Abstract
Thrombi, or clots, often occlude proximal segments of the cerebral arterial circulation in acute ischemic stroke. Thromboembolic occlusion or thrombi superimposed on atherosclerotic plaque are the principal focus of acute stroke therapies such as thrombolysis or thrombectomy. We review the imaging characteristics of thrombi on multimodal CT and MRI, angiography and ultrasonography, summarizing recent studies that facilitate therapeutic decision-making from these noninvasive studies. Information about the location, size and imaging characteristics can be ascertained using these techniques. Imaging findings in relation to occlusive thrombus have been correlated with clot pathology, response to therapeutic interventions, and clinical outcome. Diagnostic evaluation of occlusive thrombi on noninvasive studies now constitutes an integral component of acute stroke management.
Introduction
Thrombolysis, the dissolution of thrombi in the arterial supply to the brain, is the focus of treatment for acute ischemic stroke. Due to limited recanalization success with intravenous thrombolysis, endovascular thrombectomy has been developed to help open such occluded arterial segments. Findings indicative of an occlusive thrombus or therapeutic target are often evident on initial imaging studies acquired during triage of an acute ischemic stroke patient. Hyperdense vessels on CT and segmental signal abnormalities on various MRI sequences may indicate the presence of clot in a major cerebral artery. At the earliest stages of ischemic stroke, such vessel findings may be the only abnormality on an imaging study as the parenchymal or tissue changes may be unapparent or nonexistent. Imaging findings suggestive of an occlusive thrombus may facilitate triage when a differential diagnosis is uncertain or alternatively, confirm the need to consider endovascular options when persistent occlusion is noted.
Current use of various imaging studies in stroke, including multimodal CT or MRI, conventional or digital subtraction angiography (DSA) and ultrasonography, has peeked interest in understanding the implications of thrombi visualized with these diagnostic modalities. Furthermore, the absence of such findings may be seen even when an occlusion is ultimately diagnosed on DSA. The specific appearance, including location, size and imaging characteristics are therefore important to consider. Additionally, recent correlation with pathology results, response to therapeutic interventions and bearing on ultimate clinical outcomes is important as these findings are increasingly noted on routine studies.
Rapid treatment of thrombus in acute stroke patients is of great importance. “Door to needle time” has been a focal point in many emergency departments. One study determined that if the artery can be completely opened within the first few hours after symptom onset, good outcome is highly likely.(1) Therefore, rapid and efficient imaging improves the likelihood of good clinical outcomes in patients suffering from acute stroke.
We provide a comprehensive review of the various imaging findings related to occlusive thrombus on acute stroke diagnostic tests currently used in routine clinical practice. We focus on six thrombus-related factors of note: location, size or extent, imaging signal characteristics, imaging/pathological correlation, response to revascularization, and clinical outcome.
Location
An acute thrombus in an intracranial artery usually demonstrates high density when compared to an unaffected vessel or unaffected areas of the same vessel. The usual nomenclature is to use the term hyperdense followed by the artery affected. For example when the hyperdense vessel is the middle cerebral artery, it is termed the hyperdense middle cerebral artery sign (HMCAS, Figure 1).(2) The hyperdense internal carotid artery sign (HICAS) has been determined to be highly specific (100%) and have a high predictive value (100%) for distal internal carotid artery thrombus on CTA. The HMCAS on non-contrast CT (NCCT) is one of the best studied of the clot-imaging techniques and is characterized as a hyperdense tubular area within the expected course of a cerebral artery. This hyperdensity is most likely a result of densely packed red blood cells within organized thrombus.(3)
Figure 1.
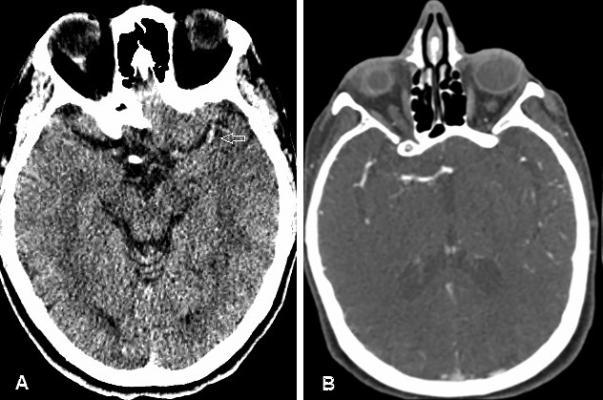
Figure A- Hyperdense MCA sign on noncontrast CT
Figure B- Demonstration of Clot on CTA with proximal and distal border outlined by contrast
Hyperdense vessels located more proximal within the arterial tree are associated with more severe neurological deficits due to their placing a greater volume of brain tissue at risk. Even location within the MCA (proximal or distal) has different implications, as does the hyperdense internal carotid artery sign (HICAS). Isolated M2 occlusions have a higher rate of revascularization in comparison to patients with M1 occlusions (82.1% vs 60%). In addition, those in the M2 occlusion group had a favorable outcome (mRS scale= 0 to 2) in 40.7% of cases vs 33.3% of cases for the M1 occlusion group and a 90-day mortality rate of 25.9% in the M2 group vs 32.9% in the M1 group.(4) Patients with the HICAS had more severe initial neurological deficit, poorer prognosis, and worse outcome despite thrombolytic therapy.(5)
Hyperdense vessels signs can also be found in the posterior circulation, and the hyperdense basilar artery sign on NCCT has been determined to be an accurate predictor of basilar artery thrombosis and short- and long-term outcomes. In fact, one study determined that the hyperdense basilar artery sign had 71% sensitivity, 98% specificity, 94% accuracy, 83% positive predictive value, and 95% negative predictive value for basilar artery occlusions.(6) However, it has been noted that identification of the hyperdense basilar artery sign is challenging because the artery is imaged in cross section as it runs along the ventral surface of the pons, and there is no other artery to compare it to.(7)
Although the hyperdense artery signs reveal good specificity for imaging thrombus, their sensitivity is lower, as not all occlusive thrombi are of the requisite density. Thus one of the most accurate means of localizing an occlusive thrombus is computed tomography angiography (CTA), which provides valuable information on status of large cervical and intracranial arteries. In addition to locating the occlusion site, CTA can help show arterial dissection, determine clot length, and grade collateral blood flow.(8) It has been determined that compared with conventional catheter-based angiography, CTA can help detect the presence of a filling defect in the vessel caused by true arterial thrombosis with 89% sensitivity.(8) Also, while CTA can show thrombi in intracranial vessels, it also has the ability to detect thrombosis of the vertebrobasilar system more clearly than NCCT.(8)
MRI can also be of use in determining the location of occlusive thrombi. Cerebrospinal fluid appears on T2* GRE imaging as high signal in the circle of Willis and provides a stark contrast to the dark flow void and the very low signal intensity caused by blooming artifact (BA) within thrombi.(2) The BA is caused by deoxyhemoglobin, which causes inhomogeneities in local magnetic field and hence signal loss on T2*sequences and its presence has a high sensitivity and specificity in the identification of acute thrombotic occlusions in the large vessels at the base of the brain such as the middle cerebral artery M1 segment.
Magnetic resonance angiography (MRA) is helpful for revealing the location and extent of occlusive thrombus. The two most commonly used methods are time-of-flight (TOF) MRA and contrast-enhanced (CE) MRA.(2) In a study where TOF MRA was used to differentiate between total occlusions and near occlusions of the internal carotid artery, it correctly depicted 92% of total occlusions and 100% of near total occlusions.(9) For the M1 segment of the MCA, it has been noted that MRA can show the occlusion quite well. However, distal clots are not well visualized on MRA.(10)
Catheter angiography is the most effective means of evaluating the cerebral vessels for arterial stenosis, dissection, and vasculopathy, with vasculitis, or occult lesions, and to provide information about collateral flow and perfusion status.(11) In acute stroke, catheter angiography is used to confirm and characterize the location of occlusive thrombus prior to endovascular thrombolyis or thrombectomy device deployment, and is considered the gold standard for defining the location of proximal occlusions and corresponding collaterals.(12) By traversing the occlusion with the micro catheter for injection distally, one can document the extent of occlusion.(12),(13)
Transcranial Color-Coded Doppler imaging (TCCD) is an emerging application of TCD technology, which allows for the visualization of the arteries at the base of the brain. Occlusive thrombus can be visualized when present in the circle of Willis as a vessel section without a characteristic back and forth flow just proximal to the occlusion. Rating scales have been determined to characterize vessels on TCD. The thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades is able to predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator.(14)
Size
Size, or clot burden, is an important factor as larger clots have been associated with poor response to therapy. Many of the modalities used for detecting the presence of occlusive thrombus, such as the hyperdense artery sign, can also be used to describe thrombotic burden.(5) Another consideration in determining clot burden is the vessel segment in which thrombus is imaged. For example more proximal occlusions as seen by the HICAS likely indicated thrombi of large in size than a proximal M1 HMCAS, which in turn is larger in size than an M2 HMCAS. Thus, length of acute occlusive thrombus on imaging will always need to be evaluated relative to the vessel segment involved.
Thrombus length as determined by vessel segment hyperdensity can be accurately measured using NCCT if the slice thickness is between 1.25mm and 2.5mm rather than the mores standard 5mm.(15) Information regarding length of thrombus in acute stroke can help predict who may benefit less from IV thrombolysis as thrombus length is greater than 8mm is associated with poor recanalization.(16)
CTA is can be used to determine clot burden, with increasing length measurements associated with worse recanalization.(17) On CTA, the clot will appear as an area of moderate density within the vessel segment in which there is no contrast present. The proximal face of the clot will be very well demarcated on the contrast-enhanced study, yet the distal end of the thrombus may be less clear. To get the best results 3-dimentional reconstruction of the vascular tree may be needed. Distal flow to a major occlusion may depend on the presence of collaterals and the ability of CTA to measure clot length may be compromised when collaterals are not present. Newer technologies such as 4D CTA may better define intracranial thrombus burden in comparison to CTA.(18) Information on length of occlusive thrombus may be of value in predicting response to particular therapies and in the future may be a means of allocating treatment options.
Clot burden can be measured using the BA found on MRI GRE sequences. Data on the prognostic value for the presence of BA as a surrogate for outcomes after arterial recanalization is mixed. Adding measures of clot burden based on extent of BA on GRE using new reproducible semiquantitative scores may help in assessing thrombi for response to therapy and long-term outcomes after intravenous thrombolysis.(19)
Imaging Characteristics
Results of studies reporting on the ability of hyperdense artery sign on NCCT to detecting occlusive thrombus have been variable. Most studies have shown that the HMCAS has a low sensitivity for detection of MCA occlusion when compared to catheter angiography, with detection rates of 26-47%. This low sensitivity can be explained by two factors: variable composition of thrombi and spatial resolution of standard NCCT.(20) The relative composition of thrombi will be addressed later in the discussion of clot pathology. Spatial resolution can be overcome by using thinner imaging slice propocols.(20) The thin-slice NCCT method allows for better contrast in density between occlusive thrombus and tissue than thick-slices would because of diminished volume averaging effects.(15)
Another aspect of evaluating arteries for hyperdensity is the ability to compare the relative density of occluded vessel to the contralateral vessel or a segment of non-occluded vessel. Density is measured using a Hounsfield unit (HU) scale of relative density in comparison to air (-1000 HU) and bone (1000 HU). Density of non-occluded blood vessels is in the range of 40-43 (mean 41.3) HU whereas occlusive thrombus is in the range of 47-61 (mean 54.0) HU.(21) Another means of identifying HMCAS is comparison of density in HU of affected side compared to the similar vessel on the unaffected side. A ratio of ≥ 1.2 was associated with HMCAS. (21) Relative density of occlusive thrombus is an important variable, which may have an effect on outcome. Factors influencing the density of occlusive thrombus may relate to histopathology, location, and size.
Compared to NCCT, CTA generally has thinner slices, allowing for more accurate localization of structures, including occlusive thrombus. In addition, by including CTA in an acute stroke evaluation, potential embolic source and plaque burden at the origins of the internal carotid arteries can be determined.(22) CTA has 100% accuracy in diagnosing total versus near occlusions of the internal carotid artery.(13) While CTA has high sensitivity for detecting occlusion of the internal carotid artery or proximal MCA, it has low specificity or at least a high false positive rate and low inter-rater reliability for more distal occlusions. This has become evident in clinical trials using CTA based identification of arterial occlusion determined locally but then adjudicated centrally.
MRI sequences can be used, as a lack of arterial flow void at the base of the brain on T2-weighted MRI may be indicative of an intracranial occlusion.(23) A clot would have altered signal intensity that is different from the normal dark flow void that is consequence of fast flowing blood. T2* shortening takes place in an acute clot because of the magnetic susceptibility differences that occur from intracellular deoxyhemoglobin.(24) This creates a non-uniform magnetic field and a quick dephasing of proton spins occurs, resulting in a loss of signal that is observed on T2*-susceptibility-weighted images, often referred to as GRE blooming artifact (BA).(24) T2*-weighted GRE MRI, a susceptibility-based sequence, is an integral part of any MRI-based acute stroke protocols as it is at least as sensitive and specific as NCCT in identifying intracerebral hemorrhage.(24),(25)
Just like the hyperdense vessel sign on CT, GRE BA is highly specific for acute arterial occlusions in acute stroke patients.(26) BA is defined as an area of hypointensity (signal loss) in the potion of the vessel containing thrombus, often distorting the margins of the vessel.(27) Presence of BA can provide information on location and extent of a clot as well as a reflection of red blood cell content.(27) A limitation of BA in assessing occlusive thrombus is its decreased sensitivity in distal arterial segments.(12) BA may be noted in about half of patients presenting to an academic medical center for acute stroke who are selected for endovascular therapy, just as half of patients in this cohort imaged with CT demonstrated the HMCAS on NCCT.(27)
Susceptibility-weighted imaging (SWI) is a relatively new fully velocity-compensated high resolution, three-dimensional GRE sequence that uses filtered-phase information and magnitude to create new sources of contrast. It differs from spin attenuation, T1, T2, and T2* by offering a unique contrast. It is currently possible to image the entire brain using SWI in about four minutes.(10) Since thrombi contain deoxyhemoglobin, acute arterial thrombosis can be detected on SWI images by the hypointense susceptibility sign.(28) Given its recent introduction, the role of SWI in determining the location and extent of occlusive thrombus remains to be determined.(12),(29)
Evaluation of the interface between the clot and vessel is an interesting and evolving area of research.(30) This interface has been referred to as the “clotface” and has been categorized as “sharp or blurred” as well as “cutoff/meniscoid type, tram track, taper, and tandem” on catherter angiography.(31) (Figure 2) Sharp clot interface as well as tram track appearance were found to have more favorable outcomes in recanalization and outcome in one study. (31)
Figure 2.
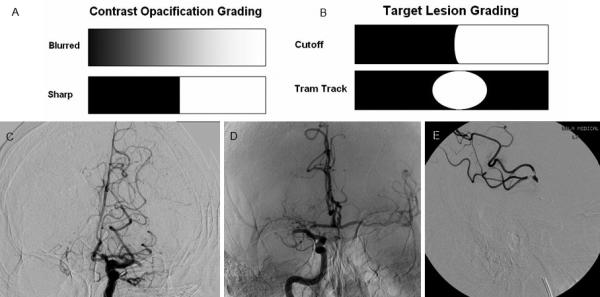
Figures A & B: Grading schemes used for contrast opacification and target lesion demarcation respectively
Figure C: Blurred Left MCA contrast opacification
Figure D: Sharp Cutoff Lesion in Right MCA
Figure E: Tandem Tram Track Lesion
Transcranial Doppler (TCD) is a noninvasive, ultrasound technique used to measure cerebral blood flow velocity.(32) TCD uses 2 MHz (low frequency) pulsed sound waves to penetrate bone and visualize intracranial vessels.(11) Normal findings for the cerebral circulation is low-resistance flow within a target velocity range. A significant increase (greater than 30%) in the flow velocity may indicate stenosis, with an inverse relationship between angiographic residual lumen diameter and flow velocity.(33),(34) Alexandrov and colleagues have developed a method for locating occlusive thrombus using TCD.(35),(14) Blood flow proximal to occlusive thrombus causes vibrations to be transmitted through the clot and a waveform. Occluded vessels can be monitored with continuous TCD and the exact moment of recanalization can be determined. The Thrombolysis In Brain Ischemia (TIBI) score based on Doppler waveform is the most widely used for method for the assessment of thrombotic occlusion, initial hemodynamics and recanalization phenomena.(35),(14) Limitation of TCD include that it is operator dependent and limited to large arteries at the base of the brain and circle of Willis.
Pathology
Non-contrast CT (NCCT) is the most frequently used modality in evaluation of acute stroke as it can be performed quickly and can detect intracranial hemorrhage with high sensitivity and specificity.(36) In addition to ruling out hemorrhage, NCCT can help detect early signs of ischemia such as obscuration of gray-white interface.(37)
Experimental models support the histopathological correlations of thrombus imaging. In one experiment two different types of thrombi, erythrocyte-rich and fibrin-rich, were injected into the common carotid artery in swine.(38) MRI was then immediately performed and the erythrocyte component showed increased signal intensity on FLAIR and isointense signal intensity on T2*-weighted signals, whereas the fibrin-rich thrombus demonstrated isointensity on FLAIR and decreased signal intensity on T2*-weighted signals.(38) Erythrocyte-rich thrombi have also been shown to display higher attenuation values than platelet-rich thrombi on non-contrast CT.(15) Thus, using CT and MRI characteristics can help differentiate clot composition within thrombi.(38)
The underlying pathophysiological mechanism of vascular arterial occlusion in acute stroke is heterogeneous.(39) White thrombi are mostly composed of platelet components and fibrin whereas red thrombi are rich in trapped erythrocytes.(40) White thrombi have been found to be more resistant to thrombolysis in comparison to red thrombi.(40) Histopathological analysis is not a definitive means of determining the origin of occlusive thrombus as both cardioembolic and arteriopathic etiologies have complex histological patterns that overlap and make differentiation difficult.(41) (Figure 3)
Figure 3.
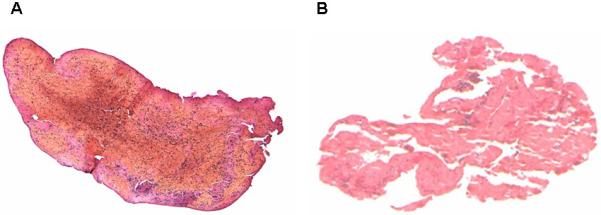
Figure A: Red blood cell-predominant thrombus retrieved from the middle cerebral artery during embolectomy
Figure B: Platelet and fibrin-predominant thrombus retrieved from the middle cerebral artery during embolectomy
Response to Therapeutic Interventions
Response to intravenous thrombolytic therapy may depend on clot size characteristics such width and length. The largest pathological study measuring size of extracted clots found that emboli extracted from MCA occlusions were typically <3 mm in width, whereas clots retrieved from the ICA were typically 3 to 5 mm in width, yet could stretch up to 30 mm in length and measured.(41) This finding complements studies showing that the more distal the occlusion is located, the higher the likelihood of recanalization after administration of TPA, the probability of complete recanalization at 2 hours of treatment is 44%, 29%, and 10% in the distal MCA, proximal MCA, and terminal internal carotid artery, respectively.(4) Proximal site of occlusion reflects large size of thrombus and better response to intravenous thrombolysis.
MRI measures of clot size and length are also predictive of outcome after intravenous thrombolysis. A novel reproducible semiquantitative score, termed the T2*-Clot burden score was tested and compared to the CTA- Clot burden score, which has been shown to be a predictor of recanalization and functional outcome. The study found that T2*-Clot burden score was inversely associated with 24-hour recanalization and 3-month outcome proceeding IV thrombolysis.(42)
Intra-arterial interventions including embolectomy and the use of retrievable stents are emerging therapies, which target a subset of patient with occlusive thrombus in the larger arteries at the base of the brain. These types of interventional therapies do not have the same graded response to therapy seen with TPA based on location of occlusion. This may be a reflection of ease in accessing the more proximal vasculature, and difficulty in accessing very distal occlusion.(43)
Other clot-related factors that may affect response to therapy include density measured in Hounsfield units (HU) on NCCT. Density may be a reflection of clot composition as platelet/fibrin predominant thrombi contain few red cells and thus have lower HU counts in comparison to red cell-predominant clots, which are composed of more dense hemoglobin.(44) Thrombus density on preintervention NCCT correlated with postintervention recanalization (measured by TICI grade) regardless of pharmacological (IV tPA r=0.69, IA tPA r=0.72, P<0.0001) or mechanical treatment (r=0.73, P<0.0001).(45) In this analysis clot density predicted recanalization whereas age, sex, baseline National Institute of Health Stroke Scale, treatment method, time to treatment, or clot volume did not. Another research group noted that successful recanalization was achieved in 79% of patients with the hyperdense vessel sign (33/42), but in only 36% (9/25) of patients without (p=0.001). (46)
The MRI equivalent to density on CT is the susceptibility vessel sign on T2*-weighted GRE, also known as blooming artifact, is found more frequently in patients with cardioembolic stroke, and is an independent predictor of recanalization.(47)
Emerging Imaging Modalities
Information about occlusive thrombi can be obtained by evaluation of standard imaging modalities looking for subtle findings. On T2-weighted fluid attenuated inversion recovery (FLAIR) sequences, the suppression of cerebrospinal fluid signal makes it difficult to identify the occlusive thrombus from the absence of a flow void. Evaluation for the presence of high signal intensity on FLAIR MRI sequences can provide information about cerebral occlusion.(2) FLAIR vascular hyperintensity (FVH), a subtle finding,(23) is usually seen in the setting of acute ischemic stroke just proximal and distal to the occlusive thrombus. It is not a means of visualizing occlusive thrombus, but rather highlighting its presence by documenting the slowed flow just proximal and distal to the occlusion.(48),(49) Thus the occlusive thrombus will demonstrate an FVH gap, and the presence of FVH distally can provide important information on collateral flow.(50) (Figure 4)
Figure 4.
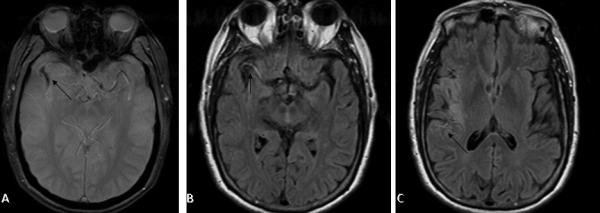
Gradient recall imaging (A) demonstrates blooming artifact within the right middle cerebral artery (arrow). Sluggish flow proximal to the clot (B) and distal to the clot through leptomeningeal collaterals (C) leads to vascular hyperintensity of Fluid Attenuation Inversion Recovery (FLAIR) sequences.
Other techniques for analysis of acute intracranial thrombi include 4-dimensional (4D) CTA.(18) The methodology of image acquisition includes non-contrast CT images of the head, near whole-brain CT perfusion (CTP), and craniocervical CTA on a 128-section multi-detector CT scanner. 4D CTA is more sensitive to delayed contrast arrival and allows for better approximation of intracranial clot burden including better identification of the distal thrombus terminus.(18)
Imaging-Pathological Correlates
Imaging properties of acute occlusive thrombus correlate with pathological findings from retrieved fragments. The presence of the hyperdense artery sign and GRE blooming artifact on pre-embolectomy MR imaging were noted in a study of consecutive cases of successful clot fragment retrieval. Histopathological studies of retrieved clots in acute ischemic stroke had revealed three principal categories, (51) predominantly platelet and fibrin, predominantly red blood cell (RBC) rich or mixed. Furthermore methods of measuring the proportion of RBC content on pathological specimens have been described.(51) When comparing pre-retrieval imaging to histopathology, larger red blood cell (RBC) concentration was associated with the hyperdense MCA sign on CT and the blooming artifact on GRE MRI.(52)The absence of these neuroimaging findings was associated with fibrin-rich thrombi. The hyperdense MCA sign was seen in 100% of subjects with RBC-dominant clots, in 67% of subjects with mixed (RBC and fibrin-rich) clots, and in just 20% of subjects with fibrin-rich clots. Similar results were found for the blooming artifact on GRE MRI where it was present in. 100% of subjects with RBC-rich clots, 63% of mixed clots, and only 22% of fibrin-rich clots.(27)
Conclusion
The neuroimaging of thrombi in acute stroke can be accurately and precisely evaluated via the use of non-contrast CT, CTA, FLAIR MR, T2*-weighted GRE and SWI MRI, MRA, catheter angiography, and TCD. Different imaging modalities can provide information on various aspects of the occlusive thrombus (Table 1).
Table 1.
Summary of the various modalities used for thrombus imaging
| Modality | Characteristics |
|---|---|
| Non-contrast CT | • Quickly performed |
| • Hyperdense MCA sign identified | |
| • Hyperdense basilar artery sign identified | |
| • Density (in HU) can be determined | |
| CT | • Uses thinner slices than noncontrast CT |
| • Useful for vertebrobasilar system | |
| • Useful for internal carotid artery plaques | |
| • Accurately detects clot length | |
| GRE MRI | • Helps to rule out hemorrhage and clearly identify occlusion |
| • Low intensity vessel signs in proximal MCA | |
| • Blooming artifact identified | |
| • Location and content of clot can be determined | |
| FLAIR MRI | • High intensity vessel signs |
| • Distinguishes between proximal MCA vs distal | |
| • Discontinuity indicates thrombus | |
| • FVH most often in Sylvian fissure | |
| MRA | • Useful for carotid bifurcation and intravascular thrombus |
| • Better for identifying occlusions of Ml segment of MCA, not distal | |
| • Useful for clot location and burden, but not characteristics of clot composition | |
| Catheter Angiography | • Helps determine internal carotid artery occlusions |
| • Noninvasive (actually quite invasive) and sensitive | |
| • Offers “in-situ” treatment | |
| • Defines the location of proximal occlusions and corresponding collaterals | |
| • Helps determine the starting point of an occlusion | |
| TCD | • Great bedside tool and continuous monitoring |
| • Useful in patients undergoing thrombolysis | |
| • Can determine location of clot | |
Imaging of the occlusions can give information on the location, size, and characteristics of the clot. In addition, imaging can give clues to the etiology of clots, response to therapy, and long term outcomes. While much data currently exists on the various imaging modalities used to diagnose thrombi in acute stroke, a more uniform and standardized procedure must be establish for radiologists and neurologists to follow. Having such a protocol would allow for more efficient analysis of patients with occlusive thrombi suffering from acute stroke.
References
- 1.Grotta J. Timing of Thrombolysis for acute ischemic stroke. Ann N Y Academy of Science. 2012;1268(1):141–4. doi: 10.1111/j.1749-6632.2012.06690.x. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan A, Goyal M, Alazri F, Lum C. State-of-the-art imaging of acute stroke. Radiographics. 2006;26:75–95. doi: 10.1148/rg.26si065501. [DOI] [PubMed] [Google Scholar]
- 3.Chehab T, Ha T. Acute middle cerebral artery thrombosis. Kansas Journal of Medicine. 2007 [Google Scholar]
- 4.Molina C. Futile recanalization in mechanical embolectomy trials: a call to improve selection of patients for revascularization. Stroke. 2010;41(5):842–3. doi: 10.1161/STROKEAHA.110.580266. [DOI] [PubMed] [Google Scholar]
- 5.Ozdemir O, Leung A, Bussiére M, Hachinski V, Pelz D. Hyperdense internal carotid artery sign: a CT sign of acute ischemia. Stroke. 2008;39(7):2011–6. doi: 10.1161/STROKEAHA.107.505230. [DOI] [PubMed] [Google Scholar]
- 6.Goldmakher G, Camargo E, Furie K. Hyperdense basilar artery sign on unenhanced CT predicts thrombus and outcome in acute posterior circulation stroke. Stroke. 2009;40(1):134–9. doi: 10.1161/STROKEAHA.108.516690. [DOI] [PubMed] [Google Scholar]
- 7.Jakanani G, Aslam M, Rao B. Basilar artery: another anatomic blind spot at brain imaging. Radiographics. 2010;30(5):1431–2. doi: 10.1148/radiographics.30.5.3051431. [DOI] [PubMed] [Google Scholar]
- 8.De lucas E, Sánchez E, Gutiérrez A, Mandly A. CT protocol for acute stroke: tips and tricks for general radiologists. Radiographics. 2008;28(6):1673–87. doi: 10.1148/rg.286085502. [DOI] [PubMed] [Google Scholar]
- 9.El-Saden S, Grant E, Hathout G, Zimmerman P. Imaging of the Internal Carotid Artery: The Dilemma of Total versus Near Total Occlusion. Radiology. 2001;221(2):301–8. doi: 10.1148/radiol.2212001606. [DOI] [PubMed] [Google Scholar]
- 10.Mittal S, Wu Z, Neelavalli J, Haacke E. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. American Journal of Neuroradiology. 2009;30(2):232–52. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira-Filho J, Koroshetz W. Neuroimaging of acute ischemic stroke. 2013 UpToDate. [Google Scholar]
- 12.Liebeskind D. Location, location, location: angiography discerns early MR imaging vessel signs due to proximal arterial occlusion and distal collateral flow. American Journal of Neuroradiology. 2005;26(9):2432–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Lee T, Hsu H, Tsang Y. Multi-Slice CT angiography in diagnosing total versus near occlusions of the internal carotid artery: comparison with catheter angiography. Stroke. 2004;35(1):83–5. doi: 10.1161/01.STR.0000106139.38566.B2. [DOI] [PubMed] [Google Scholar]
- 14.Demchuk A, Burgin W, Christou I. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke. 2001;32:89–93. doi: 10.1161/01.str.32.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Riedel C, Jensen U, Rohr A, Tietke M. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced Computed Tomography reconstructions. Stroke. 2010;41(8):1659–64. doi: 10.1161/STROKEAHA.110.580662. [DOI] [PubMed] [Google Scholar]
- 16.Riedel C, Zimmermann P, Jensen-kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42(6):1775–7. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 17.Tan I, Demchuk A, Hopyan J, Zhang L. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. American Journal of Neuroradiology. 2009;30(3):525–31. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frölich A, Schrader D, Klotz E, Schramm R. 4D CT Angiography More Closely Defines Intracranial Thrombus Burden Than Single-Phase CT Angiography. American Journal of Neuroradiology. 2013 doi: 10.3174/ajnr.A3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legrand L, Naggara O, Turc G, Mellerio C, Roca P, Calvet D, et al. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke. 2013;44(7):1878–84. doi: 10.1161/STROKEAHA.113.001026. [DOI] [PubMed] [Google Scholar]
- 20.Riedel C, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke. 2012;43(9):2319–23. doi: 10.1161/STROKEAHA.112.649921. [DOI] [PubMed] [Google Scholar]
- 21.Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10(6):419–23. doi: 10.1159/000016101. [DOI] [PubMed] [Google Scholar]
- 22.González R, Hirsch J, Koroshetz W. Acute Ischemic Stroke, Imaging and Intervention. Springerverlag; Berlin Heidelbert: 2006. [Google Scholar]
- 23.Makkat S, Vandevenne J, Verswijvel G. Signs of acute stroke seen on fluid-attenuated inversion recovery MR imaging. AJR. 2002;179(1):237–43. doi: 10.2214/ajr.179.1.1790237. [DOI] [PubMed] [Google Scholar]
- 24.Rovira A, Orellana P, Alvarez-sabín J. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004;232(2):466–73. doi: 10.1148/radiol.2322030273. [DOI] [PubMed] [Google Scholar]
- 25.Kidwell C, Chalela J, Saver J, Starkman S, Hill M. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi A, Chaudhry Z, Yoo A, Kamalian S. Degree of Clot-related susceptibility artifact is an independent risk factor for parenchymal hematoma following mechanical thrombectomy. Stroke. 2012;43(74) [Google Scholar]
- 27.Liebeskind D, Sanossian N, Yong W. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42(5):1237–43. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal P, Kalia V, Dua S. Pictorial essay: Susceptibility-weighted imaging in cerebral ischemia. Indian Journal of Radiological Imaging. 2010;20(4):250–3. doi: 10.4103/0971-3026.73530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hingwala D, Kesavadas C, Thomas B, Kapilamoorthy T. Clinical utility of susceptibility-weighted imaging in vascular diseases of the brain. Neurol India. 2010;58(4):602–7. doi: 10.4103/0028-3886.68667. [DOI] [PubMed] [Google Scholar]
- 30.Adamczyk P, Saver J, Hao Q, Sanossian N. Facing the clot: Angiographic appearance predicts recanalization and clinical outcome in acute ischemic stroke. Stroke. 2011;42(e235) [Google Scholar]
- 31.Adamczyk P, Saver JL, Hao Q, Sanossian N, Fiaz R, Ali LK, et al. Facing the Clot: Angiographic Appearance Predicts Recanalization and Clinical Outcome in Acute Ischemic Stroke. Stroke. 2011;42(3):e325. [Google Scholar]
- 32.Saqqur M, Uchino K, Demchuk A. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38(3):948–54. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 33.Demchuk A, Christou I, Wein T. Specific Transcranial Doppler Flow Findings Related to the Presence and Site of Arterial Occlusion. Stroke. 2000;31(1):140–6. doi: 10.1161/01.str.31.1.140. [DOI] [PubMed] [Google Scholar]
- 34.Lindegaard K, Bakke S, Aaslid R, Nornes H. Doppler diagnosis of Intracranial artery occlusive disorders. Journal of Neurology Neurosurgery Psychiatry. 1986;49(5):510–8. doi: 10.1136/jnnp.49.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexandrov A, Wojner A, Grotta J. Clotbust: Design of a randomized trial of ultrasound-enhanced thrombolysis for acute ischemic stroke. J Neuroimaging. 2004;14:108–12. [PubMed] [Google Scholar]
- 36.Jauch E, Saver J, Adams H, Bruno A. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 37.Barber P, Demchuk A, Zhang J, Buchan A. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Aspects study group. Alberta stroke programme early ct score. Lancet. 2000;355:1670–4. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto M, Salamon N, Mayor F. Characterization of arterial thrombus composition by magnetic resonance imaging in a Swine stroke model. Stroke. 2013;44(5):1463–5. doi: 10.1161/STROKEAHA.111.000457. [DOI] [PubMed] [Google Scholar]
- 39.Molina C. Imaging the clot: does clot appearance predict the efficacy of thrombolysis? Stroke. 2005;36(11):2333–4. doi: 10.1161/01.STR.0000185933.44619.1b. [DOI] [PubMed] [Google Scholar]
- 40.Minnerup J, Kleinschnitz C. Visualization of clot composition in ischemic stroke: do we get what we see? Stroke. 2011;42(5):1193–4. doi: 10.1161/STROKEAHA.110.612150. [DOI] [PubMed] [Google Scholar]
- 41.Marder V, Chute D, Starkman S. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37(8):2086–93. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 42.Legrand L, Naggara O, Turc G. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke. 2013;44(7):1878–84. doi: 10.1161/STROKEAHA.113.001026. [DOI] [PubMed] [Google Scholar]
- 43.Nogueira R, Schwamm L, Hirsch J. Endovascular approaches to acute stroke, part 1: Drugs, devices, and data. American Journal of Neuroradiology. 2009;30(4):649–61. doi: 10.3174/ajnr.A1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig J, Pedraza S, Demchuk A. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. American Journal of Neuroradiology. 2012;33(1):90–6. doi: 10.3174/ajnr.A2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moftakhar P, English J, Cooke D. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44(1):243–5. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 46.Froehler M, Tateshima S, Duckwiler G, Jahan R. The hyperdense vessel sign on ct predicts successful recanalization with the merci device in acute ischemic stroke. Journal of Neurointerventional Surgery. 2013;5:289–93. doi: 10.1136/neurintsurg-2012-010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho K, Kim J, Kwon S, Cho A, DW K. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36(11):2379–83. doi: 10.1161/01.STR.0000185932.73486.7a. [DOI] [PubMed] [Google Scholar]
- 48.Hohenhaus M, Schmidt W, Brunecker P. FLAIR vascular hyperintensities in acute ICA and MCA infarction: a marker for mismatch and stroke severity? Cerebrovascular Disease. 2012;34(1):63–9. doi: 10.1159/000339012. [DOI] [PubMed] [Google Scholar]
- 49.Sanossian N, Hao Q, Liebeskind D. The thrombus and discontinuity of FLAIR vascular hyperintensity. Archives of Neurology. 2011;68(7):950–1. doi: 10.1001/archneurol.2011.143. [DOI] [PubMed] [Google Scholar]
- 50.Azizyan A, Sanossian N, Mogensen M, Liebeskind D. Fluid-attenuated inversion recovery vascular hyperintensities: an important imaging marker for cerebrovascular disease. American Journal of Neuroradiology. 2011;32(10):1771–5. doi: 10.3174/ajnr.A2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marder V, Chute D, Starkman S, Abolian A. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–93. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 52.Liebeskind D, Sanossian N, Yong W, Starkman S. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–43. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]


