Abstract
Zinc (Zn2+) is one of the most important trace metals in the body. It is necessary for the normal function of a large number of proteins including enzymes and transcription factors. While extracellular fluid may contain up to micromolar Zn2+, intracellular Zn2+ concentration is generally maintained at a subnanomolar level; this steep gradient across the cell membrane is primarily attributable to Zn2+ extrusion by Zn2+ transporting systems. Interestingly, systematic investigation has revealed that activities, previously believed to be dependent on calcium (Ca2+), may be partially mediated by Zn2+. This is also supported by new findings that some Ca2+-permeable channels such as voltage-dependent calcium channels (VDCCs), N-methyl-D-aspartate receptors (NMDA), and amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors (AMPA-Rs) are also permeable to Zn2+. Thus, the importance of Zn2+ in physiological and pathophysiological processes is now more widely appreciated. In this review, we describe Zn2+-permeable membrane molecules, especially Zn2+-permeable ion channels, in intracellular Zn2+dynamics and Zn2+ mediated physiology/pathophysiology.
Keywords: calcium, fluorescence imaging, ion channel, zinc
Introduction
Following iron (Fe2+), Zn2+ is the second most abundant trace metal in human and animals. Like other metals, Zn2+ is involved in various cellular processes contributing to normal physiology. Enzymes that mediate important cellular processes, for which Zn2+ is required, number more than 200 [1-4]. It is speculated that approximately 10% of proteins utilize Zn2+ to maintain their structure and/or function [5, 6]. Therefore, either a deficiency or excess of Zn2+ is expected to be detrimental. In fact, 23% of the world's population has been estimated to be Zn2+ deficient; this deficiency may cause illness such as pneumonia and diarrhea [7, 8]. Indispensable in maintaining membrane potential, especially in excitable cells, energy-dependent Na+/K+-pumps generate a 10 to 30-fold gradient for Na+ and K+ ions [9, 10]. In comparison, the intracellular concentration of Zn2+ is only about a thousandth of the extracellular concentration [11-13]. Therefore, effective homeostatic mechanisms must exist for zinc dynamics. Though unique among other ions Zn2+ is similar to Ca2+. Ca2+ is one of the most investigated metal ions because of its diverse functions in physiological/pathophysiological processes, its widespread abundance, and its high concentration gradient across cellular membranes [14-16]. While intracellular Ca2+ dynamics are well established, it is less so for Zn2+. Recent advances in the studies of Zn2+ permeable channels and Zn2+ transporters have increased our understanding of cellular mechanism in regulating intracellular zinc homeostasis. This review will focus on the role of Zn2+-permeable ion channels in intracellular Zn2+ dynamics and cellular physiology/pathophysiology.
Physical and chemical properties of Zn2+
Zn2+ is a very common element distributed in Earth's outer crust and is handily smelted and manufactured due to its low melting point [17, 18]. The International Union of Pure and Applied Chemistry definition states that Zn2+, like cadmium, is regarded as a typical element in which the d-block of electron shell is filled with electrons [19]. Therefore, like cadmium, there is no unpaired electron in the reactive d-block, resulting in less reactivity to redox reaction compared to other divalent metal ions such as iron and cobalt, which are listed in the same period of the periodic table [20]. Considering that the redox reaction is constantly occurring in cells, less reactivity is beneficial so that molecules, such as enzymes, can perform their functions more efficiently. Zn2+ is also utilized for acid-base reactions and as a transition metal it is classified as “borderline” for its polarity [21]. Its polarity limits coordination with nitrogen, oxygen and sulfur, which are regarded as soft bases.
Zn2+ has been known to be involved in a large number of cellular processes as described below and most commonly it plays a role through its interaction with proteins. Among amino acids, the imidazole functional group of histidine, the carbonyl oxygen of aspartate and glutamate, and the thiol functional group of cysteine are prevalent molecules facilitating Zn2+ coordination [5, 22]. With such large variations of coordination, it is of no surprise that Zn2+ has a broad spectrum of functions. The functional role of Zn2+ in proteins can be classified into two main categories: structural and catalytic. Structurally, Zn2+ is normally surrounded by four amino acids preventing solvent interaction. Motifs, such as Zn2+ fingers, facilitate not only the stabilization of peptides, but also structural maintenance of the whole protein by mutual interaction with multiple local contextures [1, 4, 5]. In catalytic sites, Zn2+ is positioned in active center of the catalytic reaction. Solvents such as water occupy one ligation site of the catalytic Zn2+ [5]. The Zn2+ metalloenzymes include the family of nucleases, superoxide dismutases, and carbonic anhydrase II (see below) [4].
Physiologic and pathophysiolologic functions of Zn2+
Zn2+ is an essential factor for growth of higher organisms [23]. Its importance for growth, first established in plants in 1869 [24], has been revealed across many species [23, 25]. In humans, gonadal dysfunction and growth retardation, seen in the Middle East during the 1960's, was found to be mediated by Zn2+deficiency [26]. Meanwhile, various enzymes were found to act in a Zn2+-dependent manner and the pathophysiological symptoms related to the dysfunction of those enzymes have been described [13, 27-30]. A well recognizable pathophysiology associated with Zn2+ dependent enzymes, acrodermatitis enteropathica, is especially egregious [31]. It is characterized by initial skin lesions (e.g. eruption, erosion), subsequent intestinal disorders (e.g. diarrhea) among weaning infants, and growth and mental retardation. Recent reports suggest that high doses of Zn2+ can improve clinical outcomes in both inherited and acquired cases of acrodermatitis enteropathica, as some cases implicated ZIP4, one of the Zn2+ transporters, as an aberrant gene [32, 33]. Abnormal zinc homeostasis has also been shown to be involved in other pathophysiological conditions. Insults that result from traumatic brain injury, brain ischemia and epilepsy, for example, contribute to excessive Zn2+ levels [34-36]. Dysfunctional Zn2+ regulation in the mitochondria, leads to a disruption of cell metabolism [37-39]. The energy-generating mitochondrial oxidation-reduction reaction, on the other hand, requires the presence of intracellular Zn2+; mitochondrial complexes are not functional when Zn2+ is chelated, essentially abating ATP generation [40].
Zn2+ is relatively less toxic compared to other metals and Zn2+ poisoning rarely occur through normal dietary intake. However, absorption of Zn2+ through fumes, in gaseous form, and contamination from Zn2+-coated containers may lead to abnormally high concentrations of Zn2+ within different organ systems, which may result in disorders of nervous system, intestinal and renal systems [41]. Presently, over-the-counter oral supplementation of vitamins and minerals are common. However, excessive supplementation or iatrogenic errors may cause Zn2+ poisoning [42, 43].
Intense research of Zn2+ has been conducted in the nervous system although Zn2+ is important in other organ systems as well. One reason for systematic study of the nervous system is that neurologic symptoms are often produced by Zn2+-related disorders [26, 41, 44]. Different from other organ systems, the nervous system is surrounded by cerebrospinal fluid in which the Zn2+ concentration is much lower than the surrounding interstitial fluid [45-47]. Interestingly, neurons can release Zn2+ from their synaptic terminals [48, 49]. Low physiologic concentrations and steep gradients of Zn2+ surrounding cells may indicate a tight control mechanism of which Zn2+ plays important regulatory role in neuronal functions.
Intracellular Zn2+ balance and biochemistry
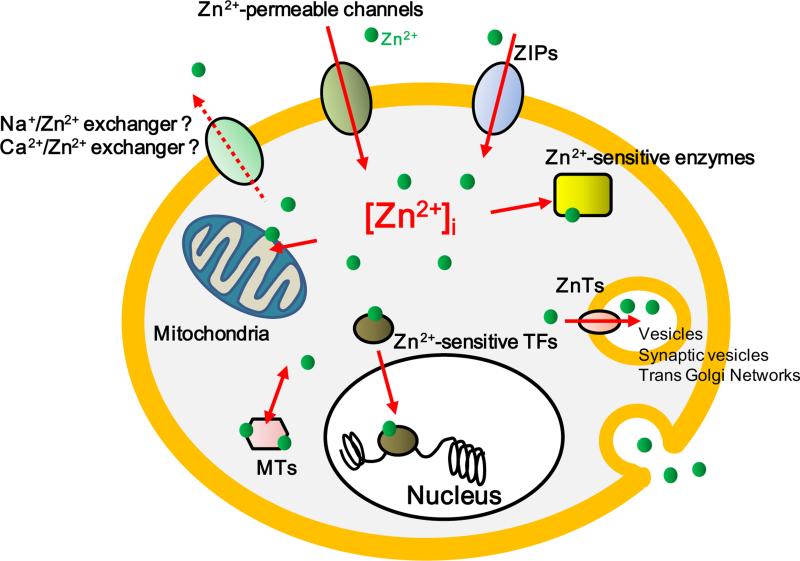
Over 90% of Zn2+ is thought to be bound tightly to intracellular molecules; the remaining intracellular Zn2+ is labile [1]. Homeostasis of intracellular Zn2+ dynamics generally include the following processes: (1) Zn2+ incorporation into intracellular organelles and molecules such as mitochondria and metallothioneins (MTs) [50]; (2) Zn2+ extrusion into extracellular space or secretion by vesicles [2, 36]; and (3) Zn2+ is transported by Zn2+ transporters and Zn2+ -permeable ion channels (Figure 1). The existence of Na+/Zn2+ and Ca2+/Zn2+ exchangers are suggested, but have not been identified at the molecular level [51, 52]. Although recent works have uncovered the importance of extracellular Zn2+, for example, in regulating Zn2+ -potentiated ectoenzymes [53-55], in Zn2+ -sensing receptors, and in Zn2+ -activated channels [56-58], intracellular Zn2+ is still considered to play a leading part in cellular functions.
Figure 1.
Schematic illustration of the molecular mechanisms involved in intracellular Zn2+ homeostasis. Over 90% of intracellular Zn2+ binds to molecules. The remaining loosely bound Zn2+ affects the equilibrium state and zinc signaling. A fraction of the variable free Zn2+ is brought through Zn2+-permeable ion channels. Excessive accumulation of labile Zn2+ disrupts cellular Zn2+ homeostasis, resulting in cellular dysfunction. Hypothetical exchangers are depicted with a dashed arrow.
MTs are small molecules, which have about 70 amino acids; many of them contain about twenty cysteine residues that can trap up to seven Zn2+ ions through metal-thiolate bonds [59, 60]. The binding or release of Zn2+ ions is regulated by redox status and intracellular pH. Besides MTs, Zn2+ is sequestered within the cytoplasm by enzymes and transcription factors. Another main system to maintain intracellular Zn2+ levels is the elimination of cytoplasmic Zn2+ constituents. In this regard, a number of molecules for Zn2+ elimination have been recently cloned [61-63]. Zrt- and Irt- like proteins (ZIPs), which mainly transport Zn2+ into cells, and the family of Zn2+ transporters (ZnTs), which transport Zn2+ out of cytoplasm, seem to work independently within different tissues, and have varied intracellular distribution and transport activity. Some reports have shown that these molecules are involved in human diseases. Although important in Zn2+ homeostasis, these transporters are beyond the scope of this review. Readers may obtain valuable information on the subject from other reviews [63-66].
Zn2+ is essential for many enzymes which are fundamental to normal cell function. One noteworthy enzyme, carbonic anhydrase II, which catalyzes the reversible hydration of carbonic dioxide, was first to be found as dependent on Zn2+ for its activity [4, 67, 68]. These reports were the first to demonstrate important physiological functions of Zn2+. Interestingly, one newly uncovered pathophysiological aspect of Zn2+ is that it may act on amyloid precursor protein (APP) and play a role in Alzheimer's disease [69]. APP excretes irons from cytoplasm as iron transporter and also works as ferroxidase, which oxidizes Fe2+ to Fe3+. It interacts with ferroportin, which binds to oxidized iron, to facilitate iron transport. Extracellular Zn2+, which is involved in aggregated β-amiloid, is suggested to inhibit the ferroxidase activity, resulting in intracellular iron retention [69].
Zn2+ -permeable channels
Zn2+ influx across the cellular membrane, through ion channels, is a key factor for the increase of intracellular Zn2+ concentration and activation of zinc signaling pathways. The activities of various channels are, in turn, modified by Zn2+. For example, NMDA and γ-aminobutric acid – type A (GABAA) receptor-gated channels are sensitive to Zn2+ inhibition, which results in reduced current flux [70-72]. While the activities of many ion channels are modified by Zn2+, some cation-permeable channels are reported to be Zn2+ permeable. For example, recent studies have demonstrated that voltage-gated Ca2+ channels and Ca2+-permeable ionotropic glutamate receptors conduct Zn2+ (see section Voltage-dependent Ca2+ channels). It has also been established that Zn2+ flux plays an important role in physiological and pathophysiological processes such as long term potentiation and ischemic stress [62, 73, 74]. Here, we provide an overview of Zn2+ -conducting ion channels and their potential functions in physiological/pathophysiological processes. The Zn2+ -permeable channels are summarized in Table 1.
Table 1.
Zinc-permeable channels in vertebrates
| Channels/receptors | Cells/organs/tissues | Endogenous function & significance | References |
|---|---|---|---|
| VDCCs | muscle, neurons, mast cells, pancreatic b cells | Zn2+ toxicity in neurons is enhanced by high K+. Zinc wave mediated by L-type calcium channel affects expression of IL-6 and TNFα genes. | [78, 80-82, 88] |
| NMDA-Rs | neurons | Zn2+ toxicity is enhanced by NMDA. Field excitatory postsynaptic potentials might be enhanced by Zn2+ entry through NMDA-Rs. | [94, 97] |
| AMPA-Rs | neurons | Zn2+ toxicity is enhanced byAMPA. | [34, 82, 84] |
| nAch-Rs | muscle endplate, chromaffin cells | N/A | [108, 110] |
| TrpM6/7 | Ubiquitous (TrpM7) Kidney (TrpM6) |
Zn2+ toxicity in neurons is inhibited by silencing TrpM7 in neurons. | [116, 118, 120, 121, 123, 126] |
| TrpM3 | Pancreatic b cells | Zn2+ is possibly taken up for subsequent exocytosis. | [134] |
| TrpA1 | DRG neurons | Zn2+ causes nociception through TRPA1 | [142] |
| TrpV6 | Intestine, kidney, placenta | N/A | [110] |
| TrpC6 | Ubiquitous | N/A | [157] |
| TRPML1 | Ubiquitous | TRPML1 knockout results in abnormal lysosomal Zn2+ homeostasis. | [166, 167] |
Voltage-dependent Ca2+ channels (VDCCs)
VDCCs are expressed mainly in excitable cells such as muscle and neuronal cells and contribute to cellular Ca2+ homeostasis [75-77]. They are inactive at resting membrane potentials but are activated when membrane potentials are depolarized, resulting in Ca2+ entry. Intracellular Ca2+ increase elicits contraction in muscles and activation of signaling pathways in neurons [77]. VDCCs were initially considered to be the main entry gate for Zn2+ from the extracellular space into cytoplasm [78, 79].
Zn2+ influx into cells was first described in invertebrates 35 years ago [80]. Shortly after that, VDCCs were found to be a route for Zn2+ entry as demonstrated by the inhibition of Zn2+ influx with VDCC blockers, such as verapamil [81]. Like invertebrates, VDCCs are also permeable to Zn2+ in mammalian neurons, cardiac myocytes, pancreatic β cells, and chromaffin cells [78, 81-85]. Variations in VDCC configuration and subunit composition likely affect the Zn2+ permeablity of these channels. Inhibition of Zn2+ influx by ω-conotoxin and nimodipine [82, 83, 86, 87], which block different types of VDCCs, suggests that the extent of Zn2+ permeability may be determined by the presence of specific subunits. Although VDCCs conduct Zn2+, interestingly, they are also known to be blocked by Zn2+ [75-79]. For example, it has been demonstrated that Zn2+ blocks Ca2+ entry through the same channels [87]. In addition, extracellular acidic pH enhances Zn2+ entry through VDCCs and modifies the ratio of Zn2+/Ca2+ flux [87], which may suggest that an increased Zn2+ influx contributes to neurotoxicity in conditions such as brain ischemia and lactic acidosis. Interestingly, recent reports have indicated that VDCCs located on intracellular endplasmic reticulum also contribute to zinc signaling and the downstream gene expression in mast cells [88].
Glutamate receptors
Glutamate is the major excitatory neurotransmitter in the central nervous system and is released into the synaptic cleft when stimulated by an action potential at the synaptic terminus. The binding of glutamate to its receptor on the postsynaptic membrane activates the receptor-gated cation channels. Glutamate receptors are divided into two major groups: ionotropic receptors, coupled to ion channels, and metabotropic receptors, coupled to G-protein mediated signaling pathways (mGluRs) [89-93]. The ionotropic receptors include N-methyl-D-aspartate receptors (NMDA-Rs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA-Rs), and kainate receptors. When these receptors are activated by glutamate, influx of cations, such as Na+ and Ca2+, is facilitated. Activation of glutamate receptor-gated channels is important for physiological functions such as neurotransmission. Zn2+ entry through NMDA-Rs has been shown to enhance field excitatory postsynaptic potentials [94]. Over activation of these channels are also known to contribute to neurotoxicity under a varitey of pathophysiological contions such as stroke and epilepsy [95].
NMDA-Rs were first reported to be blocked by Zn2+ [71, 72, 96]. Later, they were found to be Zn2+ -permeable although the permeablilty to Zn2+ is much lower than Ca2+ [97]. Physiological and pathophysiological events mediated by Zn2+ via NMDA-Rs seem to be dependent more on Zn2+ modulation of the channel rather than Zn2+ conductance [98, 99].
Early studies reported that Zn2+ can modulate the activity of AMPA-Rs by an unknown mechanism [71]. Weiss and colleagues later found that Zn2+ permeates AMPA-Rs, producing toxic effects [82]. They also demonstrated a clear relationship between Zn2+ toxicity and the activation of AMPA-Rs, which plays a role in ischemic neuronal injury [34]. Additional studies suggests that Zn2+ entry mediated by AMPA-Rs contributes to neurotoxicity more than VDCCs and NMDA-Rs [84, 100]. Interestingly, in neocortex, interneurons are more sensitive to AMPA-Rs mediated Zn2+ toxicity than other neurons [101]. Furthermore, Zn2+ inhibits gene expression of GluR2, a subunit of AMPA-Rs that regulates Ca2+ permeability; this inhibibition results in Ca2+ and Zn2+ toxicity [102, 103]. Unlike VDCCs, Ca2+ entry through AMPA-Rs are not influenced by Zn2+ [104].
Nicotinic acethylcoline receptors (nAch-Rs)
There are two types of acetylcholine receptors (Ach-R): ionotropic and metabotropic ones. Ionotropic Ach-Rs are also called nicotinic receptors (nAch-Rs). They are expressed mainly in muscle endoplate, at neuro-muscular junctions and throughout the nervous system in vertebrates [105, 106]. Neuronal nAch-Rs have a large variety of subunit components depending on their anatomical distribution. Their major role is to modulate neuronal function. In contrast, muscular nAch-Rs play an exlusive role in neuro-muscular transmission. Although neuronal nAch-Rs are in general highly Ca2+-permeable, muscular ones are less permeable to Ca2+ [107]. Therefore, secondary activation of VDCCs follwing nAch-Rs-mediated depolarization is required for Ca2+ accumulation and muscle contraction. Regardless of its relatively low Ca2+ permeability, Zn2+ conductance has been reported for nAch-Rs found on endplates in muscles [108] and in adrenal chromaffin cells [109]. Similar to native nAch-Rs, Zn2+ permeability was demonstrated in cloned nAch-Rs [110]. It has been shown that γ- and ε-subuits are Zn2+ -permeable whereas other subunits are not [110]. The physiological and/or pathophysiolological sigificance of Zn2+ permeability in nAch-Rs, however, remains to be elucidated.
Transient Receptor Potential (Trp) channels
First member of Trp channels was discovered in D. melanogaster, where a genetic mutation of the gene induced an abnormal photosensitivity [111, 112]. The Trp superfamily of ion channels now includes nearly thirty genes [113], which are divided into 6 subfamilies [113, 114]. Sequcence homology is conserved between flies and higher organisms and members of the Trp family are cation-permeable. Trp channels have widespread distribution and play important roles in various physiological and pathophysiological conditions. For example, local thermal sensation is largely attributable to certain Trp channels and genetic mutations of some Trp channels elicit a variety of diseases such as epilepsy, leukemia and hypertension [113, 115]. Recent studies of cation permeation using electrophysiology or fluorescent Zn2+ imaging have demonstrated that several members of the Trp family are Zn2+ permeable.
TrpM6/7
TrpM7 was the first among Trp channels found to be Zn2+ permeable [116]. TrpM6/7 are unique among channels because they also possess a kinase domain [117, 118]. TrpM7 plays a key role in intracellular Mg2+ homeostasis and in neuronal Ca2+ toxicity in ischemic conditions [118-122]. Monteilh-Zoller et al. demonstrated that the permeability of TrpM7 channels for Zn2+ ions is four-fold higher compared to Ca2+ [116]. TrpM7-overexpressing cells showed intracellular Zn2+ accumulation as indicated by increased fluorescence of FluoZin-3, a recently developed Zn2+ fluorescent indicator [123]. This Zn2+ accumulation contributes to, at least in part, functional gene expression in neuronal cells through the activation of metal-responsive element, which is important for monitoring intracellular metal levels [123-125]. Thus, not only because of abnormal cellular function (see above), but also because of changes in various gene expression, Zn2+ overload through TrpM7 will facilitate cytotoxicity [123, 126]. TrpM6, a homolog of TrpM7, also has a kinase domain. It has been implicated in Mg2+ homeostasis in the intestines and kidneys [120, 127]. Similar to TrpM7, TrpM6 has also been shown to be Zn2+ permeable [128].
TrpM3
TrpM3, a member of TRP channels highly expressed in brain and in pancreatic β cells [129, 130], is important for insulin release [131]. Zn2+ has been found in the vesicles of these cells, which is co-released with insulin and contributes to insulin function [131, 132]. While ZnT8 was speculated to facilitate Zn2+ uptake from cytoplasm into vesicles [133], Zn2+ permeability has recently been shown in one of the TrpM3 variants in native pancreatic β cells [134]. TrpM3 appears to contribute to Zn2+ permeation comparable to VDCCs [134]. Since activation of TrpM3 produces depolarization of β cells, which can lead to additional Zn2+ influx through VDCCs, further segregation of functional significance of Zn2+ entry between TrpM3 and VDCCs may be explored in the near future.
TrpA1
TrpA1 channels are highly expressed in dorsal root ganglia neurons [135, 136]. TrpA1 is stimulated by irritants such as mustard oil and noxious cold [135-139]. They are also expressed in the respiratory tract and play a role in hypersensitivity and inflammatory reactions associated with components of tobacco that activate the TrpA1 channels [140, 141]. Hu and colleagues demonstrated that TrpA1 channels act as a feedforward system which utilizes Zn2+ to further activate the channels [142]. TrpA1 deficient mice seem to feel less pain by Zn2+ injection whereas Zn2+ injection leads to apparent painful behavior in wild-type mice. This finding suggests that TrpA1 channels expressed in peripheral nerves are associated with pain sensation. Further studies showed that Asp 915 plays an important role in Zn2+ entry through TrpA1 while C641 and Cys 1021 are involved in the sensitivity of TrpA1 channels to intracellular Zn2+ activation [142].
TrpV6
TrpV6 is highly expressed in intestines, kidney and placenta [143-145]. Although TrpV6 is thought to contribute to Ca2+ absorption through its high Ca2+ conductance, the in vivo function of this channel still remains largely unknown. Some reports indicate that significantly higher TrpV6 expression is found on prostate cancer cells and its expression is associated with cancer progression [146-148]. Zn2+ also permeates TrpV6 channel [149], at least in TrpV6-overexpressing cells. Of note, TrpV5/6 in fishes are also known to transport Zn2+ [150, 151] and showed a transient decrease of gene expression during Zn2+ excess [152], suggesting that permeability of Zn2+ might play a role in all vertebrates.
TrpC6
TrpC6 channels are expressed ubiquitously [153, 154]. It is known that Ca2+ is much more permeable than Na+ through these channels and that intra- and extracellular Ca2+ potentiates and inhibits the channel activity, respectively [154]. Because of its broad distribution, TrpC6 channels are suggested to play a significant role in various organ systems including kidney and brain [155, 156]. The Zn2+ permeability of these channels has recently been demonstrated in neurons and in transfected HEK293 cells [157]. Interestingly, TrpC6 has been suggested to be associated with Zn2+ accumulation in the nucleus. Zn2+ may also regulate TrpC6 channel activity by modifying the effect of Ca2+. Although the physiological/pathophysiological significance of Zn2+ permeability for TrpC6 is still unclear, a recent report indicates that its overexpression facilitates Zn2+ accumulation in endo/lysosomes and upregulation of MTs [158]. However, considering that inhibition of TrpC6 degradation ameliorates ischemic brain injury [156], Zn2+ permeation through this channel does not seem to exacerbate or produce neurotoxicity.
TRPML1
TRPML1, also known as mucolipin-1, is localized to endosomes and lysosomes [159, 160]. Mutation of TRPML1 gene is associated with mucolipidosis, which is a rare autosomal recessive neurodegenerative disorder [161-163]. The clinical symptoms are severe with growth and psychomotor impairment in early stage of development (mostly 0 – 3 years). Most patients can not walk and speak over their lifetime, while half of the patients also have aberrant iron metabolism and anemia [164, 165]. Dong et al. discovered that TRPML1 is an iron-permeable channel and that changes in cellular iron level may play an important role in hereditary mucolipidosis [166]. They also found that TRPML1 conducts Zn2+ [166]. Recent findings have shown that knockdown of TRPML1 leads to abnormal cellular zinc localization and accumulation, for example, TRPML1 knockout mice have a significantly increased level of Zn2+ in brain tissues [167, 168]. These findings suggest that abnormal zinc homeostasis derived from TRPML1 mutation may contribute to neurological dysfunction.
Prospective
The activities of a number of channels are modulated by Zn2+ [169-171]. Addition of Zn2+ may enhance or inhibit the channel activity, resulting in increased or decreased current amplitude. For some channels, changes in current amplitude maybe mediated by Zn2+ permeation. As mentioned above, amino acids such as histidine, cysteine, glutamate and aspartate are likely to be preferable for Zn2+ coordination of catalytic activities and protein architecture [5, 22]. Amino acid substitution may therefore change the activity and function of channels that are modulated by Zn2+ [172-178]. However, one may also consider the possibility that certain amino acids are required for Zn2+ permeation and an examination of existing channels may facilitate the discovery of other Zn2+ -permeable channels. For instance, Zn2+ permeability might be revealed in family members of Trp channels, most of which are found to permeate various divalent cations [113, 114].
Recent advancement in fluorescent Zn2+ indicators has made the investigation of intracellular Zn2+ dynamics much easier in comparison to previous probes. Historically, fluorescent Zn2+ indicators such as zinquin have been used for many decades [83, 179-184]. However, zinquin-like indicators are limited in specificity or have low affinity for Zn2+. Some of them have high affinity for Ca2+ [51, 83, 185], or are influenced by Ca2+ [186-188]. In contrast, FluoZin-3, which was utilized to study Zn2+ permeation in TrpA1 and TrpM3, has a higher affinity (Kd = 3 ~ 15 nM) for Zn2+, which is close to the intracellular Zn2+ concentration in resting conditions [11, 189, 190]. Of special note, FluoZin-3 is insensitive to Ca2+, which has a significant advantage over previous indicators that cannot differentiate between Zn2+ and Ca2+ signals. With the emergence of new indicators, examinations of Zn2+ and Ca2+, simultaneously, using different fluorescent indicators have been reported [191, 192]. These advancements are likely to provide a better understanding of Zn2+ regulation, conductance and functional importance.
Acknowledgements
This work was supported by NIH R01NS66027, NIMHD S21-MD000101, U54NS083932, AHA 0840132N, and ALZ IIRG-10-173350.
Footnotes
Conflict of interest
The authors confirm that there is no conflict of interest.
References
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 3.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 4.McCall KA, Huang C, Fierke CA. Function and mechanism of zinc metalloenzymes. J Nutr. 2000;130:1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 5.Patel K, Kumar A, Durani S. Analysis of the structural consensus of the zinc coordination centers of metalloprotein structures. Biochim Biophys Acta. 2007;1774:1247–1253. doi: 10.1016/j.bbapap.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 7.Sandstead HH. Zinc deficiency. A public health problem? Am J Dis Child. 1991;145:853–859. doi: 10.1001/archpedi.1991.02160080029016. [DOI] [PubMed] [Google Scholar]
- 8.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 9.Skouby AP. Vascular lesions in diabetics with a special reference to the influence of treatment. Acta Med Scand Suppl. 1956;317:1–46. [PubMed] [Google Scholar]
- 10.Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJ, Lamb JF, Martin-Vasallo P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep. 2000;20:51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- 11.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 12.Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Physiol Cell Physiol. 2008;294:C726–742. doi: 10.1152/ajpcell.00541.2007. [DOI] [PubMed] [Google Scholar]
- 13.Haase H, Rink L. The physiological role of zinc ions in mammalian signal transduction. Nova Science Publishers, Inc.; 2007. [Google Scholar]
- 14.Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood L. Human physiology: from cells to systems. 7th ed. CENGAGE Learning; Belmont, CA, USA: 2010. [Google Scholar]
- 17.Fleischer M. Natural sources and distribution of zinc. In: Henkin RI, editor. Zinc. Univ Park Press; Baltimore, MA, USA: 1979. pp. 19–29. [Google Scholar]
- 18.Smith WF. Principels of material science and engineering. 2nd ed. Mc Graw-Hill Companies; Mishawaka, IN, USA: 1990. [Google Scholar]
- 19.B. JW. The place of zinc, cadmium, and mercury in the periodic table. J Chem Educ. 2003;80:952–961. [Google Scholar]
- 20.Butler A. Acquisition and utilization of transition metal ions by marine organisms. Science. 1998;281:207–209. doi: 10.1126/science.281.5374.207. [DOI] [PubMed] [Google Scholar]
- 21.Pearson RG. Hard and soft acids and bases. J Am Chem Soc. 1963;85:3533–3539. [Google Scholar]
- 22.Gregory DS, Martin AC, Cheetham JC, Rees AR. The prediction and characterization of metal binding sites in proteins. Protein Eng. 1993;6:29–35. doi: 10.1093/protein/6.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Todd WR, Elvehjem CA, Hart EB. Zinc in the nutrition of the rat. Am J Physiol. 1933;107:146–156. [Google Scholar]
- 24.Raurlin J. Etudes cliniques sur lar vegetation. Ann Sci Nat Bot Biol Veg. 1869;11:93. [Google Scholar]
- 25.Sandstead HH. Zinc and human nutrition. In: Bronner F, Coburn JW, editors. Disorders of mineral and metabolism: Trace minerals Deep River. Jonathan Grobe Books; IA, USA: 1981. pp. 93–157. [Google Scholar]
- 26.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532–546. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 27.Cunnane SC. Zinc: Clinincal and Biochemical Significance. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- 28.Hamidge KM, Casey CE, Krebs NF. Trace Elements in Human and Animal Nutrition. 5 edition ed. Academic Press; Orlando, FL: 1988. [Google Scholar]
- 29.Heyneman CA. Zinc deficiency and taste disorders. Annals of Pharmacotherapy. 1996;30:186–187. doi: 10.1177/106002809603000215. [DOI] [PubMed] [Google Scholar]
- 30.Apgar J. Zinc and reproduction. Annu Rev Nutr. 1985;5:43–68. doi: 10.1146/annurev.nu.05.070185.000355. [DOI] [PubMed] [Google Scholar]
- 31.Moynanan EJ. Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet. 1974;17:399–400. doi: 10.1016/s0140-6736(74)91772-3. [DOI] [PubMed] [Google Scholar]
- 32.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 35.Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- 36.Capasso M, Jeng JM, Malavolta M, Mocchegiani E, Sensi SL. Zinc dyshomeostasis: a key modulator of neuronal injury. J Alzheimers Dis. 2005;8:93–108. doi: 10.3233/jad-2005-8202. [DOI] [PubMed] [Google Scholar]
- 37.Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 38.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 39.Shahbaz AU, Zhao T, Zhao W, Johnson PL, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Calcium and zinc dyshomeostasis during isoproterenol-induced acute stressor state. Am J Physiol Heart Circ Physiol. 2011;300:H636–H644. doi: 10.1152/ajpheart.00900.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Kim YJ, Ra H, Kang SJ, Han S, Koh JY, Kim YH. The involvement of caspase-11 in TPEN-induced apoptosis. FEBS Lett. 2008;582:1871–1876. doi: 10.1016/j.febslet.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 41.Cooper RG. Zinc toxicology following particulate inhalation. Indian J Occup Environ Med. 2008;12:10–13. doi: 10.4103/0019-5278.40809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salzman MB, Smith EM, Koo C. Excessive oral zinc supplementation. J Pediatr Hematol Oncol. 2002;24:582–584. doi: 10.1097/00043426-200210000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Grissinger M. A fatal zinc overdose in a neonate: confusion of micrograms with milligrams. PT. 2011;36:393–407. [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad AS, Miale A, Jr., Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963;61:537–549. [PubMed] [Google Scholar]
- 45.Palm R, Sjostrom R, Hallmans G. Optimized atomic absorption spectrophotometry of zinc in cerebrospinal fluid. Clin Chem. 1983;29:486–491. [PubMed] [Google Scholar]
- 46.Molina JA, Jimenez-Jimenez FJ, Aguilar MV, Meseguer I, Mateos-Vega CJ, Gonzalez-Munoz MJ, de Bustos F, Porta J, Orti-Pareja M, Zurdo M, Barrios E, Martinez-Para MC. Cerebrospinal fluid levels of transition metals in patients with Alzheimer's disease. J Neural Transm. 1998;105:479–488. doi: 10.1007/s007020050071. [DOI] [PubMed] [Google Scholar]
- 47.Bodgen JD, Troiano RA, Joselow MM. Copper, zinc magnesium, and calcium in plasma and cerebrospinal fluid of patients with neurological diseases. Clin Chem. 1977;23:485–489. [PubMed] [Google Scholar]
- 48.Perez-Clausell J, Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985;337:91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- 49.Slomianka L, Geneser FA. Postnatal development of zinc-containing cells and neuropil in the hippocampal region of the mouse. Hippocampus. 1997;7:321–340. doi: 10.1002/(SICI)1098-1063(1997)7:3<321::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Pierrel F, Cobine PA, Winge DR. Metal Ion availability in mitochondria. Biometals. 2007;20:675–682. doi: 10.1007/s10534-006-9052-9. [DOI] [PubMed] [Google Scholar]
- 51.Ohana E, Segal D, Palty R, Dien TT, Moran A, Sensi SL, Weiss JH, Hershfinkel M, Sekler I. A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J Biol Chem. 2004;279:4278–4284. doi: 10.1074/jbc.M309229200. [DOI] [PubMed] [Google Scholar]
- 52.Simons TJ. Calcium-dependent zinc efflux in human red blood cells. J Membr Biol. 1991;123:73–82. doi: 10.1007/BF01993965. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Cooper MD. Histidine residue in the zinc-binding motif of aminopeptidase A is critical for enzymatic activity. Proc Natl Acad Sci U S A. 1993;90:1222–1226. doi: 10.1073/pnas.90.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schauder B, Schomburg L, Kohrle J, Bauer K. Cloning of a Cdna-Encoding an Ectoenzyme That Degrades Thyrotropin-Releasing-Hormone. Proc Natl Acad Sci U S A. 1994;91:9534–9538. doi: 10.1073/pnas.91.20.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zielinska W, Barata H, Chini EN. Metabolism of cyclic ADP-ribose: Zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal fluid. Life Sci. 2004;74:1781–1790. doi: 10.1016/j.lfs.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Davies PA, Wang W, Hales TG, Kirkness EF. A novel class of ligand-gated ion channel is activated by Zn2+. J Biol Chem. 2003;278:712–717. doi: 10.1074/jbc.M208814200. [DOI] [PubMed] [Google Scholar]
- 57.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148:13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda S, Miyazaki T, Munechika K, Yamashita M, Ikeda Y, Kamizono A. Isolation of Zn2+ as an endogenous agonist of GPR39 from fetal bovine serum. J Recept Signal Transduct Res. 2007;27:235–246. doi: 10.1080/10799890701506147. [DOI] [PubMed] [Google Scholar]
- 59.West AK, Hidalgo J, Eddins D, Levin ED, Aschner M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology. 2008;29:489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 61.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kambe T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem. 2011;75:1036–1043. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- 64.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 65.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 66.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophy Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Keilin D, Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J. 1940;34:1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keilin D, Mann T. Carbonic Anhydrase. Nature. 1939;144:442–443. [Google Scholar]
- 69.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI. Iron-Export Ferroxidase Activity of beta-Amyloid Precursor Protein Is Inhibited by Zinc in Alzheimer's Disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and mlecular disfunction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 71.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 72.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 73.Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J Neurosci. 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kodirov SA, Takizawa S, Joseph J, Kandel ER, Shumyatsky GP, Bolshakov VY. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc Natl Acad Sci U S A. 2006;103:15218–15223. doi: 10.1073/pnas.0607131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsien RW. calcium channels in excitable cell membranes. Ann Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- 76.Yamakage M, Namiki A. Calcium channels -basic aspects of their structure, function and gene encoding; anesthetic action on the channels -a review. Can J Anaesth. 2002;49:151–164. doi: 10.1007/BF03020488. [DOI] [PubMed] [Google Scholar]
- 77.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gyulkhandanyan AV, Lee SC, Bikopoulos G, Dai F, Wheeler MB. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J Biol Chem. 2006;281:9361–9372. doi: 10.1074/jbc.M508542200. [DOI] [PubMed] [Google Scholar]
- 79.Priel T, Hershfinkel M. Zinc influx and physiological consequences in the beta-insulinoma cell line, Min6. Biochem Biophys Res Commun. 2006;346:205–212. doi: 10.1016/j.bbrc.2006.05.104. [DOI] [PubMed] [Google Scholar]
- 80.Fukuda J, Kawa K. Permeation of manganese, cadmium, zinc, and beryllium through calcium channels of an insect muscle membrane. Science. 1977;196:309–311. doi: 10.1126/science.847472. [DOI] [PubMed] [Google Scholar]
- 81.Kawa K. Zinc-dependent action potentials in giant neurons of the snail, Euhadra quaestia. J Membr Biol. 1979;49:325–344. doi: 10.1007/BF01868990. [DOI] [PubMed] [Google Scholar]
- 82.Weiss JH, Hartley DM, Koh JY, Choi DW. AMPA receptor activation potentiates zinc neurotoxicity. Neuron. 1993;10:43–49. doi: 10.1016/0896-6273(93)90240-r. [DOI] [PubMed] [Google Scholar]
- 83.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J Biol Chem. 1995;270:2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 86.Striessnig J, Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene Knockout models. Channels. 2008;2:233–251. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- 87.Kerchner GA, Canzoniero LMT, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physiol. 2000;528:39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamasaki S, Hasegawa A, Hojyo S, Ohashi W, Fukada T, Nishida K, Hirano T. A novel role of the L-type calcium channel alpha1D subunit as a gatekeeper for intracellular zinc signaling: zinc wave. PLoS One. 2012;7:e39654. doi: 10.1371/journal.pone.0039654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111:260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Rousseaux CG. A review of glutamate receptors I: current understanding of their biology. J Toxicol Pathol. 2008;21:25–51. [Google Scholar]
- 92.Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 93.Li MH, Inoue K, Si HF, Xiong ZG. Calcium-permeable ion channels involved in glutamate receptor-independent ischemic brain injury. Acta Pharmacol Sin. 2011;32:734–740. doi: 10.1038/aps.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim TY, Hwang JJ, Yun SH, Jung MW, Koh JY. Augmentation by zinc of NMDA receptor-mediated synaptic responses in CA1 of rat hippocampal slices: mediation by Src family tyrosine kinases. Synapse. 2002;46:49–56. doi: 10.1002/syn.10118. [DOI] [PubMed] [Google Scholar]
- 95.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 96.Hori N, Galeno T, Carpenter DO. Responses of pyriform cortex neurons to excitatory amino acids: voltage dependence, conductance changes, and effects of divalent cations. Cell Mol Neurobiol. 1987;7:73–90. doi: 10.1007/BF00734991. [DOI] [PubMed] [Google Scholar]
- 97.Koh JY, Choi DW. Zinc toxicity on cultured cortical-neurons - involvement of N-methyl-D-aspartate receptors. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 98.Nozaki C, Vergnano AM, Filliol D, Ouagazzal AM, Le Goff A, Carvalho S, Reiss D, Gaveriaux-Ruff C, Neyton J, Paoletti P, Kieffer BL. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat Neurosci. 2011;14:1017–1022. doi: 10.1038/nn.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:683–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 100.Weiss JH, Sensi SL. Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci. 2000;23:365–371. doi: 10.1016/s0166-2236(00)01610-6. [DOI] [PubMed] [Google Scholar]
- 101.Martinez-Galan JR, Diaz C, Juiz JM. Histochemical localization of neurons with zinc-permeable AMPA/kainate channels in rat brain slices. Brain Res. 2003;963:156–164. doi: 10.1016/s0006-8993(02)03964-1. [DOI] [PubMed] [Google Scholar]
- 102.Calderone A, Jover T, Mashiko T, Noh K, Tanaka H, Bennett MVL, Zukin RS. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pellegrini-Giampietro DE, Gorter JA, Bennett MVL, Zukin RS. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 104.Jia Y, Jeng JM, Sensi SL, Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol. 2002;543:35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordero-Erausquin M, Marubio LM, Klink R, Changeux JP. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- 106.Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 107.Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann N Y Acad Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- 108.Adams DJ, Dwyer TM, Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980;75:493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vega MT, Villalobos C, Garrido B, Gandia L, Bulbena O, Garciasancho J, Garcia AG, Artalejo AR. Permeation by Zinc of Bovine Chromaffin Cell Calcium Channels - Relevance to Secretion. Pflug Arch Eur J Phy. 1994;429:231–239. doi: 10.1007/BF00374317. [DOI] [PubMed] [Google Scholar]
- 110.Ragozzino D, Giovannelli A, Degasperi V, Eusebi F, Grassi F. Zinc permeates mouse muscle ACh receptor channels expressed in BOSC 23 cells and affects channel function. J Physiol. 2000;529:83–91. doi: 10.1111/j.1469-7793.2000.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mikne B. Drosophila mutant with a transducer defect. Biophys Struct Mech. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- 112.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 113.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 114.Padinjat R, Andrews S. TRP channels at a glance. J Cell Sci. 2004;117:5707–5709. doi: 10.1242/jcs.01343. [DOI] [PubMed] [Google Scholar]
- 115.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 116.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 118.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 119.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 120.Schlingmann KP, Weber S, Peters M, Nejsum LN, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 121.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 122.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 123.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.LaRochelle O, Gagne V, Charron J, Soh JW, Seguin C. Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J Biol Chem. 2001;276:41879–41888. doi: 10.1074/jbc.M108313200. [DOI] [PubMed] [Google Scholar]
- 125.Laity JH, Andrews GK. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch Biochem Biophys. 2007;463:201–210. doi: 10.1016/j.abb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 126.Leng TD, Lin J, Sun HW, Zeng Z, O'Bryant Z, Inoue K, Xiong ZG. Local Anesthetic Lidocaine Inhibits TRPM7 Current and TRPM7-Mediated Zinc Toxicity. CNS Neurosci Ther. 2015;21:32–39. doi: 10.1111/cns.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nair AV, Hocher B, Verkaart S, van Zeeland F, Pfab T, Slowinski T, Chen YP, Schlingmann KP, Schaller A, Gallati S, Bindels RJ, Konrad M, Hoenderop JG. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci U S A. 2012;109:11324–11329. doi: 10.1073/pnas.1113811109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- 130.Oberwinkler J, Lis A, Giehl KM, Flockerzi V, Philipp SE. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem. 2005;280:22540–22548. doi: 10.1074/jbc.M503092200. [DOI] [PubMed] [Google Scholar]
- 131.Michael DJ, Ritzel RA, Haataja L, Chow RH. Pancreatic beta-cells secrete insulin in fast- and slow-release forms. Diabetes. 2006;55:600–607. doi: 10.2337/diabetes.55.03.06.db05-1054. [DOI] [PubMed] [Google Scholar]
- 132.Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 133.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wagner TF, Drews A, Loch S, Mohr F, Philipp SE, Lambert S, Oberwinkler J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflugers Arch. 2010;460:755–765. doi: 10.1007/s00424-010-0838-9. [DOI] [PubMed] [Google Scholar]
- 135.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Jilius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 136.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 137.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 138.Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci. 2007;27:4443–4451. doi: 10.1523/JNEUROSCI.0557-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 140.Bessac BF, Sivula M, Von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee LY. TRPA1 ion channels: a gateway to airway irritation and reflex responses induced by inhaled oxidants. Journal of Physiology-London. 2010;588:747–748. doi: 10.1113/jphysiol.2010.187286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu HZ, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nature Chemical Biology. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 144.Moreau R, Daoud G, Bematchez R, Simoneau L, Masse A, Lafond J. Calcium uptake and calcium transporter expression by trophoblast cells from human term placenta. Biochimica Et Biophysica Acta-Biomembranes. 2002;1564:325–332. doi: 10.1016/s0005-2736(02)00466-2. [DOI] [PubMed] [Google Scholar]
- 145.Peng JB, Brown EM, Hediger MA. Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics. 2001;76:99–109. doi: 10.1006/geno.2001.6606. [DOI] [PubMed] [Google Scholar]
- 146.Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 147.Lehen'kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene. 2007;26:7380–7385. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- 148.Lehen'kyi V, Raphael M, Oulidi A, Flourakis M, Khalimonchyk S, Kondratskyi A, Gordienko DV, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N. TRPV6 determines the effect of vitamin D3 on prostate cancer cell growth. PLoS One. 2011;6:e16856. doi: 10.1371/journal.pone.0016856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kovacs G, Tamas D, Bergeron MJ, Bernadett B, Suzuki Y, Akos Z, Hediger MA. Heavy metal cations permeate the TRPV6 epithelial cation channel. Cell Calcium. 2011;49:43–55. doi: 10.1016/j.ceca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Qiu A, Hogstrand C. Functional characterisation and genomic analysis of an epithelial calcium channel (ECaC) from pufferfish, Fugu rubripes. Gene. 2004;342:113–123. doi: 10.1016/j.gene.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 151.Hogstrand C, Verbost PM, Bonga SE, Wood CM. Mechanisms of zinc uptake in gills of freshwater rainbow trout: interplay with calcium transport. Am J Physiol. 1996;270:R1141–1147. doi: 10.1152/ajpregu.1996.270.5.R1141. [DOI] [PubMed] [Google Scholar]
- 152.Zheng D, Feeney GP, Handy RD, Hogstrand C, Kille P. Uptake epithelia behave in a cell-centric and not systems homeostatic manner in response to zinc depletion and supplementation. Metallomics. 2014;6:154–165. doi: 10.1039/c3mt00212h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 154.Dietrich A, Gudermann T. TRPC6. Handb Exp Pharmacol. 2007:1251–1241. doi: 10.1007/978-3-540-34891-7_7. [DOI] [PubMed] [Google Scholar]
- 155.Heeringa SF, Moller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One. 2009;4:e7771. doi: 10.1371/journal.pone.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120:3480–3492. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gibon J, Tu P, Bohic S, Richaud P, Arnaud J, Zhu MK, Boulay G, Bouron A. The over-expression of TRPC6 channels in HEK-293 cells favours the intracellular accumulation of zinc. Biochim Biophys Acta. 2011;1808:2807–2818. doi: 10.1016/j.bbamem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 158.Chevallet M, Jarvis L, Harel A, Luche S, Degot S, Chapuis V, Boulay G, Rabilloud T, Bouron A. Functional consequences of the over-expression of TRPC6 channels in HEK cells: impact on the homeostasis of zinc. Metallomics. 2014;6:1269–1276. doi: 10.1039/c4mt00028e. [DOI] [PubMed] [Google Scholar]
- 159.Manzoni M, Monti E, Bresciani R, Bozzato A, Barlati S, Bassi MT, Borsani G. Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: effects on the late endocytic compartment organization. FEBS Lett. 2004;567:219–224. doi: 10.1016/j.febslet.2004.04.080. [DOI] [PubMed] [Google Scholar]
- 160.Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A. TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem. 2005;280:43218–43223. doi: 10.1074/jbc.M508210200. [DOI] [PubMed] [Google Scholar]
- 161.Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- 162.Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Human Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr., Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 164.Schiffmann R, Dwyer NK, Lubensky IA, Tsokos M, Sutliff VE, Latimer JS, Frei KP, Brady RO, Barton NW, Blanchette-Mackie EJ, Goldin E. Constitutive achlorhydria in mucolipidosis type IV. Proc Natl Acad Sci U S A. 1998;95:1207–1212. doi: 10.1073/pnas.95.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bach G. Mucolipidosis type IV. Mol Genet Metab. 2001;73:197–203. doi: 10.1006/mgme.2001.3195. [DOI] [PubMed] [Google Scholar]
- 166.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–9926. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eichelsdoerfer JL, Evans JA, Slaugenhaupt SA, Cuajungco MP. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J Biol Chem. 2010;285:34304–34308. doi: 10.1074/jbc.C110.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kukic I, Lee JK, Coblentz J, Kelleher SL, Kiselyov K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem J. 2013;451:155–163. doi: 10.1042/BJ20121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 170.Elinder F, Arhem P. Metal ion effects on ion channel gating. Q Rev Biophysics. 2003;36:373–427. doi: 10.1017/s0033583504003932. [DOI] [PubMed] [Google Scholar]
- 171.Smart TG, Hosie AM, Miller PS. Zn2+ ions: Modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- 172.Dunne EL, Hosie AM, Wooltorton JRA, Duguid IC, Harvey K, Moss SJ, Harvey RJ, Smart TG. An N-terminal histidine regulates Zn2+ inhibition on the murine GABAA receptor beta 3 subunit. Br J Pharmacol. 2002;137:29–38. doi: 10.1038/sj.bjp.0704835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Horenstein J, Akabas MH. Location of a high affinity Zn2+ binding site in the channel of alpha 1 beta 1 gamma-aminobutyric acidA receptors. Mol Pharmacol. 1998;53:870–877. [PubMed] [Google Scholar]
- 174.Fisher JL, Macdonald RL. The role of an alpha subtype M2-M3 his in regulating inhibition of GABAA receptor current by zinc and other divalent cations. J Neurosci. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang TL, Hackam A, Guggino WB, Cutting GR. A single histidine residue is essential for zinc inhibition of GABA Rho-1 receptors. J Neurosci. 1995;15:7684–7691. doi: 10.1523/JNEUROSCI.15-11-07684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Biasi M, Drewe JA, Kirsch GE, Brown AM. Histidine substitution identifies a surface position and confers Cs+ selectivity on a K+ pore. Biophys J. 1993;65:1235–1242. doi: 10.1016/S0006-3495(93)81154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jiang Q, Inoue K, Wu X, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zinc-mediated inhibition. Neuroscience. 2011;193:89–99. doi: 10.1016/j.neuroscience.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kurz LL, Klink H, Jakob I, Kuchenbecker M, Benz S, Lehmann-Horn F, Rudel R. Identification of three cysteines as targets for the Zn2+ blockade of the human skeletal muscle chloride channel. J Biol Chem. 1999;274:11687–11692. doi: 10.1074/jbc.274.17.11687. [DOI] [PubMed] [Google Scholar]
- 179.Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987;20:91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- 180.Canzoniero LM, Turetsky DM, Choi DW. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J Neurosci. 1999;19:RC31. doi: 10.1523/JNEUROSCI.19-19-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Snitsarev V, Budde T, Stricker TP, Cox JM, Krupa DJ, Geng L, Kay AR. Fluorescent detection of Zn2+-rich vesicles with zinquin: Mechanism of action in lipid environments. Biophys J. 2001;80:1538–1546. doi: 10.1016/S0006-3495(01)76126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng C, Reynolds IJ. Calcium-sensitive fluorescent dyes can report increases in intracellular free zinc concentration in cultured forebrain neurons. J Neurochem. 1998;71:2401–2410. doi: 10.1046/j.1471-4159.1998.71062401.x. [DOI] [PubMed] [Google Scholar]
- 183.Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II). Biochem J. 1993;296:403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 185.Simons TJ. Measurement of free Zn2+ ion concentration with the fluorescent probe mag-fura-2 (furaptra). J Biochem Biophys Methods. :25–37. doi: 10.1016/0165-022x(93)90065-v. 1993/08/01 ed1993. [DOI] [PubMed] [Google Scholar]
- 186.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 187.Stork CJ, Li YV. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci. 2006;26:10430–10437. doi: 10.1523/JNEUROSCI.1588-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tsien R, Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- 189.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 190.Naik HB, Beshire M, Walsh BM, Liu J, Soybel DI. Secretory state regulates Zn2+ transport in gastric parietal cell of the rabbit. Am J Physiol Cell Physiol. 2009;297:C979–C989. doi: 10.1152/ajpcell.00577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Devinney MJ, 2nd, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium. 2005;37:225–232. doi: 10.1016/j.ceca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 192.Kiedrowski L. Cytosolic acidification and intracellular zinc release in hippocampal neurons. J Neurochem. 2012;121:438–450. doi: 10.1111/j.1471-4159.2012.07695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]