Abstract
Importance
Among HIV-infected patients, visceral adiposity is associated with metabolic dysregulation and ectopic fat accumulation. Tesamorelin, a growth hormone-releasing hormone analogue, specifically targets visceral fat reduction, but its effects on liver fat are unknown.
Objective
To investigate the effect of tesamorelin on visceral and liver fat.
Design, Setting, and Participants
Fifty antiretroviral-treated HIV-infected men and women with abdominal fat accumulation were recruited for this double-blind, randomized, placebo-controlled trial at Massachusetts General Hospital. The first patient was enrolled on 1/10/2011; the final patient completed his 6 month study visit on 9/6/2013.
Intervention
Tesamorelin 2mg vs. placebo SC daily for 6 months
Main Outcomes
Primary endpoints were changes in visceral adipose tissue (VAT) and liver fat. Secondary endpoints included glucose and other metabolic endpoints.
Results
76 patients were screened and 54 randomized. 50 presented for baseline assessment and 48 received treatment with study drug. Tesamorelin significantly reduced VAT (Δ −34 [−53, −15] vs. 8 [−14, 30] cm2, mean [95% CI], tesamorelin vs. placebo, treatment effect −42cm2 [95% CI −71, −14], P = 0.005) and liver fat (Δ −2.0 [−6.4, 0.1] vs. 0.9 [−0.6, 3.7] lipid-to-water %, median [IQR], tesamorelin vs. placebo, P=0.003) over 6 months, for a net treatment effect of −2.9 lipid-to-water %. Fasting glucose increased in the tesamorelin group at 2 weeks (Δ 9 [5, 13] vs. 2 [−3, 8] mg/dL, mean [95% CI], treatment effect 7mg/dL [95% CI 1, 14], P=0.03), but overall changes over 6 months in fasting glucose (Δ 4 [−2, 10] vs. 2 [−4, 7] mg/dL, mean [95% CI], treatment effect 2mg/dL [95% CI −6, 10], P=0.72) and 2 hour glucose (Δ −1 [−18, 15] vs. −8 [−24, 8] mg/dL, mean [95% CI], treatment effect 7mg/dL [95% CI −16, 29], P=0.53) were not significant.
Conclusions and Relevance
In this preliminary study of HIV-infected patients with abdominal fat accumulation, tesamorelin administered for 6 months was associated with reductions in visceral fat and, additionally, with modest reductions in liver fat. Further studies are needed to determine the clinical importance and long-term consequences of these findings.
Trial Registration
clinicaltrials.gov NCT01263717
Keywords: liver fat, visceral fat, growth hormone releasing hormone, HIV
Introduction
In HIV-infection, visceral adipose tissue (VAT) accumulation is associated with ectopic fat accumulation in the liver 1–3. Indeed, HIV-infected patients demonstrate a high prevalence of nonalcoholic fatty liver disease (NAFLD), estimated at 30–40% 1,2,4, which is seen often in the context of increased VAT 1,2. NAFLD encompasses simple steatosis, characterized by triglyceride accumulation in hepatocytes (“liver fat”), as well as steatohepatitis, characterized by inflammation, hepatocellular injury, and fibrosis that may progress to end stage liver disease and hepatocellular carcinoma. To date, there are no approved pharmacologic strategies to reduce liver fat, and no strategies have proven successful in HIV-infected patients. A sub-study of HIV-infected individuals participating in a trial of growth hormone (GH) and rosiglitazone 5 showed no change in liver fat with rosiglitazone and a trend for reduction in liver fat with GH 6.
The current study investigates changes in liver fat using a different treatment approach, in which a growth hormone releasing hormone (GHRH) analogue, tesamorelin, is administered to increase endogenous pulsatile GH. Tesamorelin reduces VAT, with minimal effects on subcutaneous fat 7,8, but its effects on other ectopic fat depots and detailed metabolic indices have not been investigated.
Methods
Patient Selection
Potential participants were identified through referral from infectious disease physicians, advertisements in community centers and health clinics, and the clinical research study volunteer program. Patients underwent screening, and eligible patients were invited to participate. Fifty men and women with HIV-infection and increased abdominal adiposity participated in a baseline assessment. Recruitment began in December, 2010. The first patient was enrolled on 1/10/2011, and the final study visit was completed on 9/6/2013. The study was approved by the MGH Institutional Review Board (IRB), and written informed consent was obtained from each patient prior to study procedures.
HIV-infected patients 18–65 years of age with stable use of antiretroviral therapy (ART) for ≥3 months who noted body fat changes including abdominal fat accumulation in the context of ART, and who had objective evidence of abdominal adiposity as determined by gender-specific waist circumference (WC) and waist-to-hip ratio (WHR) criteria (WC ≥95 cm and WHR ≥0.94 for male, WC ≥94 cm and WHR ≥0.88 for female9) were included. Patients with a history of pituitary disease or cranial irradiation, use of GH or GHRH during the past 6 months, or use of supraphysiologic corticosteroids, gonadal steroids except physiologic testosterone replacement, or antidiabetic agents were excluded. Lipid-lowering and anti-hypertensive medications were allowed if doses were stable for ≥3 months prior to baseline. Patients were excluded for pregnancy, inability to undergo magnetic resonance imaging, severe chronic illness, any active malignancy, and history of colon cancer, prostate cancer, or pituitary malignancy. Laboratory exclusion criteria were fasting glucose >126 mg/dL, aspartate aminotransferase (AST) > 2.5 times the upper limit of normal, hemoglobin < 12 g/dL, creatinine >1.4 mg/dL, CD4+ T-cell count <200 copies/mL, and, for males, prostate specific antigen >5 ng/mL. Patients with increased PSA were excluded to avoid enrolling patients with abnormal prostate growth. Three patients had participated in previous randomized controlled trials of tesamorelin in our research group 7,10,11, but, as per protocol, none of these individuals had received tesamorelin in the 6 months prior to enrollment.
Study Design
After screening, eligible volunteers underwent two independent randomizations – a double-blind, 1:1 randomization to tesamorelin 2mg subcutaneously daily vs. identical placebo, and, independently, a 1:1 randomization to undergo euglycemic hyperinsulinemic clamp in addition to other study procedures. Randomization was stratified by gender and, for males, by physiologic testosterone use, using a permuted block algorithm within each stratum, with randomly varying block sizes of 2, 4, or 8. Baseline assessment included fasting blood sampling for lipids, IGF-1, CBC, T-cell subsets, HIV viral load, hemoglobin A1c (HbA1c), c-reactive protein (CRP), adiponectin, AST, and alanine aminotransferase (ALT); 75g oral glucose tolerance test (OGTT); waist and hip circumferences; dual-energy x-ray absorptiometry (DXA, Hologic, Discovery A) for total body and regional fat mass; single-slice computed tomography (CT) at L4 for assessment of visceral and subcutaneous adipose tissue (SAT) area12,13; 1H magnetic resonance spectroscopy (MRS) for hepatocellular lipid-to-water percent (HCL/W%) and intramyocellular lipid (IMCL) of the tibialis anterior and soleus muscles 14,15; overnight frequent sampling for GH concentrations; and neck ultrasound for measurement of carotid intima-media thickness (cIMT) 16. MRS was performed in the morning following an 8-hour fast. Two patients did not follow instructions to fast for their 6 month scans. According to the intent-to-treat design of the study, data from these patients were retained in the analyses; changes in liver fat remained significant between groups in sensitivity analyses excluding these patients (see results). All images were performed on the same scanner. Calculation of liver fat from spectroscopy data was automated, and results were reviewed by a single radiologist, blinded to treatment assignment, to ensure quality control. With regard to reproducibility, Bland-Altman analysis of scans repeated using our technique shows a mean difference between same-day scans of 0.29% (95% CI: −1.46 and 2.05%) 14. The diagnostic accuracy of MRS for liver steatosis is high with an area under the receiver operating characteristic curve of 0.94 (95% CI 0.88–1.0) compared to assessment of liver biopsy by an experienced pathologist 17. For measurement of VAT and SAT, single-slice CT has an estimated correlation between repeat measurements of 0.99, with errors in precision estimated at 1.9% for SAT and 3.9% for VAT 18. Dietary intake, including alcohol, was assessed by 4-day food record (Nutrition Data System). Physical activity was assessed using the Modifiable Activity Questionnaire (MAQ) 19. For assessment of overnight GH, patients had dinner at 1700 and began fasting at 1800. Blood samples were drawn every 20 minutes from 2000 until 0740. At the conclusion of the baseline assessment, patients received their first dose of study drug, which they administered daily for the next 6 months. Patients returned for a safety visit 2 weeks after baseline, a 3-month assessment including oral glucose tolerance test, and a 6-month assessment identical to baseline. Patients randomized to the euglycemic hyperinsulinemic clamp subset (n=13 in tesamorelin group and n=11 in placebo group) also underwent clamp procedure at baseline, 3 months, and 6 months (see Supplemental Methods). Full clamp data were not available for three patients in the tesamorelin group and two patients in the placebo group. Adherence to the study medication was measured by patient-completed study diary and by vial count of returned study drug. Data on self-reported race and ethnicity were collected as these characteristics may affect fat distribution.
Laboratory Methods
GH (Beckman Access Ultrasensitive Assay), insulin (Beckman Access), total adiponectin (Alpco), and hsCRP (Labcorp) were measured by immunoassays. IGF-I was measured by liquid chromatography/mass spectroscopy (Quest Diagnostics). Lipids, glucose and transaminases were measured by standard clinical assays (Labcorp). Homeostasis model assessment – insulin resistance (HOMA-IR) was calculated 20.
Statistical Analysis
Given the absence of prior data on hepatic fat, the study was powered for VAT reduction, with the hypothesis that tesamorelin would reduce visceral fat in the abdomen and related ectopic depots. The protocol was therefore initially designed with VAT as the primary endpoint, but prior to trial initiation, due to increasing interest in liver fat as a critical endpoint, we reconsidered the endpoints and made hepatic fat a co-primary endpoint, with secondary endpoints including IMCL, measures of glucose homeostasis, lipid, cIMT, transaminases and systemic inflammatory markers as listed in the initial clinicaltrials.gov posting dated 12/15/2010, prior to enrollment of the first patient. The protocol was initially planned to enroll 60 patients, with an estimated 48 planned to complete the study, providing 80% power to detect a treatment effect of 16.5% change in VAT. Due to issues with drug supply, recruitment stopped a few months earlier than anticipated, resulting in 43 patients completing the study. Based on this change in enrollment and more recent data regarding the SD of change in VAT with tesamorelin (41 cm2) from the combined Phase 3 studies 8, post hoc power calculations showed that the sample size of 43 patients had 85% power to detect a treatment difference of 38.5 cm2 in ΔVAT at a two sided α=0.05.
Data were tested for normality using the Shapiro-Wilk test. Normally distributed variables are presented as mean ± standard deviation (SD) or, for changes over time, as mean [95% CI]; variables that are not normally distributed are presented as median [interquartile range (IQR)]. At baseline, comparisons between treatment groups for categorical variables were made using Pearson Chi-Square. For continuous variables, comparisons were made using Student’s t-test for normally distributed variables or Wilcoxon rank sum test for variables that were not normally distributed.
Analysis for treatment effect was based on a modified intention to treat population among patients with available baseline and 6 month follow-up data. For variables measured only at baseline and 6 months, including the primary endpoints of VAT and hepatic fat, between-group comparisons of changes over time were made using Student’s t-test for normally distributed variables or Wilcoxon rank sum test for non-normally distributed variables. For hepatic fat, data were missing for one patient at baseline in the tesamorelin group and for 8 patients at follow up (3 in the placebo group, 5 in the tesamorelin group). For VAT, follow up data were missing in 6 patients (2 in the placebo group, 4 in the tesamorelin group). Sample sizes for each analysis are provided in each table. To handle missing data, analyses using an imputation approach confirmed the results of the analyses using all available data for hepatic fat and VAT, as well as for secondary endpoints assessed at baseline and 6 months (Table 2 and see Supplemental Table 1). An additional analysis was performed using logistic regression to assess the significance of treatment group in predicting liver fat reduction controlling for age, duration of HIV, and lipid lowering therapy. Secondarily, within-group comparisons were made using paired t-test for normally distributed variables and Wilcoxon signed rank test for non-normally distributed variables.
Table 2.
Effects of Tesamorelin on Body Composition, Ectopic Fat, Carotid Intima-Media Thickness, and Immunologic Parameters.
| Baseline | Six Months | Change after Six Months | Treatment Effect – all available data‡ | P-value - all available data‡ | P-value - imputation‡‡ (range of estimates) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tesamorelin n=28 |
Placebo n=22 |
Tesamorelin n=23 |
Placebo n=20 |
Tesamorelin | Placebo | ||||
| Body Composition | |||||||||
| VAT (cm2) | 208 ± 98 n=28 |
237 ± 127 n=22 |
165 ± 59 n=24† |
252 ± 131 n=20 |
−34 [−53, −15]* n=24 |
8 [−14, 30] n=20 |
−42 [−71, −14] | 0.005 | 0.005 (−54, −35) |
| SAT (cm2) | 258 ± 116 n=28 |
256 ± 123 n=21 |
247 ± 121 n=24† |
272 ± 149 n=20 |
2 [−5, 10] n=24 |
8 [−3, 20] n=19 |
−6 [−19, 7] | 0.37 | 0.29 (−14, −1) |
| BMI (kg/m2) | 28.1 (25.8, 32.7) n=28 |
30.1 (27.0, 33.2) n=22 |
28.5 (25.4, 30.7) n=23 |
30.3 (27.2, 35.1) n=20 |
0.3 (−0.3, 0.8) n=23 |
0.3 (−0.2, 0.8) n=20 |
0.0 | 0.89 | 0.62 |
| Lean mass (kg) | 60.7 ± 10.1 n=28 |
62.4 ± 9.7 n=22 |
61.5 ± 9.3 n=23 |
62.6 ± 9.8 n=20 |
0.4 [−0.8, 1.5] n=23 |
−0.5 [−1.6, 0.5] n=20 |
0.9 [−0.6, 2.4] | 0.23 | 0.21 (0.4, 1..6) |
| Fat mass (kg) | 24.4 (20.3, 30.6) n=28 |
26.4 (22.4, 33.3) n=22 |
23.3 (19.8, 30.9) n=23 |
27.2 (24.4, 33.8) n=20 |
−0.2 (−1.6, 1.4) n=23 |
1.2 (0.3, 3.4)* n=20 |
−1.4 | 0.04 | 0.02 |
| Trunk fat (kg) | 13.9 (11.1, 16.8) n=28 |
15.4 (13.8, 18.1) n=22 |
12.6 (11.0, 16.7) n=23 |
15.9 (15.2, 18.2) n=20 |
−0.4 (−1.4, 0.7) n=23 |
0.6 (0.1, 1.7)* n=20 |
−1.0 | 0.01 | 0.004 |
| Ectopic Fat | |||||||||
| Liver Fat (HCL/W%) | 4.5 (2.0, 19.3) n=27 |
6.2 (2.1, 20.6) n=22 |
4.2 (1.8, 11.2) n=23† |
7.2 (4.6, 19.8) n=19 |
−2.0 (−6.4, 0.1)* n=22 |
0.9 (−0.6, 3.7) n=19 |
−2.9 | 0.004 | 0.003 |
| Soleus IMCL/Cr | 13.7 (7.1, 18.6) n=28 |
16.1 (8.5, 27.7) n=22 |
10.6 (7.7, 14.8) n=23† |
11.4 (7.0, 24.8) n=20 |
−1.7 (−3.9, 0.7) n=23 |
−0.2 (−5.2, 5.5) n=20 |
−1.5 | 0.46 | 0.19 |
| Tibialis IMCL/Cr | 4.3 (2.8, 5.8) n=28 |
3.3 (2.5, 5.8) n=22 |
3.9 (2.3, 5.7) n=24† |
4.1 (2.9, 6.0) n=20 |
−0.3 (−2.1, 0.8) n=24 |
0.2 (−2.3, 2.0) n=20 |
−0.5 | 0.39 | 0.39 |
| Carotid IMT (mm) | 0.76 ± 0.16 n=27 |
0.84 ± 0.18 n=22 |
0.71 ± 0.15 n=23 |
0.82 ± 0.20 n=20 |
−0.03 [−0.07, − 0.00]* n=23 |
−0.00 [−0.03, 0.03] n=20 |
−0.03 [−0.08, 0.01] | 0.15 | 0.14 (−0.05, −0.02) |
| Immunologic/Virologic Parameters | |||||||||
| % CD4 | 33 ± 10 n=28 |
31 ± 10 n=22 |
33 ± 9 n=22 |
32 ± 11 n=20 |
0 [−1, 2] n=22 |
1 [0, 3]* n=20 |
−1 [−3, 1] | 0.26 | 0.32 (−2, 0) |
| % CD8 | 43 ± 12 n=28 |
46 ± 10 n=22 |
42 ± 11 n=22 |
46 ± 9 n=20 |
−1 [−2, 0] n=22 |
−1 [−2, 0]* n=20 |
0 [−1, 2] | 0.62 | 0.73 (−1, 1) |
| Log10 VL (log10 copies/mL) | 0 (0, 1.7) n=28 |
0 (0, 1.7) n=22 |
0 (0, 1.4) n=22 |
0 (0, 1.0) n=20 |
0 (−1.0, 0.0) n=22 |
0 (−0.5, 0) n=20 |
0 | 0.97 | 0.51 |
Normally distributed data are presented as the mean±standard deviation for baseline and 6 months, and the mean [95% CI] for change after 6 months. Data that are not normally distributed are presented as median (interquartile range).
Between group testing for comparison of baseline values utilized Students t-test for normally distributed data and Wilcoxon rank sum for non-normally distributed data. No statistically significant differences were seen between groups at baseline. Sample size for each variable at each timepoint is given. Data on hepatic fat from one patient were excluded due to a technical problem with scan acquisition.
Treatment effect and p-value for a modified intention to treat analysis using all available data. Student’s t-test was used to compare changes between groups for normally distributed endpoints, and Wilcoxon rank sum test was used to compare changes between groups for endpoints that were not normally distributed. Treatment effect is mean [95% CI] for normally distributed endpoints and the net difference between median changes in each group for endpoints that were not normally distributed.
p-value for imputation analyses. For normally distributed endpoints, multiple imputation was performed by replacing missing values with imputed values calculated over 100 iterations, using longitudinal mixed effects modeling, and discarding the first 10 iterations. The p-value is the average of the p-values from the individual runs of the multiply imputed data sets. The values in parentheses provide a range (2.5th percentile, 97.5th percentile) of the estimated effect sizes for the imputation analyses. For non-normally distributed endpoints, imputation analysis was performed by replacing the missing data for the 0 to 6 month changes using the median of the change in the combined groups. The p-value given is for Wilcoxon rank sum test for comparison of change between groups using the imputed data set. For these data, range of estimates is not available.
Within group testing utilized paired t-test for normally distributed data and Wilcoxon signed rank test for non-normally distributed data.
indicates significant within-group difference between baseline and 6 months (P < 0.05).
MRI and CT data from one patient who was discontinued between the 3 and 6 month visits for adverse event are included. These data were obtained at a termination visit.
Abbreviations: VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; BMI: body mass index; HCL/W%: hepatocellular lipid-to-water percent; IMCL/Cr: intramyocellular lipid-to-creatinine ratio; IMT: intima-media thickness; VL: viral load.
For outcomes measured at >2 timepoints (e.g., baseline, 3, and 6 months), random intercept mixed effects modeling using restricted maximum likelihood was applied to assess the significance of time×randomization interaction. Two analyses were performed: mixed effects analysis using all available data, and a mixed effects analysis performed to handle missing data using imputation for missing values (see Supplemental Table 1).
Treatment effect (with 95% CI) is shown for normally distributed data. For non-normally distributed data, statistical determination of a treatment effects and associated 95% CI is not possible, but an approximate net treatment effect was determined by subtracting the median changes in each group. Changes within each group for non-normally distributed data are presented showing the median [IQR] of the paired changes over time in each group, whereas the data presented at each time point represent the median [IQR] at such points for each group. Subtraction of the group medians may differ from the medians of the paired changes due to normality of data.
Relationships between continuous variables were assessed using Pearson correlation coefficient (denoted as r) when both variables were normally distributed and Spearman’s rank correlation coefficient (denoted as ρ) when one or both variables was not normally distributed. For comparisons of interest (e.g., change in VAT by change in liver fat), we performed multivariable linear regression modeling, including treatment group and a (group × x-variable) interaction term, to assess whether associations were different between treatment groups.
P-values shown in the text for aggregate changes over time between groups in primary and secondary endpoints are those for imputation analyses. In tables, p-values from both imputation and from analysis using all available data are shown. All statistical analyses were two-sided, with alpha = 0.05 as the pre-defined threshold for statistical significance. Data analysis was performed with SAS 9.3 and JMP 10.0.0 (SAS Institute, Cary, NC).
Results
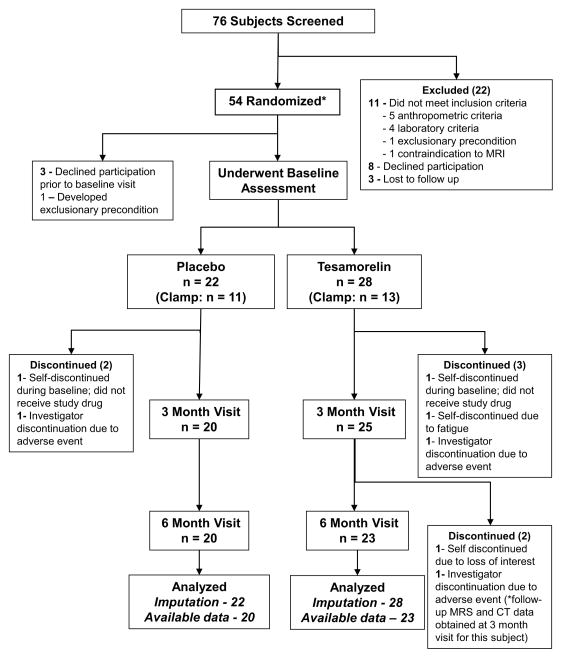
Of 76 patients who completed eligibility screening, 50 were randomized and underwent baseline assessment (Figure 1). Reasons for patient exclusion are listed in Figure 1. Two patients participated in the baseline visit but discontinued before starting study drug. Patient disposition during the study is shown in Figure 1. Overall adherence by vial count was 98% [87, 100] in the tesamorelin group and 99% [88, 99] in the placebo group (P = 0.95). Adherence by study diary was similar: 99% [97, 100] in tesamorelin and 99% [97, 100] in placebo, P = 0.51. One patient in the placebo group and 2 patients in the tesamorelin group had compliance of <80% (P = 0.65).
Figure 1.
CONSORT Diagram detailing participant flow. *Two randomization events – a double-blind 1:1 randomization to tesamorelin vs. placebo, and a 1:1 randomization to euglycemic hyperinsulinemic clamp – occurred simultaneously prior to the baseline visit. These randomization events were independent of each other. Two statistical analyses were performed using (i) an imputation approach to handle missing data and (ii) all available data with missing data treated as missing.
Baseline Characteristics
There were no differences between treatment groups in baseline demographics, alcohol use, or hepatitis C status (Table 1). No patient reported consuming alcohol equivalent to 3 or more drinks per day. Menopausal status did not differ (75% post-menopausal in both groups, P = 1.00). Duration of HIV, antiretroviral therapy use, and lipid lowering therapy use did not differ at baseline (Table 1). Body composition did not differ at baseline (Table 2), nor were there differences between groups in measures of glucose homeostasis (Table 3); lipids, transaminases, or inflammatory markers (Supplemental Table 2); immunologic measures (Table 2); or dietary intake and activity (Supplemental Table 3).
Table 1.
Baseline Characteristics
| Tesamorelin (N= 28) | Placebo (N=22) | |
|---|---|---|
| Demographics | ||
| Age (years) | [49 (46, 54)] | [53 (49, 58]) |
| Gender-M/F (% male) | 24/4 (86) | 18/4 (82) |
| Race/Ethnicity* (N [%]) | ||
| White | 20 [71%] | 14 [64%] |
| Black | 6 [21%] | 3 [14%] |
| Hispanic | 1 [4%] | 3 [14%] |
| Other | 1 [4%] | 2 [9%] |
| Smoking Status (N [%]) | ||
| Never | 14 [50%] | 9 [41%] |
| Past | 9 [32%] | 10 [45%] |
| Current | 5 [18%] | 3 [14%] |
| Alcohol Use (g/day) | [0 (0, 6)] | [0 (0, 0.3)] |
| Duration of HIV (years) | 17 ± 7 | 20 ± 6 |
| Hepatitis C (N [%]) | 7 [25%] | 5 [23%] |
| Medication use at Baseline | ||
| NRTI (N [%]) | 28 [100%] | 21 [95%] |
| NNRTI (N [%]) | 14 [50%] | 15 [68%] |
| PI (N [%]) | 11 [39%] | 10 [45%] |
| Other ART (N [%]) | 7 [25%] | 6 [27%] |
| Lipid lowering therapy (N [%]) | 13 [46%] | 13 [59%] |
| Statin (N [%]) | 8 [29%] | 11 [50%] |
| Testosterone (N [%]) | 7 [25%] | 6 [27%] |
Continuous data are presented as mean±standard deviation for normally distributed variables and median (interquartile range) for variables that are not normally distributed. No data are missing.
Race and ethnicity data collected via patient self-report.
Abbreviations: HIV: human immunodeficiency virus; NRTI: nucleoside reverse transcriptase inhibitors; NNRTI: non-nucleoside reverse transcriptase inhibitors; PI: protease inhibitors; Other ART: antiretroviral therapy including entry inhibitors and integrase inhibitors
Table 3.
Glucose Homeostasis
| Baseline | 2 Weeks | 3 Months | 6 Months | Δ after 6months | Treatment effect – all available data‡ |
P-value - all available data‡ |
P-value - imputation‡‡ (range of estimates) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tesamorelin | Placebo | Tesamorelin | Placebo | Tesamorelin | Placebo | Tesamorelin | Placebo | Tesamorelin | Placebo | ||||
| Fasting glucose (mg/dl) | 87 ± 8 n=28 |
91± 13 n=22 |
97 ± 12 † n=26 |
92 ± 11 n=21 |
94 ± 9 n=25 |
92 ± 10 n=20 |
91 ± 13 n=23 |
92 ± 16 n=20 |
4 (−2, 10) n=23 |
2 (−4, 7) N=20 |
0 [−6, 6] | 0.94 | 0.72 (−3, 3) |
| 2 hour glucose (mg/dl) | 114 ± 31 n=28 |
130 ± 29 n=22 |
(not measured) | 119 ± 43 n=25 |
125 ± 29 n=20 |
118 ± 44 n=22 |
123 ± 36 n=18 |
−1 (−18, 15) n=22 |
−8 (−24, 8) n=18 |
8 [−11, 27] | 0.43 | 0.53 (−6, 18) | |
| Fasting Insulin (μIU/ml) | 7.9 (4.7, 11.1) n=28 |
8.0 (3.9, 16.8) n=22 |
8.1 (4.7, 12.8) n=25 |
10.8 (6.8, 13.7) n=20 |
7.9 (4.8, 11.3) n=22 |
7.6 (4.5, 11.7) n=20 |
−0.2 (−2.6, 1.8) n=22 |
0.5 (−4.9, 3.0) n=20 |
0.6 [−3.5, 4.8] | 0.76 | 0.68 (−0.7, 2.3) | ||
| HOMA-IR | 1.6 (1.1, 2.5) n=28 |
1.9 (0.9, 3.6) n=22 |
1.8 (1.0, 3.0) n=25 |
2.6 (1.5, 2.9) n=20 |
1.7 (1.0, 2.5) n=22 |
1.7 (1.1, 3.0) n=20 |
0.0 (−0.8, 0.5) n=22 |
0.2 (−1.1, 0.8) n=20 |
0.4 [−0.7, 1.4] | 0.48 | 0.45 (0.0, 0.8) | ||
No statistically significant differences between groups at baseline. For normally distributed data, results are presented as the mean ± standard deviation at each timepoint, and change after 6 months is presented as mean (95% CI). Non-normally distributed data are presented as median with interquartile range (25%, 75%).
P-value and treatment effect [95% CI] for mixed effects model (time×randomization) using all available data over six months.
P-value for imputation analyses. Multiple imputation was performed by replacing missing values with imputed values calculated over 100 iterations, using longitudinal mixed effects modeling, and discarding the first 10 iterations. The p-value is the average of the p-values from the individual runs of the multiply imputed data sets. The values in parentheses provide a range (2.5th percentile, 97.5th percentile) of the estimated effect sizes for the imputation analyses.
Indicates significant change from baseline in tesamorelin vs. placebo groups, P < 0.05. Change from baseline at each timepoint was assessed using Student’s t-test.
Abbreviations: HOMA-IR: homeostasis model assessment of insulin resistance.
SI conversion factors: To convert glucose to mmol/L, multiply values by 0.0555. To convert insulin to pmol/L, multiple values by 6.945.
Baseline measures of visceral fat and liver fat were positively associated (ρ = 0.42, P = 0.003), and both showed associations with measures of glucose homeostasis and lipids (Supplemental Table 4). Both VAT (ρ = −0.43, P = 0.003) and liver fat (ρ = −0.44, P = 0.003) were negatively associated with baseline overnight mean GH concentrations and showed no association with baseline IGF-1.
Changes in Body Composition and Ectopic Fat
The tesamorelin group experienced a significant decrease in abdominal VAT area (−34 [−53, −15] vs. 8 [−14, 30] cm2, tesamorelin vs. placebo, mean [95% CI], treatment effect −42cm2 [95% CI −71, −14], P = 0.005) without effects on SAT area (2 [−5, 10] vs. 8[−3, 20] cm2, mean [95% CI], treatment effect −6cm2 [95% CI −19, 7], P = 0.29 (Table 2). Percentage change in VAT was −9.9 [−19.7, −0.2] vs. 6.6% [−4.1, 17.3], mean [95% CI], for a net treatment effect of −16.6% [95% CI −30.6, −2.6], similar to that seen in previous studies 7,8. Hepatic lipid-to-water percent decreased significantly in the tesamorelin group as compared to placebo (−2.0 [−6.4, 0.1]) vs. 0.9 [−0.6, 3.7] lipid-to-water %, tesamorelin vs. placebo, P = 0.003, for a net effect between groups of −2.9 lipid-to-water %, Table 2). This effect of tesamorelin on liver fat remained statistically significant (P = 0.005) controlling for age, duration of HIV, and lipid lowering therapy. In a sensitivity analysis excluding 2 patients who were not fasting for MRS, both in the placebo group, the change in liver fat remained significant (P=0.0005). For the 3 patients with poor compliance, change in hepatic fat was within the interquartile range for the respective treatment groups. Both total fat and trunk fat as measured by DXA decreased significantly compared to placebo (Table 2). IMCL did not change (Table 2).
Changes in Glucose Homeostasis
Fasting glucose increased in the tesamorelin group compared to placebo between baseline and two weeks (Δ 9 [5, 13] vs. 2 [−3, 8] mg/dL, mean [95% CI]; treatment effect 7mg/dL [95% CI 1, 14], P=0.03, Table 3) but was not different from baseline at subsequent assessments (3 months: Δ 6 [2, 10] vs. 2 [−4, 7] mg/dL, mean [95% CI], treatment effect 4mg/dL [95% CI −2, 11], P = 0.20; 6 months: Δ 4 [−2, 10] vs. 2 [−4, 7] mg/dL, mean [95% CI], treatment effect 2mg/dL [95% CI −6, 10], P = 0.56; Table 3). Mixed effects modeling showed no significant effects of tesamorelin on fasting glucose (P = 0.72), fasting insulin (P = 0.68), or HOMA-IR (P = 0.45, Table 3) over the six month period. There was a slight but statistically significant increase in HbA1c from baseline to 6 months (Δ 0.20 [0.04, 0.36] vs. 0.02 [−0.07, 0.10] %, mean [95% CI], treatment effect 0.19% [95% CI 0.01, 0.36], P = 0.03). One patient in each treatment group progressed from impaired fasting glucose to diabetes by fasting glucose, whereas 1 additional patient in each group progressed from impaired glucose tolerance to diabetes by 2hr OGTT (see Supplemental Table 5 for distribution of glucose values). During the 6-month treatment period, no patient in either group experienced fasting blood glucose > 150mg/dL, which was the pre-determined cutoff for study discontinuation.
In the euglycemic hyperinsulinemic clamp subgroup, there was a significant difference in the change from baseline to 3 months in insulin stimulated glucose uptake (M) (Δ −0.5 [−1.7, 0.7] vs. 1.3 [0.6, 2.1] mg/kg/min, mean [95% CI], treatment effect −1.8mg/kg/min [−3.3, −0.4], P = 0.02, whereby the insulin sensitivity decreased in the tesamorelin group and increased in the placebo group). In contrast, the change from baseline was not significant at 6 months (0.4 [−1.2, 1.9] vs. 0.7 [−0.6, 2.1] mg/kg/min, mean [95% CI], treatment effect −0.4 mg/kg/min [−2.3, 1.5], P = 0.68). Results were similar when M was corrected for steady state insulin level and, at six months, for lean body mass.
Changes in Transaminases
There were no significant overall changes in ALT, whereas AST decreased with tesamorelin compared to placebo −4 [−12, 2] vs. 0 [−6, 5] U/L, tesamorelin vs. placebo, (P = 0.046) (Supplemental Table 2).
Changes in Cardiovascular Risk Measures
Intima-media thickness of the left carotid artery decreased in the tesamorelin group (Δ −0.03 [−0.07, −0.00] mm, mean [95% CI], P = 0.04) but did not change in the placebo group (Δ −0.00 [−0.03, 0.03] mm, P = 0.89), though the primary comparison between groups was not significant, treatment effect −0.03 mm [95% CI −0.08, 0.01], P=0.14 (Table 2). Blood pressure and lipids did not significantly change (Supplemental Table 2). CRP did not significantly change, whereas tesamorelin tended to increase adiponectin (P = 0.07) (Supplemental Table 2).
Changes in Growth Hormone and IGF-1
Changes from baseline in IGF-1 and IGF-1 Z-scores were significantly different between treatment groups at 2 weeks, 3 months, and 6 months of treatment (see Supplemental Table 6). Mean overnight GH (Δ 0.35 [0.15, 0.57] vs. −0.01 [−0.07, 0.06] ng/mL, tesamorelin vs. placebo, P = 0.0003) also increased significantly in the tesamorelin group compared to placebo. Supplemental Figure 1 shows the median [IQR] GH at each overnight sampling time point.
Nutrition and Physical Activity
There were no significant changes in dietary intake or physical activity (Supplemental Table 3). Alcohol intake also did not significantly change over six months (0 [0, 4] vs. 0 [0, 0] grams/day, tesamorelin vs. placebo, P = 0.79).
Interrelationship of Reductions in Ectopic Fat with Metabolic Changes and Glucose
Among all patients, changes in hepatic lipid were significantly associated with changes in VAT (ρ = 0.31, P = 0.047) (Supplemental Figure 2), HOMA-IR (ρ = 0.50, P = 0.001) and fasting insulin (ρ = 0.50, P = 0.001). See Supplemental Table 7 for correlations with change in liver fat by treatment group.
Change in VAT was significantly associated with change in mean GH (ρ = −0.46, P = 0.005), whereas change in HCL/W% was not associated with change in mean GH (ρ = −0.22, P = 0.21).
Safety and Adverse Events
Adverse events that occurred in greater than 5 percent of patients are reported in Table 4. There were three serious adverse events (SAEs) in both the treatment and placebo arms of the study. SAEs in the tesamorelin arm consisted of one hospitalization due to exacerbation of existing congestive heart failure, one hospitalization for pneumonia, and one diagnosis of basal cell carcinoma in a patient with a prior history of the same. SAEs in the placebo arm consisted of one hospitalization for acute stroke, one hospitalization for Heller myotomy, and one diagnosis of basal cell carcinoma in a patient with a prior history of the same. Two patients underwent blinded dose reductions, see Supplemental Results and Supplemental Table 6. For further discussion of AE’s, see Table 4 and Supplemental Results.
Table 4.
Adverse Events
| Event | Tesamorelin Number (%) | Placebo Number (%) | P value |
|---|---|---|---|
| Any adverse event | 25 (89%) | 21 (95%) | 0.42 |
| Resulted in discontinuation from study† | 3 (11%) | 1 (5%) | 0.42 |
| Serious adverse event | 3 (11%) | 3 (14%) | 0.75 |
| Hospitalization for CHF exacerbation | 1 | 0 | |
| Hospitalization for pneumonia | 1 | 0 | |
| Basal cell carcinoma | 1 | 1 | |
| Hospitalization for Heller myotomy | 0 | 1 | |
| Hospitalization for acute stroke | 0 | 1 | |
| Adverse events occurring in >5% of patients | |||
| Injection site bruising | 10 (36%) | 11 (50%) | 0.31 |
| Paresthesias | 6 (21%) | 1 (5%) | 0.09 |
| Injection site erythema | 4 (14%) | 2 (9%) | 0.57 |
| Arthralgias | 4 (14%) | 4 (18%) | 0.71 |
| Injection site stinging | 3 (11%) | 0 (0%) | 0.11 |
| Myalgias | 3 (11%) | 0 (0%) | 0.11 |
| Hyperglycemia* | 2 (7%) | 2 (9%) | 0.80 |
| Edema | 2 (7%) | 1 (5%) | 0.70 |
| Sinusitis | 2 (7%) | 1 (5%) | 0.70 |
| Dose adjustment‡ | 2 (7%) | 0 (0%) | 0.20 |
Hyperglycemia defined as fasting glucose > 126 mg/dl or 2 hour OGTT glucose > 200 mg/dl at any visit.
Reasons for study discontinuation in the tesamorelin group were as follows: (1) investigator discontinuation due to CHF exacerbation, (2) investigator discontinuation due to basal cell carcinoma, and (3) a self-discontinuation due to fatigue. The reason for study discontinuation in the placebo group was investigator discontinuation due to stroke.
IRB-approved dose reductions to 1mg daily were performed for 2 patients due to complaints of paresthesias. The study-blind was maintained.
There were no significant changes in immunologic parameters in the tesamorelin group (Table 2).
Discussion
In this preliminary study, our data demonstrate a modest but statistically significant decrease in liver fat with tesamorelin in HIV-infected individuals selected for abdominal fat accumulation, although the clinical importance of this finding is uncertain. Liver fat and visceral fat were closely associated at baseline, and the reduction in liver fat during the study was significantly associated with the reduction in VAT.
To our knowledge, the data from this study are the first to demonstrate in a clinical trial that an agent selectively reducing visceral fat simultaneously reduced liver fat independent of changes in weight. Thus our data support the hypothesis that visceral fat accumulation is linked to liver fat accumulation and suggest that selective targeting of VAT reduction can lead to reductions in liver fat. The mechanisms by which GH augmentation reduced liver fat are unknown. GH augmentation by tesamorelin may increase oxidation of visceral fat. In addition, GH may reduce liver fat through inhibition of hepatic de novo lipogenesis21,22 or other mechanisms. Two prior papers have investigated GH replacement in non-HIV hypopituitary models showing mixed results on hepatic fat 23,24. In contrast, the current study used GHRH to augment endogenous GH secretion as a strategy to reduce visceral fat in an HIV model selected for excess VAT.
The decrease in liver fat in this study suggests that strategies to reduce visceral adiposity merit further investigation in HIV-infected patients with NAFLD, a condition for which there are no approved treatments. Importantly, NAFLD is associated with visceral adiposity and other metabolic abnormalities in HIV1,25. Although the causal pathways underlying these interrelationships are not yet clear, visceral adiposity results in increased inflammatory cytokine production and increased portal free fatty acid flux, either or both of which may contribute to steatohepatosis and hepatic insulin resistance26–28.
In this study, tesamorelin resulted in reductions in VAT, without reductions in SAT. Subcutaneous fat is thought to represent a beneficial depot that may serve as a buffer to protect ectopic fat distribution into other organs 26,29–31. Strategies such as tesamorelin, which are selective to VAT and do not simultaneously reduce SAT, may be optimal to reduce ectopic fat. Further studies of the effects of tesamorelin on other depots linked to VAT, including epicardial fat, should be performed in HIV-infected patients.
Our data also further elucidate effects of tesamorelin on glucose homeostasis. Administration of GH increases glucose 13,32. In contrast, studies to date have suggested that tesamorelin has limited adverse effect on glucose homeostasis7,8,33. Our data demonstrate that tesamorelin initially perturbed glucose as well as insulin sensitivity, assessed by clamp. However, these initial changes were reversed and glucose returned to baseline over longer duration of treatment. We showed a modest increase in HbA1c, consistent with data from larger studies of tesamorelin7,8, which may reflect initial increases in glucose.
Our study has limitations. First, the purpose of this study was to determine detailed metabolic endpoints, including 1H MR spectroscopy and euglycemic hyperinsulinemic clamp, limiting sample size. Thus the study may have been underpowered to detect changes in secondary endpoints. Nonetheless, trends toward improvement in adiponectin and significant improvements in AST suggest additional metabolic effects of VAT reduction in the HIV-infected population. In this study, we chose to enroll patients based on the FDA approved indication for tesamorelin to reduce abdominal fat, and we determined benefits to liver fat and metabolic indices. As the cohort was not specifically chosen for increased liver fat, and the absolute change in lipid-to-water % was modest, the clinical significance of our data is not known. Changes in liver fat may have been more pronounced in a cohort specifically selected for NAFLD. NAFLD may have a benign clinical course and may not progress to liver disease. Liver biopsies, which are the gold standard for assessing features of steatohepatitis and advanced liver disease, were not performed in this study. Our population was primarily male and had been living with HIV and under ART treatment for a long period, consistent with many patients exhibiting lipodystrophic changes in fat. Though abdominal hypertrophy may be less common with newer ART, there exists a substantial group of patients with abdominal fat accumulation in the context of long term prior ART. Further, we did not collect data following discontinuation of tesamorelin. Previous studies have shown that visceral fat may re-accumulate after discontinuation of tesamorelin34, and future studies will be necessary to determine if reductions in liver fat with tesamorelin are maintained following treatment discontinuation. Moreover, tesamorelin is expensive, which is a barrier to its use.
Conclusion
In this preliminary study of HIV-infected patients with abdominal fat accumulation, tesamorelin administered for 6 months was associated with reductions in visceral fat and, additionally, with modest reductions in liver fat. Further studies are needed to determine the clinical importance and long-term consequences of these findings.
Supplementary Material
Acknowledgments
We gratefully acknowledge the research volunteers for their participation in the study, the MGH bionutrition and nursing staffs for their dedicated patient care, and the members of the Data and Safety Monitoring Board for their time and commitment to patient safety.
Footnotes
Author Contributions: Dr. Stanley: study design, data acquisition, data analysis and interpretation, manuscript preparation and editing, final manuscript review. Ms. Feldpausch: data acquisition, data analysis and interpretation, manuscript preparation, final manuscript review. Ms. Oh: data acquisition, data analysis, manuscript preparation, final manuscript review. Ms. Branch: data acquisition, final manuscript review. Dr. Lee: study design, data analysis and interpretation, manuscript preparation, final manuscript review. Dr. Torriani: data acquisition, data analysis and interpretation, manuscript preparation, final manuscript review. Dr. Grinspoon: study conception and study design, data acquisition, data analysis and interpretation, manuscript preparation and editing, final manuscript review. Dr. Grinspoon had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Previous presentations: Data from this study were presented at the 2014 Conference on Retroviruses and Opportunistic Infections (CROI, Boston, MA, March 3–6, 2014). Data will be presented at the 2014 Endocrine Society meeting (Chicago, IL, June 21–24, 2014).
Disclosures and Funding: Funding was provided by NIH R01DK063639 to S.K.G., K23DK089910 to T.L.S. and by NIH M01-RR-01066 and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. Also supported in part by Nutrition Obesity Research Center at Harvard, NIH Grant P30 DK40561. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Study drug was provided by Theratechnologies, Inc. Theratechnologies had no role in design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. Theratechnologies reviewed the manuscript prior to submission, but submission was not contingent on approval by Theratechnologies, Inc. Dr. Grinspoon has served as a consultant to Aileron Therapeutics, Inc., Ferrer, Sanofi-Aventis, Navidea, Astra-Zeneca and EMD Serono Inc. all unrelated to this manuscript, and received investigator initiated research funds from Theratechnologies, Inc., BMS, Gilead, Amgen/Immunex, Serono unrelated to this project. T.L.S., M.N.F., J.O., K.L.B., H.L, and M.T. have nothing to disclose.
References
- 1.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008 Jul 15;47(2):250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 2.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007 Nov 1;46(3):312–317. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 3.Perseghin G. Lipids in the wrong place: visceral fat and nonalcoholic steatohepatitis. Diabetes Care. 2011 May;34(Suppl 2):S367–370. doi: 10.2337/dc11-s249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009 Apr 15;50(5):464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glesby MJ, Albu J, Chiu YL, et al. Recombinant human growth hormone and rosiglitazone for abdominal fat accumulation in HIV-infected patients with insulin resistance: a randomized, double-blind, placebo-controlled, factorial trial. PLoS One. 2013;8(4):e61160. doi: 10.1371/journal.pone.0061160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q, Engelson ES, Kotler DP, Albu JB, Chiu YL, Glesby MJ. Effect of Recombinant Human Growth Hormone (rhGH) and Rosiglitazone (rosi) on Liver Fat in People with HIV-Associated Abdominal Obesity and Insulin Resistance. Antiviral Therapy; 14th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV; Washington, D.C. 2012. p. A31. [Google Scholar]
- 7.Falutz J, Allas S, Blot K, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007 Dec 6;357(23):2359–2370. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 8.Falutz J, Mamputu JC, Potvin D, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010 Sep;95(9):4291–4304. doi: 10.1210/jc.2010-0490. [DOI] [PubMed] [Google Scholar]
- 9.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. American Journal of Clinical Nutrition. 1996;64(5):685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 10.Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S. Growth Hormone-Releasing Hormone in HIV-Infected Men With Lipodystrophy: A Randomized Controlled Trial. JAMA. 2004 Jul 14;292(2):210–218. doi: 10.1001/jama.292.2.210. [DOI] [PubMed] [Google Scholar]
- 11.Falutz J, Allas S, Kotler D, et al. A placebo-controlled, dose-ranging study of a growth hormone releasing factor in HIV-infected patients with abdominal fat accumulation. AIDS. 2005 Aug 12;19(12):1279–1287. doi: 10.1097/01.aids.0000180099.35146.30. [DOI] [PubMed] [Google Scholar]
- 12.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982 Jul;36(1):172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 13.Lo J, You SM, Canavan B, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. JAMA. 2008 Aug 6;300(5):509–519. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredella MA, Ghomi RH, Thomas BJ, et al. Breath-hold 1H-magnetic resonance spectroscopy for intrahepatic lipid quantification at 3 Tesla. J Comput Assist Tomogr. 2010 May-Jun;34(3):372–376. doi: 10.1097/RCT.0b013e3181cefb89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torriani M, Thomas BJ, Halpern EF, Jensen ME, Rosenthal DI, Palmer WE. Intramyocellular lipid quantification: repeatability with 1H MR spectroscopy. Radiology. 2005 Aug;236(2):609–614. doi: 10.1148/radiol.2362041661. [DOI] [PubMed] [Google Scholar]
- 16.Chan R, Kaufhold J, Hemphill LC, Lees RS, Karl WC. Anisotropic Edge-Preserving Smoothing in Carotid B-mod Ultrasound for Improved Segmentation and Intima-Media Thickness (IMT) Measurement. Computers in Cardiology. 2000;27:37–40. [Google Scholar]
- 17.Georgoff P, Thomasson D, Louie A, et al. Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. AJR Am J Roentgenol. 2012 Jul;199(1):2–7. doi: 10.2214/AJR.11.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaete FL, Colberg SR, Burke T, Kelley DE. Reproducibility of computed tomography measurement of visceral adipose tissue area. Int J Obes Relat Metab Disord. 1995 Jul;19(7):464–467. [PubMed] [Google Scholar]
- 19.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990 Apr;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz JM, Mulligan K, Lee J, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002 Feb;87(2):942. doi: 10.1210/jcem.87.2.8391. [DOI] [PubMed] [Google Scholar]
- 22.Goodman HM. Effects of chronic growth hormone treatment on lipogenesis by rat adipose tissue. Endocrinology. 1963 Jan;72:95–99. doi: 10.1210/endo-72-1-95. [DOI] [PubMed] [Google Scholar]
- 23.Nishizawa H, Iguchi G, Murawaki A, et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol. 2012 Jul;167(1):67–74. doi: 10.1530/EJE-12-0252. [DOI] [PubMed] [Google Scholar]
- 24.Gardner CJ, Irwin AJ, Daousi C, et al. Hepatic steatosis, GH deficiency and the effects of GH replacement: a Liverpool magnetic resonance spectroscopy study. Eur J Endocrinol. 2012 Jun;166(6):993–1002. doi: 10.1530/EJE-12-0002. [DOI] [PubMed] [Google Scholar]
- 25.Crum-Cianflone N, Krause D, Wessman D, et al. Fatty liver disease is associated with underlying cardiovascular disease in HIV-infected persons(*) HIV Med. 2011 Sep;12(8):463–471. doi: 10.1111/j.1468-1293.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec 14;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 27.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013 Jan;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 28.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010 Sep 30;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential Fat Deposition in Subcutaneous Versus Visceral Depots Is Associated with Insulin Sensitivity. Journal of Clinical Endocrinology & Metabolism. 2011;96(11):E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010 Jun;34(6):949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007 Sep;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen S, Moller N, Christiansen JS, Jorgensen JO. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes. 2001 Oct;50(10):2301–2308. doi: 10.2337/diabetes.50.10.2301. [DOI] [PubMed] [Google Scholar]
- 33.Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a Growth Hormone-Releasing Hormone Analog on Endogenous GH Pulsatility and Insulin Sensitivity in Healthy Men. J Clin Endocrinol Metab. 2011 Jan;96(1):150–158. doi: 10.1210/jc.2010-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falutz J, Allas S, Mamputu JC, et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS. 2008 Sep 12;22(14):1719–1728. doi: 10.1097/QAD.0b013e32830a5058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.