and snap-frozen in methyl butane. Sections 50 μm thick were cut with a cryostat and mounted on gelatin-coated glass slides. Sections were equilibrated at room temperature for 10 min in assay buffer (30 mM MgCl2, 150 mM NaCl, 2.7 mM KCl, and 37.5 mM HEPES, pH 7.4) and for 15 min in 2 mM GDP. Agonist-stimulated binding was then performed with 2 mM GDP and 0.1 nM [35S]GTPγS (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and one of four serial dilutions ranging from 45 nM to 45 μM [Leu31,Pro34]-NPY (Sigma-Aldrich) in water treated with a peptidase inhibitor cocktail containing 0.170 mg/ml bacitracin, 17 μg/ml leupeptin, 17 μg/ml chymostatin, and 0.850 mg/ml bovine serum albumin. Drug was omitted and replaced with either peptidase-treated water for basal determinations or 10 μM unlabeled GTPγS in water for nonspecific binding determinations. Slides were incubated at 37°C for 45 min and then rinsed for 2 min twice with ice-cold 50 mM Tris-Cl, pH 7.4 and twice with deionized water at room temperature. Slides were exposed to Kodak X-OMAT autoradiographic film (Sigma-Aldrich) for 18–24 h. Densitometric analysis of images was performed by measuring the mean density of pixels in the superficial laminae of the left and right lumbar dorsal horn and subtracting the mean dorsal column background value. Percent stimulation over basal was calculated using the following equation:
2.13 Data Analysis
Data were analyzed using Prism software (GraphPad, San Diego CA). All data are expressed as mean ± SEM. Statistical significance was set at P < 0.05. Behavioral data were analyzed by two-way ANOVA with treatment as the between-subjects factor and time as the within-subjects factor. Other data were analyzed by one-way or two way ANOVA and Holm-Sidak's or Tukey's post-hoc tests.
Concentration-response data were fitted using non-linear regression by a sigmoidal dose-response function: Y = Emin + (Emax-Emin) / (1 + 10^(Log EC50-Log X)), where X is the concentration of drug, EC50 (IC50 for inhibition) is the concentration of drug that produces half of the maximal effect or inhibition, Emin is the effect at diminishingly low drug concentrations and Emax is the effect at saturating concentrations of drug. In analyzing the Y1 receptor stimulation data, simultaneous fitting of two sets of data to the dose-response function and the `extra sum of squares F-test' were used to determine whether their EC50 and Emax were different. For analysis of the antagonist-displacement data, the “zero antagonist concentration” baseline condition was included in the non-linear regression by assigning it a concentration value three log units lower than the estimated EC50.
3.0 RESULTS
3.1 NPY inhibits capsaicin-induced SP release from the spinal cord
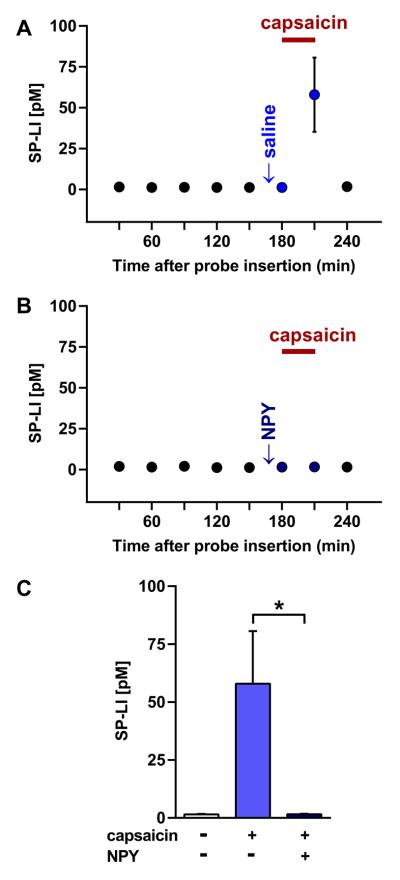
To test the hypothesis that NPY inhibits SP release in the dorsal horn, we used microdialysis to measure SP release in vivo induced by perfusing capsaicin (25 μM) through the dialysis tube for 30 min. As shown in Fig. 1, basal SP release in the dorsal horn was low, as previously reported (Afrah et al., 2001). Reverse dialysis administration of capsaicin increased the extracellular concentration of SP-LI (Fig. 1 A). NPY (1 μg/μl) applied to the dorsal surface of the spinal cord decreased the capsaicin-induced SP release (Fig. 1 B–C; ANOVA: p = 0.0165).
Fig. 1. NPY reduces capsaicin-evoked SP release from the rat spinal cord in vivo.
Microdialysis probes were inserted into dorsal horn at L5. Following a 1 hr washout period, 5 baseline samples were collected at 30 min intervals. Twenty min later, 50 μl of either saline (A, n = 6 rats) or 1 μg/μl NPY (B, n = 5 rats) was directly applied to the dorsal surface of the spinal cord. Ten min later, capsaicin (25 μM, bar) was infused by reverse microdialysis for 30 min during sample collection. SP-LI was detected in 100 μl samples with a high sensitivity RIA. (C) Comparison of SP-LI released during capsaicin or vehicle infusion, with and without NPY. ANOVA, p = 0.0165, n = 5–6; Tukey's post-hoc test, * p< 0.05.
3.2 NPY inhibits NK1R internalization induced by a noxious stimulus
As an alternative measure of SP release in vivo, we used NK1R internalization in dorsal horn neurons evoked by a noxious stimulus delivered to the hindpaw (Mantyh et al., 1995, Abbadie et al., 1997b). The noxious stimulus consisted of clamping one of the hind paws with a hemostat under anesthesia. This evoked a substantial amount of NK1R internalization in lamina I neurons at the ipsilateral dorsal horn (62 ± 8 % NK1R neurons with internalization, n = 4), and much less internalization at the contralateral side (16 ± 3 %, n = 4). An intrathecal injection of 10 μg NPY, 60 min before paw clamp, reduced the evoked NK1R internalization in the ipsilateral side to 30 ± 4 % (n = 7). NPY produced no effect in the contralateral side (16 ± 4 %, n = 7). Two-way ANOVA: NPY vs. saline, p = 0.0051; ipsilateral vs. contralateral, p < 0.0001; interaction, p = 0.0055.
3.3 NPY inhibits hyperalgesia and NK1R internalization induced by a non-noxious stimulus in the carrageenan model of inflammatory pain
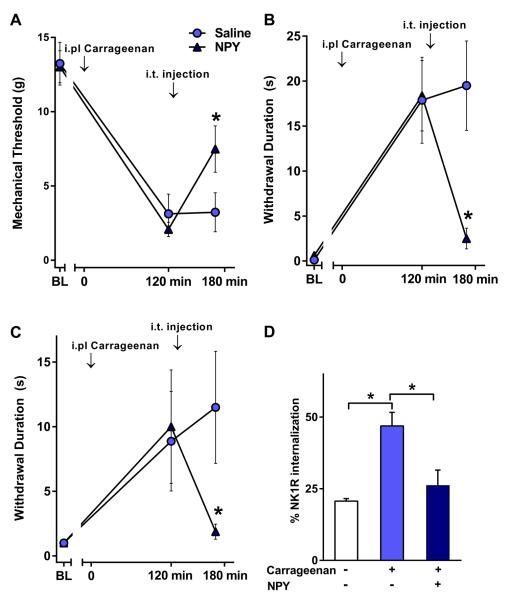
Next, we investigated the effect of NPY on the hyperalgesia produced by carrageenan injected in the hind paw and compared it with its ability to inhibit NK1R internalization in the dorsal horn. In addition, the mechanical allodynia produced by carrageenan provided an opportunity to study the effect of NPY on SP release produced by a non-noxious stimulus in the awake rat (Abbadie et al., 1997b, Honore et al., 1999a). Carrageenan produced multiple signs of hypersensitivity within 2 hr of its injection, including mechanical allodynia to von Frey hair stimulation (Fig. 2 A), cold allodynia to acetone application (Fig. 2 B) and mechanical hyperalgesia to pin prick (Fig. 2 C). Intrathecal administration of NPY (but not saline) produced a robust reduction of all these responses (p < 0.05). A non-noxious stimulus, consisting of application of a toothbrush to the affected plantar skin, induced a substantial amount of NK1R internalization in lamina I neurons in carrageenan-injected rats but not in control rats injected with saline (Fig. 2 D). This is consistent with previous studies (Honore et al., 1999b). NPY significantly (p < 0.05) reduced the NK1R internalization induced by non-noxious stimulation in carrageenan-injected rats.
Fig. 2. Intrathecal NPY inhibits nociception and NK1R internalization induced by non-noxious stimulation in the rat carrageenan model.
Paw withdrawal responses to von Frey hairs (A), acetone (B) and pin prick (C) were evaluated before (BL) and 2 hr after intraplantar carrageenan. Five minutes later, rats received an intrathecal injection of saline or 30 μg NPY. Forty five minutes later, behavior was again assessed followed by perfusion for NK1R internalization. (D) NK1R internalization in lamina I of the L5 spinal segment induced by a non-noxious stimulus (brush). n=6–8, except n=3 for the group not given carrageenan in panel D; *p<0.05 vs. 2 hr, or as indicated.
3.4 Immunohistochemistry for Y1 receptors in the DRG and the dorsal horn
Next, we investigated whether the inhibition of SP release produced by NPY was mediated by Y1 receptors. We started by confirming that Y1 receptors are present in the population of primary afferents that contain SP, which largely overlap with the ones that contain CGRP.
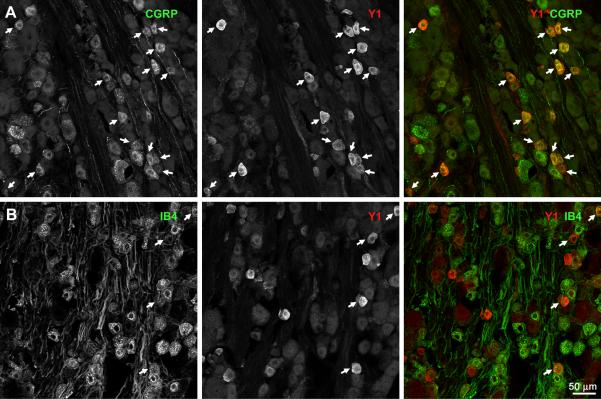
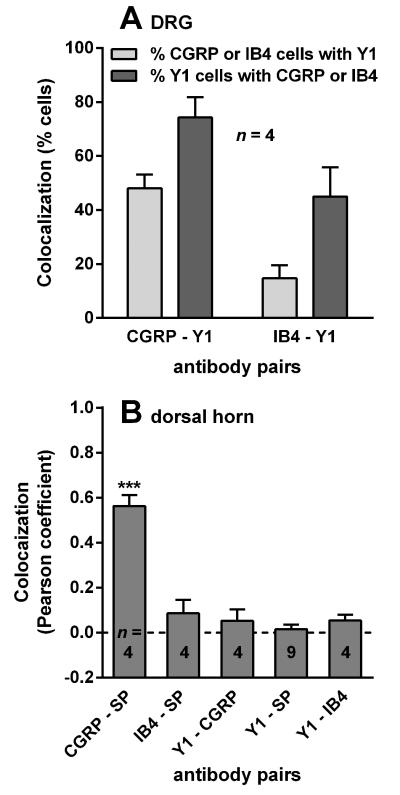
Double-labeling of DRG sections with antibodies against CGRP and Y1 receptors showed that most Y1 receptor-positive DGR neurons also expressed CGRP (Fig. 3 A). In contrast, few Y1 receptor-positive DRG neurons were labeled with isolectin B4, which binds to a different population of C-fibers relative to the CGRP- and SP-containing population (Fig. 3 B). A quantitative analysis of confocal images is shown in Fig. 4 A: about 75% of Y1 receptor-positive DRG cells were also CGRP-positive, and 50% of CGRP-positive cells were Y1 receptor-positive. In contrast, less than 20% of IB4-positive cells expressed Y1 receptors, although about 40% of Y1 receptor cells stained for IB4.
Fig. 3. Presence of Y1 receptors in rat DRG neurons.
Sections from lumbar DRG (L1–L6) were labeled with antibodies against CGRP or the Y1 receptor, or with isolectin B4-biotin (IB4). Each image consists of 4 confocal sections spaced 0.99 μm, taken with a 20x objective. A) CGRP and Y1 receptor immunoreactivities. B) IB4 staining and Y1 receptor immunoreactivity. Arrows indicate double-labeled cell bodies.
Fig. 4. Quantification of Co-localization.
A) DRG sections from rats were double-labeled as indicated, and confocal images were obtained with a 20x objective in 4 confocal stacks of 4–8 optical sections. Co-localization was measured by calculating the percentage of double-labeled profiles relative to each of the single-labeled profiles. ANOVA (2-way): p = 0.0013 comparing antibody pairs, p = 0.0027 for direction of comparison, p = 0.79 for interaction. B) Sections from the rat lumbar spinal cord (L4–L6, transversal and sagittal) were double-labeled as indicated: CGRP, calcitonin gene-related peptide; SP, substance P; Y1, Y1 receptor; IB4, isolectin B4-biotin. Confocal images were obtained from laminae I-II with a 63x objective in n confocal stacks of 10–18 optical sections. Co-localization of the indicated label pairs was measured with Imaris Co-localization as the Pearson correlation coefficient in all voxels in a confocal stack above intensity thresholds of 20 or 30 (in the scale of 0–255). ANOVA, p < 0.0001, F4,20 = 43.02, ***p < 0.001 compared to CGRP - SP (Holm-Sidak's post-hoc test).
In transverse spinal cord sections under low magnification, Y1 receptor staining appeared as a narrow band that overlapped both the area stained by CGRP (Fig. 5 A) and the area stained by IB4 (Fig. 5 B), which largely corresponded to different laminae of the dorsal horn (Fig. 5 C). To determine whether Y1 receptors were present in primary afferents that contained CGRP, SP or IB4, we assessed co-localization of immunoreactivity in laminae I and II of sagittal spinal cord sections using high magnification confocal microscopy. As established previously (Lawson, 1995, Marvizon et al., 2009), we observed substantial co-localization of CGRP and SP immunoreactivity in fibers and puncta (Fig. 6 A). Y1 receptor immunoreactivity was largely restricted to lamina II neurons and their dendrites. We could not find co-localization of Y1 receptor immunoreactivity with either CGRP- and SP-containing fibers, although we occasionally observed them to be in close proximity. SP immunoreactivity and IB4 staining did not co-localize with each other or with Y1 receptors (Fig. 6 B).
Fig. 5. Distribution of Y1 receptors in the rat dorsal horn.
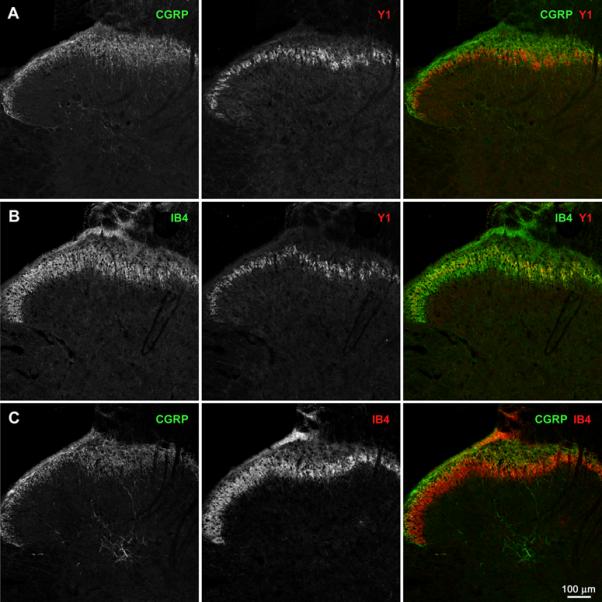
Transverse sections of lumbar spinal cord (L4–L6) were labeled with antibodies against CGRP or the Y1 receptor, or with isolectin B4-biotin (IB4). Each image consists of a single confocal section taken with a 10x objective at the middle of the histological section. Dorsal side is up. A) CGRP and Y1 receptor immunoreactivities. B) IB4 staining and Y1 receptor immunoreactivity. C) CGRP immunoreactivity and IB4 staining.
Fig. 6. Localization of Y1 receptors relative to substance P, CGRP and IB4 staining in the rat superficial dorsal horn.
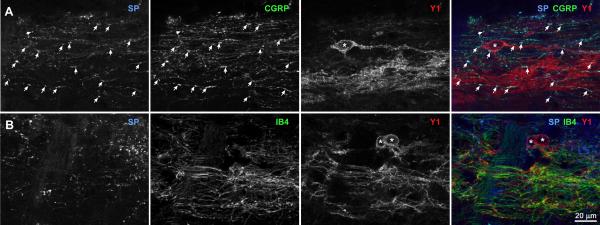
Sagittal sections from the lumbar spinal cord (L4–L6) were labeled with antibodies against substance P (SP), CGRP or the Y1 receptor, or with isolectin B4-biotin (IB4). Confocal images were taken with a 63x objective from the laminae I-II. Optical section separation is 0.44 μm. Dorsal side is up. Arrows indicate instances of SP / CGRP co-localization. Asterisks indicate the cell bodies of Y1 receptor neurons. A) Substance P, CGRP and Y1 receptor immunoreactivities; 7 optical sections. B) Substance P immunoreactivity, IB4 staining and Y1 receptor immunoreactivity; 4 optical sections.
To confirm these qualitative observations, we quantified co-localization in confocal images similar to those shown in Fig. 6. As shown in Fig. 4 B, Pearson coefficients for CGRP-SP were above 0.5, indicating a high level of co-localization. In contrast, Pearson coefficients for IB4-SP, Y1-CGRP, Y1-SP and Y1-IB4 were close to 0.0, indicating that Y1 receptor immunoreactivity is not robust in fibers that stain for CGRP, SP, or IB4.
3.5 A selective Y1 receptor agonist partially inhibits NK1R internalization in spinal cord slices
We next determined whether Y1 are the receptors that mediate the inhibition of SP release by NPY. SP release (measured as NK1R internalization) was elicited in spinal cord slices by electrically stimulating one dorsal root with 1000 pulses of 20 V and 0.4 ms, delivered in a single train at 100 Hz. These stimulation parameters are optimal to induce SP release from Aδ and C fibers (Adelson et al., 2009) and have been previously used to study the pharmacological modulation of SP release (Lao et al., 2003b, Marvizon et al., 2003a, Lao and Marvizon, 2005a, Zhang et al., 2010a). As shown in Fig. 7 A, this stimulus produced NK1R internalization in >50% of NK1R-positive neuronal profiles in lamina I. An example of NK1R internalization in a lamina I neuron ipsilateral to dorsal root stimulation is shown in Fig. 8 A. Negligible NK1R internalization was observed in the contralateral dorsal horn (not shown).
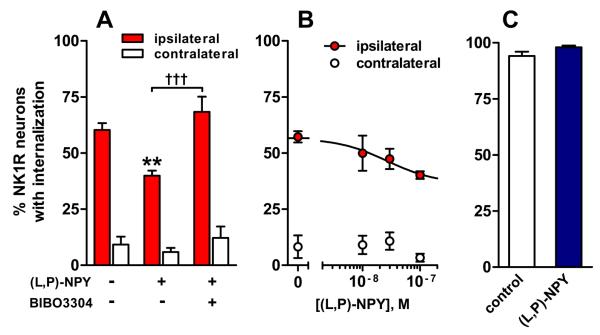
Fig. 7. Y1 receptors decrease NK1R internalization evoked by dorsal root stimulation in rat spinal cord slices.
Spinal cord slices were electrically stimulated at the dorsal root with 1000 pulses of 20 V, 0.4 ms, delivered at 100 Hz, to evoke SP release, which was measured as NK1R internalization in lamina I neurons in the dorsal horns ipsilateral and contralateral to the stimulated root. A) The Y1 receptor agonist [Leu31, Pro34]-NPY [(L,P)-NPY] (100 nM) inhibited the evoked NK1R internalization, and this inhibition was reversed by the Y1 antagonist BIBO3304 (100 nM). ANOVA (2-way): drug, p = 0.0005; stimulus, p < 0.0001; interaction, p = 0.018, n = 3–7. Tukey's post-hoc tests: **, p < 0.01; †††, p<0.001. B) Concentration-response of the Y1 agonist [Leu31, Pro34]-NPY. C) NK1R internalization evoked by incubating the slices with 1 μM substance P (no dorsal root stimulation) was not affected by [Leu31, Pro34]-NPY (100 nM). T-test: n = 3, p > 0.05.
Fig. 8. Examples of NK1R neurons in rat spinal cord slices.
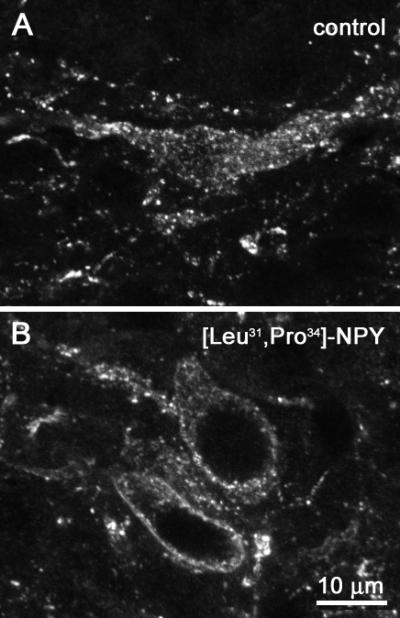
Representative images of lamina I neurons labeled with the NK1R antibody. Dorsal root stimulation of spinal cord slices resulted in extensive NK1R internalization in these neurons in control conditions (A). Superfusion with the Y1 receptor agonist [Leu31, Pro34]-NPY (100 nM) produced a decrease in NK1R internalization (B).
The selective Y1 agonist [Leu31,Pro34]NPY decreased stimulus-evoked NK1R internalization from 57±11 % to 36±5% (Fig. 7 A) with an IC50 of 27 nM (95% CI=0.6-1152 nM, Fig. 7 B). An example of an NK1R neuron in lamina I in a slice superfused with 100 nM [Leu31,Pro34]NPY is shown in Fig. 8 B. To confirm that this effect is mediated by Y1 receptors, we determined whether the selective Y1 antagonist BIBO3304 (Wieland et al., 1998, Dumont et al., 2000) could reverse the inhibition produced by 100 nM [Leu31,Pro34]NPY. As shown in Fig. 7 A, BIBO3304 (100 nM) significantly prevented the inhibition produced by the Y1 agonist.
[Leu31,Pro34]NPY could reduce NK1 internalization either by inhibition of SP release or by direct inhibition of NK1R internalization. To rule out the latter possibility, we induced NK1R internalization in spinal cord slices by incubating them with exogenous SP (1 μM) in the presence and absence of 100 nM [Leu31,Pro34]NPY. Fig. 7 C shows that the Y1 receptor agonist did not affect NK1R internalization induced by SP.
Taken together, these observations indicate that NPY activation of spinal Y1 receptors leads to the inhibition of SP release.
3.6 In the CFA model of inflammation, Y1 receptors reduce hyperalgesia and NK1R internalization induced by noxious stimulation
Next, we studied the inhibition of SP release by Y1 receptors in vivo. As before, SP release was induced with noxious clamp of the hind paw and measured as NK1R internalization in the lumbar spinal cord (L5). [Leu31,Pro34]NPY (30 μg, i.t.) was injected 45 min prior to hind paw clamp to determine whether it inhibits SP release. In contrast to the inhibitory effects of NPY described above, [Leu31,Pro34]NPY did not inhibit NK1R internalization (Fig. 9 B, left).
Fig. 9. [Leu31, Pro34]-NPY inhibits CFA-induced hyperalgesia and NK1R internalization.
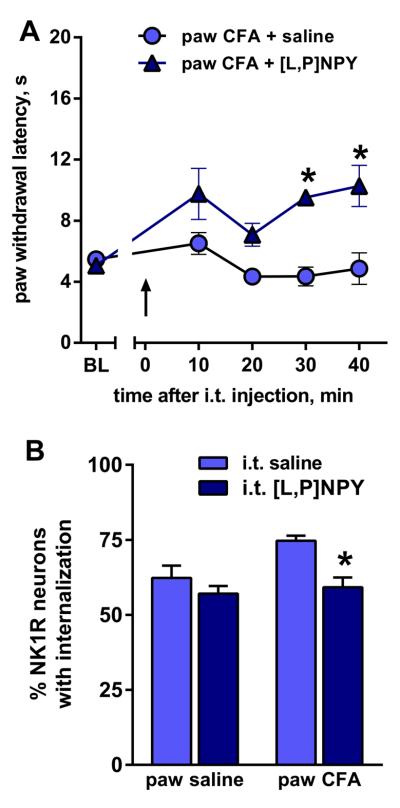
Rats were injected under the plantar skin of one hind paw with saline or CFA; 3 days later they received an intrathecal (i.t.) injection of saline or [Leu31, Pro34]-NPY ([L,P]NPY, 30 μg). A) Withdrawal latencies to heat stimulation in the paw ipsilateral to CFA injection (BL: baseline). n = 3, * p < 0.05. B) NK1R internalization in lamina I of the L5 spinal segment induced by noxious pinch applied to the inflamed paw 45 min after the intrathecal injection. ANOVA (2-way): n = 3, p = 0.0436 for CFA, p = 0.009 for [L,P]NPY, p = 0.129 for interaction. Holm-Sidak's post-hoc test: * p < 0.05 compared to i.t. saline.
We then investigated whether the inhibitory effect of Y1 receptors increased during chronic nociception, using the CFA model of inflammatory hyperalgesia. Three days following CFA, paw withdrawal latency to radiant heat dropped from approximately 10 s to 5 s. Rats then received intrathecal [Leu31,Pro34]NPY (30 μg), and withdrawal latencies were measured every 10 min (Fig. 9 A). Intrathecal [Leu31, Pro34] NPY increased ipsilateral withdrawal latency to baseline values of approximately 10 s (Fig. 9 A), with no effect at the contralateral side (not shown). The effect of NPY on NK1R internalization in the lumbar (L5) spinal cord was studied in these same rats, measured 45 min after intrathecal injection. Compared with rats that received intrathecal saline, [Leu31, Pro34] NPY significantly reduced NK1R internalization (Fig. 9 B). In sham-injured rats, [Leu31, Pro34] NPY had no effects (data not shown). Taken together, these results suggest that inflammation amplifies the ability of Y1 receptors to exert anti-hyperalgesic effects through inhibition of SP release.
3.7 CFA inflammation increases coupling between Y1 receptors and G-proteins in dorsal horn
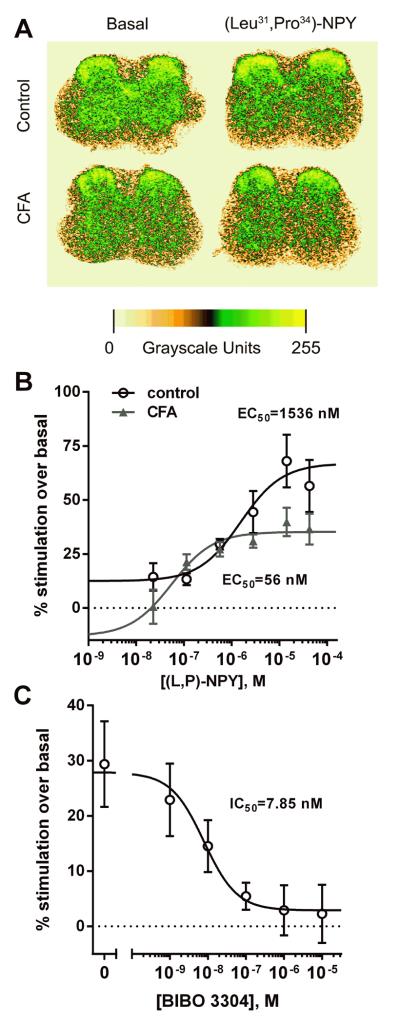
Next, we investigated changes in Y1 receptor-mediated inhibitory signaling using functional G-protein coupling assays ([35S]GTPγS binding) during inflammation. Two days before the experiment, mice received an intraplantar injection of saline (control) or CFA in the left hindpaw. Spinal cord sections were incubated with 2 mM GDP, 0.1 nM [35S]GTPγS and the Y1 receptor agonist [Leu31, Pro34] NPY. Figure 10 A shows representative autoradiograms of [35S]GTPγS binding stimulated by 42.5 μM [Leu31, Pro34] NPY in sections of the spinal cord of control and CFA-injected mice. Figure 10 B shows quantitative autoradiography results from [Leu31, Pro34] NPY concentration-response experiments in the left dorsal horn (ipsilateral to the injected paw). In control mice, [Leu31, Pro34] NPY stimulated [35S]GTPγS binding with an EC50 of 1536 nM (95% CI = 321–7344 nM) and an Emax of 67 ± 6% (Emin = 12 ± 6%). In the CFA-injected mice, the EC50 of [Leu31, Pro34] NPY was decreased to 56 nM (95% CI = 8 nM–407 nM) and its Emax was decreased to 35 ± 3% (Emin = −13 ± 18%). Simultaneous fitting of the two concentration-responses using an `extra sum of squares F-test' to compare their parameters revealed significant differences between control and CFA mice in both the EC50 (p = 0.0236, F1,76 = 5.339) and the Emax (p = 0.0002, F1,76 = 15.86) for [Leu31, Pro34] NPY. The Emin parameter was not significantly different from 0% for either set of data: control, p = 0.0898, F1,29 = 3.079; CFA, p = 0.3568, F1,47 = 0.866, according to an `extra sum of squares F-test' comparing the fitting of each set of data to a dose-response function with and without Emin constrained to 0%. In summary, the inflammation produced by CFA resulted in an increase in Y1-stimulated GTPγS sensitivity and a decrease in the density of coupled receptors.
Fig. 10. [35S]GTPγS binding stimulated by the Y1 receptor agonist [Leu31,Pro34]NPY in spinal cord sections of control and CFA-treated mice.
A) Representative pseudo-color images of [35S]GTPγS binding quantitative autoradiography in mouse spinal cord sections. Binding assays were performed in the absence (basal) or presence of 42.5 μM [Leu31,Pro34]NPY in sections obtained from mice given intraplantar saline (control) or CFA two days before the experiment. B) Concentration-responses of [Leu31,Pro34]NPY-stimulated [35S]GTPγS binding. Mice were injected i.pl. in the left hind paw with vehicle (control, n = 6) or CFA (n = 9) two days before the experiment. EC50 (control) = 1536 nM (95% CI = 321–7344 nM), EC50 (CFA) = 56 nM (95% CI = 8 nM-407 nM); Emax (control) = 67 ± 6 %, Emax (CFA) = 35 ± 3 %; Emin (control) = 12 ± 6 %, Emin (CFA) = -13 ± 18 %. C) Concentration-response of the Y1 receptor antagonist BIBO3304 to inhibit [35S]GTPγS binding induced by 2 μM [Leu31,Pro34]NPY (n=4–5). IC50 (BIBO3304) = 7.85 nM (95% CI = 1.4 – 59.4 nM).
[35S]GTPγS binding assays require inclusion of GDP to reduce non-specific [35S]GTPγS binding and thus improve signal/noise ratios. This often shifts the agonist concentration-response curve to the right, and can contribute to an EC50 measurement that is greater than the Kd for receptor-ligand binding. Therefore, we conducted additional [35S]GTPγS binding experiments in control mice to confirm receptor specificity. We co-incubated spinal cord sections with [Leu31, Pro34] NPY and with varying concentrations of the Y1 receptor antagonist BIBO3304. We used a concentration of [Leu31, Pro34] NPY of 2 μM, just slightly higher than its EC50. As illustrated in Fig. 10C, BIBO3304 inhibited GTPγS binding elicited by [Leu31, Pro34] NPY with an IC50 of 7.85 nM (95% CI = 1.04 nM–59.4 nM). The inhibition was complete, reaching a Emin value at the highest antagonist concentration of 2.9 ± 3.3 %, which was not significantly different from 0% (p = 0.41, F1,27 = 0.71) according to a `extra sum of squares F-test' comparing fittings to a dose-response curve with and without Emin constrained to 0%. The Ki for BIBO3304 was 0.595 nM, calculated from its IC50 using the Cheng-Prusoff equation. Alternatively, a Gaddum-Schild analysis of the combined concentration-response data for [Leu31, Pro34] NPY and BIBO03304 in control mice yielded a Ki for BIBO03304 of 0.762 nM (95% CI = 0.14 nM – 4.15 nM) and a EC for [Leu31, Pro34] NPY of 1.09 μM (95% CI = 0.477 μM – 2.49 μM). This Ki value is consistent with the Ki of 0.7 nM in receptor binding studies for BIBO3304 (Wieland et al. 1998).
4.0 DISCUSSION
This study establishes that the anti-hyperalgesic effects of NPY result from Y1-G-protein signaling, leading to inhibition of SP release from primary afferent terminals in the spinal dorsal horn. Our results indicate that inflammation increases the ability of Y1 agonists to couple with high affinity to activated G-proteins and thus inhibit spinal SP release and nociception. The inhibitory actions of the Y1 agonist were incomplete, indicating that Y1 accounts for only part of the effects of NPY on NK1 internalization and SP release. The high concentration of NPY Y2 receptors on primary afferent neurons and their central terminals (Brumovsky et al., 2005) point to Y2 as in important target for future studies. Indeed, Y2 contributes to the antihyperalgesic actions of both exogenous and endogenous NPY in models of chronic inflammatory and neuropathic pain (Intondi et al., 2008, Solway et al., 2011).
4.1 NPY inhibits SP release in the dorsal horn
We demonstrated that NPY inhibits SP release in the spinal dorsal horn using several in vivo approaches. First, we used microdialysis to measure SP release induced by capsaicin, which was perfused through the dialysis tube. Capsaicin releases SP from primary afferent terminals in the spinal cord by triggering Ca2+ entry through TRPV1 channels present in the presynaptic terminals (Lao et al., 2003a). Spinal application of NPY inhibited capsaicin-evoked SP-LI in microdialysate. These results are consistent with the antibody microprobe studies of Duggan et al., who reported that microinjection of NPY into the substantia gelatinosa reduced SP-LI that was released by electrical stimulation of unmyelinated primary afferent nerves (Duggan et al., 1991). Our results also support the findings of Gibbs and Hargreaves, who reported that Y1 activation attenuated capsaicin-evoked mechanical allodynia and release of calcitonin gene-related peptide in dorsal horn slices, and attenuated bradykinin/prostaglandin E2-evoked increases in intracellular calcium levels in trigeminal ganglion neurons (Gibbs et al., 2004, Gibbs et al., 2006, Gibbs et al., 2007). Second, we measured SP release in terms of NK1R internalization, and induced the release by applying a noxious stimulus to one hind paw of the rat. This widely-used approach (Mantyh et al., 1995, Abbadie et al., 1997b, Kondo et al., 2005, Zhang et al., 2010a) recruits the release of SP from primary afferents by triggering high frequency action potentials (Adelson et al., 2009). In this case, intrathecal NPY given one hour before the hind paw clamp inhibited the evoked NK1R internalization by 50%. Third, SP release (as measured as NK1R internalization) was evoked by a non-noxious stimulus (brushing) applied to hind paw inflamed with carrageenan. Again, intrathecal NPY inhibited the evoked NK1R internalization by nearly 50%. Moreover, in the carrageenan model, intrathecal NPY produced a dramatic inhibition of mechanical allodynia, mechanical hyperalgesia and cold allodynia. We suggest that these anti-hyperalgesic effects of NPY are mediated through its action on presynaptic terminals of primary afferent terminals; however, further studies are needed to evaluate the involvement of other cell types such as interneurons and projection neurons in the dorsal horn.
4.2 Presence of Y1 receptors in dorsal horn neurons and SP-containing primary afferents
To determine whether the inhibition of SP by NPY is mediated by Y1 receptors, we started by asking whether Y1 receptors are present in SP-containing primary afferents. Using immunohistochemistry, we found that Y1 receptors are indeed present in neuronal somas in DRG. Moreover, 75% of the Y1 receptor-positive DRG cells also contained the neuropeptide CGRP, which is largely co-expressed with SP in these neurons (Lawson, 1995, Marvizon et al., 2009). In addition, a substantial proportion (40%) of the Y1 receptor-expressing DRG neurons turned out to belong to the IB4-binding class of C-fibers. This type of DRG neurons is considered to be largely complementary to the CGRP- and SP-containing population, though there is in fact some overlap between the two classes (Price and Flores, 2007). However, despite the clear presence of Y1 receptor immunoreactivity in DRG neurons, we could not identify Y1 receptor immunoreactive primary afferent axons or terminals in the dorsal horn, due to lack of co-localization with CGRP, SP or IB4. The same problem was reported by Brumovsky et al. (2002b): they found Y1 receptor immunoreactivity in CGRP-positive DRG cell bodies and fibers in the sciatic nerve and dorsal roots, but almost no Y1 receptor and CGRP co-localization in fibers in the dorsal horn. Moreover, dorsal rhizotomy did not change Y1 receptor staining in the dorsal horn, but completely depleted CGRP staining. To explore this issue in depth, we obtained high magnification confocal images of laminae I–II from sections double- and triple-labeled for Y1 receptors, CGRP, SP and IB4, and analyzed the co-localization of label pairs voxel-by-voxel in the whole confocal stacks. This state-of-the-art quantitative analysis failed to reveal any co-localization of Y1 receptors with CGRP or SP, whereas the co-localization of CGRP with SP (used as a positive control) was high, as expected. One possible explanation is that Y1 receptors are expressed in DRG neurons but not transported centrally into the spinal cord. However, this is unlikely because Brumovsky et al., (2002b) found Y1 receptor immunoreactivity in the dorsal roots. A second possibility is that Y1 receptors bind to proteins in primary afferent terminals that hinder the binding of the antibody used to label it, as happens with NMDA receptors (Nagy et al., 2004). A third possibility, which we favor, is that the lack of co-localization is an artifact caused by the masking of Y1 receptors in primary afferent axons by the stronger immunoreactivity signal of Y1 receptors in dorsal horn neurons (Ji et al., 1994). Indeed, while DRG and spinal cord sections were stained using the same antibody concentrations, a higher photomultiplier gain of the confocal microscope was used to acquire images from the DRG (880–985) than from the dorsal horn (540–800), indicating that Y1 receptors are more dense in dorsal horn neurons. This, together with the thinness of the primary afferent axons, may have hindered the identification of Y1 receptors in them.
Our immunohistochemical studies are consistent with previous studies describing Y1 receptors in dorsal root ganglion (DRG) neurons that contain calcitonin gene-related peptide (CGRP) and in small dorsal horn neurons in laminae I–III (Ji et al., 1994, Zhang et al., 1994, Brumovsky et al., 2002a). Some of the Y1 receptor neurons in lamina I project to the brain, and most seem to be excitatory neurons under the inhibitory influence of NPY and Y1 receptors (Brumovsky et al., 2006, Polgar et al., 2013). We found that the volume of primary afferent axons in the dorsal horn is very small compared with the volume of Y1 receptor-expressing dorsal horn neurons. Furthermore, the density of Y1 receptors in primary afferents was small compared with their density in dorsal horn neurons - so small indeed that they could not be detected with immunohistochemistry. Therefore, the majority of Y1 receptors in the dorsal horn are most likely located in dorsal horn neurons. However, even scant Y1 receptors on afferent terminals, if avidly coupled, could account for a significant or even large proportion of overall NPY signaling in dorsal horn.
4.3 NPY receptor inhibition of NK1R internalization in the absence of inflammation
To determine the contribution of Y1 receptors to the inhibition of SP release, we used a selective agonist and antagonist of the Y1 receptor, and measured NK1R internalization in rat spinal cord slices. The Y1 receptor selective agonist [Leu31, Pro34]-NPY produced a concentration-dependent inhibition of DRS-evoked NK1R internalization, albeit with low efficacy. The effect of [Leu31, Pro34]-NPY was completely reversed by the selective Y1 receptor antagonist BIBO3304, showing that it was indeed mediated by Y1 receptors. In vivo, however, intrathecal NPY but not [Leu31, Pro34]-NPY inhibited the NK1R internalization induced by noxious hind paw clamp. Of the 4 characterized receptors for NPY in the rat, Y1 and Y2 are predominantly expressed in the dorsal horn. We argue that NPY acts in part at Y2 receptors to inhibit in vivo NK1R internalization in the uninjured animal. Indeed, the classic study of NPY and pain by Yaksh and colleagues suggested that Y2 agonists inhibit transient nociception (Hua et al., 1991). Further studies involving a dose-response analysis with Y1 and Y2 receptor agonists and antagonists could resolve the relative contribution of Y1 and Y2 to SP release. Albeit, our results suggest that Y1 receptors contribute to the inhibition of SP release produced by NPY in the uninjured state.
4.4 SP release and hyperalgesia are decreased by Y1 receptors during inflammation
Using the CFA model of inflammation, we found that [Leu31, Pro34]-NPY has anti-hyperalgesic properties similar to NPY: it reversed the thermal hyperalgesia produced by CFA (Fig. 9 A). Hence, we suggest that Y1 receptors contribute to the anti-hyperalgesic effects of NPY. Furthermore, although intrathecal [Leu31, Pro34]-NPY did not inhibit SP release elicited by noxious hind paw clamp in control rats, it produced a noticeable inhibition 3 days after the CFA injection. This suggests that the function of presynaptic Y1 receptors in primary afferent terminals is augmented by inflammation.
4.5 Y1 receptor functional coupling in the dorsal horn
The functional coupling of Y1 receptors to G proteins in the dorsal horn was measured in this study as [Leu31, Pro34]-NPY-stimulated [35S]GTPγS binding. Due to the relatively large volume of Y1 receptors in dorsal horn, it is likely that spinal neurons provide a major contribution to overall [35S]GTPγS binding. However, we cannot rule out a contribution to NPY signaling of the central terminals of primary afferents, which likely remain viable in our assay system. Our results revealed that the affinity of coupling between Y1 receptors and activated G-proteins was increased dramatically during CFA-induced inflammation, albeit its efficacy was reduced by roughly half. This profound increase in the avidity of Y1 receptor-G protein interactions could amplify the coupling events between Y1 receptors and their effectors. We suggest that the resultant potentiation of inhibition of intracellular signaling contributes to enhanced NPY anti-hyperalgesia after inflammation.
4.6 Conclusion
In summary, NPY produces anti-hyperalgesia in several rodent models of chronic pain and inhibits SP release from primary afferent terminals. Both of these effects increase during inflammation. As a result, inflammation-evoked enhancement of Y1 receptor signaling could vastly increase the pain inhibitory potency of NPY during inflammation as compared to the normal state. This may serve as a compensatory mechanism whereby NPY homeostatically regulates persistent pain and impedes the transition from acute to chronic inflammatory pain (Solway et al., 2011).
Highlights
NPY acts at Y1 receptors in dorsal horn to decrease inflammatory hyperalgesia.
NPY inhibits substance P release from primary afferent neurons.
Y1 is broadly co-expressed with CGRP in DRG neurons
Inflammation increases coupling between Y1 receptors and G-proteins in dorsal horn of spinal cord.
Inflammation enhances NPY-Y1 mediated inhibition of substance P release and hyperalgesia.
Acknowledgments
Supported by NIH R01NS45954 and K02DA19656 to BKT and NICHD HD02528 to KEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the patter of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997a;69:101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997b;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson DW, Lao L, Zhang G, Kim W, Marvizón JC. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increases with the firing frequency of C-fibers. Neuroscience. 2009;161:538–553. doi: 10.1016/j.neuroscience.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrah AW, Gustafsson H, Olgart L, Brodin E, Stiller CO, Taylor BK. Capsaicin-evoked substance P release in rat dorsal horn increases after peripheral inflammation: a microdialysis study. Neuroscience letters. 2004;368:226–230. doi: 10.1016/j.neulet.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H. Involvement of spinal N-methyl-D-aspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neuroscience letters. 2001;316:83–86. doi: 10.1016/s0304-3940(01)02380-1. [DOI] [PubMed] [Google Scholar]
- Aguado F, Pozas E, Blasi J. Colchicine administration in the rat central nervous system induces SNAP-25 expression. Neuroscience. 1999;93:275–283. doi: 10.1016/s0306-4522(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997a;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. The J Neurosci. 1997b;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin E, Lindefors N, Dalsgaard CJ, Theodorsson-Norheim E, Rosell S. Tachykinin multiplicity in rat central nervous system as studied using antisera raised against substance P and neurokinin A. Regul Pept. 1986;13:253–272. doi: 10.1016/0167-0115(86)90044-3. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hokfelt T. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138:1361–1376. doi: 10.1016/j.neuroscience.2005.11.069. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol. 2005;489:328–348. doi: 10.1002/cne.20639. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hokfelt T. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp Neurol. 2002a;174:1–10. doi: 10.1006/exnr.2001.7845. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hokfelt T. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp Neurol. 2002b;174:1–10. doi: 10.1006/exnr.2001.7845. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Stiller C, Gustafsson H, malmberg AB. Elevated substance-P-like immunoreactivity levels in spinal dialysates during the formalin test in normal and diabetic rats. Brain Res. 2000;856:20–27. doi: 10.1016/s0006-8993(99)02345-8. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Cortes R, hokfelt T. Effect of reserpine and colchicine on neuropeptide mRNA levels in the rat hypothalamic paraventricular nucleus. Brain Res Mol Brain Res. 1991a;9:57–69. doi: 10.1016/0169-328x(91)90130-p. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Ernfors P, Villar MJ, Persson H, Hokfelt T. Expanded distribution of mRNA for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the rat brain after colchicine treatment. Proc Natl Acad Sci U S A. 1991b;88:10352–10356. doi: 10.1073/pnas.88.22.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Marvizon JC. Acute inflammation induces segmental, bilateral, supraspinally mediated opioid release in the rat spinal cord, as measured by μ-opioid receptor internalization. Neuroscience. 2009a;161:157–172. doi: 10.1016/j.neuroscience.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Marvizon JC. Acute inflammation induces segmental, bilateral, supraspinally mediated opioid release in the rat spinal cord, as measured by mu-opioid receptor internalization. Neuroscience. 2009b;161:157–172. doi: 10.1016/j.neuroscience.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Song B, Lao L, Perez OA, Kim W, Marvizon JCG. Comparing analgesia and μ-opioid receptor internalization produced by intrathecal enkephalin: Requirement for peptidase inhibition. Neuropharmacology. 2007;53:664–667. doi: 10.1016/j.neuropharm.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP. Characterization of hind paw licking and lifting to noxious radiant heat in the rat with and without chronic inflammation. J Neurosci Methods. 2006;155:122–125. doi: 10.1016/j.jneumeth.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Cortes R, Ceccatelli S, Schalling M, Hokfelt T. Differential effects of intracerebroventricular colchicine administration on the expression of mRNAs for neuropeptides and neurotransmitter enzymes, with special emphasis on galanin: an in-situ hybridization study. Synapse. 1990;6:369–391. doi: 10.1002/syn.890060410. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Hope PJ, Lang CW. Microinjection of neuropeptide Y into the superficial dorsal horn reduces stimulus-evoked release of immunoreactive substance P in the anaesthetized cat. Neuroscience. 1991;44:733–740. doi: 10.1016/0306-4522(91)90092-3. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Cadieux A, Doods H, Fournier A, Quirion R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIB03304 (Y1) and CGP71683A (Y5) Canadian Journal of Physiology & Pharmacology. 2000;78:116–125. [PubMed] [Google Scholar]
- Gibbs J, Flores CM, Hargreaves KM. Neuropeptide Y inhibits capsaicin-sensitive nociceptors via a Y1-receptor-mediated mechanism. Neuroscience. 2004;125:703–709. doi: 10.1016/j.neuroscience.2004.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Diogenes A, Hargreaves KM. Neuropeptide Y modulates effects of bradykinin and prostaglandin E2 on trigeminal nociceptors via activation of the Y1 and Y2 receptors. British journal of pharmacology. 2007;150:72–79. doi: 10.1038/sj.bjp.0706967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Flores CM, Hargreaves KM. Attenuation of capsaicin-evoked mechanical allodynia by peripheral neuropeptide Y Y1 receptors. Pain. 2006;124:167–174. doi: 10.1016/j.pain.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. Journal of Comparative Neurology. 1984;227:78–91. doi: 10.1002/cne.902270109. [DOI] [PubMed] [Google Scholar]
- Gustafsson H, de Araujo Lucas G, Schott E, Stiller CO, Alster P, Wiesenfeld-Hallin Z, Brodin E. Measurement of cholecystokinin release in vivo in the rat spinal dorsal horn. Brain Research Brain Research Protocols. 1999;4:192–200. doi: 10.1016/s1385-299x(99)00016-1. [DOI] [PubMed] [Google Scholar]
- Hibbs AR, MacDonald G, Garsha K. Practical Confocal Microscopy. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. Springer; New York, NY: 2006. pp. 650–671. [Google Scholar]
- Hokfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, Landry M, Bao L, Schalling M, Koistinaho J, DeArmond SJ, Prusiner S, Gong J, Walsh JH. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal cord substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999a;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. Journal of Neuroscience. 1999b;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Boublik JH, Spicer MA, Rivier JE, Brown MR, Yaksh TL. The antinociceptive effects of spinally administered neuropeptide Y in the rat: systematic studies on structure-activity relationship. Journal of Pharmacology & Experimental Therapeutics. 1991;258:243–248. [PubMed] [Google Scholar]
- Hunt SP, Kelly JS, Emson PC, Kimmel JR, Miller RJ, Wu JY. An immunohistochemical study of neuronal populations containing neuropeptides or gamma-aminobutyrate within the superficial layers of the rat dorsal horn. Neuroscience. 1981;6:1883–1898. doi: 10.1016/0306-4522(81)90029-4. [DOI] [PubMed] [Google Scholar]
- Intondi AB, Dahlgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137:352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. Journal of Neuroscience. 1994;14:6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Hisano S, Kawano H, Kagotani Y, Daikoku S. Light- and electron-microscopic evidence of costoring of immunoreactive enkephalins and substance P in dorsal horn neurons of rat. Cell Tissue Res. 1988;253:297–303. doi: 10.1007/BF00222285. [DOI] [PubMed] [Google Scholar]
- Kjorsvik Bertelsen A, Warsame Afrah A, Gustafsson H, Tjolsen A, Hole K, Stiller CO. Stimulation of spinal 5-HT(2A/2C) receptors potentiates the capsaicin-induced in vivo release of substance P-like immunoreactivity in the rat dorsal horn. Brain research. 2003;987:10–16. doi: 10.1016/s0006-8993(03)03216-5. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal KE, Solway B, Pedrazzini T, Taylor BK. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition. 2008;24:885–891. doi: 10.1016/j.nut.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao L, Marvizon JC. GABA(A) receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord. Neuroscience. 2005a;130:1013–1027. doi: 10.1016/j.neuroscience.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Lao L, Marvizon JCG. GABAA receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord. Neuroscience. 2005b;130:1013–1027. doi: 10.1016/j.neuroscience.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Lao L, Song B, Marvizon JCG. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with NMDA and GABAB receptors. Neuroscience. 2003a;121:667–680. doi: 10.1016/s0306-4522(03)00501-3. [DOI] [PubMed] [Google Scholar]
- Lao LJ, Song B, Marvizon JC. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with N-methyl-D-aspartate and GABA(B) receptors. Neuroscience. 2003b;121:667–680. doi: 10.1016/s0306-4522(03)00501-3. [DOI] [PubMed] [Google Scholar]
- lawson SN. Neuropeptides in morphologically and functionally identified primary afferent neurons in dorsal root ganglia: substance P, CGRP and somatostatin. Prog Brain Res. 1995;104:161–173. doi: 10.1016/s0079-6123(08)61790-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Mahinda TB, Taylor BK. Intrathecal neuropeptide Y inhibits behavioral and cardiovascular responses to noxious inflammatory stimuli in awake rats. Physiol Behav. 2004;80:703–711. doi: 10.1016/j.physbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Gates T, Mantyh CR, Maggio JE. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. Journal of Neuroscience. 1989;9:258–279. doi: 10.1523/JNEUROSCI.09-01-00258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Chen W, Murphy N. Enkephalins, dynorphins and β-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. J Comp Neurol. 2009;517:51–68. doi: 10.1002/cne.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. European Journal of Neuroscience. 1999;11:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. Journal of Neuroscience. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003a;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Marvizon JCG, Wang X, Lao L, Song B. Effect of peptidases on the ability of exogenous and endogenous neurokinins to produce neurokinin 1 receptor internalization in the rat spinal cord. Br J Pharmacol. 2003b;140:1389–1398. doi: 10.1038/sj.bjp.0705578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Thoren P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. Increased analgesic potency of mu agonists after continuous naloxone infusion in rats. J Pharmacol Exp Ther. 1991;259:582–589. [PubMed] [Google Scholar]
- Polgar E, Sardella TC, Tiong SY, Locke S, Watanabe M, Todd AJ. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain. 2013 doi: 10.1016/j.pain.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan V, henry JL. Novel substance P antagonist, CP-96,345, blocks responses of cat spinal dorsal horn neurons to noxious cutaneous stimulation and to substance P. Neuroscience letters. 1991;132:39–43. doi: 10.1016/0304-3940(91)90428-v. [DOI] [PubMed] [Google Scholar]
- Ren K, Iadarola MJ, Dubner R. An isobolographic analysis of the effects of N-methyl-D-aspartate and NK1 tachykinin receptor antagonists on inflammatory hyperalgesia in the rat. Br J Pharmacol. 1996;117:196–202. doi: 10.1111/j.1476-5381.1996.tb15174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, Pioro EP, Cuello AC. Substance P- and enkephalin-like immunoreactivities are colocalized in certain neurons of the substantia gelatinosa of the rat spinal cord: an ultrastructural double-labeling study. J Neurosci. 1991;11:1068–1080. doi: 10.1523/JNEUROSCI.11-04-01068.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RC, Trafton JA, Chi SI, Basbaum AI. Presynaptic regulation of spinal cord tachykinin signaling via GABA(B) but not GABA(A) receptor activation. Neuroscience. 2001;103:725–737. doi: 10.1016/s0306-4522(00)00571-6. [DOI] [PubMed] [Google Scholar]
- Senba E, Yanaihara C, Yanaihara N, Tohyama M. Co-localization of substance P and Met-enkephalin-Arg6-Gly7-Leu8 in the intraspinal neurons of the rat, with special reference to the neurons in the substantia gelatinosa. Brain Res. 1988;453:110–116. doi: 10.1016/0006-8993(88)90148-5. [DOI] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce μ-opioid receptor internalization in the rat spinal cord. J Neurosci. 2003;23:9171–9184. doi: 10.1523/JNEUROSCI.23-27-09171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. NMDA receptors and large conductance calcium-sensitive potassium channels inhibit the release of opioid peptides that induce μ-opioid receptor internalization in the rat spinal cord. Neuroscience. 2005;136:549–562. doi: 10.1016/j.neuroscience.2005.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. Journal of Neuroscience Methods. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Taylor BK. Antihyperalgesic effects of intrathecal neuropeptide Y during inflammation are mediated by Y1 receptors. Pain. 2002;96:353–363. doi: 10.1016/S0304-3959(01)00481-X. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? Journal of Neuroscience. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherer MM, Seybold VS. A quantitative study of the coexistence of peptides in varicosities within the superficial laminae of the dorsal horn of the rat spinal cord. J Neurosci. 1989;9:195–205. doi: 10.1523/JNEUROSCI.09-01-00195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin J, Schott E. Substance P release in the spinal dorsal horn following peripheral nerve injury. Neuropeptides. 2002;36:252–256. doi: 10.1016/s0143-4179(02)00024-0. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Stereospecific effects of a nonpeptidic NK1 selective antagonist, CP,96–345: antinociception in the absence of motor dysfunction. Life sciences. 1991;49:1955–1963. doi: 10.1016/0024-3205(91)90637-q. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen W, Lao L, Marvizon JC. Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as NK1R internalization. Eur J Neurosci. 2010a;31:225–237. doi: 10.1111/j.1460-9568.2009.07075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen W, Marvizon JC. Src family kinases mediate the inhibition of substance P release in the rat spinal cord by μ-opioid receptors and GABAB receptors, but not α2 adrenergic receptors. Eur J Neurosci. 2010b;32:963–973. doi: 10.1111/j.1460-9568.2010.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, Hokfelt T. Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc Natl Acad Sci U S A. 1994;91:11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorman G, Belcher G, Adams JE, Fields HL. Lumbar intrathecal naloxone blocks analgesia produced by microstimulation of the ventromedial medulla in the rat. Brain Res. 1982;236:77–84. doi: 10.1016/0006-8993(82)90035-x. [DOI] [PubMed] [Google Scholar]