Abstract
Sudden cardiac death (SCD) is a leading cause of mortality in patients with cardiomyopathy. While angiotensin converting enzyme inhibitors (ACEi) and receptor blockers (ARB) decrease cardiac mortality in these cohorts, their role in preventing SCD has not been well established. We sought to determine whether the use of ACEi or ARB in patients with cardiomyopathy is associated with a lower incidence of appropriate implantable cardiac defibrillator (ICD) shocks in the Genetic Risk Assessment of Defibrillator Events (GRADE) study which included subjects with an ejection fraction of ≤30% and ICDs. Treatment with ACEi/ARB versus no ACEi/ARB was physician dependent. There were 1509 patients (mean age [SD] 63[12] years, 80% male, mean [SD] EF 21% [6%]) with 1213 (80%) on ACEi/ARB, and 296 (20%) not on ACEi/ARB. We identified 574 propensity matched patients (287 in each group). After a mean (SD) of 2.5(1.9) years, there were 334 (22%) appropriate shocks in the entire cohort. The use of ACEi/ARB was associated with lower incidence of shocks at 1, 3 and 5 years in the matched cohort (7.7%, 16.7%, 18.5% vs. 13.2%, 27.5%, and 32.0% (RR= 0.61[0.43–0.86], p =0.005). Among patients with GFR >60 and 30–60 ml/min/1.73m2, those on no-ACEi/ARB were at 45% and 77% increased risk of ICD shock as compared to those on ACEi/ARB, respectively. ACEi/ARB were associated with significant lower incidence of appropriate ICD shock in patients with cardiomyopathy and GFR ≥30 ml/min/1.73m2, and with neutral effect among those GFR <30 ml/min/1.73m2.
Keywords: ACEi/ARB, cardiomyopathy, appropriate ICD shock
Introduction
Sudden cardiac death (SCD) is a leading cause of cardiovascular mortality in patients with left ventricular (LV) systolic dysfunction1. Angiotensin converting enzyme inhibitors (ACEi) and receptor blockers (ARB) antagonize the action of angiotensin II, a known precursor of interstitial fibrosis2, 3 that is associated with ventricular arrhythmia4–8. While ACEi/ARB decrease cardiac mortality in LV dysfunction patients9–11, their role in preventing SCD has not been well established. In one study, Obeyesekere et al. showed that absence of ACEi/ARB therapy was a predictor of appropriate ICD shock; however, the study was of small sample size, limited events, and excluded patients in the secondary prevention population12. Hence, the aim of the study is to explore the role of ACEi/ARB in predicting appropriate implantable cardiac defibrillator (ICD) shocks in a large multicenter registry of patients with severe systolic dysfunction. We hypothesized that ACEi/ARB usage is associated with a decreased incidence of appropriate shock in patients with cardiomyopathy. We also sought to elucidate the role of ACEi/ARB in predicting appropriate ICD shocks in a) distinct glomerular filtration rate (GFR) strata, b) in ischemic versus non-ischemic cardiomyopathy, and lastly c) based on indication for ICD implantation cohorts (primary versus secondary prevention).
Methods
Subjects included in this study are from the NHLBI sponsored prospective observational multi-center GRADE (The Genetic Risk Assessment of Defibrillator Events) study, designed to identify genetic modifiers of arrhythmic risk13. Inclusion criteria were: patients who were ≥18 years of age with a diagnosis of at least moderate systolic left ventricular dysfunction (EF ≤30%), and who had an ICD at the University of Pittsburgh Medical Center (coordinating center; Pittsburgh, PA), Emory University Medical Center, (Atlanta, GA), Massachusetts General Hospital, (Boston, MA), Ohio State University Medical Center, (Columbus, OH), Mid-Ohio Cardiology (Columbus, OH) or the Pittsburgh Veterans Affairs Medical Center (Pittsburgh, PA). Subjects were excluded if they had intractable Class IV heart failure, and conditions (other than HF) that were expected to limit survival to less than 6 months. The institutional review boards of participating medical centers approved the study and each patient gave written informed consent prior to participation. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and the trial was registered at www.clinicaltrials.gov (NCT 02045043).
A total of 1808 GRADE patients, enrolled between March 2002 and July 2010 within 5 years of ICD implantation, were considered for the current analysis. Of these, 252 patients with no available follow-up data on first appropriate shock outcome and 47 patients without ACEi/ARB medication use data were excluded. The final study population consisted of 1509 subjects and was divided to two primary comparison groups: 1213 ACEi/ARB (80%) and 296 No-ACEi/ARB (20%). Baseline measurements recorded at the first visit included demographic characteristics, left ventricular EF (by echocardiography, nuclear study, or left ventriculogram), New York Heart Association functional class, medication profile, serum electrolytes, electrocardiographic parameters, echocardiographic parameters, hemodynamic measurements, model and settings of the ICD, etiology of heart failure (ischemic versus non ischemic), and indication for device (primary versus secondary prevention). The left ventricular EF was determined by 2-dimensional echocardiography in the majority of subjects.
Ischemic HF patients included those with a documented history of myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft or ≥50% diameter stenosis of any of the 3 major coronary epicardial arteries.
Duration of follow-up was defined as the interval from the date of enrollment or ICD implantation (whichever came later) to the date of the first endpoint or last follow-up when the data were censored. Clinical follow-up was done yearly by telephone by the research coordinator and ICD interrogation was performed. ICD shocks, implantation of ventricular assist device, heart transplantation and mortality data were collected and the validity of these data ascertained by ICD interrogation and hospital medical record documentation. ICD telemetry from all device downloads was sent to the coordinating center for review. Appropriate ICD shocks were adjudicated by two cardiologists, and a third in cases of disagreement. ICD programming was left to the discretion of the local electrophysiologist to select the cutoff rate for fast ventricular tachycardia (VT) and ventricular fibrillation (VF). Shocks for supraventricular tachycardia (SVT) were excluded from analyses. Episodes that only required anti-tachycardia pacing (ATP) were excluded from analyses. Episodes that required both ATP and appropriate ICD shocks were included.
The primary endpoint in this study was time to first appropriate ICD shock for ventricular tachycardia or ventricular fibrillation. Secondary endpoints included all-cause death, and the composite endpoint of death, ventricular assist device or cardiac transplantation.
Patients with missing appropriate ICD shock follow-up or discharge ACEi/ARB status were excluded from the study. A total of 25 other patient factors (Table 1) were considered in this analysis and 669 of 1509 study patients had 100% complete data (44%). Only 5 patient factors (QRS interval, QTC interval, systolic Blood Pressure, body mass index, and creatinine) had missing data in more than 5%. Data on medical history and medications intake were imputed assuming normal condition and no medication, respectively. Missing values were imputed using the median or the mode of the variable as applicable. Few patients had missing GFR levels and they were categorized as having normal GFR (> 60 ml/min/1.73 m2). There was no difference in results when missing GFR data were excluded and only unimputed data were used.
Table 1.
Baseline characteristics stratified by ACEi/ARB
| Un Matched Cohort | Propensity Matched Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACEi/ARB (N=1213) |
No ACEi/ARB (N=296) |
ACEi/ARB (N=287) |
No ACEi/ARB (N=287) |
|||||||||
| Variable | Mean | SD | Mean | SD | Diff | p-value | Mean | SD | Mean | SD | Diff | |
| Age (years) | 63 | 12 | 64 | 12 | 8.9% | 0.2 | 65 | 12 | 64 | 12 | 8.1% | |
| Systolic Blood Pressure (mm Hg) | 120 | 19 | 129 | 18 | 2.6% | 0.7 | 120 | 19 | 120 | 18 | 1.8% | |
| Glomerular filtration rate (imputed), (ml/min/1.73m2) | 63 | 20 | 58 | 20 | 25% | <.001 | 60 | 18 | 59 | 19 | 3.6% | |
| Glomerular filtration rate(unimputed), (ml/min/1.73m2) | 64 | 24 | 57 | 23 | 32% | <.001 | 59.0 | 22 | 58 | 23 | 4.4% | |
| Ejection Fraction (%) | 21 | 6 | 20 | 6.0 | 9.0% | 0.2 | 20 | 6 | 20 | 6 | 2.3% | |
| QRS interval (ms) | 136 | 36 | 135 | 33 | 3.0% | 0.7 | 135 | 36 | 136 | 34 | 2.3% | |
| QTC interval (ms) | 472 | 52 | 471 | 54 | 2.7% | 0.6 | 468 | 49 | 471 | 54 | 6.3% | |
| N | % | N | % | Diff | p-value | N | % | N | % | Diff | ||
| Men | 981 | 81% | 230 | 78% | 7.8% | 0.22 | 229 | 80% | 224 | 78% | 4.3% | |
| White | 1035 | 85% | 239 | 81% | 12% | 0.05 | 235 | 82% | 234 | 82% | 0.9% | |
| Age (years) | < 50 | 152 | 13% | 31 | 11% | 6.5% | 0.36 | 28 | 10% | 30 | 11% | 2.3% |
| 50–59 | 296 | 24% | 71 | 24% | 1.0% | 61 | 21% | 69 | 24% | 6.7% | ||
| 60–69 | 407 | 34% | 92 | 31% | 5.3% | 97 | 34% | 88 | 31% | 6.7% | ||
| ≥ 70 | 358 | 30% | 102 | 35% | 11% | 101 | 35% | 100 | 35% | 0.7% | ||
| Body mass index (kg/m2) | <21 | 46 | 4% | 12 | 4% | 1.4% | 0.92 | 8 | 3% | 12 | 4% | 7.6% |
| 21–25 | 194 | 16% | 50 | 17% | 2.4% | 54 | 19% | 49 | 17% | 4.5% | ||
| 25–30 | 512 | 42% | 132 | 45% | 4.8% | 127 | 44% | 129 | 45% | 1.4% | ||
| 30–35 | 286 | 24% | 64 | 22% | 4.7% | 69 | 24% | 60 | 21% | 7.5% | ||
| 35–40 | 120 | 10% | 25 | 8% | 5.0% | 19 | 7% | 25 | 9% | 7.9% | ||
| >40 | 55 | 5% | 13 | 4% | 0.7% | 10 | 4% | 12 | 4% | 3.6% | ||
| Glomerular filtration rate (ml/min/1.73m2) | >60 | 177 | 60% | 845 | 70% | 21% | <.001 | 184 | 64% | 177 | 62% | 5.0% |
| 30–60 | 93 | 31% | 327 | 27% | 9.6% | 87 | 30% | 90 | 31% | 2.3% | ||
| <30 | 26 | 9% | 41 | 3% | 23% | 16 | 6% | 20 | 7% | 6% | ||
| New York Heart Association class | I | 151 | 12% | 52 | 18% | 14% | 0.045 | 42 | 15% | 52 | 18% | 9.4% |
| II | 728 | 60% | 156 | 53% | 15% | 160 | 56% | 152 | 53% | 5.6% | ||
| III | 324 | 27% | 87 | 29% | 6.0% | 85 | 30% | 82 | 29% | 2.3% | ||
| IV | 10 | .8% | 1 | .3% | 6.4% | 0 | 0% | 1 | 0.3% | 8.4% | ||
| Ischemic etiology | 854 | 70% | 221 | 75% | 9.6% | 0.15 | 229 | 80% | 213 | 74% | 13.3% | |
| Device Indications | Secondary | 292 | 24% | 77 | 26% | 4.5% | 0.49 | 74 | 26% | 76 | 27% | 1.6% |
| Defibrillator with biventricular pacing | 523 | 43% | 136 | 46% | 5.7% | 0.38 | 123 | 43% | 131 | 46% | 5.6% | |
| Prior myocardial infarction | 649 | 54% | 169 | 57% | 7.2% | 0.27 | 173 | 60% | 162 | 56% | 7.8% | |
| Diabetes mellitus | 404 | 33% | 116 | 39% | 12% | 0.06 | 119 | 42% | 110 | 38% | 6.4% | |
| Hypertension | 778 | 64% | 181 | 61% | 6.2% | 0.34 | 179 | 62% | 176 | 61% | 2.2% | |
| Hyperlipidemia | 784 | 65% | 188 | 64% | 2.3% | 0.7 | 184 | 64% | 182 | 63% | 1.5% | |
| Smoker | 632 | 52% | 154 | 52% | 0.2% | 0.99 | 149 | 52% | 148 | 52% | 0.70% | |
| Medications | Beta Blockers | 1043 | 86% | 243 | 82% | 11% | 0.09 | 233 | 81% | 238 | 83% | 4.5% |
| Diuretics | 881 | 73% | 207 | 70% | 6.0% | 0.35 | 189 | 66% | 203 | 71% | 10.5% | |
| Aldactone/Eplerenone | 336 | 28% | 69 | 23% | 10% | 0.13 | 69 | 24% | 68 | 24% | 0.82% | |
| Digoxin | 572 | 47% | 107 | 36% | 23% | 0.001 | 108 | 38% | 105 | 37% | 2.2% | |
| Anti-Arrhythmics | 249 | 21% | 63 | 21% | 1.9% | 0.77 | 62 | 22% | 62 | 22% | 0.0% | |
| Amiodarone | 194 | 16% | 55 | 19% | 6.9% | 0.28 | 51 | 18% | 54 | 19% | 2.7% | |
Diabetes mellitus was defined as fasting blood glucose >126 mg/dl or HbA1c ≥6.5 or taking hypoglycemic agents or insulin. Hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or taking anti-hypertensive medication. Hyperlipidemia was defined as elevated total cholesterol or low-density lipoprotein levels above the goals set by the ATPIII or taking cholesterol lowering agents.
The ACEi/ARB and No-ACEi/ARB patient groups exhibited significant differences in demographic and risk factors (Table 1) that may confound any association between the outcome (development of appropriate shock after ICD insertion) and ACEi/ARB medication intake. To minimize such confounding, we used propensity score matching to derive matched sub-cohorts of equal size. No-ACEi/ARB propensity score was derived via a non-parsimonious logistic multivariate regression model that considered No-ACEi/ARB as the dependent outcome variable. A total of 25 risk factors (identified from prior published trials and existing literature) were entered into the model. The resulting propensity scores were distinctly different for ACEi/ARB (Yes) versus ACEi/ARB (No) patients (mean [SD]: 0.813[0.079] vs. 0.768[0.097] respectively; p<.001]. The corresponding C-statistic values (area under the ROC curve) was 0.64±0.02 indicating fair-to-moderate discrimination. We obtained 1-to-1 matched cohorts where a given ACEi/ARB was always matched to the closest available No-ACEi/ARB counterpart to within ±1% difference. Adequacy of patient group matching was assessed by calculating the standardized difference, d(%), separately for each factor and based on whether they were continuous or categorical as previously published14.
Continuous data were expressed as mean (standard deviation) and categorical data were expressed as counts and percentages. Univariate comparisons were done with chi-square (X2) for categorical factors, while continuous factors were compared by independent t-test or Mann Whitney rank sum test based on normality. Survival comparisons were done via Kaplan-Meier analysis (Log rank test). The corresponding hazard ratios (95% confidence interval) were derived by proportional hazard Cox regression analysis.
Here, to control for potential interaction between ACEi/ARB medication and kidney function, a composite variable of 6 categories was developed as follows: (1) ACEi/ARB (yes), GFR:>60 ml/min/1.73m2, (2) ACEi/ARB (yes), GFR:30–60 ml/min/1.73m2 (3) ACEi/ARB (yes), GFR:<30 ml/min/1.73m2, (4) ACEi/ARB (no), GFR:>60 ml/min/1.73m2, (5) ACEi/ARB (no), GFR:30–60 ml/min/1.73m2, and (6) ACEi/ARB (no), GFR:<30 ml/min/1.73m2. A p<0.05 was used to indicate significance. Analyses were done using SPSS version 21.0 software (IBM, Armonk, NY).
Results
There were 1509 patients (mean age [SD]: 63[12] years, 80% male) with 1213 (80%) on ACEi and/or ARB after enrollment. Compared to ACEi/ARB patients, the patients not on ACEi/ARB (N=296, 20%) had worse kidney function, more advanced heart failure symptoms, and were less likely to be taking digoxin while other patient factors were similar (Table 1). After propensity matching, there were 287 patients in each group that were well matched (Table 1).
At a mean (SD) follow-up of 2.5(1.9) years, a total of 334 patients had experienced one or more appropriate shock (22%) in the entire study population. Patients who had an appropriate shock had more co-morbidities and were less likely to be on ACEi/ARB (Table 2).
Table 2.
Baseline characteristics stratified by presence or absence of appropriate shock
| Variable | SHOCK (N=334) | NO SHOCK (N=1175) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | ||
| Age (years) | 61 | 12 | 63 | 12 | 0.006 | |
| Systolic Blood Pressure (mm Hg) | 117 | 18 | 120 | 19 | 0.004 | |
| Glomerular filtration rate (ml/min/1.73m2) | 61 | 20 | 62 | 20 | 0.290 | |
| Ejection Fraction (%) | 20 | 6 | 21 | 6 | <.001 | |
| QRS interval (ms) | 140 | 35 | 135 | 35 | 0.015 | |
| QTC interval (ms) | 475 | 52 | 471 | 53 | 0.330 | |
| N | % | N | % | p-value | ||
| Men | 285 | 85% | 926 | 79% | 0.01 | |
| White | 272 | 81% | 1002 | 85% | 0.10 | |
| Age (years) | < 50 | 50 | 15% | 133 | 11% | 0.16 |
| 50–59 | 88 | 26% | 279 | 24% | ||
| 60–69 | 101 | 30% | 398 | 34% | ||
| ≥ 70 | 95 | 28% | 365 | 31% | ||
| Body mass index (kg/m2) | <21 | 11 | 3.3% | 47 | 4.0% | 0.16 |
| 21–25 | 43 | 13% | 201 | 17% | ||
| 25–30 | 155 | 46% | 489 | 42% | ||
| 30–35 | 71 | 21% | 279 | 24% | ||
| 35–40 | 40 | 12% | 105 | 8.9% | ||
| >40 | 14 | 4.2% | 54 | 4.6% | ||
| Glomerular filtration rate (ml/min/1.73m2) | >60 | 213 | 64% | 809 | 69% | 0.08 |
| 30–60 | 100 | 30% | 320 | 27% | ||
| <30 | 21 | 6.3% | 46 | 3.9% | ||
| New York Heart Association class | I | 45 | 14% | 158 | 13% | 0.76 |
| II | 189 | 57% | 695 | 59% | ||
| III | 97 | 29% | 314 | 27% | ||
| IV | 3 | 0.9% | 8 | 0.7% | ||
| Ischemic etiology | 252 | 75% | 823 | 70% | 0.05 | |
| Device Indications | Secondary | 102 | 31% | 267 | 23% | 0.00 |
| Defibrillator with biventricular pacing | 138 | 41% | 521 | 44% | 0.33 | |
| Prior myocardial infarction | 190 | 57% | 628 | 53% | 0.27 | |
| Diabetes mellitus | 112 | 34% | 408 | 35% | 0.69 | |
| Hypertension | 196 | 59% | 763 | 65% | 0.04 | |
| Hyperlipidemia | 221 | 66% | 751 | 64% | 0.45 | |
| Smoker | 178 | 53% | 608 | 52% | 0.62 | |
| Medications | ACEi/ARB | 252 | 75% | 961 | 82% | 0.01 |
| Beta Blockers | 281 | 84% | 1005 | 86% | 0.53 | |
| Diuretics | 253 | 76% | 835 | 71% | 0.09 | |
| Aldactone/Eplerenone | 99 | 30% | 306 | 26% | 0.19 | |
| Digoxin | 170 | 51% | 509 | 43% | 0.01 | |
| Antiarrythmics | 96 | 29% | 216 | 18% | <.001 | |
| Amiodarone | 77 | 23% | 172 | 15% | <.001 | |
Definitions as in table 1.
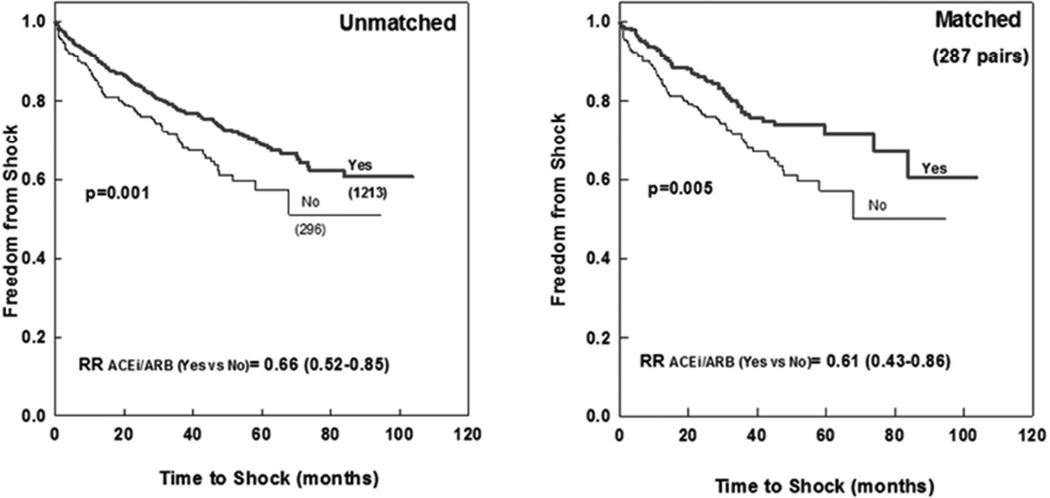
The 1, 3 and 5 years incidence of ICD shock in the matched cohort were 7.7%, 17%, 19% for patients on ACEi/ARB vs. 13%, 28%, and 32% for patients on no-ACEi/ARB (RR=0.61[0.43–0.86], p =0.005; Figure 1). On multivariate analysis, independent predictors of ICD shock included no-ACEi/ARB, lower GFR, younger age, reduced ejection fraction, wider QRS duration, and ischemic etiology (Table 3). The use of beta-blockers and biventricular pacing were not independent predictors of outcomes (p-value >0.2), and forcing them as factors in the multivariate model did not significantly alter the findings.
Figure 1.
Freedom from appropriate ICD shock in ACEi/ARB versus No-ACEi/ARB patient subcohorts: All patients (left) and propensity matched sub-cohorts (right).
Numbers in brackets represent the number of patients in each group. p-value represents log rank significance level ACEi (angiotensin converting enzyme inhibitor); ARB (angiotensin receptor blocker); ICD (implantable cardiac defibrillator)
Table 3.
Independent Predictors of Shock on Multivariate Cox Proportional Hazard Model
| Unmatched (N=1509) | Propensity Matched (N=574) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| ACEi/ARB *GFR | <.001 | 0.046 | ||
| ACEi/ARB (+), GFR (>60 ml/min/1.73m2) | 1.0 (Ref) | --- | 1.0 (Ref) | --- |
| ACEi/ARB (+), GFR (30–60 ml/min/1.73m2) | 1.28 (0.96–1.69) | 0.09 | 1.17 (0.65–2.11) | 0.61 |
| ACEi/ARB (+), GFR (<30 ml/min/1.73m2) | 2.35 (1.32–4.19) | 0.004 | 1.81 (0.69–4.78) | 0.23 |
| ACEi/ARB (−), GFR (>60 ml/min/1.73m2) | 1.45 (1.05–2.01) | 0.02 | 1.68 (1.08–2.62) | 0.02 |
| ACEi/ARB (−), GFR (30–60 ml/min/1.73m2) | 2.05 (1.32–3.16) | 0.001 | 2.15 (1.26–3.66) | 0.005 |
| ACEi/ARB (−), GFR (<30 ml/min/1.73m2) | 2.41 (1.18–4.95) | 0.02 | 2.34 (0.96–5.66) | 0.06 |
| Age (years) | 0.98 (0.97–0.99) | <.001 | 0.98 (0.97–0.99) | 0.04 |
| Systolic Blood Pressure (mm Hg) | 0.99 (0.99–1.00) | 0.03 | --- | --- |
| Ejection Fraction (%) | 0.98 (0.96–0.99) | 0.04 | 0.97 (0.94–0.99) | 0.03 |
| QRS (ms) | 1.004(1.001–1.007) | 0.02 | 1.005 (1.00–1.01) | 0.05 |
| Women | 0.70 (0.51–0.97) | 0.03 | --- | --- |
| Race (African American) | 1.65 (1.23–2.23) | 0.001 | --- | --- |
| Ischemic etiology | 1.39 (1.05–1.84) | 0.02 | 2.16 (1.29–3.62) | 0.003 |
| Digoxin | 1.24 (0.97–1.55) | 0.054 | --- | --- |
| Anti-arrhythmics | 1.60 (1.25–2.04) | <.001 | 1.44 (0.98–2.10) | 0.06 |
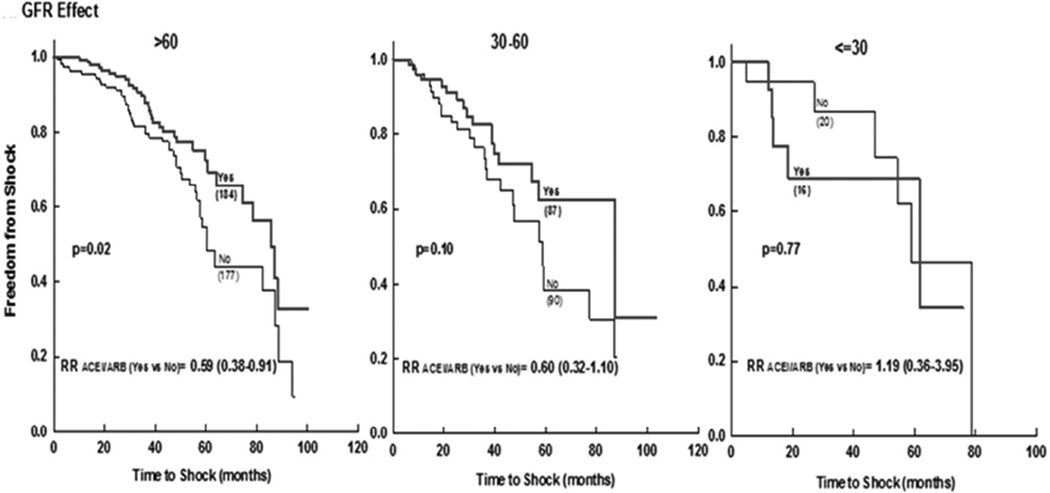
Patients with lower GFR were more likely to have received an ICD shock (Supplement figure 1/efigure 1). When stratified to GFR categories, the use of ACEi/ARB was associated with significantly fewer shocks for patients with GFR >60 ml/min/1.73m2 and with a trend for those with GFR 30–60 ml/min/1.73m2, but was not significant for those with GFR <30 ml/min/1.73m2 (Figure 2). There was a significant interaction between ACEi/ARB and GFR (p =0.046 in the matched cohorts). Among patients with normal GFR, those on no-ACEi/ARB were at 45% increased risk of ICD shock as compared to those on ACEi/ARB (Table 3). Similarly, patients with GFR 30–60 ml/min/1.73m2 on no-ACEi/ARB had more than 77% increased risk in shock as compared to those on ACEi/ARB (HR 2.05 [1.32–3.16] vs. 1.28 [0.96–1.69]). Those with GFR <30 ml/min/1.73m2 had the worst outcome irrespective of the use of ACEi/ARB (Table 3).
Figure 2.
Freedom from appropriate ICD shock in ACEi/ARB versus No-ACEi/ARB in matched patient subcohorts stratified by glomerular filtration rate.
ACEi (angiotensin converting enzyme inhibitor); ARB (angiotensin receptor blocker); GFR (glomerular filtration rate); ICD (implantable cardiac defibrillator).
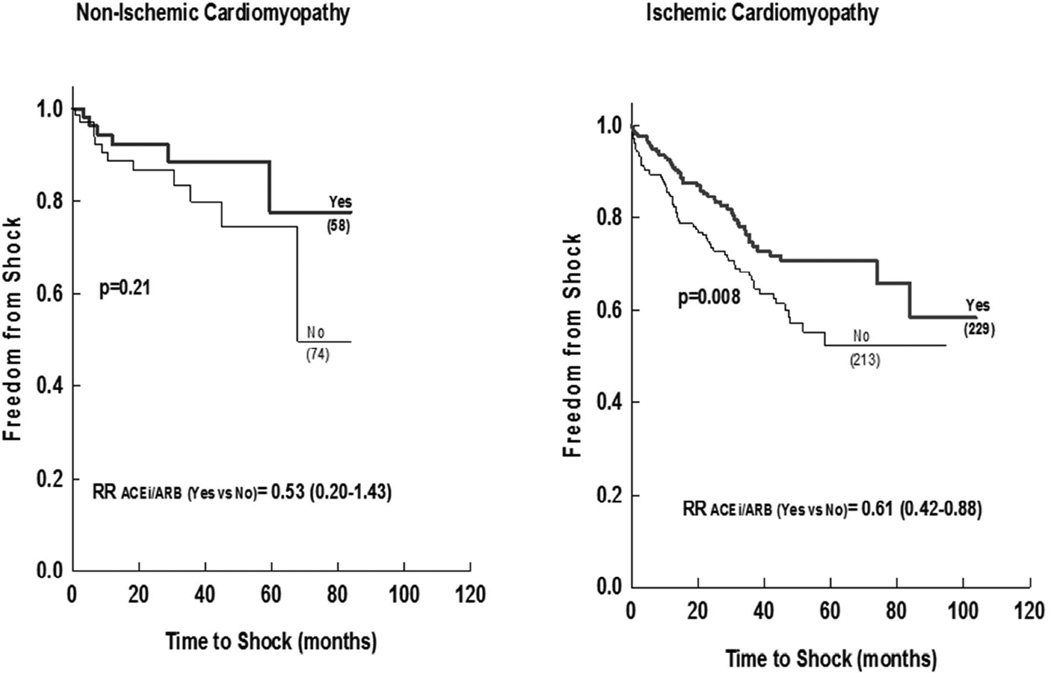
Furthermore, ACEi/ARB were associated with lower incidence of appropriate ICD shocks among patients with ischemic cardiomyopathy (matched cohort), but not among those with non-ischemic etiology, although the magnitude of the risk reduction was similar and with smaller number of non-ischemic patients (Figure 3).
Figure 3.
Freedom from appropriate ICD shock in ACEi/ARB versus No-ACEi/ARB in matched patient subcohorts stratified to Ischemic versus Non ischemic cardiomyopathy.
Numbers in brackets represent the number of patients in each group. p-value represents log rank significance level ACEi (angiotensin converting enzyme inhibitor); ARB (angiotensin receptor blocker); ICD (implantable cardiac defibrillator) GFR (glomerular filtration rate).
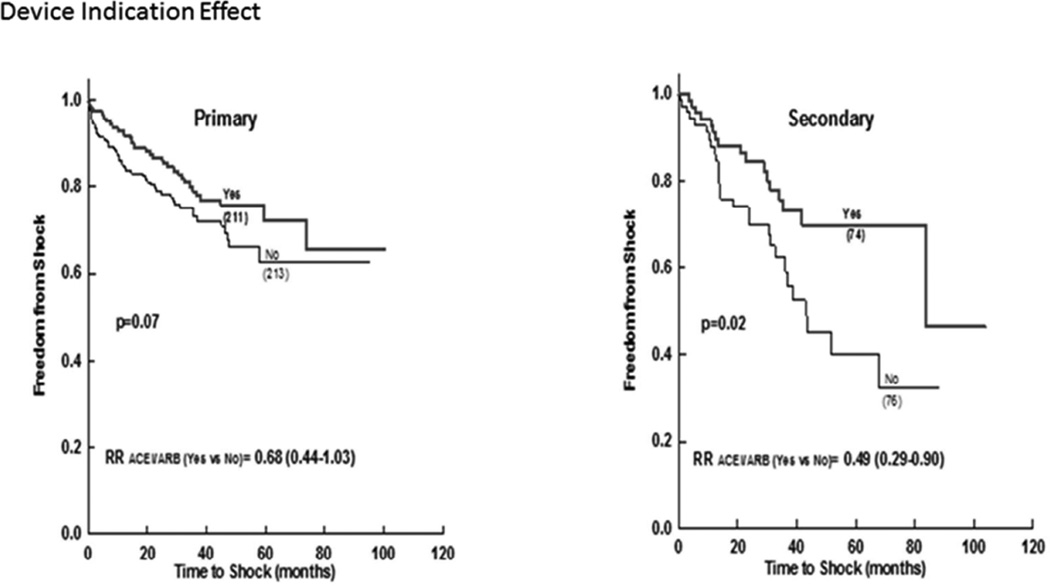
Patients receiving ICD for secondary prevention had significant more shocks as compared to those for primary prevention (Supplement figure 1/efigure 1). The use of ACEi/ARB was associated with lower incidence of appropriate ICD shocks in the secondary prevention group and with a trend in the primary prevention group (Figure 4).
Figure 4.
Freedom from appropriate ICD shock in ACEi/ARB versus No-ACEi/ARB in matched patient subcohorts stratified by indication for device implantation.
ACEi (angiotensin converting enzyme inhibitor); ARB (angiotensin receptor blocker); ICD (implantable cardiac defibrillator).
At the end of study follow-up, there were 388 total deaths (26%) and 479 (32%) subjects who reached the combined endpoint of death, transplant or ventricular assist device. In the matched cohorts, patients on ACEi/ARB had significantly lower mortality rate as compared to those without ACEi/ARB (23% vs. 29%, HR 0.74 [0.53–1.02], p =0.07), and similarly lower combined secondary endpoint with ACEi/ARB (27% vs. 34%, HR 0.75 [0.55–1.01], p=0.06). Patients who received any appropriate ICD shock during the follow-up period had significantly higher mortality as compared to those who did not (37% vs. 23%, p <0.001), and similarly for the combined endpoint. There was significant interaction between ACEi/ARB and GFR (p<0.001 for both secondary outcomes). On multivariate analysis, patients receiving ACEi/ARB and with normal GFR had lowest mortality, while those not receiving ACEi/ARB and/or with chronic kidney disease had worse outcome (supplement table1). Patients with GFR <30 ml/min/1.73m2 had highest mortality and secondary endpoint irrespective of the use of ACEi/ARB. Similar results were found for combined endpoint of death, transplant and ventricular assist device (supplement table 2).
Discussion
In our large cohort of subjects with severe cardiomyopathy and heart failure, we found that ACEi/ARB use is associated with significant lower incidence of appropriate ICD shocks in patients with normal or mild to moderate decrease in GFR. The results were most significant for patients with ischemic cardiomyopathy, those receiving the ICD for secondary prevention, and GFR ≥30ml/min/1.73m2; there was also a trend for lower ICD shock for patients receiving ACEi/ARB therapy with non-ischemic cardiomyopathy and device placement for primary prevention. Patients with GFR <30 ml/min/1.73m2 had the highest arrhythmic risk irrespective of the use of ACEi/ARB.
ACEi/ARB decrease cardiovascular mortality and all-cause mortality in patients with CHF, in post-MI patients with or without LV dysfunction, and also in patients with stable CAD. Several studies and secondary analyses of high risk patients and those with severe ischemic cardiomyopathy, showed significant 30–35% reduction in the risk of SCD, VT/VF or arrhythmic death with the use of ACEi15,16,17, 18, 19, while others showed no significant benefit20, 21. On the other hand, the absence of ACEi/ARB therapy was shown to be a predictor of appropriate ICD shock in a small study with limited events of 126 patients who had an ICD placed for primary prevention for severe cardiomyopathy12.
The reduction of ICD shock could be secondary to the role of ACEi/ARB in reducing interstitial fibrosis and scar formation. Indeed, recent studies showed that ACEi increases Connexin-43 (Cx43) levels in cardiomyopathy, a potential link for the effect seen22, 23. Also, ACEi/ARB prevent left ventricular remodeling, reduce concentrations of circulating angiotensin II and noradrenaline, increase baroreflex sensitivity and vagal tone, which might explain the relatively greater incidence of appropriate shocks in patients who were on no inhibitors of the renin-angiotensin system compared to patients to those who were.
We considered a number of potential subgroups including those with ischemic versus non-ischemic cardiomyopathy, renal dysfunction, and indication for device implantation. We found that patients with ischemic cardiomyopathy had more benefit if taking ACEi/ARB as compared to the non-ischemic subgroup (although the trend was present in the latter group), which could be partly related to larger scar burden24. Myocardial scarring however, is also seen in non-ischemic cardiomyopathy25, and may explain the trend we have.
When we pursued stratification by GFR, patients with lower GFR had more ICD shocks which is consistent with the literature reporting chronic kidney disease as an independent predictor of ICD shock26. The use of ACEi/ARB therapy, however, resulted in less ICD shock among patients with preserved and mild or moderate impairment in GFR but with neutral effect among those with GFR <30 ml/min/m2. Only 67 patients had eGFR < 30 ml/min/m2, so the absence of an association in this group likely is a reflection of inadequate statistical power. The lower incidence of appropriate ICD shocks from ACEi/ARB is not merely due to afterload reduction. Indeed, we did adjust for the use of other afterload reducing agents that are commonly used in HF when ACEi/ARB are contraindicated such as hydralazine and nitrates, and similarly for systolic blood pressure to account for afterload effect; there was no significant change in the overall results.
Recent data from the MADIT-CRT showed that the use of β-blockers is associated with less inappropriate ICD shocks and with preferential effect (carvedilol better than metoprolol)27. In fact, carvedilol was associated with a 36% lower rate of inappropriate ATP and shock therapy compared with metoprolol. However, the use of β-blockers was not predictive of appropriate ICD shocks in our cohort.
Strengths of our study include the large sample size, multicenter nature, and propensity matching. Yet, it is important to acknowledge several potential limitations. First, appropriate ICD shock is not a surrogate for SCD, but remains an important endpoint as ICD shocks are associated with quality of life, syncope and SCD. Second, ventricular tachycardia and fibrillation detection rates and number of patients programmed with anti-tachycardia pacing as initial therapy (both measures are important factors in reducing ICD shocks), were not available. Third, there were few missing values, particularly GFR, QRS and QTc that had to be imputed; however, running the analysis with and without the imputed values did not alter our results (Supplement Figure 2/efigure 2). We have assessed the use of ACEi/ARB at one time only; however, we could not account for the dose of the medication, duration of being on the medication, time of discontinuation if any, and the fact that some patients who were not on ACEi/ARB could have been placed on it during one of the follow-up visits. Finally, additional useful parameters such as brain natriuretic peptide and low-density lipoprotein levels were not available.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by a research grant from the National Heart, Lung, and Blood Institute (Grant # R01 HL77398).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 2.Schnee JM, Hsueh WA. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc Res. 2000;46:264–268. doi: 10.1016/s0008-6363(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 3.McEwan PE, Gray GA, Sherry L, Webb DJ, Kenyon CJ. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation. 1998;98:2765–2773. doi: 10.1161/01.cir.98.24.2765. [DOI] [PubMed] [Google Scholar]
- 4.Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324–330. doi: 10.1161/CIRCEP.110.959544. [DOI] [PubMed] [Google Scholar]
- 5.Scott PA, Rosengarten JA, Murday DC, Peebles CR, Harden SP, Curzen NP, Morgan JM. Left ventricular scar burden specifies the potential for ventricular arrhythmogenesis: A LGE-CMR study. J Cardiovasc Electrophysiol. 2013;24:430–436. doi: 10.1111/jce.12035. [DOI] [PubMed] [Google Scholar]
- 6.Karagueuzian HS. Targeting cardiac fibrosis: A new frontier in antiarrhythmic therapy? Am J Cardiovasc Dis. 2011;1:101–109. [PMC free article] [PubMed] [Google Scholar]
- 7.Massare J, Berry JM, Luo X, Rob F, Johnstone JL, Shelton JM, Bassel-Duby R, Hill JA, Naseem RH. Diminished cardiac fibrosis in heart failure is associated with altered ventricular arrhythmia phenotype. J Cardiovasc Electrophysiol. 2010;21:1031–1037. doi: 10.1111/j.1540-8167.2010.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. J Am Med Assoc. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 10.Kjekshus J, Swedberg K, Snapinn S. Effects of enalapril on long-term mortality in severe congestive heart failure. CONSENSUS trial group. Am J Cardiol. 1992;69:103–107. doi: 10.1016/0002-9149(92)90683-p. [DOI] [PubMed] [Google Scholar]
- 11.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The solvd investigattors. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 12.Obeyesekere MN, Chan W, Stub D, Prabhu S, Teo EP, Toogood G, Mariani J, Broughton A, Kistler PM. Left ventricular ejection fraction and absence of ace inhibitor/angiotensin II receptor blocker predicts appropriate defibrillator therapy in the primary prevention population. Pacing Clin Electrophysiol. 2010;33:696–704. doi: 10.1111/j.1540-8159.2009.02669.x. [DOI] [PubMed] [Google Scholar]
- 13.Refaat MM, Lubitz SA, Makino S, Islam Z, Frangiskakis JM, Mehdi H, Gutmann R, Zhang ML, Bloom HL, MacRae CA, Dudley SC, Shalaby AA, Weiss R, McNamara DM, London B, Ellinor PT. Genetic variation in the alternative splicing regulator rbm20 is associated with dilated cardiomyopathy. Heart Rhythm. 2012;9:390–396. doi: 10.1016/j.hrthm.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul C. 2009;38:1228–1234. [Google Scholar]
- 15.Das MK, Zipes DP. Antiarrhythmic and nonantiarrhythmic drugs for sudden cardiac death prevention. J Cardiovasc Pharmacol. 2010;55:438–449. [PubMed] [Google Scholar]
- 16.Cleland JG, Erhardt L, Murray G, Hall AS, Ball SG. Effect of ramipril on morbidity and mode of death among survivors of acute myocardial infarction with clinical evidence of heart failure. A report from the AIRE study investigators. Eur Heart J. 1997;18:41–51. [PubMed] [Google Scholar]
- 17.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong Ml. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 18.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril cardiac evaluation (TRACE) study group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 20.Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative north scandinavian enalapril survival study (CONSENSUS). The CONSENSUS trial study group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 21.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 22.Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC. Inhibition of c-src tyrosine kinase prevents angiotensin ii-mediated connexin-43 remodeling and sudden cardiac death. J Am Coll Cardiol. 2011;58:2332–2339. doi: 10.1016/j.jacc.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sovari AA, Rutledge CA, Jeong EM, Dolmatova E, Arasu D, Liu H, Vahdani N, Gu L, Zandieh S, Xiao L, Bonini MG, Duffy HS, Dudley SC., Jr Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ Arrhythm Electrophysiol. 2013;6:623–631. doi: 10.1161/CIRCEP.112.976787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong-Sit P, Klein GJ, Stirrat J, Fine N, Pallaveshi L, Wisenberg G, Thompson TR, Prato F, Drangova M, White JA. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: Evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:448–456. doi: 10.1161/CIRCIMAGING.111.971549. [DOI] [PubMed] [Google Scholar]
- 26.Hage FG, Aljaroudi W, Aggarwal H, Bhatia V, Miller J, Doppalapudi H, Wazni O, Iskandrian AE. Outcomes of patients with chronic kidney disease and implantable cardiac defibrillator: Primary versus secondary prevention. Int J Cardiol. 2013;165:113–116. doi: 10.1016/j.ijcard.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 27.Ruwald MH, Abu-Zeitone A, Jons C, Ruwald AC, McNitt S, Kutyifa V, Zareba W, Moss AJ. Impact of carvedilol and metoprolol on inappropriate implantable cardioverter-defibrillator therapy: The MADIT-CRT trial (multicenter automatic defibrillator implantation with cardiac resynchronization therapy) J Am Coll Cardiol. 2013;62:1343–1350. doi: 10.1016/j.jacc.2013.03.087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.