Abstract
Background
Vascular endothelial growth factor (VEGF), a well-characterized regulator of angiogenesis, has been mechanistically implicated in retinal neovascularization and in the pathogenesis of ROP. However, the ontogeny of VEGF expression in the human fetal retina is not well known. Because retinal vasculature grows with gestational maturation, we hypothesized that VEGF expression also increases in the midgestation human fetal eye as a function of gestational age.
Methods
To identify changes in VEGF gene expression during normal human development, we measured VEGF mRNA by quantitative PCR and measured VEGF protein by ELISA and western blots in 10-24 week gestation fetal vitreous, retina, and serum.
Results
VEGF mRNA expression in the retina increased with gestational age. VEGF isoform A, particularly its VEGF121 splice variant, contributed to this positive correlation. Consistent with these findings, we detected increasing VEGF121 protein concentrations in vitreous humor from fetuses of 10-24 weeks gestation, while VEGF concentrations decreased in fetal serum.
Conclusions
VEGF121 mRNA and protein concentrations increase with increasing gestational age in the developing human retina. We speculate that VEGF plays an important role in normal retinal vascular development, and that preterm delivery affects production of this vascular growth factor.
Introduction
Infants born at the limits of viability are susceptible to morbidities involving many organ systems. Retinopathy of prematurity (ROP) is a well-known morbidity specific to the developing eye. This disease involves the abnormal maturation of the retinal vasculature, and it is one of the most common causes of irreversible childhood blindness today (1). Although epidemiological evidence indicates ROP to be associated with multiple risk factors such as prematurity, oxygen use, low birth weight, infections, and poor postnatal weight gain, the etiopathogenesis of this disorder remains unclear (1-5).
Vascular endothelial growth factor (VEGF), a well-characterized regulator of angiogenesis, has been mechanistically implicated in retinal neovascularization and in the pathogenesis of ROP (6-9). Understanding the ontogeny of VEGF expression in the normal human fetal retina is an important step in the study of angiogenic factors operative in ROP (10, 11). In this study, we hypothesized that VEGF expression increases in the midgestation human fetal eye as a function of gestational age, and measured VEGF isoforms and splice variants in retinal tissue and vitreous fluid obtained from fetuses of 10-24 weeks gestation.
Results
Endogenous controls
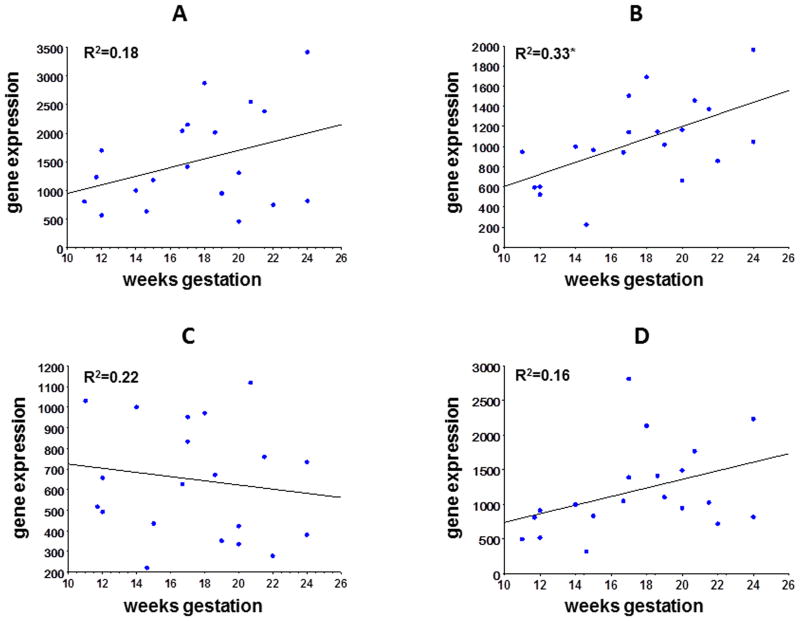
We evaluated beta-actin (β-actin), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), hypoxia inducible factor 1 alpha (HIF-1α) and ribosomal 18s as possible genes to serve as normalizing controls in quantitative PCR (qPCR) reactions (figure 1). We found no statistical difference in gene expression across all gestational ages tested (10-24 weeks) with GAPDH (p=0.108), β-actin (p=0.522) or HIF-1α (p=0.077), while 18s gene expression increased significantly from early to late mid-gestation with 18s (p=0.006). We chose GAPDH to serve as our endogenous control because of its relative abundance in relation to VEGF.
Figure 1. Gene expression of GAPDH, β-actin, 18s and HIF-1α.
Gene expression in retina for GAPDH (panel A), 18s (panel B) β-actin (panel C), and HIF-1α (panel D) from 10-24 week samples is shown. Gene expression of GAPDH, β-actin and HIF-1a was not significantly different across the range of gestational age tested, while gene expression of 18s increased with increasing gestational age (p=0.006).
VEGF concentrations in serum and vitreous
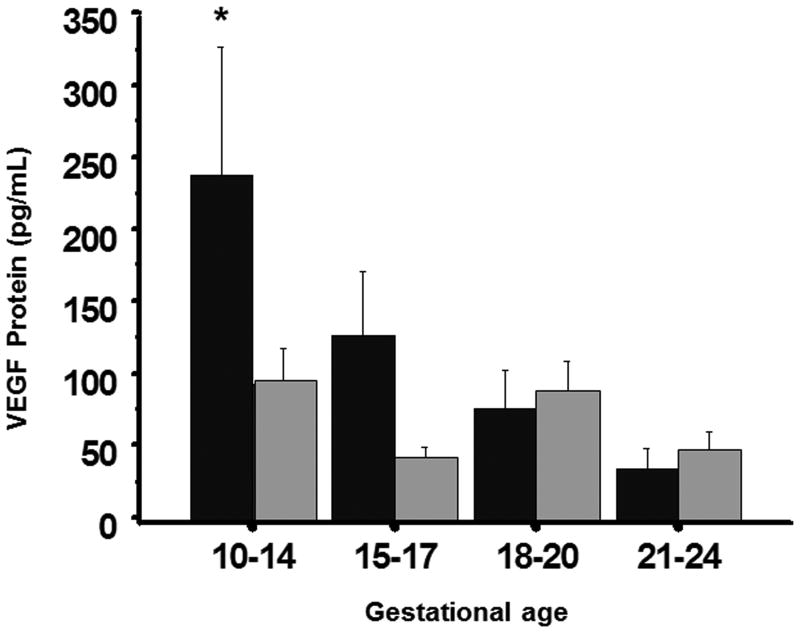
We initially measured VEGF concentrations in vitreous and serum in 10-24 week fetal samples (figure 2). The ELISA primarily measured VEGF165 but could not distinguish VEGF165 from VEGF121. Serum VEGF165/121 concentrations were significantly higher at 10-14 weeks gestation than concentrations at other gestational ages, and were higher than vitreous concentrations at 10-14 weeks (p<0.05). Vitreous VEGF165/121 concentrations were similar among gestational age groups, and were similar to serum concentrations at 15-17, 18-21, and 22-24 weeks gestation.
Figure 2. VEGF protein concentrations in fetal serum and vitreous.
VEGF165/121 protein concentrations in serum (black bars) and vitreous (grey bars) measured from 10 to 24 weeks gestation. VEGF165/121 protein concentrations were significantly greater in serum at 10-14 weeks (p<0.05) than in serum at other gestational ages, while concentrations in vitreous were similar at all gestational ages tested. Serum VEGF165/121 protein concentrations were similar to vitreous concentrations from 15-24 weeks.
VEGF-A expression increases in the 2nd trimester retina with advancing gestation
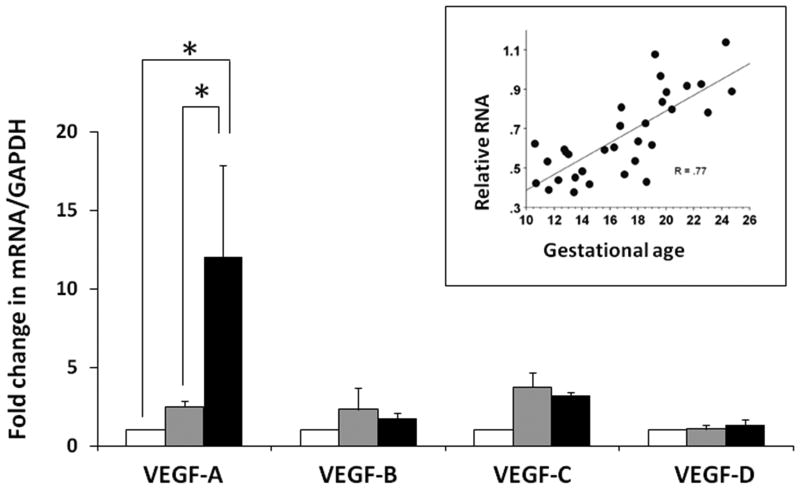
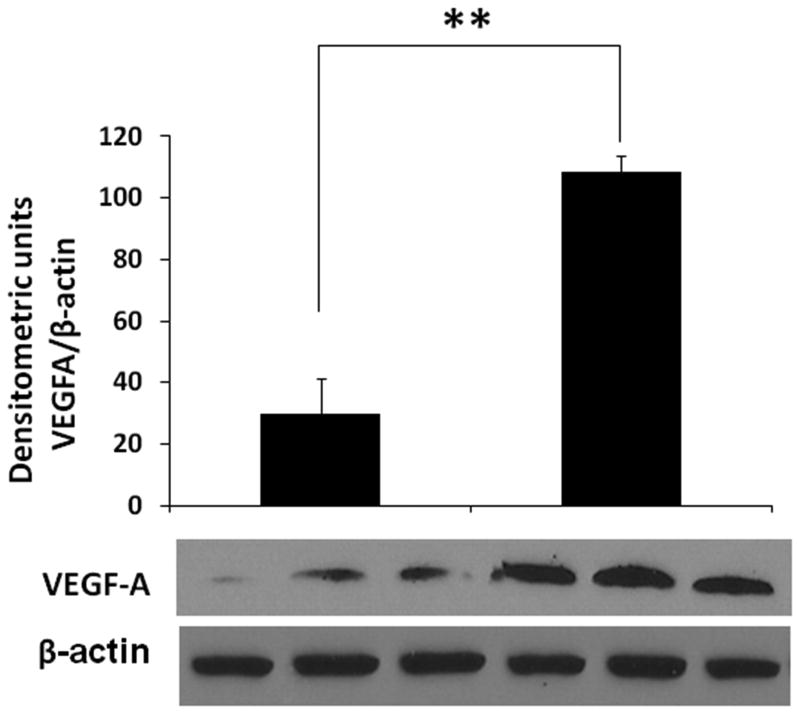
We measured mRNA expression of VEGF-A, -B, -C, and -D isoforms in fetal retinal tissue by RT-qPCR. As shown in figure 3, the expression of VEGF-A, but not VEGF-B, -C, or D, increased in the midgestation retina as a function of gestation age. VEGF mRNA expression showed a strong positive correlation with gestational age (inset; Spearman's r = 0.77, p<0.05). Consistent with these findings, we measured VEGF in retinal tissue and showed higher VEGF-A protein expression in tissue from 20-24 week fetuses than 11-14 week fetuses (figure 4).
Figure 3. VEGF-A mRNA expression in the 2nd trimester retina with advancing gestation.
Bar diagrams (means ± SE) show fold change in the mRNA expression of VEGF isoforms, VEGF-A, -B, -C, and -D, in retinal tissue from fetuses of 10-14 weeks, 15-19 weeks, and 20-24 weeks gestation. Inset: Scatter-plot of relative RNA expression of VEGF/18S ribosomal RNA vs. gestational age. R = Spearman's correlation coefficient). * indicates P<0.05.
Figure 4. Tissue-bound VEGF-A protein expression in retinal tissue from fetuses at 10-14 weeks and 20-24 weeks gestation.
Representative Western blots show tissue-bound VEGF-A and β-actin. Bar-diagrams (means ± SE) summarize densitometric data.
VEGF121 is the predominant splice variant of VEGF-A in the midgestation fetal retina
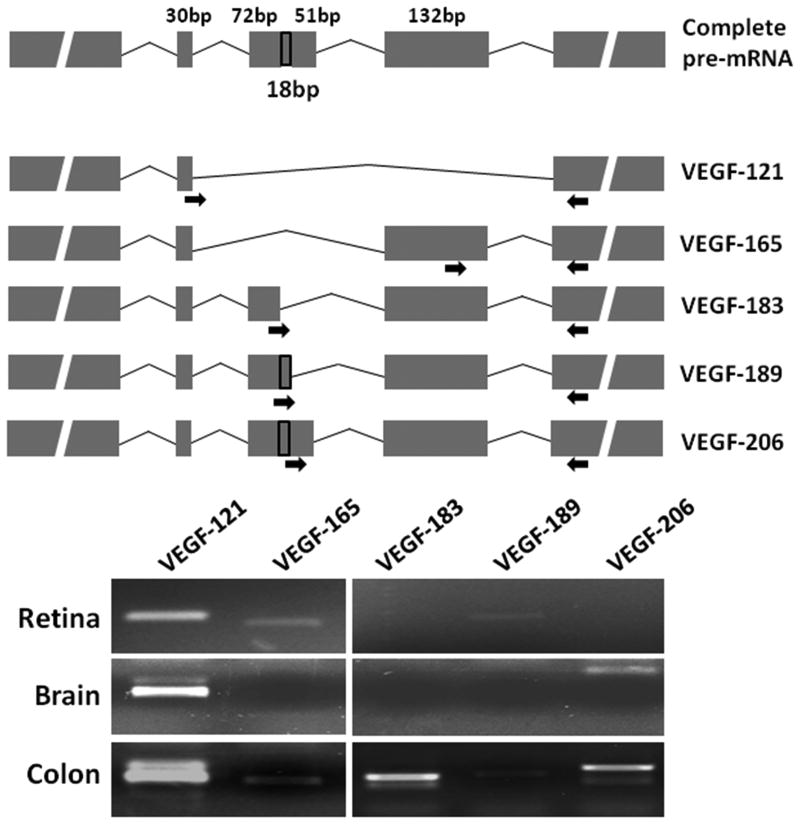
We next PCR-amplified major splice variants of VEGF-A mRNA in retinal tissue from fetuses of 20-24 weeks' gestation. As shown in figure 5, VEGF121was the predominant splice variant of VEGF-A, but we also detected faint bands of VEGF165 and VEGF189. These findings were specific to the retina; we detected only VEGF121and VEGF206 in the fetal brain, but all the 5 splice variants in the fetal colon at the same gestational age.
Figure 5. VEGF-A splice variants in the midgestation fetal retina.
Top: Schematic shows the complete pre-mRNA and the major splice variants of VEGF-A. The site of the primers is indicated by arrows. Bottom: Representative agarose gels showing PCR products corresponding to the major splice variants in fetal retina, brain, and colon.
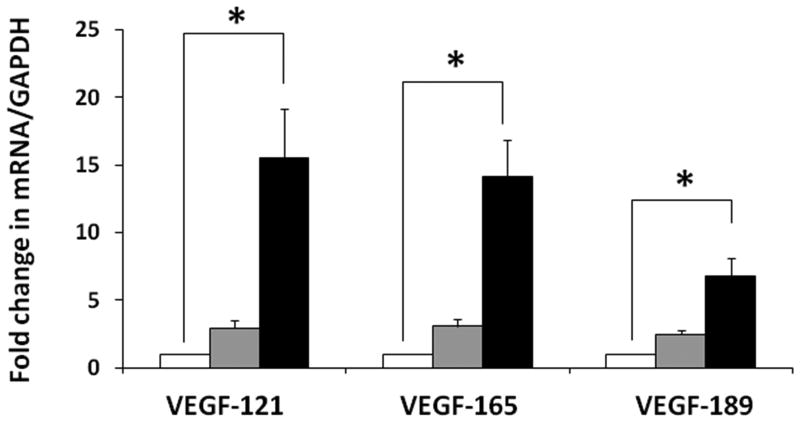
We next used our SYBR green I-based qPCR protocol to measure the developmental changes in VEGF121, VEGF165, and VEGF189. Although all the three splice variants showed increased expression with advancing gestation, VEGF121 and VEGF165 showed a greater developmental change than VEGF189 (figure 6).
Figure 6. Developmental change in VEGF-A splice variants in the midgestation fetal retina.
Bar diagrams (means ± SE) show fold change in the mRNA expression of VEGF-A splice variants, VEGF121, VEGF165, and VEGF189, in retinal tissue from fetuses of 10-14 weeks, 15-19 weeks, and 20-24 weeks. * indicates P<0.05.
VEGF concentrations increase in the vitreous humor with advancing gestation
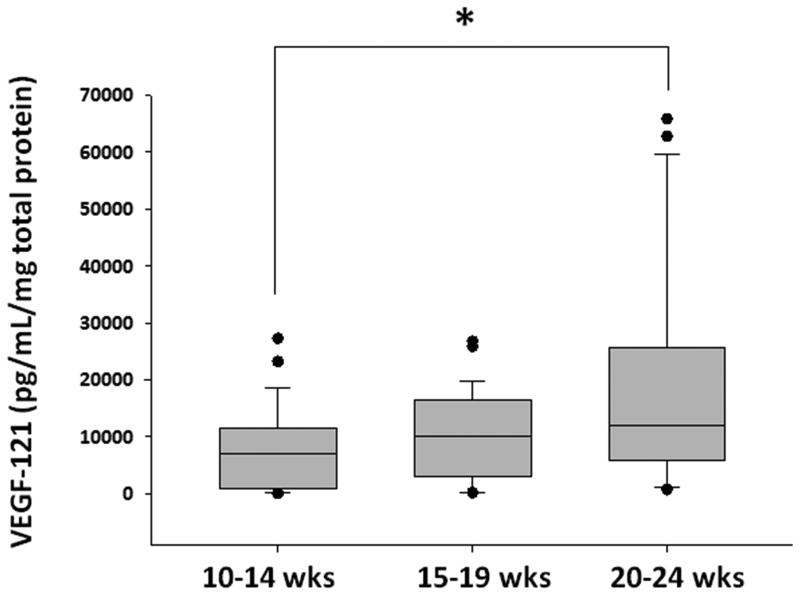
Existing information indicates that VEGF121 and VEGF165 are diffusible proteins, in contrast to the larger, tissue-bound VEGF189 protein (12). Therefore, to confirm the biological relevance of our findings at the protein level, we next measured VEGF121concentrations in vitreous humor from these fetuses. As shown in figure 7, VEGF121 concentrations increased in vitreous humor with advancing gestation. Compared to fetuses of 10-14 weeks' gestation, there was a trend towards higher vitreous VEGF121 concentrations at 15-19 weeks, and significantly higher VEGF121 concentrations at 20-24 weeks.
Figure 7. VEGF121 concentrations increase in the vitreous humor with advancing gestation.
Box-plots show VEGF121 concentrations in vitreous humor from fetuses of 10-14 weeks, 15-19 weeks, and 20-24 weeks. * indicates P<0.05.
Discussion
The normal role of VEGF in the developing human eye continues to be evaluated. In this study we found increasing concentrations of retinal VEGF-A mRNA with increasing gestational age in the human fetal eye. VEGF121 was the predominant splice variant of VEGF-A in fetal retina, and consistent with these findings, we detected increasing VEGF121 concentrations in vitreous humor with increasing gestational age. Our findings of increasing retinal VEGF mRNA and total VEGF protein with increasing gestational age are consistent with the developing vasculature of the eye.
Vascularization in the eye begins during the fifth week of gestation and is not completed until after birth (2, 8). The eye has an initial vascular system that develops but then regresses when the permanent retinal vascular system, limited to the inner two-thirds of the retina, starts to develop in the fourth month of gestation (8). Due to its tissue origins, the retina is thought to be primarily vascularized by angiogenesis, the formation of vessels from existing vessels, rather than vasculogenesis, the de novo formation of vessels (8, 10). VEGF is the primary hypoxically-regulated growth factor responsible for angiogenesis, which includes formation of the hyaloid vascular system early in development as well as later retinal vessel formation. However, before it assumes its role as a angiogenic factor, VEGF may also serve as a neurogenic factor for progenitors and newly postmitotic cells in the prevascular retina (13).
The term VEGF is often used synonymously with its isoform A. The gene is organized in 8 exons (6, 14, 15), where differential splicing results in two families of isoforms, one that is pro-angiogenic involved in neovascularization and the other that is anti-angiogenic inhibiting blood vessel proliferation (16). The isoforms formed from alternative splicing of the VEGF-A gene that are present in both families, include at least 4 transcripts containing 121, 165, 189, and 206 amino acid residues. These VEGF-A isoforms have functionally unique, biological activity and are distinguished by their affinity for heparin (9, 14, 17). VEGF-A has also been shown to be necessary for the development and stability of retinal vessels (18). The smaller isoforms, 121 and 165, are diffusible proteins; whereas, the larger isoforms, 189 and 206, have a high affinity for heparin and are bound to tissue. We detected VEGF121 to be the predominant splice variant of VEGF-A in the fetal retina. These findings show an interesting contrast with the adult retina, where the predominant splice variant is VEGF165 (19). Further study is needed to determine whether this developmental dichotomy in VEGF-A splice variants remains true in ROP and other retinal disorders.
The mechanisms for the observed maturational increase in VEGF expression in the developing retina are unclear. However, existing data indicate a role for several growth factors and cytokines as regulators of VEGF-A expression and splicing (20). For instance, transforming growth factor-beta (TGF-β) has been shown to act via the p38 MAPK to activate SR protein splicing factors such as SRp55, which binds the VEGF pre-RNA in the exon 8b region (21). Similarly, insulin-like growth factor (IGF)-1 can phosphorylate ASF/SF2 (alternative splicing factor/splicing factor 2) through the SR protein kinase SRPK1 (serine/arginine domain protein kinase 1) (22). ASF/SF2 can bind to VEGF pre-RNA around proximal splice-site selection) and results in differential expression of various splice variants of VEGF-A. IGF-1 may also regulate VEGF expression via hypoxia-induced factor (HIF)-1α -dependent and -independent pathways. HIF-1α is known to be abundant in hypoxic tissues with active angiogenesis (22). While HIF 1 expression did not significantly increase with development, there was a trend. This fits with our understanding of the role of “physiological hypoxia” in retinal vessel development.
Previous studies have evaluated the concentration of VEGF protein in the eye. Gogat and colleagues (8) determined the distribution of VEGF on retinal sections during development and noted that VEGF transcripts had a temporal and spatial correlation with normal development of human ocular vasculature. That study did not detect VEGF mRNA in older mid-gestational tissues, but did detect VEGF protein. In another study, Kim et al. (23) evaluated the levels of VEGF mRNA transcripts and protein in samples from normal monkeys, and detected constitutive expression of VEGF, VEGFR-1, and VEGFR-2. Pierce et al. (24) showed that VEGF mRNA levels, followed by VEGF protein, increase in a mouse model with relative retinal hypoxia. In another study, Pe'er et al. (25) showed that abnormal retinal vessel growth in human and rabbit neovascular retinas was associated with dramatic elevations of retinal VEGF expression compared to control retinas. These findings are consistent with our hypothesis that VEGF has an integral role in the normal development of the human eye, and abnormal levels may be associated with ROP.
Our findings provide important physiological information on the normal expression of VEGF in the developing retina. Although the pathophysiology of ROP is not well- understood because of the complexity of this disease, the role of VEGF is now widely-accepted (10, 26, 27). ROP has a well-recognized progression in the retina that is divided into two discrete, but associated, stages: cessation of normal retinal angiogenesis and a subsequent hyperproliferative neovascular response to retinal ischemia where the 1st phase occurs from 22 to 30 weeks postmenstrual age, and phase 2 from 31 to 44 weeks' postmenstrual age (2, 28, 29). Phase 1 occurs when the premature infant is exposed to relative hyperoxia, often associated with supplemental oxygen, resulting in reduced VEGF expression. Understanding these relationships between oxygen and VEGF can help improve the management of ROP, including both the prevention as well as early (i.e., in phase 1) and later treatment (i.e., in phase 2) (30). The role of growth factors, including erythropoietin (31, 32), IGF-1 (2), and matrix metalloproteinases (33), on VEGF expression in the normal retina and in ROP needs further evaluation. The efficacy of anti-VEGF agents such as intravitreal bevacizumab in ROP has also emphasized the important pathophysiological role of VEGF in ROP (34). Identification of the specific isoforms of VEGF expressed in the developing retina could help reduce adverse effects and facilitate the development of more specific anti-VEGF agents. In the adult retina, the anti-angiogenic VEGF165 antibody inhibits hypoxia-induced neovascularization (16), although our findings indicate a need to confirm whether VEGF121 or VEGF165 is the predominant splice variant of VEGF-A in ROP.
Methods
Fetal tissues
This study was evaluated by the Human Research Review Committee at the University of New Mexico and deemed not to constitute human subject research as no identifiable human subject data were collected. Vitreous, serum, and retinal tissue samples were collected from fetuses between 10 and 24 weeks gestation for measurement of VEGF mRNA and protein. The gestational age grouping was chosen based on availability of the tissue. Samples were divided into early, mid and late second trimester based on distribution across the 10-24 week gestation range. All biological specimens were handled and stored similarly accordingly to a standardized checklist. Gestational age was estimated based on fetal foot length. Gender could not be determined on most samples, and this information was not collected. The contents of the globe were extracted, retinal tissue (including choroid) isolated (figure 8), and the aqueous and vitreous collected from both fetal eyes and combined for each sample. Vitreous samples were clarified by centrifugation at 200g × 5 min and then frozen until the time of analysis. Retinal tissue from both eyes was triturated using serially smaller syringes in a commercially-available lysis buffer (T-PER tissue protein extraction reagent with added protease inhibitors; Thermo Scientific, Rockford, IL) for measurement of total protein concentration by the Bradford assay (Thermo Scientific).
Figure 8. Fetal eyes and retina.
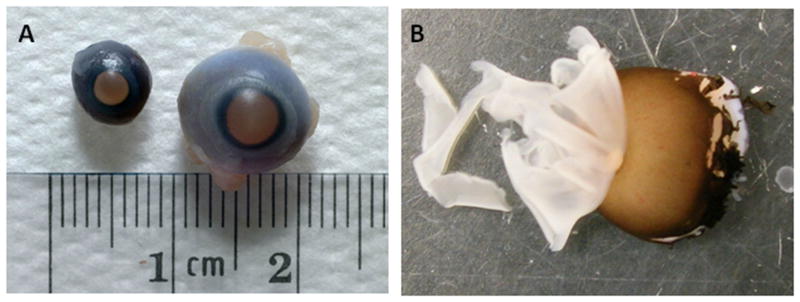
Fetal eyes at 14 weeks and 18 weeks gestation (panel A) are shown. Dissection of the 18 week fetal eye revealing the retina and choroid is shown (panel B).
Western blots
Tissue-bound VEGF-A protein was measured in retinal samples in Western blots using a mouse monoclonal IgG1 that detects the -189, -165 and -121 amino acid splice variants (Santa Cruz Biotech, Santa Cruz, CA) and established methods (35). This antibody detects VEGF as a dimer of 43 kDa molecular weight.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from fetal retinal samples using the single-step acid guanidinium thiocyanate-phenol-chloroform extraction (36) and was then reverse-transcribed using a commercially-available kit (cDNA Archive kit, Applied Biosystems, Foster City, CA). The expression of VEGF isoforms A-D was measured using standard SYBR green I-based RT-qPCR (37).
Isoform-specific primers were designed using the Beacon Design software (Bio-Rad, Hercules, CA): VEGF-A forward: CCTTGCCTTGCTGCTCTAC, reverse: TTCTGCCCTCCTCCTTCTG; VEGF-B forward: GGACAGAGTTGGAAGAGGAGAC, reverse: GGAAGAGCCAGTTGTAAGATGC; VEGF-C forward: CAGACAAGTTCATTCCATTATTAG, reverse: AGTCATCTCCAGCATCCG; VEGF-D forward: TCCAGACCAACCTTCCATTCAC, reverse: CAGCACACCTTTCTCATTCACC. For quantitative measurements, we developed a standard curve by reverse-transcribing serial dilutions of total RNA from Hep3B cells, an immortalized cell line that constitutively expresses VEGF (38).
The architecture of VEGF-A was analyzed using the databases aceview (19), GeneCard (39), and Ensembl (29), and splice variant-specific amplicons were identified as previously described by Lamani et al. (40). We used forward primers designed specifically for each splice variant: VEGF121: GAGCAAGACAAGAAAAATGTG; VEGF165: CAGACTCGCGTTGCAAGATG; VEGF-183: GTATAAGTCCTGGAGCGTTCC; VEGF189: AAGCGCAAGAAATCCCGTCC; and VEGF206: TGCTGTCTAATGCCCTGGAG, and combined these with a common reverse primer: GTCTTCACTGGATGTATTTGAC. PCR products were resolved in agarose gels for qualitative analysis. In some experiments, fetal brain and colonic tissue were included for control.
VEGF121 enzyme-linked immunosorbent assay (ELISA)
VEGF protein concentrations were initially quantified by ELISA that had a linear range of 15.6-1000 pg/mL (R&D Systems, Minneapolis, MN). The commercially available kit was designed to measure VEGF165, but per manufacturers information could not distinguish VEGF165 from VEGF121. Additional analyses were therefore performed on available vitreous samples. VEGF121 was measured in vitreous humor using an ELISA kit that includes a capture antibody specific for VEGF121 and does not display cross-reactivity for other splice variants of VEGF (MyBiosource, San Diego, CA). This assay had a linear range of 15.625-1000 pg/mL. Samples containing VEGF concentrations greater than the upper limit of the assay were re-tested in dilution. Data were normalized against the total protein concentration in each vitreous sample.
Statistical methods
Parametric and non-parametric tests were applied using the the Sigma Stat 3.1.1 software (Systat, Point Richmond, CA). Regression analyses and ANOVA were performed on endogenous control data. For PCR data, crossing-threshold (ΔΔCT) values were compared across experimental groups using analysis of variance or the Kruskal-Wallis H test/Dunn's multiple post-test. In all tests, p<0.05 was accepted as significant.
Acknowledgments
The authors thank Dr. Curtis Boyd and staff at Southwestern Women's Options, as well as Mabel Padilla for technical assistance.
Statement of financial support: Supported by a grant from the Department of Pediatrics, University of New Mexico (RKO) and the National Institutes of Health award HD059142 (AM)
Footnotes
Conflicts of interest: The authors have no financial or other conflicts to disclose.
Category of study: Basic Science
References
- 1.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellstrom A, Ley D, Hansen-Pupp I, et al. New insights into the development of retinopathy of prematurity--importance of early weight gain. Acta Paediatr. 2010;99:502–508. doi: 10.1111/j.1651-2227.2009.01568.x. [DOI] [PubMed] [Google Scholar]
- 3.Carlo WA, Bell EF, Walsh MC, et al. Oxygen-saturation targets in extremely preterm infants. N Engl J Med. 2013;368:1949–1950. doi: 10.1056/NEJMc1304827. [DOI] [PubMed] [Google Scholar]
- 4.Eckert GU, Fortes Filho JB, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond) 2012;26:400–406. doi: 10.1038/eye.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlo WA, Finer NN, Walsh MC, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gammons MV, Dick AD, Harper SJ, Bates DO. SRPK1 inhibition modulates VEGF splicing to reduce pathological neovascularisation in a rat model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2013;54:5797–806. doi: 10.1167/iovs.13-11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Wu D, Zhou W, et al. Association of VEGF gene polymorphisms with advanced retinopathy of prematurity: a meta-analysis. Mol Biol Rep. 2012;39:10731–10737. doi: 10.1007/s11033-012-1964-6. [DOI] [PubMed] [Google Scholar]
- 8.Gogat K, Le Gat L, Van Den Berghe L, et al. VEGF and KDR gene expression during human embryonic and fetal eye development. Invest Ophthalmol Vis Sci. 2004;45:7–14. doi: 10.1167/iovs.02-1096. [DOI] [PubMed] [Google Scholar]
- 9.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–1228. [PubMed] [Google Scholar]
- 10.Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 11.Bautch VL. VEGF-directed blood vessel patterning: from cells to organism. Cold Spring Harb Perspect Med. 2012;2:a006452. doi: 10.1101/cshperspect.a006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–118. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Keyt B. Vascular endothelial growth factor: basic biology and clinical implications. EXS. 1997;79:209–232. doi: 10.1007/978-3-0348-9006-9_9. [DOI] [PubMed] [Google Scholar]
- 16.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- 17.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 18.Curatola AM, Moscatelli D, Norris A, Hendricks-Munoz K. Retinal blood vessels develop in response to local VEGF-A signals in the absence of blood flow. Exp Eye Res. 2005;81:147–158. doi: 10.1016/j.exer.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;2006;7(Suppl 1):S12, 11–14. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba T, McLeod DS, Edwards MM, et al. VEGF 165 b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241:595–607. doi: 10.1002/dvdy.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–675. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- 23.Kim I, Ryan AM, Rohan R, et al. Constitutive expression of VEGF, VEGFR-1, and VEGFR-2 in normal eyes. Invest Ophthalmol Vis Sci. 1999;40:2115–2121. [PubMed] [Google Scholar]
- 24.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72:638–645. [PubMed] [Google Scholar]
- 26.Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–5182. doi: 10.1167/iovs.08-2584. [DOI] [PubMed] [Google Scholar]
- 27.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 28.Bossi E, Koerner F. Retinopathy of prematurity. Intensive Care Med. 1995;21:241–246. doi: 10.1007/BF01701481. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard TJ, Aken BL, Beal K, et al. Ensembl 2007. Nucleic Acids Res. 2007;35:D610–617. doi: 10.1093/nar/gkl996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mintz-Hittner HA, Kennedy KA, Chuang AZ BEAT_ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel S, Rowe MJ, Winters SA, Ohls RK. Elevated erythropoietin mRNA and protein concentrations in the developing human eye. Pediatr Res. 2008;63:394–397. doi: 10.1203/PDR.0b013e318165b8d1. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 33.Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 34.Mititelu M, Chaudhary KM, Lieberman RM. An evidence-based meta-analysis of vascular endothelial growth factor inhibition in pediatric retinal diseases: part 1. Retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012;49:332–40. doi: 10.3928/01913913-20120821-03. [DOI] [PubMed] [Google Scholar]
- 35.Kurien BT, Scofield RH. Western blotting. Methods. 2006;38:283–293. doi: 10.1016/j.ymeth.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954–958. 960, 962. [PubMed] [Google Scholar]
- 38.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safran M, Chalifa-Caspi V, Shmueli O, et al. Human Gene-Centric Databases at the Weizmann Institute of Science: GeneCards, UDB, CroW 21 and HORDE. Nucleic Acids Res. 2003;31:142–146. doi: 10.1093/nar/gkg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamani E, Wu YX, Dong J, Litaker MS, Acevedo AC, MacDougall M. Tissue- and Cell-Specific Alternative Splicing of NFIC. Cells Tissues Organs. 2009;189:105–110. doi: 10.1159/000152912. [DOI] [PMC free article] [PubMed] [Google Scholar]