Abstract
Receptor tyrosine kinases (RTKs) bind to a subset of growth factors on the surface of cells and elicit responses with broad roles in developmental and postnatal cellular processes. Receptors in this subclass consist of an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular domain harboring a catalytic tyrosine kinase and regulatory sequences that are phosphorylated either by the receptor itself or various interacting proteins. Once activated, RTKs bind signaling molecules and recruit effector proteins to mediate downstream cellular responses through various intracellular signaling pathways. In this chapter, we will highlight the role of a subset of RTK families in regulating the activity of neural crest cells (NCCs) and the development of their derivatives in mammalian systems. NCCs are migratory, multipotent cells that can be subdivided into four axial populations, cranial, cardiac, vagal and trunk. These cells migrate throughout the vertebrate embryo along defined pathways and give rise to unique cell types and structures. Interestingly, individual RTK families often have specific functions in a subpopulation of NCCs that contribute to the diversity of these cells and their derivatives in the mammalian embryo. We will additionally discuss current methods used to investigate RTK signaling, including genetic, biochemical, large-scale proteomic and biosensor approaches, which can be applied to study intracellular signaling pathways active downstream of this receptor subclass during NCC development.
Keywords: growth factor, receptor tyrosine kinase, neural crest, mouse, allelic series, phospho-antibodies, proteomics, mass spectrometry, biosensors
1. Introduction
Growth factors encompass an expansive range of proteins, cytokines and hormones that bind to receptors on the surface of cells and stimulate various cellular activities. A subset of these factors signal through membrane-spanning RTKs and elicit wide-ranging responses with broad roles in developmental and postnatal cellular processes, such as proliferation, growth, survival, apoptosis, adhesion, migration, differentiation, metabolism, cell cycle progression, transcription, RNA processing, protein synthesis, vesicle trafficking and autophagy. All RTKs share a basic structural framework consisting of an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular domain harboring a catalytic tyrosine kinase (Figure 1) and regulatory sequences that are phosphorylated either by the receptor itself or various interacting proteins. RTKs are activated by ligand binding that, in the case of most receptors, induces receptor dimerization and promotes tyrosine kinase activity. In some instances, such as for the fibroblast growth factor (FGF) receptor, interactions with additional extracellular proteins including heparan sulfate proteoglycans are required for maximal receptor dimerization and activation (reviewed in Schlessinger, 2000; Lemmon and Schlessinger, 2010).
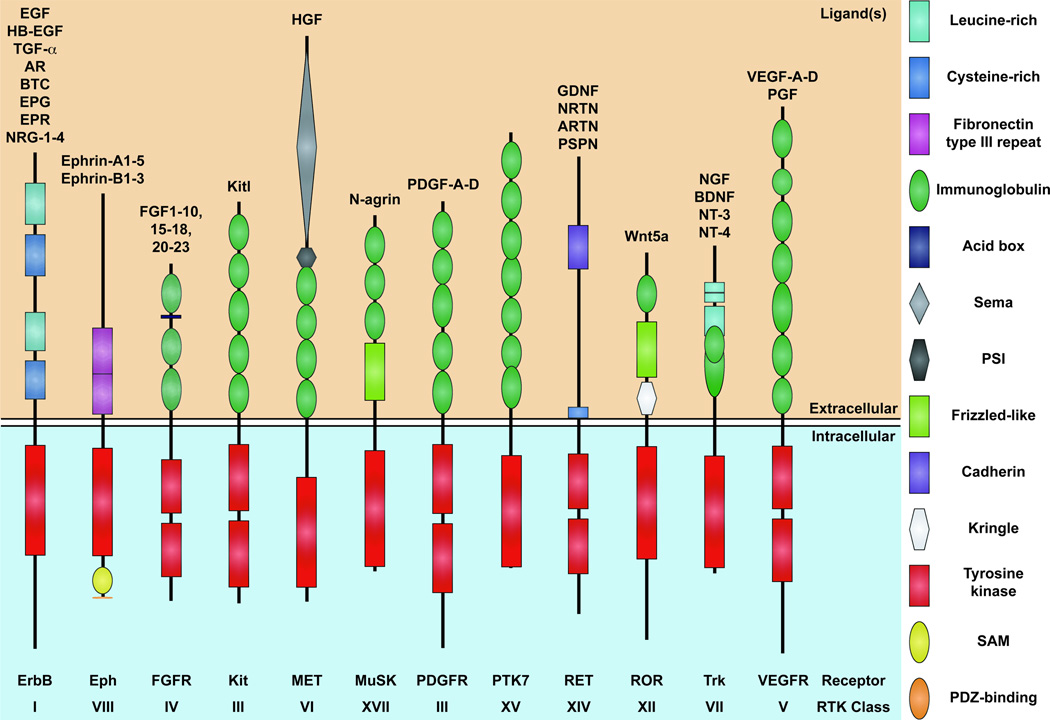
Figure 1.
Schematic representation of the 12 RTK families involved in mammalian NCC development. The family name and RTK class are listed below each receptor, while the interacting ligands are listed above. Structural domains in the extracellular and intracellular portions of the receptors are depicted according to the key at right.
Tyrosine autophosphorylation sites in the noncatalytic portions of the receptor intracellular domains act as docking sites for signaling molecules containing phosphotyrosine recognition motifs such as Src homology 2 (SH2) or phosphotyrosine-binding (PTB) domains. SH2 domain-containing interacting proteins include a subset with intrinsic enzymatic activity, including Src family tyrosine kinases, phosphatidylinositol 3-kinase (PI3K), the tyrosine phosphatase SHP-2, phospholipase Cγ (PLCγ) and GTPase activating protein (GAP); the signal transducer and activator of transcription (Stat) family of transcription factors; and adaptor proteins such as Shc, growth factor receptor-bound proteins (Grb) 2/7/10/14, Crk/Crkl and Nck. PTB domain-containing proteins that bind RTKs include fibroblast growth factor receptor substrate (Frs) 2/3 and insulin receptor substrate (IRS) 1/2. Once bound, these molecules employ various additional domains (pleckstrin homology, FYVE, SH3, WW, PDZ, etc.) to establish multiprotein signaling complexes. Upon recruitment, effector proteins are activated by several mechanisms, such as membrane translocation, conformational changes and/or tyrosine phosphorylation, and subsequently mediate downstream cellular responses through a variety of intracellular signaling pathways (reviewed in Schlessinger, 2000; Lemmon and Schlessinger, 2010).
Despite the fact that the various RTKs interact with similar subsets of signaling molecules and utilize an overlapping network of intracellular signaling pathways to stimulate a range of cellular activities, biological specificity is introduced at several levels to generate a unique response downstream of receptor activation. First, the expression of individual growth factors and their corresponding RTKs is neither ubiquitous nor continuous within the embryo, thereby localizing their activity to specific tissues and/or timepoints during development. Similarly, the inherent binding properties of individual RTKs combined with enrichment of interacting proteins and their effectors at particular sites together dictate the unique combination of signaling molecules that are engaged by the various RTKs. Second, the requirement in some cases for additional interactions with extracellular proteins to optimize receptor dimerization and activation adds an extra layer of constraint for a subset of RTKs, for example, FGFR. Additionally, the ability of some RTK families, such as the erythroblastic leukemia viral oncogene homolog (ErbB) receptor and the platelet-derived growth factor (PDGF) receptor families, to induce signaling downstream of both homodimeric and heterodimeric receptor complexes may impart distinct effects on cellular behavior. Finally, differences in the strength and duration of signaling pathway activation induced by various RTKs have been shown to alter downstream biological responses (reviewed in Schlessinger, 2000; Lemmon and Schlessinger, 2010).
Here, we will discuss the role of a subset of RTK families in mediating the activity of NCCs and the development of their derivatives in mammalian systems, with a particular emphasis on their role in the mouse embryo (Table 1). NCCs are migratory, multipotent cells that play a critical role in vertebrate development. During mammalian embryogenesis, NCCs arise at the border of the neural ectoderm, undergo an epithelial to mesenchymal transition and subsequently delaminate from the cranial neural folds or dorsal neural tube. They can be subdivided into four axial populations, cranial, cardiac, vagal and trunk, which migrate throughout the embryo along defined pathways and contribute to diverse derivatives (Figure 2). Cranial, or cephalic, NCCs originate from the forebrain to the hindbrain, which is segmented into seven transient neuroepithelial rhombomeres, and populate the frontonasal prominence and pharyngeal arches 1–4. These cells give rise to the bone and cartilage of the frontonasal skeleton and cartilages of the jaw, middle ear, hyoid and thyroid. Cranial NCCs additionally generate smooth muscle, tendons, connective tissue, melanocytes and cranial sensory ganglia of the peripheral nervous system as well as contribute to the formation of the eye, teeth, thyroid gland, parathyroid gland and thymus. Cardiac NCCs are a subpopulation of cranial NCCs that arise as far rostrally as the otic vesicle and contribute to the aorticopulmonary septum and the caudal pharyngeal arch arteries. Vagal and sacral NCCs generate the enteric ganglia of the gut peripheral nervous system. Finally, trunk NCCs, which originate caudally to the cranial NCC domain, give rise to melanocytes, the dorsal root and sympathetic ganglia of the peripheral nervous system, Schwann cells and the adrenal medulla (reviewed in Trainor, 2005; Mayor and Theveneau, 2013).
Table 1.
RTKs contributing to murine NCC development. The RTK families and individual receptors are listed, along with the NCC phenotype(s) of their corresponding mouse models and their functional role(s), if known, in regulating NCC activity.
| Family | Receptor | Mouse model NCC phenotype(s) | Function |
|---|---|---|---|
| ErbB receptors | EGFR | craniofacial abnormalities (cleft palate; misshapen snout; micrognathia; abnormal Meckel’s cartilage development) | |
| defective cardiac semilunar valvulogenesis | |||
| ErbB2 | hypoplastic cranial sensory and sympathetic ganglia; loss of sensory and motor neurons; absent Schwann cells in the peripheral nerves | migration | |
| absence of cardiac ventricular trabeculae | |||
| ErbB3 | hypoplastic cranial, dorsal root, sympathetic and enteric ganglia; absent Schwann cells in sensory and motor neurons | ||
| absence of mesenchyme in cardiac cushions; hypoplastic cardiac valves | |||
| ErbB4 | misprojections of cranial sensory ganglia | migration | |
| absence of cardiac ventricular trabeculae | |||
| Eph receptors | EphA4 | coronal synostosis | migration |
| FGF receptors | FGFR1 | craniofacial abnormalities (cleft palate; cleft lip; micrognathia; abnormal tooth bud development; inner ear malformations; hypoplastic hyoid) | survival, migration |
| FGFR2 | craniofacial abnormalities (domed head; dysmorphic cranial base; craniosynostosis; cleft palate; misshapen snout; abnormal tooth bud development; inner ear malformations) | survival, migration | |
| Kit receptor | Kit | defective pigmentation | survival, migration |
| absent small- and medium-diameter sensory neurons in dorsal root ganglia | differentiation | ||
| MET receptor | MET | ectopic melanoblasts | survival, differentiation |
| MuSK receptor | MuSK | impaired segmental trunk NCC migration | migration |
| PDGF receptors | PDGFRα | craniofacial bone hypoplasia (cleft palate; cleft lip; shortening of the premaxilla; malformations of the basisphenoid, alisphenoid and pterygoid bones) | migration, proliferation |
| thymus hypoplasia | |||
| cardiac outflow tract defects | migration | ||
| defective pigmentation | |||
| PDGFRβ | cardiac ventricular septal defects | migration | |
| PTK7 receptor | PTK7 | misshapen cranial and dorsal root ganglia | distribution |
| cardiac outflow tract defects; cardiac ventricular septal defects | |||
| RET receptor | RET | absent enteric ganglia | survival, migration, proliferation, differentiation |
| neuronal loss in dorsal root and sympathetic ganglia | |||
| ROR receptors | Ror2 | craniofacial bone hypoplasia (cleft palate; short snout; micrognathia) | migration, proliferation |
| cardiac ventricular septal defects | |||
| Trk receptors | TrkA | neuronal loss in trigeminal, dorsal root and sympathetic ganglia | |
| defective thymus development | differentiation | ||
| TrkB | neuronal loss in trigeminal and dorsal root ganglia | survival | |
| defective enteric glial cell development | |||
| TrkC | neuronal loss in trigeminal and dorsal root ganglia | ||
| cardiac atrial and ventricular septal defects; valvular defects |
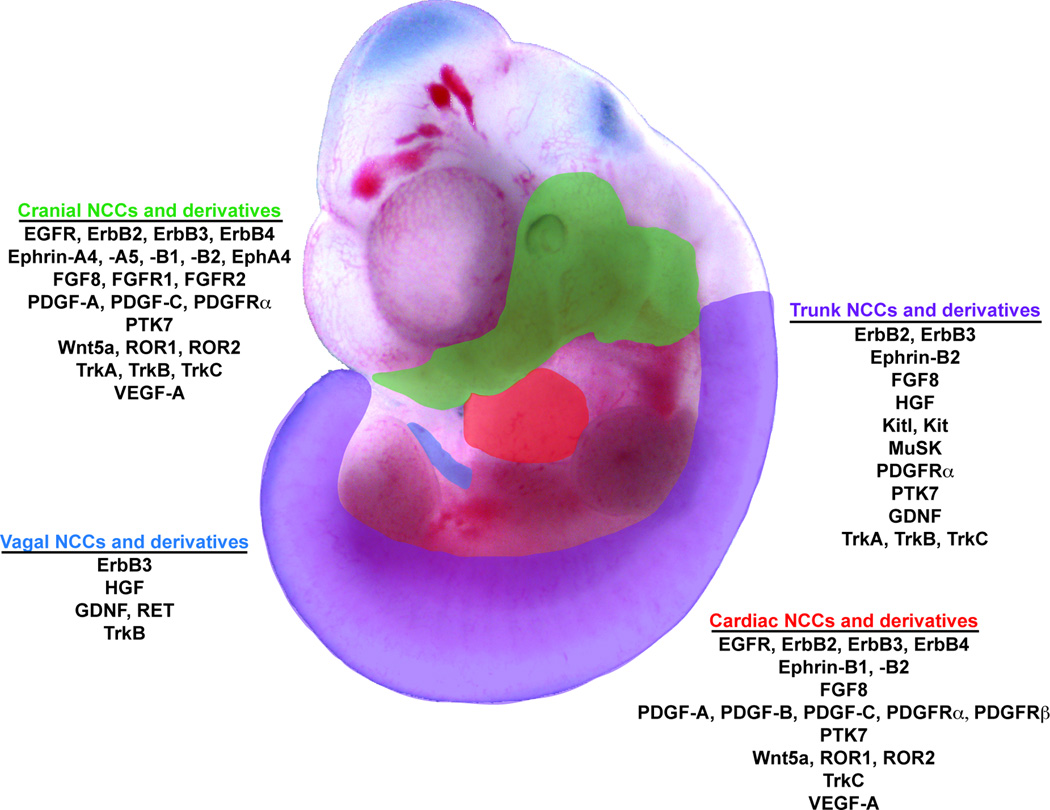
Figure 2.
Schematic diagram of an E11.5 mouse embryo in which the target tissues of the four axial NCC populations are broadly highlighted. Ligands and corresponding RTKs contributing to the development of each population and its derivatives are listed.
In humans, diseases stemming from defects in NCC activity are collectively referred to as neurocristopathies (Bolande, 1974). These diseases fall under two broad categories: congenital malformations and neoplasms. Dysgenetic neurocristopathies encompass craniofacial malformations; pigmentary disorders; diseases of the peripheral nervous system, such as Hirschsprung’s disease; and syndromes affecting multiple sites through the body, such as DiGeorge, Kallmann and craniofrontonasal syndromes (reviewed in Bolande, 1996; Etchevers et al., 2006).
While not all RTK families have been shown to play a role in mammalian NCC development, those that do often have specific functions in a subpopulation of NCCs that contribute to the diversity of these cells and their derivatives in the mammalian embryo (Table 1; Figure 2). The combined application of genetic, proteomic and in vivo biosensor approaches to investigate RTK signaling promises to shed further light on the intracellular signaling pathways active downstream of this receptor subclass during NCC development.
2. Receptor Tyrosine Kinase Signaling in Mammalian Neural Crest Cell Development
2.1 ErbB receptors
In mammals, the ErbB family is composed of 11 ligands, epidermal growth factor (EGF), heparin-binding EGF-like growth factor (HB-EGF), transforming growth factor-α (TGF-α), amphiregulin, betacellulin, epigen, epiregulin, and neuregulin 1–4, which variously bind and activate three receptors, ErbB1 (also known as Her1, EGFR); ErbB3 (Her3) and ErbB4 (Her4). A fourth receptor, ErbB2 (Her2, Neu), does not directly bind ligands (Stein and Staros, 2000). The ErbB receptors are composed of an extracellular region harboring four subdomains organized as a tandem repeat of homologous domains, leucine-rich 1 (LR1), cysteine-rich 1 (CR1), LR2 and CR2, and a cytoplasmic tyrosine kinase domain (Ullrich et al., 1984; Bajaj et al., 1987) (Figure 1). While the neuregulins mainly activate ErbB3 and ErbB4, the remaining ligands in the family primarily activate EGFR (Leahy, 2004). ErbB2, which lacks a known ligand, and ErbB3, which lacks an active kinase domain (Guy et al., 1994), are incapable of signaling on their own and heterodimerize with other receptors in the family to potentiate a signal (Klapper et al., 1999; Citri et al., 2003).
EGFR is expressed in various epithelial tissues throughout the developing embryo (Sibilia and Wagner, 1995). Homozygous null mice show strain-dependent phenotypes ranging from peri-implantation lethality stemming from inner cell mass defects, to midgestation lethality owing to placental defects and perinatal lethality approximately three weeks after birth (Threadgill et al., 1995; Sibilia and Wagner, 1995). In the latter case, mice display abnormalities in the development of several organs, including the brain, eye, lung, kidney, liver, gastrointestinal tract, skin and hair follicles (Threadgill et al., 1995; Sibilia and Wagner, 1995; Miettinen et al., 1995). Homozygous null neonates additionally exhibit defects in NCC-derived structures in the face and heart. These include craniofacial abnormalities such as cleft palate, misshapen snouts, micrognathia and abnormal Meckel’s cartilage development, which are caused, at least in part, by decreased matrix metalloproteinase secretion (Miettinen et al., 1999), as well as defects in semilunar valvulogenesis mediated through signaling of the tyrosine phosphatase SHP-2 (Chen et al., 2000).
Targeted disruption of Erbb2, Erbb3 or Erbb4 receptors in mice results in embryonic lethality during midgestation and a subset of overlapping NCC phenotypes (Lee et al., 1995; Riethmacher et al., 1997; Erickson et al., 1997; Gassmann et al., 1995). ErbB2 is expressed in the mouse nervous system and cardiac myocytes during development, and Erbb2 homozygous null embryos display defects in cranial sensory ganglia, sympathetic ganglia, motor nerve and heart development, due in part to defects in NCC migration (Lee et al., 1995; Britsch et al., 1998). Genetic rescue of the cardiac defects of Erbb2 mutant mice revealed an absence of NCC-derived Schwann cells in the peripheral nerves and loss of motor and sensory neurons (Morris et al., 1999; Woldeyesus et al., 1999). Embryos homozygous for a kinase-dead Erbb2 allele recapitulate the null phenotypes, indicating that the catalytic activity of ErbB2 is required for proper development (Chan et al., 2002). Moreover, analysis of an allelic series of autophosphorylation mutant knock-in mice at the Erbb2 locus revealed a role for Shc adaptor signaling in mediating the activity of the receptor in cutaneous sensory neurons (Chan et al., 2004). Consistent with expression of Erbb3 in the murine NCCs, brain, Schwann cells, various ganglia and heart, among other sites (Meyer et al., 1997; Britsch et al., 1998), Erbb3 homozygous null embryos exhibit brain defects, a lack of Schwann-cell precursors accompanying sensory and motor neurons, defects in cranial ganglia, dorsal root ganglia, sympathetic ganglia and enteric ganglia, and heart abnormalities (Riethmacher et al., 1997; Erickson et al., 1997). Erbb4 is expressed in the brain and myocardium, and Erbb4 homozygous null embryos accordingly display defects in innervation of the hindbrain and heart development (Gassmann et al., 1995). Moreover, abnormal migration of hindbrain-derived cranial NCCs in Erbb4 mutant embryos results in misprojections, and in some cases fusions, of the cranial sensory ganglia (Golding et al., 2000).
2.2 Eph receptors
The erythropoietin-producing hepatocellular carcinoma (Eph) receptors are the largest subfamily of RTKs in vertebrates and are subdivided into two classes, A-type and B-type, based on homology and ligand binding affinities (Gale et al., 1996). In mammals, the family consists of eight Eph receptor interacting (ephrin) proteins, ephrin-A1–5 and ephrin-B1–3, and 14 Eph receptors, EphA1–8, EphA10, EphB1–4 and EphB6, which are capable of bidirectional signaling. Signaling downstream of Ephs upon ephrin binding constitutes forward signaling, while activation of signal transduction pathways downstream of ephrins upon interaction with Eph receptors comprises reverse signaling (Holland et al., 1996; Brückner et al., 1997; Davy and Soriano, 2005). Ephrin-A proteins are tethered to the membrane via a glycosylphosphatidyl inositol anchor, while ephrin-B proteins possess a transmembrane domain and a cytoplasmic domain harboring a PDZ-domain-binding motif (Davis et al., 1994; Lin et al., 1999). Eph receptors are composed of an extracellular region with a globular domain, a cysteine-rich region and two fibronectin type III repeats, and an intracellular region consisting of a tyrosine kinase domain, a SAM domain and a carboxyl-terminal PDZ-domain-binding motif (Hirai et al., 1987) (Figure 1). Ephrin proteins and Eph receptors typically interact within their subclass (Gale et al., 1996), with few exceptions: ephrin-A5 can also bind EphB2 and EphA4 is also capable of binding to all B-type ephrins.
Despite the considerable number of proteins in the Eph receptor family and their extensive roles during mammalian development, relatively few, ephrins-A4, -A5, -B1, -B2 and EphA4, have been demonstrated to regulate NCC activity in the embryo. The majority of the functional studies in mice have addressed the roles of ephrins-B1 and –B2 in controlling the migration of various NCC populations. Ephrin-B1 and its cognate receptors are expressed in cranial NCCs. Efnb1 null embryos as well as those with conditional inactivation of the gene in NCCs using the Wnt1-Cre driver exhibit abnormalities in cranial and cardiac NCC migration and partially penetrant defects in the palate and tympanic ring (Davy et al., 2004). Mice harboring point mutations in Efnb1 that disrupt reverse signaling pathways do not display craniofacial abnormalities, indicating that the NCC defects observed in Efnb1 null mutants are due to impaired forward signaling (Bush and Soriano, 2009). Similarly, Ephrin-B2 forward signaling from the branchial arch surface ectoderm through its cognate receptors in cranial NCCs is required for proper cranial NCC migration and subsequent branchial arch morphogenesis. Whereas Efnb2 null embryos variably exhibit hypoplastic first and second branchial arches and reduced aortic arches and fifth (trigeminal) cranial ganglia due to impaired NCC migration (Adams et al., 2001; Davy and Soriano, 2007), these defects are rescued in embryos hemizygous for a null allele in combination with an allele encoding an ephrin-B2 protein lacking tyrosine resides required for reverse signaling (Davy and Soriano, 2007). Ephrin-B2 is also expressed in the posterior half of somites (Wang and Anderson, 1997; Davy and Soriano, 2007) while its cognate receptors are expressed in migrating trunk NCCs (Wang and Anderson, 1997). As such, Efnb2 null embryos additionally exhibit trunk NCC migration defects with an invasion of NCCs into the posterior half of somites (Davy and Soriano, 2007). Furthermore, conditional ablation of Efnb2 in NCCs using the Wnt1-Cre driver impairs migration of the thymus into the thoracic cavity due to defects in the motility of NCC-derived thymic mesenchymal cells (Foster et al., 2010). Efnb1/Efnb2 compound heterozygous embryos display further abnormalities in tissues composed of NCC-derived mesenchyme, including the salivary gland and eyelid, indicating that the two proteins act redundantly at these sites (Davy and Soriano, 2007), though their exact mechanism of action on these tissues is unknown.
Additionally, both ephrin-A4 and EphA4 have been shown to play a role in establishing calvarial boundaries. In a Twist1 haploinsufficiency model of coronal synostosis, typified by premature fusion of the suture between the NCC-derived frontal bone and the mesoderm-derived parietal bone, reduced ephrin-A4 distribution in the suture accompanies a boundary defect wherein the NCCs invade the undifferentiated mesoderm (Merrill et al., 2006). This same coronal synostosis phenotype is observed in Epha4 null mice, and is associated with reduced phospho-Erk1/2 expression in the ectocranial layer of the suture (Ting et al., 2009). Consistent with the mouse model data, heterozygous germline mutations of EFNA4 in humans are associated with several cases of non-syndromic coronal synostosis (Merrill et al., 2006).
Functional roles for ephrin-A5 and ephrin-B1 in regulating survival of cranial NCCs and proliferation of cranial NCC-derived mesenchyme, respectively, have also been demonstrated. Wnt1-Cre-mediated expression of ephrin-A5-Fc along the dorsal midline of the mouse midbrain results in craniofacial hypoplasia. Accompanying NCC explant experiments revealed that ephrin-A5-Fc hinders cranial NCC survival in the dorsal midline (Noh et al., 2014), providing a potential mechanism for the phenotype. In the case of X-linked ephrin-B1, heterozygous loss of the gene in mice results in sorting out of ephrin-B1-expressing and -non-expressing cells due to X inactivation, disruption of cell proliferation in the anterior palatal shelf mesenchyme and perturbations in downstream Erk/MAPK signaling. These defects result in a cleft palate phenotype that mirrors a subset of craniofacial abnormalities in human X-linked craniofrontonasal syndrome (Bush and Soriano, 2010) caused by heterozygous loss-of-function mutations in EFNB1 (Twigg et al., 2004).
Finally, additional analyses of Efnb1+/− mice revealed that the observed calvarial defects in this model stem from inhibition of gap junction communication and impaired differentiation of NCCs into osteogenic precursors (Davy et al., 2006).
2.3 FGF receptors
The mammalian FGF family consists of 22 FGF proteins, 18 of which variously signal through four receptors, FGFR1–4. Most FGF ligands additionally bind heparin or heparan sulfate proteoglycans, an interaction that serves to increase the affinity between the ligands and FGFRs and contribute to receptor dimerization and activation (Yayon et al., 1991; Rapraeger et al., 1991; Spivak-Kroizman et al., 1994). The FGFRs are composed of an extracellular portion containing three immunoglobulin-like domains (D1–D3) and an acid box, and a cytoplasmic portion with a split tyrosine kinase domain (Lee et al., 1989) (Figure 1). The FGFRs are subject to extensive alternative splicing which produce, among other forms, FGFR1–3 isoforms containing an alternatively-spliced C-terminal half of D3 depending on the inclusion of exon 8 (“b” isoforms) or exon 9 (“c” isoforms) (Johnson et al., 1991; Miki et al., 1992; Yayon et al., 1992). Importantly, this alternative splicing produces receptor isoforms with different tissue-specific expression as well as unique ligand-binding properties (Miki et al., 1992; Yayon et al., 1992).
The FGFRIIIb isoforms are generally expressed by the epithelia during development, while the FGFRIIIc isoforms commonly localize to the mesenchyme (Orr-Urtreger et al., 1993; Avivi et al., 1993), with their respective ligands expressed in the adjacent compartment (Ornitz et al., 1996). To date, only a handful of FGF family members – the ligand FGF8 and the receptors FGFR1 and FGFR2 – have been shown to regulate mammalian NCC activity in vivo. Fgf8 is expressed in the craniofacial, central nervous system and limb bud epithelia as well as the pharyngeal arch ectoderm and endoderm during development and binds to FGFR isoforms expressed in the mesenchyme (MacArthur et al., 1995). Targeted disruption of Fgf8 in mice results in embryonic lethality before E10.5 and a loss of all mesoderm-derived structures (Meyers et al., 1998). Mice homozygous for an Fgf8 hypomorphic allele die perinatally and exhibit impaired development of the midbrain, cerebellum and olfactory bulbs (Meyers et al., 1998). Compound heterozygous mice harboring one null and one hypomorphic Fgf8 allele have variable defects, a subset of which phenocopy human 22q11 deletion syndromes, including DiGeorge syndrome. These defects include abnormalities in craniofacial, pharyngeal gland, brain, cardiac and posterior axis development (Meyers et al., 1998; Frank et al., 2002; Abu-Issa et al., 2002). Analyses of mutant mouse models have revealed that FGF8 regulates several aspects of NCC activity during development of various craniofacial, pharyngeal, cardiac and neural structures. The ligand promotes survival of the NCC-derived ectomesenchymal cells of the first branchial arch (Trumpp et al., 1999) and pharyngeal and cardiac NCCs (Abu-Issa et al., 2002; Frank et al., 2002). FGF8 expression from the ectoderm establishes and maintains rostral-caudal polarity in the ectomesenchymal cells of the first branchial arch (Tucker et al., 1999) and is required for outgrowth of this structure (Trumpp et al., 1999). Furthermore, upregulation of craniofacial Fgf8 expression has been shown to result in an expansion of cranial NCCs leading to an enlarged first branchial arch, maxillary hyperplasia and a high arched palate in two ciliopathic mutant mouse models (Tabler et al., 2013). In pharyngeal arches 3–6, FGF8 expression in both the ectoderm and endoderm is required for proper thymus, parathyroid and cardiac development (Macatee et al., 2003; Park et al., 2006). Moreover, a requirement for FGF8 has been demonstrated in promoting the survival of NCCs that will give rise to postganglionic neurons (Chen et al., 2012)
Targeted disruption of Fgfr1 results in embryonic lethality between E9.5–E12.5, defects in cell migration out of the posterior primitive streak and defective patterning of axial structures (Yamaguchi et al., 1994; Deng et al., 1994). While mice homozygous for an inactivating mutation in the FGFR1IIIb isoform are viable and fertile, those deficient in the FGFR1IIIc isoform phenocopy the Fgfr1 null mutant (Partanen et al., 1998). Mice homozygous for Fgfr1 hypomorphic alleles die perinatally and exhibit defects in craniofacial and limb development as well as abnormalities in the formation of the anterior-posterior axis (Partanen et al., 1998). Further analyses of the Fgfr1 hypomorphic allele in combination with conditional deletion of the receptor in NCCs revealed that FGFR1 is required for the entry of NCCs into the second branchial arch and for proper lip and secondary palate formation (Trokovic et al., 2003; Wang et al., 2013). While the former requirement for the receptor is non-cell-autonomous in NCCs (Trokovic et al., 2003), the latter is cell-autonomous in the palatal mesenchyme and non-cell-autonomous in the palatal and medial edge epithelia (Wang et al., 2013).
Targeted disruption of Fgfr2 results in embryonic lethality at E10–E11 and defects in placenta and limb bud formation (Xu et al., 1998). Mice lacking FGFR2IIIb die perinatally and exhibit defects in the skull, palate, teeth, inner ear, salivary gland, anterior pituitary gland, limb, lung and skin (De Moerlooze et al., 2000), while those deficient in the FGFR2IIIc isoform are viable and display delayed ossification, craniosynostosis and dwarfism of the long bones and axial skeleton (Eswarakumar et al., 2002). Mice heterozygous for an Fgfr2 gain-of-function allele expressed exclusively in NCCs exhibit prematurely fused cranial sutures and dysmorphologies affecting the snout, cranial base and cranial vault (Heuze et al., 2014).
Additional studies have revealed roles for various adaptor proteins, specifically Crkl and Frs2, in mediating NCC activity downstream of FGFR activation. FGF8 binding induces the phosphorylation of both FGFR1 and FGFR2 and their subsequent binding to Crkl, which in turn promotes NCC survival and migration (Moon et al., 2006). Similar to mutant mouse models of Fgf8, targeted disruption of the gene encoding the Crkl adaptor protein also results in a combination of craniofacial, glandular and cardiovascular phenotypes reminiscent of human 22q11 deletion syndromes (Guris et al., 2001), indicating that Crkl signaling downstream of FGF8-mediated FGFR activation regulates NCC activity in several locations throughout the embryo. In regards to Frs2, homozygous mutant mice in which the Frs2/3 binding site on FGFR1 is deleted die during late embryogenesis with defects in spinal neural tube closure and development of the second pharyngeal arch and tail bud (Hoch and Soriano, 2006). The pharyngeal arch defect was shown to stem from impaired NCC migration into the first and second arches and ectopic cell death along the migratory path, and further resulted in hypoplastic second pharyngeal arch derivatives in the middle ear and hyoid (Hoch and Soriano, 2006).
Point mutations in FGFR1, FGFR2 and FGFR3 underlie a series of human syndromes characterized by craniosynostosis and/or midface hypoplasia, among other associated defects (reviewed in Kelleher et al., 2013). A recent study using mesoderm- or NCC-specific Cre drivers in combination with a Cre-inducible activating mutation in Fgfr2 revealed that mesodermal expression of the mutation is necessary and sufficient to generate a craniosynostosis phenotype (Holmes and Basilico, 2012). Finally, heterozygous mutations in FGFR1 and FGF8 cause different forms of Kallmann syndrome typified by hypogonadotropic hypogonadism and a variable frequency of cleft palate, sensorineural hearing loss and anosmia (Dodé et al., 2003; Falardeau et al., 2008).
2.4 Kit receptor
The Kit family is composed of the Kit ligand (Kitl, also known as MGF, SCF, Steel factor) and its receptor, Kit (Williams et al., 1990; Zsebo et al., 1990; Huang et al., 1990). Kitl is alternatively spliced to generate membrane-bound and soluble ligands, both of which are able to bind and activate Kit (Anderson et al., 1991). The Kit receptor consists of an extracellular portion harboring five immunoglobulin-like domains and an intracellular portion containing a split tyrosine kinase domain (Yarden et al., 1987; Qiu et al., 1988) (Figure 1).
Kitl is encoded at the Sl (Steel) locus (Copeland et al., 1990; Zsebo et al., 1990; Huang et al., 1990) and is expressed in the mesenchyme lining the migratory pathways and destinations of melanoblast precursors, hematopoietic cells and germ cells, with additional expression in the brain and spinal cord (Matsui et al., 1990). Kit is encoded at the W (Dominant white spotting) locus (Chabot et al., 1988; Geissler et al., 1988) and is reciprocally expressed in melanoblasts, hematopoietic cells and germ cells, as well as in ectoderm-derived structures of the central nervous system and craniofacial region, and the endoderm-derived intestinal tract (Orr-Urtreger et al., 1990). Mutations at both loci result in defects in pigmentation, hematopoiesis and reproduction (Bennett, 1956; Dunn, 1937; Sarvella and Russell, 1956; Gruneberg, 1942; McCoshen and McCallion, 1975; Geissler et al., 1981). As the names of their loci suggest, these mutants were prized for their coat color and collected in Asia (Tokuda, 1935) and eventually the European “Mouse Fancy”, ultimately contributing to the establishment of inbred mouse strains.
Signaling through the Kit receptor has been shown to regulate the survival and migration of NCC-derived melanoblast precursors in mice. Membrane-bound Kitl and Kit kinase activity are required for melanoblast survival, as melanoblasts of KitlSl-d mutants lacking both the transmembrane and cytoplasmic domains of the ligand and KitlSl-17H mutants with reduced cell surface expression of the membrane-bound ligand, as well as those of KitW-v and KitW-41J mutants carrying point mutations in the receptor tyrosine kinase domain of the receptor, are observed along migratory pathways but are subsequently absent from their target sites in the inner ear and dermis (Steel et al., 1992; Wehrle-Haller and Weston, 1995; Cable et al., 1995; MacKenzie et al., 1997; Wehrle-Haller and Weston, 1999). Conversely, melanoblast precursors are absent from the migratory pathways of embryos homozygous for the KitlSl null allele, indicating that soluble ligand is necessary for melanoblast precursor dispersal (Wehrle-Haller and Weston, 1995). By rescuing melanoblast precursor survival in KitlSl null embryos, Kit signaling was shown to regulate the entrance and migration of these cells along a dorsolateral pathway (Wehrle-Haller et al., 2001). The role of Kit in melanogenesis is dependent, at least in part, on signaling through Src family kinases, SHP-1 and/or SHP-2, as mice homozygous for two tyrosine to phenylalanine mutations at residues known to mediate association of the receptor with these proteins have a white coat devoid of melanocytes. Further analysis revealed that these mutations result in reduced receptor autophosphorylation and attenuated downstream Erk/MAPK signaling (Kimura et al., 2004). Examination of an additional mouse model harboring a targeted mutation in one of these residues demonstrated that homozygous mutant animals exhibit variably penetrant loss of ventral pigment that is exacerbated in hemizygous animals carrying one copy of a Kit null allele, confirming a role for Src signaling downstream of the receptor in pigmentation (Agosti et al., 2004). These authors also revealed a potential role for PI3K-mediated Kit signaling in regulating melanogenesis, as mice hemizygous for an allele abrogating binding of the receptor to PI3K in combination with a Kit null allele have a mild pigmentation phenotype (Agosti et al., 2004). Finally, signaling through the Kit receptor has also been shown to contribute to the development of a subset of primary sensory neurons, as both small- and medium-diameter sensory neurons are absent in the dorsal root ganglia of KitW mutant mice (Zhang and Sieber-Blum, 2009).
2.5 MET receptor
The hepatocyte growth factor (HGF) family consists of the HGF/scatter factor (SF) ligand and its receptor, MET (Bottaro et al., 1991). The MET receptor consists of an extracellular portion composed of a Sema domain, a cysteine-rich PSI domain and four immunoglobulin-like repeat domains, as well as an intracellular portion containing a tyrosine kinase domain and a carboxy-terminal binding domain (Graveel et al., 2013) (Figure 1).
HGF/SF is generally expressed in the mesenchyme of various organs in the developing mouse embryo and the MET receptor is reciprocally expressed in the adjacent epithelia, yet expression of the two transcripts overlaps in NCCs, among other sites (Sonnenberg et al., 1993; Andermarcher et al., 1996). Both Hgf and Met homozygous null embryos die during midgestation with liver and placental defects, and Met null embryos additionally exhibit skeletal muscle abnormalities (Schmidt et al., 1995; Uehara et al., 1995; Bladt et al., 1995). While neither knockout model has a NCC phenotype, analyses of transgenic mice ubiquitously overexpressing HGF/SF uncovered a role for this signaling pathway in NCC derivatives. Overexpression of HGF/SF induces the presence of ectopic melanoblasts in the embryonic neural tube and dorsal root ganglia, as well as ectopic melanocyte formation in the adult central nervous system and skin (Takayama et al., 1996; Kos et al., 1999). Additionally, HGF/SF was shown to promote melanoblast survival and melanoctye differentiation in NCC explant cultures (Kos et al., 1999). Finally, HGF/SF transgenic mice have a high incidence of gastrointestinal obstruction, which may stem from abnormal development of the enteric ganglia, thus pointing to a potential additional role for this pathway in regulating NCC derivatives (Takayama et al., 1996).
2.6 MuSK receptor
The mammalian muscle-specific kinase (MuSK) family consists of one bona fide ligand, the heparan-sulfate proteoglycan N-agrin, which activates the MuSK receptor (Glass et al., 1996). The receptor is composed of an extracellular portion harboring three immunoglobulin-like domains and a Frizzled-like cysteine-rich domain, and an intracellular portion containing a tyrosine kinase domain (Valenzuela et al., 1995; Xu and Nusse, 1998; Masiakowski and Yancopoulos, 1998) (Figure 1). While Wnt11r, the zebrafish orthologue of the mammalian secreted glycoprotein Wnt11, has been shown to bind the MuSK receptor via its cysteine-rich domain (Jing et al., 2009), N-agrin does not bind MuSK, but instead interacts with MuSK-bound LRP4 to enhance the LRP4-MuSK association and activate MuSK (Kim et al., 2008; Zhang et al., 2008).
MuSK is expressed in developing muscle, at the neuromuscular junction, in the brain and in sperm (Valenzuela et al., 1995; Garcia-Osta et al., 2006; Kumar et al., 2006), and mutant mouse models of both MuSK and N-agrin die perinatally and exhibit defective neuromuscular synaptogenesis (DeChiara et al., 1996; Gautam et al., 1996). While studies of MuSK function during murine development have primarily focused on its role in neuromuscular junction formation, a recent study revealed an additional requirement for the receptor in maintaining segmental NCC migration. In Musk homozygous null mouse embryos, trunk NCCs fail to be restricted to the anterior somite and instead spread throughout the entire somite (Banerjee et al., 2011). In zebrafish, the identical role for MuSK is mediated through the Wnt11r ligand and Dishevelled signaling downstream of the receptor (Banerjee et al., 2011).
2.7 PDGF receptors
The mammalian PDGF family is composed of four ligands, PDGF-A-D, which variously signal through two receptors, PDGFRα and PDGFRβ. The PDGF receptors consist of five extracellular immunoglobulin-like loops and a split intracellular tyrosine kinase domain (Williams, 1989) (Figure 1). While numerous ligand and receptor interactions have been demonstrated in vitro, relatively few functional interactions have been demonstrated in vivo. The homodimers PDGF-AA and PDGF-CC have been shown to exclusively activate PDGFRα signaling during mammalian development (Boström et al., 1996; Ding et al., 2004; Soriano, 1997), while PDGF-BB solely activates PDGFRβ signaling (Levéen et al., 1994; Soriano, 1994). The role of PDGF-DD has yet to be investigated using mouse models.
To date, PDGFRα signaling has been shown to contribute to both cranial and cardiac NCC development, while a more restricted role in cardiac NCC development has been demonstrated for PDGFRβ. PDGFRα is expressed in the embryonic mesenchyme, specifically in the non-neuronal derivatives of NCCs, while its ligands PDGF-A and PDGF-C are reciprocally expressed in the surface ectoderm and epithelium (Morrison-Graham et al., 1992; Orr-Urtreger and Lonai, 1992; Ding et al., 2000). Targeted disruption of Pdgfra in mice results in embryonic lethality during midgestation, with homozygous null embryos exhibiting a cleft face, subepidermal blebbing, edema, hemorrhaging, cardiac outflow tract defects, abnormalities in neural tube development, abnormally patterned somites and skeletal defects (Soriano, 1997). These defects are phenocopied in mice lacking both PDGF-A and PDGF-C ligands (Ding et al., 2004). Conditional ablation of Pdgfra in NCCs using the Wnt1-Cre driver results in a subset of the null phenotypes, specifically, facial clefting, midline hemorrhaging, aortic arch defects and thymus hypoplasia (Tallquist and Soriano, 2003). Additional analyses exploring the cellular mechanism of the facial clefting phenotype demonstrated that Pdgfrafl/fl;Wnt1-Cre embryos exhibit a delay in the migration of NCCs into the frontonasal prominence and decreased proliferation in this structure (He and Soriano, 2013). PDGFRβ is also expressed in the embryonic mesenchyme, with high expression levels in the heart, among other sites (Soriano, 1994). Pdgfrb-deficient mice die perinatally and exhibit hemorrhaging, thrombocytopenia, anemia and kidney defects (Soriano, 1994). Furthermore, both Pdgfrb and Pdgfb null mouse embryos exhibit cardiac defects associated with impaired cardiac NCC development (Richarte et al., 2007; Van den Akker et al., 2008). Conditional ablation of both Pdgfra and Pdgfrb in NCCs using the Wnt1-Cre driver results in defects in multiple cardiac NCC derivatives that are more severe than those observed in either single conditional homozygous mutant alone, stemming from impaired cardiac NCC migration into the outflow tract (Richarte et al., 2007).
Analysis of an allelic series of autophosphorylation mutant knock-in mice at the Pdgfra locus identified PI3K signaling as the main intracellular pathway downstream of PDGFRα signaling during embryogenesis in the mouse (Klinghoffer et al., 2002). Embryos homozygous for an allele (PdgfraPI3K) harboring two tyrosine to phenylalanine mutations at residues that mediate the ability of PDGFRα to bind PI3K (Yu et al., 1991) die perinatally and exhibit a cleft palate, among other defects (Klinghoffer et al., 2002). Moreover, the full range of Pdgfra−/− phenotypes, including complete facial clefting, is observed in PdgfraPI3K/PI3K;PdgfrbPI3K/PI3K double homozygous mutant embryos in which PI3K signaling cannot be engaged through PDGFRα/β heterodimers (Klinghoffer et al. 2002). We recently employed a mass spectrometry-based phosphoproteomic approach to identify intracellular effectors downstream of PI3K/Akt-mediated PDGFRα signaling in mouse embryonic NCC-derived palatal mesenchyme. Our analysis identified both established and novel Akt phosphorylation target proteins, a subset of which we demonstrated regulate cell survival and proliferation downstream of PDGFRα activation in part through modulation of p53 activity (Fantauzzo and Soriano, 2014).
While PDGFRβ does not have an established role in cranial NCC development, limited evidence, including the phenotypes of Pdgfrafl/fl;Pdgfrbfl/fl;Wnt1-Cre and PdgfraPI3K/PI3K;PdgfrbPI3K/PI3K double homozygous mutant embryos described above (Richarte et al., 2007; Klinghoffer et al., 2002), indicate that the two receptors may be able to form functional heterodimers in vivo. Previous in vitro studies suggest that PDGFRα/β heterodimers have distinct properties from homodimeric receptor complexes. For example, PDGFRα is phosphorylated at Y754 exclusively upon heterodimer formation, resulting in preferential binding of SHP-2 (Rupp et al., 1994). Conversely, phosphorylation of PDGFRβ at Y771 is decreased in heterodimer receptor complexes, leading to reduced association with Ras-GAP and prolonged activation of Ras and MAP kinase (Ekman et al., 1999). Furthermore, heterodimers have been shown to generate an enhanced mitogenic response over that of either homodimer receptor complex (Rupp et al., 1994). The prevalence of these heterodimers, as well as their effect on cellular behavior during development, has yet to be determined.
Finally, PDGFRα may also play a cell-autonomous role in melanocyte development. Chimeric mice derived from embryonic stem cells heterozygous for either the Pdgfra null or PdgfraPI3K allele exhibit poor coat color chimerism, indicating a particular dosage requirement for PI3K-mediated PDGFRα signaling in contributing to coat color pigmentation (Soriano, 1997; Klinghoffer et al., 2002).
2.8 PTK7 receptor
Mammalian protein tyrosine kinase 7 (PTK7) is an orphan receptor composed of an extracellular portion containing seven immunoglobulin-like loops and an intracellular portion with an inactive tyrosine kinase domain (Park et al., 1996) (Figure 1). Ptk7 transcripts are detected in the primitive streak, craniofacial region, heart, gut, lung, somites, limbs and tail in the mouse embryo (Jung et al., 2004; Paudyal et al., 2010), while the protein has been shown to localize specifically to the neuroepithelium and mesenchyme during early development (Paudyal et al., 2010). Mice homozygous for a gene trap insertion in the second intron of the gene die perinatally and display defects in neural tube closure and stereociliary bundle orientation in the ear (Lu et al., 2004). Chuzhoi mice, which are homozygous for an ENU-induced splice site mutation in the Ptk7 gene, also die perinatally and similarly exhibit severe neural tube defects and altered planar cell polarity in the ear, as well as cardiac outflow tract and ventricular septal defects, omphalocele, abnormal lung development, and skeletal defects affecting the ribs and limbs (Paudyal et al., 2010). Chuzhoi mutant embryos additionally have abnormal NCC distribution, displaying misshapen cranial and dorsal root ganglia (Paudyal et al., 2010).
2.9 RET receptor
Signaling through the rearranged during transfection (RET) receptor involves the formation of a multicomponent receptor complex consisting of a glial cell line-derived neurotrophic factor (GDNF) family ligand (GFL) (GDNF, neurturin, artemin or persephin), a ligand-binding glycosyl-phosphatidylinositol (GPI)-anchored coreceptor (GFRα1–4) and the RET receptor tyrosine kinase. The RET receptor consists of an extracellular domain composed of a cadherin-related motif and a cysteine-rich region, and an intracellular domain harboring a split tyrosine kinase domain (Iwamoto et al., 1993) (Figure 1). GDNF, neurturin (NRTN), artemin (ARTN) and persephin (PSPN) primarily bind and signal through GFRα1, GFRα2, GFRα3 and GFRα4, respectively (Jing et al., 1996; Treanor et al., 1996; Klein et al., 1997; Buj-Bello et al., 1997; Baloh, et al., 1998; Enokido et al., 1998).
In the developing mouse embryo, Gdnf is expressed in the anterior neuroectoderm during early neurogenesis and later localizes to the mesenchyme at several sites throughout the embryo, including the gastrointestinal tract, kidney, testes, facial prominences, eye, tongue, tooth primordia, vibrissae (whisker) follicles, ear, paravertebral mesenchyme and limbs (Hellmich et al., 1996). Ret is expressed in NCCs, several lineages of the peripheral and central nervous systems, including the cranial, autonomic, dorsal root and enteric ganglia, the nephric duct, the epithelia of the ureteric bud and the renal collecting ducts (Pachnis et al., 1993). Targeted disruption of both Gdnf and Ret in mice results in perinatal lethality, with homozygous null embryos lacking enteric neurons and displaying renal agenesis due to defective induction of the ureteric bud, among other defects (Sanchez et al., Pichel et al., 1996; Moore et al., 1996; Schuchardt et al., 1994). Conditional ablation of Ret in NCCs using the Wnt1-Cre driver similarly results in intestinal aganglionosis (Luo et al., 2007). Gdnf null neonates additionally exhibit neuronal loss in dorsal root and sympathetic ganglia (Moore et al., 1996).
Studies using rodent models have demonstrated that perturbations of the GDNF-GFRα1-RET signaling pathway disrupt several aspects of enteric NCC development, including survival, migration, proliferation and/or neuronal differentiation, which contribute to the pathology of the intestinal aganglionosis phenotype in mammals (Heuckeroth et al., 1998; Taraviras et al., 1999; Uesaka et al., 2008; Uesaka et al., 2010). Furthermore, analysis of RET phosphorylation mutant knock-in mice have revealed roles for the MAPK, PI3K/Akt and JNK signaling cascades downstream of Shc adaptor and cAMP-dependent PKA interactions in mediating the role of the receptor during enteric nervous system development (Jijiwa et al., 2004; Wong et al., 2005; Asai et al., 2006; Jain et al., 2010).
In humans, heterozygous germline mutations in GDNF and/or RET underlie a significant subset of cases of Hirschsprung’s disease (Eketjäll and Ibáñez, 2002; Romeo et al., 1994; Edery et al., 1994), characterized by a congenital absence of enteric ganglia in a portion of the gastrointestinal tract.
2.10 ROR receptors
The mammalian receptor tyrosine kinase-like orphan receptor (ROR) family consists of two receptors, Ror1 and Ror2. While a ligand has not been identified for Ror1, the secreted glycoprotein Wnt5a has been shown to bind and signal through Ror2 to activate the non-canonical Wnt pathway, thereby inducing cell migration (Oishi et al., 2003; Nishita et al., 2006; Yamamoto et al., 2007; Nomachi et al., 2008; He et al., 2008) and regulating cell proliferation (He et al., 2008). The extracellular portions of the receptors are composed of an immunoglobulin-like domain, Frizzled-like cysteine-rich domain and a kringle domain, while the intracellular portions contain a tyrosine kinase domain and a carboxy-terminal proline-rich domain (Masiakowski and Carroll, 1992; Oishi et al., 1999) (Figure 1).
In the developing mouse embryo, Ror1 is expressed in the anterior part of the embryo at E7.5, while Ror2 is detected throughout the primitive streak. One day later, Ror1 transcripts localize to the cephalic mesenchyme, with particularly high expression levels in NCCs, while Ror2 is more broadly expressed in neural and non-neural tissues, including cephalic NCCs. At later stages, the two receptors have largely overlapping expression patterns at several sites throughout the embryo, with particularly high transcript levels in NCCs and their derivatives in the face and heart (Oishi et al., 1999; Al-Shawi et al., 2001; Matsuda et al., 2001). Similar to Ror2, Wnt5a is expressed in the primitive streak and later in the facial primordia and heart, among other sites (Yamaguchi et al., 1999; Schleiffarth et al., 2007).
Both Ror1 and Ror2 homozygous null embryos die perinatally with respiratory defects (Nomi et al., 2001; Takeuchi et al., 2000; DeChiara et al., 2000). Ror2-deficient mice additionally exhibit widespread skeletal defects, including craniofacial bone hypoplasia, and ventricular septal defects of the heart (Takeuchi et al., 2000; DeChiara et al., 2000). Double homozygous mutant mice also die perinatally and exhibit enhanced skeletal phenotypes over those observed in Ror2 null mice, as well as transposition of the great arteries, indicating that the two receptors interact genetically during skeletal and cardiac development (Nomi et al., 2001). Wnt5a homozygous null embryos also display craniofacial bone truncations and cardiac outflow tract abnormalities, among other defects (Yamaguchi et al., 1999; Schleiffarth et al., 2007). Furthermore, Ror2+/−;Wnt5a+/− double heterozygous embryos exhibit a cleft palate, confirming an interaction between this ligand and receptor pair in the palatal mesenchyme (He et al., 2008).
In humans, a subset of mutations in ROR2 result in autosomal recessive Robinow syndrome, characterized by skeletal dysplasia affecting the craniofacial bones, limbs and vertebra, as well as genital hypoplasia (Afzal et al., 2000; van Bokhoven et al., 2000), while the phenotypically less severe autosomal dominant form of the syndrome stems from mutations in WNT5A (Person et al., 2010).
2.11 Trk receptors
The mammalian tropomyosin-related kinase (Trk) receptor family is composed of four ligands, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and NT-4, which variously bind and activate three receptors, TrkA, TrkB and TrkC. The vertebrate Trk receptors consist of an extracellular portion harboring a cysteine-rich cluster, three leucine-rich repeats, a second cysteine-rich cluster and two immunoglobulin-like domains, as well as an intracellular portion containing a tyrosine kinase domain (Benito-Gutiérrez et al., 2006) (Figure 1). NGF binds to TrkA (Klein et al., 1991; Kaplan et al., 1991); BDNF and NT-4 interact with TrkB (Squinto et al., 1991; Klein et al., 1991); and NT-3 binds with high affinity to TrkC and with lower affinity to other receptors of the family (Lamballe et al., 1991).
Ntrk1, encoding TrkA, is expressed exclusively in the NCC-derived dorsal root and cranial sensory ganglia of the peripheral nervous system during murine development (Martin-Zanca et al. 1990). Ntrk2 and Ntrk3, encoding TrkB and TrkC, respectively, are expressed at the same sites as TrkA, with broader expression in the brain, the spinal cord, the non-sensory cranial ganglia and placode-derived sensory ganglia of the peripheral nervous system, and the dorsal aorta, among other non-neural sites (Klein et al., 1990; Tessarollo et al., 1993). Ntrk2 and Ntrk3 are additionally expressed in the NCC-derived enteric nervous system, with Ntrk2 expressed in the enteric glial cells (Levanti et al., 2009) and Ntrk3 expressed in the enteric ganglia (Tessarollo et al., 1993). The expression of specific Trk receptors in various neuronal populations appears to specify their subtype, as expression of Ntrk3 from the Ntrk1 locus alters the fate of a subset of dorsal root ganglia neurons (Moqrich et al., 2004).
Targeted disruption of Ntrk1 in mice results in premature death, typically within the first month after birth, with homozygous null mice exhibiting neuronal loss in dorsal root, trigeminal and sympathetic ganglia (Smeyne et al., 1994), as well as defects in thymus development (García-Suárez et al., 2000). Both the Ntrk2 and Ntrk3 loci undergo alternative splicing to generate either a full-length receptor or a truncated receptor lacking the tyrosine kinase domain. Mice homozygous for a germline mutation in the tyrosine kinase domain of Ntrk2 die within the first two weeks after birth and display neuronal loss in both the central and peripheral nervous systems, including in the trigeminal and dorsal root ganglia, among other defects (Klein et al., 1993). Mutant mice lacking all isoforms of Ntrk2 exhibit similar timing of premature death, yet less severe neuronal loss in sensory ganglia, indicating that the truncated receptor may negatively regulate neuron survival (Luikart et al., 2003). Ntrk2-deficient mice additionally display defects in enteric glial cell development (Levanti et al., 2009). Mice homozygous for a targeted mutation in the tyrosine kinase domain of Ntrk3 usually die within the first month of birth and lack a subset of dorsal root ganglia neurons (Klein et al., 1994). Mutant mice lacking all isoforms of Ntrk3 die within one week of birth and display more severe neuronal phenotypes affecting the trigeminal, vestibular, cochlear, petrosal-nodose and dorsal root ganglia. These mice also exhibit cardiac defects such as atrial and ventricular septal defects and valvular defects, among other abnormalities, suggesting a kinase-independent role for TrkC during mouse development (Tessarollo et al., 1997). Analysis of mice harboring a mutation of the Shc-binding site in TrkB or TrkC receptors revealed a role for this pathway in regulating target innervation of sensory neurons downstream of the TrkB, but not the TrkC, receptor (Postigo et al., 2002).
In humans, mutations in NTRK1 underlie the autosomal recessive disorder congenital insensitivity to pain with anhidrosis (Indo et al., 1996), which is characterized by defective NCC differentiation into a subset of sensory neurons as well as neuronal loss in the sympathetic ganglia. Furthermore, polymorphisms in NTRK3 have been found in several patients with Hirschsprung’s disease, which is typified by defective NCC activity during development of the enteric nervous system, though a causal role for these variants in the disease phenotype has not yet been demonstrated (Ruiz-Ferrer et al., 2008; Fernández et al., 2009).
2.12 VEGF receptors
The mammalian vascular endothelial growth factor (VEGF) family consists of five ligands that are subject to alternative splicing and/or processing, VEGF-A-D and placental growth factor (PGF), which variously signal through three receptors, VEGFR1 (also known as Flt-1), VEGFR2 (KDR/Flk-1) and VEGFR-3 (Flt-4), as well as two neuropilin (Nrp) co-receptors, Nrp1 and Nrp2. The VEGF receptors consist of an extracellular portion with seven immunoglobulin-like domains and an intracellular portion with a split tyrosine kinase domain (Shibuya et al., 1990) (Figure 1). The NRP co-receptors, which also bind semaphorins (He et al., 1997; Kolodkin et al., 1997), are quite distinct from the VEGF receptors and contain an extracellular portion with three interaction domains designated a1/a2, b1/b2 and c, and a negligible cytoplasmic domain that lacks catalytic function (Kawakami et al., 1996). Binding of VEGF ligand to a VEGF receptor induces receptor homo- or heterodimerization. VEGF-A binds VEGFR1 and VEGFR2 homodimers, VEGFR1/2 and VEGFR2/3 heterodimers, as well as Nrp1 homodimers; VEGF-B and PGF bind VEGFR1 homodimers, VEGFR1/2 heterodimers and Nrp1 homodimers; and VEGF-C and VEGF-D bind VEGFR2 and VEGFR3 homodimers, VEGFR2/3 heterodimers and Nrp2 homodimers (reviewed in Koch and Claesson-Welsh, 2012).
While all members of the family function in vascular development, only the interaction of VEGF-A with Nrp1 has been implicated in NCC biology. Vegfa is widely expressed by parenchymal cells throughout the embryo, including the cardiac outflow tract, pharyngeal arch endoderm, thymus, facial prominences and palate, among other sites, while Nrp1 is expressed in neighboring, often endothelial, cells at each of these sites (Stalmans et al., 2003). Consistent with its expression, mouse embryos devoid of the major, Nrp1-binding isoform of Vegfa exhibit cardiac outflow tract, pharyngeal arch artery, thymic, parathyroid and craniofacial defects, reminiscent of human DiGeorge syndrome (Stalmans et al., 2003). Moreover, endothelial-specific disruption of Nrp1 similarly results in a combination of phenotypes typical of DiGeorge syndrome, such as defects in the cardiac outflow tract (Gu et al., 2003; Zhou et al., 2012), pharyngeal organ hypoplasia and cleft palate (Zhou et al., 2012). The defects observed in the above mouse models are not due to defective NCC migration, but have instead been attributed to vascular dysgenesis and endothelial cell dysfunction (Stalmans et al., 2003; Zhou et al., 2012). In contrast, a study in chick revealed that VEGFR2 and Nrp1 are expressed in cranial NCCs while VEGF-A is expressed in the surface ectoderm adjacent to the rhombomere 4 NCC migratory route, and furthermore, that the VEGF-A-Nrp1 interaction was required for proper cranial NCC invasion from the rhombomere 4 migratory stream into branchial arch 2 (McLennan et al., 2010).
3. Current Methods to Investigate Receptor Tyrosine Kinase Signaling
3.1 Receptor allelic series
As highlighted above, beyond the analysis of null mouse models, the use of conditional, floxed alleles in conjunction with NCC-specific Cre driver alleles has allowed researchers to examine the roles of various receptors and the signaling proteins with which they interact exclusively in NCCs. This approach has been utilized with a Wnt1-Cre driver (Danielian et al., 1998) in combination with Efnb1, Efnb2, Fgfr1, Pdgfra and Ret conditional alleles to demonstrate cell autonomous functions of these receptors in NCCs (Davy et al., 2004; Foster et al., 2010; Wang et al., 2013; Tallquist and Soriano, 2003; He and Soriano, 2013; Luo et al., 2007). While these studies have provided critical data on the roles of each of these RTKs in NCCs, it should be noted that the original Wnt1-Cre driver (Danielian et al., 1998) ectopically activates Wnt signaling, resulting in defects in midbrain development in heterozygous animals which are even more severe in Wnt1-CreTg/Tg mice (Lewis et al., 2013). However, the development of a new tool, the Wnt1-Cre2 transgenic mouse line (Lewis et al., 2013), circumvents these issues and will likely be of considerable use to the field going forward. Further NCC-specific Cre drivers include the P0-Cre (Yamauchi et al., 1999), P3Pro-Cre (Li et al., 2000), Ht-PA-Cre (Pietri et al., 2003) and S4F:Cre (Stine et al., 2009) alleles. Moreover, by employing additional, tissue-specific Cre drivers active in NCC target sites, the cell-autonomous role of a particular protein can be assessed in the various layers of tissues populated by NCCs. Using the pharyngeal arch as an example, the Foxg1-Cre transgene (Hébert and McConnell, 2000) can be used to inactivate gene expression throughout the arch, while Crect (Reid et al., 2011), Foxa2mcm (Park et al., 2008) and Myf5-Cre (Tallquist et al., 2000) drivers can be used to specifically target the pharyngeal arch ectoderm, pharyngeal pouch endoderm and paraxial mesoderm, respectively (Tavares et al., 2012). Further tissue-specific Cre drivers of potential interest include Ap2-Cre alleles, which drive expression in the pharyngeal arch ectoderm (Macatee et al., 2003) or frontonasal process (Nelson and Williams, 2004); the Mesp1-Cre allele, targeting the cranial mesoderm and myocardium of the heart tube (Saga et al., 1999); and the Tyr-Cre allele, which drives expression in the melanocytes and peripheral nerves (Delmas et al., 2003; Tonks et al., 2003). Lastly, it is possible to perform tissue-specific, in vivo lineage tracing by combining Cre drivers with lacZ (Soriano, 1999) or fluorescent (Muzumdar et al., 2007; Prigge et al., 2013) Cre reporter alleles, such that all cells of a particular lineage are permanently marked for detection.
One approach that has yielded a wealth of functional information for a subset of RTK families to which it has been applied is the use of homologous recombination to generate series of knock-in alleles that disrupt either particular domains of a receptor or individual tyrosine autophosphorylation sites necessary for interaction with specific proteins. In contrast to the analysis of null alleles for these signaling molecules and their effectors, which can be obscured by the fact that multiple RTKs may utilize the same molecule as well as redundancy in intracellular signaling networks, the knock-in approach addresses the role of these molecules downstream of an individual receptor. As discussed above, such studies have revealed roles for Shc adaptor signaling in mediating the activity of ErbB2 in cutaneous sensory neurons (Chan et al., 2004); Frs2 downstream of FGFR1 in regulating NCC migration and survival in the second pharyngeal arch (Hoch and Soriano, 2006); Src family kinases and PI3K downstream of Kit in regulating melanogenesis in the coat (Kimura et al., 2004; Agosti et al., 2004); PI3K-mediated PDGFRα signaling in contributing to NCC-derived skeletal development (Klinghoffer et al., 2002); MAPK, PI3K and JNK signaling cascades downstream of Shc adaptor and PKA interactions in mediating the role of RET during enteric nervous system development (Jijiwa et al., 2004; Wong et al., 2005; Asai et al., 2006; Jain et al., 2010); and Shc in regulating target innervation of sensory neurons downstream of TrkB activation (Postigo et al., 2002).
Finally, genetic knock-in approaches in which the domain of one RTK is replaced with that of another have begun to address questions of receptor functional specificity during mouse development. For example, swapping of the PDGFRα and PDGFRβ intracellular signaling domains revealed that signaling downstream of the two receptors is largely conserved, despite differences in expression and ligand binding affinities, such that null phenotypes are mostly rescued in both knock-in lines. Interestingly however, this analysis demonstrated that sustained MAPK signaling specifically downstream of PDGFRβ is required for proper vascular development (Klinghoffer et al., 2001). A second study using a knock-in approach to fuse the extracellular domain of PDGFRα to the intracellular domain of either the Drosophila RTK Torso or mouse FGFR1 revealed that neither replacement can completely rescue proper development, due to alterations in MAPK and/or PI3K signaling, further indicating that strict regulation of downstream signaling pathways is required in some instances to mediate the specific biological function of individual RTKs (Hamilton et al., 2003).
3.2 Phospho-specific reagents
The development of phospho-specific reagents, particularly antibodies recognizing individual phosphorylated residues within signaling molecules or directed against phosphorylated consensus recognition motifs, has greatly enhanced the biochemical analysis of intracellular events downstream of RTK activation. Within the embryo, whole mount or section immunohistochemistry can be performed using phospho-specific antibodies to assess the spatiotemporal expression of activated downstream effector proteins. This approach has revealed localization of several such molecules to discrete domains during murine development, indicating a role for these signaling molecules at particular sites and/or timepoints during embryogenesis (Corson et al., 2003; Fantauzzo and Soriano, 2014).
For in vitro studies, cells can be serum-starved, stimulated with ligand and a particular receptor immunoprecipitated from the cell lysates. Western blotting can then be performed using an antibody recognizing phosphorylated tyrosine residues such as 4G10 or pY20 (Druker et al., 1989; Glenney et al, 1988) to examine receptor activation, or antibodies specific to signaling proteins known to interact with the receptor, such as those against Src, p85, SHP-2, PLCγ, Ras-GAP, Stat3, Shc, Grb2, Crk, Nck, Frs2, IRS-1, etc. Similarly, whole cells lysates can be subjected to Western blotting with antibodies directed against various activated downstream effector proteins, such as phospho-SAPK/JNK, phospho-Akt, phospho-p44/42 (Erk1/2), phospho-PLCγ, etc, to assess the status of these pathways downstream of receptor stimulation.
Furthermore, phosphosubstrate-specific antibodies have allowed for detection of phosphorylation events downstream of particular intracellular signaling molecules in response to RTK activation. One widely used example is the anti-Akt-phosphosubstrate antibody (Manning et al., 2002) generated against the phosphorylated AGC kinase family consensus recognition motif RXRXXS/T recognized by Akt, RSK and p70 S6 kinases (Alessi et al., 1996; Obata et al., 2000). This antibody has been used to identify individual Akt substrates downstream of RTK signaling using standard immunoprecipitation and Western blotting techniques (Manning et al., 2002), as well as to perform larger, mass spectrometry-based screens in response to growth factor stimulation in both cancer (Moritz et al., 2010) and primary (Fantauzzo and Soriano, 2014) cell lines.
Notably, these approaches can be combined with the use of pharmacological inhibitors that allow researchers to target RTK signaling pathway components at several levels, with the caveat that several of these inhibitors target more than one protein. Using the PDGFRα pathway as an example, Gleevec (imatinib mesylate) can be used to inhibit the receptor itself (Buchdunger et al., 1996), LY294002 can be used to inhibit the receptor-binding protein PI3K (Vlahos et al., 1994) and rapamycin can be used to inhibit the intracellular signaling molecule mTOR (Brown et al., 1994).
Finally, a recent study has combined several techniques to investigate signaling networks commonly engaged downstream of individual RTKs (Wagner et al., 2013). Six isogenic transformed human embryonic kidney cell lines expressing EGFR, FGFR1, IGF-1R, MET, PDGFRβ or TRKB were used in combination with lentiviral shRNA expression vectors to alter the levels of intracellular signaling proteins. Upon stimulation with relevant growth factors, phosphorylation of downstream proteins was evaluated at several timepoints by probing lysate microarrays with phospho-specific antibodies. Intriguingly, analysis of the resulting data highlighted three distinct RTK classes with conserved downstream signaling networks: 1) EGFR, FGFR1 and MET; 2) IGF-1R and TRKB; and 3) PDGFRβ (Wagner et al., 2013). Such multi-faceted approaches will no doubt identify further commonalities and differences in signaling downstream of the various RTK families.
3.3 Proteomics
Mass spectrometry-based proteomic approaches have been employed to identify protein phosphorylation targets downstream of growth factor stimulation and new technologies have allowed for the quantification of these post-translational modifications. One shotgun proteomics strategy that has been used in two studies examining phosphorylation targets downstream of RTK signaling in NCC-derived primary mouse embryonic palatal mesenchyme cells is the immunoprecipitation of target proteins from whole cell lysates using either an anti-phosphotyrosine or anti-Akt-phosphosubstrate antibody, analysis of the tryptic peptides by nanoliquid chromatography coupled to tandem mass spectrometry (nano-LC-MS/MS) and assessment of phosphorylation changes in response to ephrin-B1-Fc or PDGF-AA treatment, respectively, by spectral counting (Bush and Soriano, 2010; Fantauzzo and Soriano, 2014). In these scenarios, summing the number of tandem mass spectra obtained for a given protein, a process known as spectral counting, approximates the abundance of the protein in the sample within over two orders of magnitude (Liu et al., 2004).
Alternative isotope labeling approaches have been more commonly used with transformed or cancer cell lines in the RTK field and allow for quantitative proteomics analyses. One such technique, iTRAQ (isobaric tag for relative and absolute quantitation) (Ross et al., 2004), has successfully been used to investigate, for example, the dynamics of tyrosine phosphorylation in response to EGF treatment in a transformed human mammary epithelial cell line (Zhang et al., 2005). For this study, tryptic peptides from four growth factor stimulation timepoints were separately labeled with one of four covalent tags of the same mass, mixed, immunoprecipitated with an anti-phosphotyrosine antibody and analyzed by LC-MS/MS (Zhang et al., 2005). In the case of iTRAQ, individual peptides are quantitated by comparing the relative ratios of reporter ions generated by fragmentation of the covalent tags in tandem mass spectrometry (Ross et al., 2004). Two additional studies used a related approach, SILAC (stable isotope labeling with amino acids in cell culture) (Ong et al., 2002), to identify phosphorylation targets downstream of EGFR, MET and/or PDGFRα signaling in various human cancer cell lines (Olsen et al., 2006; Moritz et al., 2010). Here, cells were grown in the presence of isotope-substituted forms of arginine and lysine, stimulated with growth factor or treated with various inhibitors and mixed. Tryptic peptides were then enriched for phosphopeptides and analyzed by LC-MS/MS (Olsen et al., 2006; Moritz et al., 2010). With SILAC, peptides are subsequently quantitated by assessing the relative intensities of isotopic forms detected by mass spectrometry (Ong et al., 2002). Importantly, each of the mass spectrometry-based proteomics techniques discussed here has unique benefits and drawbacks (reviewed in Brewis and Brennan, 2010; Ahmad and Lamond, 2014) that should be considered when designing a relevant experimental strategy.
3.4 Biosensors
Lastly, various biosensors have been utilized both in vitro and in vivo to examine the spatiotemporal dynamics of RTK signaling. Bioluminescence resonance energy transfer (BRET) involves the transfer of energy from a luminescent donor (such as Renilla luciferase) to a fluorescent acceptor (such as GFP or EYFP). Upon co-expression of fusion molecules in live cells, protein-protein interactions or conformational changes can be assessed by measuring the ratio of emissions from the donor and acceptor (reviewed in Siddiqui et al., 2013). One such study utilizing this technology examined the interactions between RTKs of the ErbB, Kit, PDGF, Trk and VEGF receptor families with the signaling molecules Grb2, p85, Stat5a, Shc46 and PLCγ1 in transformed human embryonic kidney cells, revealing specific receptor-signaling molecule interactions in response to growth factor treatment (Tan et al., 2007). Additional studies have employed BRET to examine receptor conformational changes upon ligand treatment. For example, BRET assays conducted in Chinese hamster ovary cells demonstrated that the association between TrkB and Shc is constitutive and that the complex undergoes a conformational rearrangement in response to BDNF stimulation (De Vries et al., 2010).
More recently, biosensor mouse models have been developed that allow for the assessment of intracellular signaling molecule activity downstream of RTK signaling in vivo. To date, a single study has employed this technology in the examination of neural crest-derived cell activity, using transgenic mouse lines expressing Förster (or fluorescence) resonance energy transfer (FRET) biosensors in conjunction with live imaging by two-photon excitation microscopy (Goto et al., 2013). The authors used transgenic lines harboring PKA, Erk, Rac1, Cdc42 and JNK FRET biosensors (Kamioka et al., 2012; Komatsu et al., 2011; Goto et al., 2013) to demonstrate that PKA activity in migrating enteric neural crest-derived cells is positively correlated with the distribution of GDNF and inversely correlated with Rac1 and Cdc42 activity (Goto et al., 2013). Similar application of in vivo biosensors will likely provide a profusion of information on the activity of signaling molecules downstream of RTK induction during NCC development, migration and differentiation.
4. Concluding Remarks
Over the past two decades, numerous advances have been made in the growth factor signaling field using biochemical, expression and genetic knockout approaches that have highlighted the mechanism and function of RTK signaling during murine embryogenesis. A role for several of these receptor families has thus been demonstrated in regulating NCC activity and the development of their derivatives in mammalian embryogenesis. The application of additional techniques, including receptor allelic series, large-scale, quantitative proteomics and biosensor imaging, promises to reveal novel aspects of RTK signaling during development. Furthermore, the in vivo analysis of transcriptional readout in response to individual RTK stimulation will likely provide a wealth of knowledge on the mechanisms by which extracellular growth factors mediate diverse cellular activities.
Acknowledgements
We thank our laboratory colleagues for their helpful discussions and comments on this manuscript. We apologize to authors whose work we were unable to cite due to space limitations. Work in the Soriano laboratory is supported by National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) grants R01DE022363 and R01DE022778 and NYSTEM grant IIRP N11G-131 to P.S. K.A.F. is additionally supported by NIH/NIDCR Ruth L. Kirschstein NRSA Individual Postdoctoral Fellowship F32DE022719.
References
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nature Genetics. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- Agosti V, Corbacioglu S, Ehlers I, Waskow C, Sommer G, Berrozpe G, et al. Critical role of Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. Journal of Experimental Medicine. 2004;199:867–878. doi: 10.1084/jem.20031983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Y, Lamond AI. A perspective on proteomics in cell biology. Trends in Cell Biology. 2014;24:257–264. doi: 10.1016/j.tcb.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Letters. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Development Genes and Evolution. 2001;211:161–171. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- Andermarcher E, Surani MA, Gherardi E. Co-expression of the HGF/SF and c-met genes during early mouse embryogenesis precedes reciprocal expression in adjacent tissues during organogenesis. Developmental Genetics. 1996;18:254–266. doi: 10.1002/(SICI)1520-6408(1996)18:3<254::AID-DVG6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Williams DE, Tushinski R, Gimpel S, Eisenman J, Cannizzaro LA, et al. Alternate splicing of mRNAs encoding human mast cell growth factor and localization of the gene to chromosome 12q22-q24. Cell Growth & Differentiation. 1991;2:373–378. [PubMed] [Google Scholar]
- Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, et al. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development. 2006;133:4507–4516. doi: 10.1242/dev.02616. [DOI] [PubMed] [Google Scholar]
- Avivi A, Yayon A, Givol D. A novel form of FGF receptor-3 using an alternative exon in the immunoglobulin domain III. FEBS Letters. 1993;330:249–252. doi: 10.1016/0014-5793(93)80882-u. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Waterfield MD, Schlessinger J, Taylor WR, Blundell T. On the tertiary structure of the extracellular domains of the epidermal growth factor and insulin receptors. Biochimica et Biophysica Acta. 1987;916:220–226. doi: 10.1016/0167-4838(87)90112-9. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, et al. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signaling through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Gordon L, Donn TM, Berti C, Moens CB, Burden SJ, et al. A novel role for MuSK and non-canonical Wnt signaling during segmental neural crest cell migration. Development. 2011;138:3287–3296. doi: 10.1242/dev.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Gutiérrez E, Garcia-Fernàndez J, Comella JX. Origin and evolution of the Trk family of neurotrophic receptors. Molecular and Cellular Neuroscience. 31:179–192. doi: 10.1016/j.mcn.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bennett D. Developmental analysis of a mutation with pleiotropic effects in the mouse. Journal of Morphology. 1956;98:199–233. [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bolande RP. The neurocristopathies: A unifying concept of disease arising in neural crest maldevelopment. Human Pathology. 1974;5:409–429. doi: 10.1016/s0046-8177(75)80115-8. [DOI] [PubMed] [Google Scholar]
- Bolande RP. Neurocristopathy: its growth and development in 20 years. Pediatric Pathology & Laboratory Medicine. 1997;17:1–25. [PubMed] [Google Scholar]
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Brewis IA, Brennan P. Proteomics technologies for the global identification and quantification of proteins. Advances in Protein Chemistry and Structural Biology. 2010;80:1–44. doi: 10.1016/B978-0-12-381264-3.00001-1. [DOI] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, et al. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes & Development. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Brückner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Research. 1996;56:100–104. [PubMed] [Google Scholar]
- Buj-Bello A, Adu J, Piñón LG, Horton A, Thompson J, Rosenthal A, et al. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997;387:721–724. doi: 10.1038/42729. [DOI] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Ephrin-B1 regulates axon guidance by reverse signaling through a PDZ-dependent mechanism. Genes & Development. 2009;23:1586–1599. doi: 10.1101/gad.1807209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes & Development. 2010;24:2068–2080. doi: 10.1101/gad.1963210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable J, Jackson IJ, Steel KP. Mutations at the W locus affect survival of neural crest-derived melanocytes in the mouse. Mechanisms of Development. 1995;50:139–150. doi: 10.1016/0925-4773(94)00331-g. [DOI] [PubMed] [Google Scholar]
- Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Chan R, Hardy WR, Dankort D, Laing MA, Muller WJ. Modulation of Erbb2 signaling during development: a threshold level of Erbb2 signaling is required for development. Development. 2004;131:5551–5560. doi: 10.1242/dev.01425. [DOI] [PubMed] [Google Scholar]
- Chan R, Hardy WR, Laing MA, Hardy SE, Muller WJ. The catalytic activity of the ErbB-2 receptor tyrosine kinase is essential for embryonic development. Molecular and Cellular Biology. 2002;22:1073–1078. doi: 10.1128/MCB.22.4.1073-1078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, et al. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nature Genetics. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- Chen Y, Moon AM, Gaufo GO. Influence of mesodermal Fgf8 on the differentiation of neural crest-derived postganglionic neurons. Developmental Biology. 2012;361:125–136. doi: 10.1016/j.ydbio.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: The biology of ErbB-2 and ErbB-3. Experimental Cell Research. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Copeland NG, Gilbert DJ, Cho BC, Donovan PJ, Jenkins NA, Cosman D, et al. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Current Biology. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes & Development. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biology. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Developmental Dynamics. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Developmental Biology. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nature Genetics. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- Delmas V, Martinozzi S, Bourgeois Y, Holzenberger M, Larue L. Cre-mediated recombination in the skin melanocyte lineage. Genesis. 2003;36:73–80. doi: 10.1002/gene.10197. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J-M, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signaling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Deng C-X, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes & Development. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- De Vries L, Finana F, Cachoux F, Vacher B, Sokoloff P, Cussac D. Cellular BRET assay suggests a conformational rearrangement of preformed TrkB/Shc complexes following BDNF-dependent activation. Cellular Signaling. 2010;22:158–165. doi: 10.1016/j.cellsig.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Ding H, Wu X, Boström H, Kim I, Wong N, Tsoi B, et al. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nature Genetics. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- Ding H, Wu X, Kim I, Tam PP, Koh GY, Nagy A. The mouse Pdgfc gene: dynamic expression in embryonic tissues during organogenesis. Mechanisms of Development. 2000;96:209–213. doi: 10.1016/s0925-4773(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Dû N, Soussi-Yanicostas N, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nature Genetics. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Dono R, Texido G, Dussel R, Ehmke H, Zeller R. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. EMBO Journal. 1998;17:4213–4225. doi: 10.1093/emboj/17.15.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Mamon BS, Roberts TM. Oncogenes, growth factors, and signal transduction. The New England Journal of Medicine. 1989;321:1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Dunn LC. Studies on Spotting Patterns II. Genetic Analysis of Variegated Spotting in the House Mouse. Genetics. 1937;22:43–64. doi: 10.1093/genetics/22.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, et al. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- Eketjäll S, Ibáñez CF. Functional characterization of mutations in the GDNF gene of patients with Hirschsprung disease. Human Molecular Genetics. 2002;11:325–329. doi: 10.1093/hmg/11.3.325. [DOI] [PubMed] [Google Scholar]
- Ekman S, Thuresson ER, Heldin CH, Rönnstrand L. Increased mitogenicity of an alphabeta heterodimeric PDGF receptor complex correlates with lack of RasGAP binding. Oncogene. 1999;18:2481–2488. doi: 10.1038/sj.onc.1202606. [DOI] [PubMed] [Google Scholar]
- Enokido Y, de Sauvage F, Hongo JA, Ninkina N, Rosenthal A, Buchman VL. GFR alpha-4 and the tyrosine kinase Ret form a functional receptor complex for persephin. Current Biology. 1998;8:1019–1022. doi: 10.1016/s0960-9822(07)00422-8. [DOI] [PubMed] [Google Scholar]
- Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Monsonego-Ornan E, Pines M, Antonopoulou I, Morriss-Kay GM, Lonai P. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development. 2002;129:3783–3793. doi: 10.1242/dev.129.16.3783. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Amiel J, Lyonnet S. Molecular basis of human neurocristopathies. Advances in Experimental Medicine and Biology. 2006;589:213–234. doi: 10.1007/978-0-387-46954-6_14. [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. Journal of Clinical Investigation. 118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantauzzo KA, Soriano P. PI3K-mediated PDGFRα signaling regulates survival and proliferation in skeletal development through p53-dependent intracellular pathways. Genes & Development. 2014;28:1005–1017. doi: 10.1101/gad.238709.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández RM, Sánchez-Mejías A, Mena MD, Ruiz-Ferrer M, López-Alonso M, Antiñolo G, et al. A novel point variant in NTRK3, R645C, suggests a role for this gene in the pathogenesis of Hirschsprung disease. Annals of Humam Genetics. 2009;73:19–25. doi: 10.1111/j.1469-1809.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, Grigorieva E, et al. EphB-ephrin-B2 interactions are required for thymus migration during organogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13414–13419. doi: 10.1073/pnas.1003747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, et al. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. Journal of Neuroscience. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Suárez O, Germanà A, Hannestad J, Ciriaco E, Laurà R, Naves J, et al. TrkA is necessary for the normal development of the murine thymus. Journal of Neuroimmunology. 2000;108:11–21. doi: 10.1016/s0165-5728(00)00251-4. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Geissler EN, McFarland EC, Russell ES. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981;97:337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Jr, Zokas L, Kamps MP. Monoclonal antibodies to phosphotyrosine. Journal of Immunological Methods. 1988;109:277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Golding JP, Trainor P, Krumlauf R, Gassmann M. Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor ErbB4. Nature Cell Biology. 2000;2:103–109. doi: 10.1038/35000058. [DOI] [PubMed] [Google Scholar]
- Goto A, Sumiyama K, Kamioka Y, Nakasyo E, Ito K, Iwasaki M, et al. GDNF and endothelin 3 regulate migration of enteric neural crest-derived cells via protein kinase A and Rac1. Journal of Neuroscience. 2013;33:4901–4912. doi: 10.1523/JNEUROSCI.4828-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveel CR, Tolbert D, Vande Woude GF. MET: a critical player in tumorigenesis and therapeutic target. Cold Spring Harbor Perspectives in Biology. 2013;5:a009209. doi: 10.1101/cshperspect.a009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg H. Inherited macrocytic anaemias in the house mouse. II. Dominance relationships. Journal of Genetics. 1942;43:285–293. [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Developmental Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guris DL, Fantes J, Tara D, Druker BJ, Imamoto A. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nature Genetics. 2001;27:293–298. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Molecular and Cellular Biology. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Soriano P. A critical role for PDGFRα signaling in medial nasal process development. PLoS Genetics. 2013;9:e1003851. doi: 10.1371/journal.pgen.1003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, et al. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin II. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Developmental Biology. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiological Reviews. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mechanisms of Development. 1996;54:95–105. doi: 10.1016/0925-4773(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Developmental Biology. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Heuzé Y, Singh N, Basilico C, Jabs EW, Holmes G, Richtsmeier JT. Morphological comparison of the craniofacial phenotypes of mouse models expressing the Apert FGFR2 S252W mutation in neural crest- or mesoderm-derived tissues. Bone. 2014;63:101–109. doi: 10.1016/j.bone.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Soriano P. Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development. 2006;133:663–673. doi: 10.1242/dev.02242. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signaling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Holmes G, Basilico C. Mesodermal expression of Fgfr2S252W is necessary and sufficient to induce craniosynostosis in a mouse model of Apert syndrome. Developmental Biology. 2012;368:283–293. doi: 10.1016/j.ydbio.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Nocka K, Beier DR, Chu T-Y, Buck J, Lahm H-W, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand for the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature Genetics. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Taniguchi M, Asai N, Ohkusu K, Nakashima I, Takahashi M. cDNA cloning of mouse ret proto-oncogene and its sequence similarity to the cadherin superfamily. Oncogene. 1993;8:1087–1091. [PubMed] [Google Scholar]
- Jain S, Knoten A, Hoshi M, Wang H, Vohra B, Heuckeroth RO, et al. Organotypic specificity of key RET adaptor-docking sites in the pathogenesis of neurocristopathies and renal malformations in mice. Journal of Clinical Investigation. 2010;120:778–790. doi: 10.1172/JCI41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, et al. A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Molecular and Cellular Biology. 2004;24:8026–8036. doi: 10.1128/MCB.24.18.8026-8036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Lefebvre JL, Gordon LR, Granato M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron. 2009;61:721–733. doi: 10.1016/j.neuron.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Hoist PL, Luo Y, Fang M, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Lu J, Chen H, Werner S, Williams LT. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Molecular and Cellular Biology. 1991;11:4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JW, Shin WS, Song J, Lee ST. Cloning and characterization of the full-length mouse Ptk7 cDNA encoding a defective receptor protein tyrosine kinase. Gene. 2004;328:75–84. doi: 10.1016/j.gene.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Kamioka Y, Sumiyama K, Mizuno R, Sakai Y, Hirata E, Kiyokawa E, et al. Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Structure and Function. 2012;37:65–73. doi: 10.1247/csf.11045. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 252:554–558. doi: 10.1126/science.1850549. (1001). [DOI] [PubMed] [Google Scholar]
- Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. Journal of Neurobiology. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kelleher FC, O’Sullivan H, Smyth E, McDermott R, Viterbo A. Fibroblast growth factor receptors, developmental corruption and malignant disease. Carcinogenesis. 2013;34:2198–2205. doi: 10.1093/carcin/bgt254. [DOI] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. 2008 doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Jones N, Klüppel M, Hirashima M, Tachibana K, Cohn JB, et al. Targeted mutations of the juxtamembrane tyrosines in the Kit receptor tyrosine kinase selectively affect multiple cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6015–6020. doi: 10.1073/pnas.0305363101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Jing SQ, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R, Martin-Zanca D, Barbacid M, Parada LF. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, et al. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, et al. Disruption of the neurotrophin-3 receptor gene trkC eliminates Ia muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, Vandlen R, et al. A GPI-liked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997;387:717–721. doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Hamilton TG, Hoch R, Soriano P. An allelic series at the PDGFalphaR locus indicates unequal contributions of distinct signaling pathways during development. Developmental Cell. 2002;2:103–113. doi: 10.1016/s1534-5807(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Molecular Cell. 2001;7:343–354. doi: 10.1016/s1097-2765(01)00182-4. [DOI] [PubMed] [Google Scholar]
- Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, et al. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Molecular Biology of the Cell. 2011;22:4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos L, Aronzon A, Takayama H, Maina F, Ponzetto C, Merlino G, et al. Hepatocyte growth factor/scatter factor-MET signaling in neural crest-derived melanocyte development. Pigment Cell Research. 1999;12:13–21. doi: 10.1111/j.1600-0749.1999.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Kumar P, Ferns MJ, Meizel S. Identification of agrinSN isoform and muscle-specific receptor tyrosine kinase (MuSK) in sperm. Biochemical and Biophysical Research Communications. 2006;342:522–528. doi: 10.1016/j.bbrc.2006.01.161. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Leahy DJ. Structure and function of the epidermal growth factor (EGF/ErbB) family of receptors. Advances in Protein Chemistry. 2004;68:1–27. doi: 10.1016/S0065-3233(04)68001-6. [DOI] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Lee PL, Johnson DE, Cousens LS, Fried VA, Williams LT. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989;245:57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanti MB, Esteban I, Ciriaco E, Pérez-Piñera P, Cabo R, García-Suarez O, et al. Enteric glial cells express full-length TrkB and depend on TrkB expression for normal development. Neuroscience Letters. 2009;454:16–21. doi: 10.1016/j.neulet.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes & Development. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lewis AE, Vasudevan HN, O’Neill AK, Soriano P, Bush JO. The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Developmental Biology. 2013;379:229–234. doi: 10.1016/j.ydbio.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen F, Epstein JA. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis. 2000;26:162–164. doi: 10.1002/(sici)1526-968x(200002)26:2<162::aid-gene21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. Journal of Biological Chemistry. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Analytical Chemistry. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Luikart BW, Nef S, Shipman T, Parada LF. In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience. 2003;117:847–858. doi: 10.1016/s0306-4522(02)00719-4. [DOI] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- MacArthur CA, Lawshé A, Xu J, Santos-Ocampo S, Heikinheimo M, Chellaiah AT, et al. FGF-8 isoforms activate receptor splice forms that are expressed in mesenchymal regions of mouse development. Development. 1995;121:3603–3613. doi: 10.1242/dev.121.11.3603. [DOI] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Frances L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie MAF, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Developmental Biology. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous-sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Molecular Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D, Barbacid M, Parada LF. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes & Development. 1990;4:683–694. doi: 10.1101/gad.4.5.683. [DOI] [PubMed] [Google Scholar]
- Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. Journal of Biological Chemistry. 1992;267:26181–26190. [PubMed] [Google Scholar]
- Masiakowski P, Yancopoulos GD. The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Current Biology. 1998;8:R407. doi: 10.1016/s0960-9822(98)70263-5. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, et al. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mechanisms of Development. 2001;105:153–156. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo KM, Hogan BL. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- Mayor R, Theveneau E. The neural crest. Development. 2013;140:2247–2251. doi: 10.1242/dev.091751. [DOI] [PubMed] [Google Scholar]
- McCoshen JA, McCallion DJ. A study of the primordial germ cells during their migratory phase in Steel mutant mice. Experientia. 1975;31:589–590. doi: 10.1007/BF01932475. [DOI] [PubMed] [Google Scholar]
- McLennan R, Teddy JM, Kasemeier-Kulesa JC, Romine MH, Kulesa PM. Vascular endothelial growth factor (VEGF) regulates cranial neural crest migration in vivo. Developmental Biology. 2010;339:114–125. doi: 10.1016/j.ydbio.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AE, Bochukova EG, Brugger SM, Ishii M, Pilz DT, Wall SA, et al. Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Human Molecular Genetics. 2006;15:1319–1328. doi: 10.1093/hmg/ddl052. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nature Genetics. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Chin JR, Shum L, Slavkin HC, Shuler CF, Derynck R, et al. Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nature Genetics. 1999;22:69–73. doi: 10.1038/8773. [DOI] [PubMed] [Google Scholar]
- Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, et al. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Guris DL, Seo J, Li L, Hammond J, Talbot A, et al. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Developmental Cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Earley TJ, Watson J, Andahazy M, Backus C, Martin-Zanca D, et al. Expressing TrkC from the TrkA locus causes a subset of dorsal root ganglia neurons to switch fate. Nature Neuroscience. 2004;7:812–818. doi: 10.1038/nn1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A, Li Y, Gui A, Villén J, Wang Y, MacNeill J, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Science Signaling. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Morrison-Graham K, Schatteman GC, Bork T, Bowen-Pope DF, Weston JA. A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells. Development. 1992;115:133–142. doi: 10.1242/dev.115.1.133. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nelson DK, Williams T. Frontonasal process-specific disruption of AP-2alpha results in postnatal midfacial hypoplasia, vascular anomalies, and nasal cavity defects. Developmental Biology. 2004;267:72–92. doi: 10.1016/j.ydbio.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. Journal of Cell Biology. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Park E, Park S. In vivo expression of ephrinA5-Fc in mice results in cephalic neural crest agenesis and craniofacial abnormalities. Molecules and Cells. 2014;37:59–65. doi: 10.14348/molcells.2014.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. Journal of Biological Chemistry. 2008;283:27973–27981. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- Nomi M, Oishi I, Kani S, Suzuki H, Matsuda T, Yoda A, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Molecular and Cellular Biology. 2001;21:8329–8335. doi: 10.1128/MCB.21.24.8329-8335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, et al. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. Journal of Biological Chemistry. 2000;17:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkaware B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signaling pathway. Genes to Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Oishi I, Takeuchi S, Hashimoto R, Nagabukuro A, Ueda T, Liu ZJ, et al. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes to Cells. 1999;4:41–56. doi: 10.1046/j.1365-2443.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Ong SE, Blagoev B, Kratchmarovak I, Kristensen DB, Steen H, Pandey A, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Molecular & Cellular Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, et al. Receptor specificity of the fibroblast growth factor family. Journal of Biological Chemistry. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, et al. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2) Developmental Biology. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Lonai P. Platelet-derived growth factor-A and its receptor are expressed in separate, but adjacent cells layers of the mouse embryo. Development. 1992;115:1045–1058. doi: 10.1242/dev.115.4.1045. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai C-L, Black BL, et al. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Sun X, Nichol P, Saijoh Y, Martin JF, Moon AM. System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Developmental Dynamics. 2008;237:447–453. doi: 10.1002/dvdy.21415. [DOI] [PubMed] [Google Scholar]
- Park SK, Lee HS, Lee ST. Characterization of the human full-length PTK7 cDNA encoding a receptor protein tyrosine kinase-like molecule closely related to chick KLG. Journal of Biochemistry. 1996;119:235–239. doi: 10.1093/oxfordjournals.jbchem.a021228. [DOI] [PubMed] [Google Scholar]
- Partanen J, Schwartz L, Rossant J. Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes & Development. 1998;12:2332–2344. doi: 10.1101/gad.12.15.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, et al. The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Developmental Biology. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Developmental Dynamics. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm A-C, Drago J, Grinberg A, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Developmental Biology. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Postigo A, Calella AM, Fritzsch B, Knipper M, Katz D, Ellers A, et al. Distinct requirements for TrkB and TrkC signaling in target innervation by sensory neurons. Genes & Development. 2002;16:633–645. doi: 10.1101/gad.217902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge JR, Wiley JA, Talago EA, Young EM, Johns LL, Kundert JA, et al. Nuclear double-fluorescent reporter for in vivo and ex vivo analyses of biological transitions in mouse nuclei. Mammalian Genome. 2013;24:389–399. doi: 10.1007/s00335-013-9469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui FH, Ray P, Brown K, Barker PE, Jhanwar S, Ruddle FH, et al. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family – oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO Journal. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Reid BS, Yang H, Melvin VS, Taketo MM, Williams T. Ectodermal Wnt/β-catenin signaling shapes the mouse face. Developmental Biology. 2011;349:261–269. doi: 10.1016/j.ydbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarte AM, Mead HB, Tallquist MD. Cooperation between the PDGF receptors in cardiac neural crest cell migration. Developmental Biology. 2007;306:785–796. doi: 10.1016/j.ydbio.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molecular & Cellular Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ferrer M, Fernandez RM, Antiñolo G, Lopez-Alonso M, Borrego S. NTF-3, a gene involved in the enteric nervous system development, as a candidate gene for Hirschsprung disease. Journal of Pediatric Surgery. 2008;43:1308–1311. doi: 10.1016/j.jpedsurg.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Rupp E, Siegbahn A, Rönnstrand L, Wernstedt C, Claesson-Welsh L, Heldin CH. A unique autophosphorylation site in the platelet-derived growth factor alpha receptor from a heterodimeric receptor complex. European Journal of Biochemistry. 1994;225:29–41. doi: 10.1111/j.1432-1033.1994.00029.x. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sarvella PA, Russell LB. Steel, a new dominant gene in the house mouse. Journal of Heredity. 1956;47:123–128. [Google Scholar]
- Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, et al. Wnt5a is required for cardiac outflow tract septation in mice. Pediatric Research. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Cong W-N, Daimon CM, Martin B, Maudsley S. BRET biosensor analysis of receptor tyrosine kinase functionality. Frontiers in Endocrinology. 2013;4:46. doi: 10.3389/fendo.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. Journal of Cell Biology. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes & Development. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman T, Lemmon MA, Dikic I, Ladbury JE, Pinchasi D, Huang J, et al. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994;79:1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Lambrechts D, De Smet F, Jansen S, Wang J, Maity S, et al. VEGF: A modifier of the del22q11 (DiGeorge) syndrome? Nature Medicine. 2003;9:173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Stein RA, Staros JV. Evolutionary analysis of the ErbB receptor and ligand families. Journal of Molecular Evolution. 2000;50:397–412. doi: 10.1007/s002390010043. [DOI] [PubMed] [Google Scholar]
- Stine ZE, Huynh JL, Loftus SK, Gorkin DU, Salmasi AH, Novak T, et al. Oligodendroglial and pan-neural crest expression of Cre recombinase directed by Sox10 enhancer. Genesis. 2009;47:765–770. doi: 10.1002/dvg.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler JM, Barrell WB, Szabo-Rogers HL, Healy C, Yeung Y, Perdiguero EG, et al. Fuz mutant mice reveal shared mechanisms between ciliopathies and FGF-related syndromes. Developmental Cell. 2013;25:623–635. doi: 10.1016/j.devcel.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, La Rochelle WJ, Anver M, Bockman DE, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5866–5871. doi: 10.1073/pnas.93.12.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes to Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130:507–518. doi: 10.1242/dev.00241. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Weismann KE, Hellström M, Soriano P. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 2000;127:5059–5070. doi: 10.1242/dev.127.23.5059. [DOI] [PubMed] [Google Scholar]
- Tan PK, Wang J, littler P-LH, Wong KK, Sweetnam TA, Keefe W, et al. Monitoring interactions between receptor tyrosine kinases and their downstream effector proteins in living cells using bioluminescence resonance energy transfer. Molecular Pharmacology. 2007;72:1440–1446. doi: 10.1124/mol.107.039636. [DOI] [PubMed] [Google Scholar]
- Taravira S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, et al. Signaling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- Tavares AL, Garcia EL, Kuhn K, Woods CM, Williams T, Clouthier DE. Ectodermal-derived Endothelin1 is required for patterning the distal and intermediate domains of the mouse mandibular arch. Developmental Biology. 2012;371:47–56. doi: 10.1016/j.ydbio.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, et al. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Martin-Zanca D, Gilbert DJ, Jenkins NA, Copeland NG, et al. trkC, a receptor for neurotropin-3, is widely expressed in the developing nervous system and in non-neuronal tissues. Development. 1993;118:463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Ting M-C, Wu NL, Roybal PG, Sun J, Lui L, Yen Y, et al. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development. 2009;136:855–864. doi: 10.1242/dev.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda M. An eighteenth century Japanese guide-book on mouse-breeding. Journal of Heredity. 1935;26:481–484. [Google Scholar]
- Tonks ID, Nurcombe V, Paterson C, Zournazi A, Prather C, Mould AW, et al. Tyrosinase-Cre mice for tissue-specific gene ablation in neural crest and neuroepithelial-derived tissues. Genesis. 2003;37:131–138. doi: 10.1002/gene.10242. [DOI] [PubMed] [Google Scholar]
- Trainor PA. Specification of neural crest cell formation and migration in mouse embryos. Seminars in Cell & Developmental Biology. 2005;16:683–693. doi: 10.1016/j.semcdb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Mai P, Partanen J. Fgfr1 regulates patterning of the pharyngeal region. Genes & Development. 2003;17:141–153. doi: 10.1101/gad.250703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JLR, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes & Development. 1999;13:3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AS, Yamada G, Grigoriou M, Pachnis V, Sharpe PT. Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development. 1999;126:51–61. doi: 10.1242/dev.126.1.51. [DOI] [PubMed] [Google Scholar]
- Twigg SRF, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Uesaka T, Enomoto H. Neural precursor death is central to the pathogenesis of intestinal aganglionosis in Ret hypomorphic mice. Journal of Neuroscience. 2010;30:5211–5218. doi: 10.1523/JNEUROSCI.6244-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka T, Nagashimada M, Yonemura S, Enomoto H. Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. Journal of Clinical Investigation. 2008;118:1890–1898. doi: 10.1172/JCI34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam A, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattsson K, Compton DL, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15:573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nature Genetics. 2000;25:423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- Van den Akker NM, Winkel LC, Nisancioglu MH, Maas S, Wisse LJ, Armulik A, et al. PDGF-B signaling is important for murine cardiac development: its role in developing atrioventricular valves, coronaries, and cardiac innervation. Developmental Dynamics. 2008;237:494–503. doi: 10.1002/dvdy.21436. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) Journal of Biological Chemistry. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wagner JP, Wolf-Yadlin A, Sevecka M, Grenier JK, Root DE, Lauffenburger DA, et al. Receptor tyrosine kinases fall into distinct classes based on their inferred signaling networks. Science Signaling. 2013;6:ra58. doi: 10.1126/scisignal.2003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chang JYF, Yang C, Huang Y, Liu J, You P, et al. Type 1 fibroblast growth factor receptor in cranial neural crest cell-derived mesenchyme is required for palatogenesis. Journal of Biological Chemistry. 2013;288:22174–22183. doi: 10.1074/jbc.M113.463620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 18:383–396. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Meller M, Weston JA. Analysis of melanocyte precursors in Nf1 mutants reveals that MGF/KIT signaling promotes directed cell migration independent of its function in cell survival. Developmental Biology. 2001;232:471–483. doi: 10.1006/dbio.2001.0167. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Altered cell-surface targeting of stem cell factor causes loss of melanocytes precursors in Stell17H mutant mice. Developmental Biology. 1999;210:71–86. doi: 10.1006/dbio.1999.9260. [DOI] [PubMed] [Google Scholar]
- Williams DE, Eisenman J, Baird A, Rauch C, Van Ness K, March CJ, et al. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Williams LT. Signal transduction by the platelet-derived growth factor receptor. Science. 1989;243:1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, et al. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes & Development. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Bogni S, Kotka P, de Graaff E, D’Agati V, Costantini F, et al. Phosphotyrosine 1062 is critical for the in vivo activity of the Ret9 receptor tyrosine kinase isoform. Molecular and Cellular Biology. 2005;25:9661–9673. doi: 10.1128/MCB.25.21.9661-9673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, et al. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Xu YK, Nusse R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Current Biology. 1998;8:R405–R406. doi: 10.1016/s0960-9822(98)70262-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes & Development. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes to Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, et al. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Developmental Biology. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Kuang W-J, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO Journal. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yayon A, Zimmer Y, Shen GH, Avivi A, Yarden Y, Givol D. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO Journal. 1992;11:1885–1890. doi: 10.1002/j.1460-2075.1992.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JC, Heidaran MA, Pierce JH, Gutkind JS, Lombardi D, Ruggiero M, et al. Tyrosine mutations within the alpha platelet-derived growth factor receptor kinase insert domain abrogate receptor-associated phosphatidylinositol-3 kinase activity without affecting mitogenic or chemotactic signal transduction. Molecular and Cellular Biology. 1991;11:3780–3785. doi: 10.1128/mcb.11.7.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wolf-Yadlin A, Ross PL, Pappin DJ, Rush J, Lauffenburger DA, et al. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Molecular & Cellular Proteomics. 2005;4:1240–1250. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang Z-J, Sieber-Blum M. Essential role of stem cell factor signaling in primary sensory neuron development. Developmental Neuroscience. 2009;31:202–211. doi: 10.1159/000193396. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pashmforoush M, Sucov HM. Endothelial neuropilin disruption in mice causes DiGeorge syndrome-like malformations via mechanisms distinct to those caused by loss of Tbx1. PLoS One. 2012;7:e32429. doi: 10.1371/journal.pone.0032429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, et al. Fibroblast growth factor 2 control of vascular tone. Nature Medicine. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]