Abstract
Brain activation associated with normal and speeded comprehension of expository texts on familiar and unfamiliar topics was investigated in reading and listening. The goal was to determine how brain activation and the comprehension processes it reflects are modulated by comprehension speed and topic familiarity. Passages on more familiar topics differentially activated a set of areas in the anterior temporal lobe and medial frontal gyrus, areas often associated with text-level integration processes, which we interpret to reflect integration of previous knowledge with the passage content. Passages presented at the faster presentation resulted in more activation of a network of frontal areas associated with strategic and working-memory processes (as well as visual or auditory sensory-related regions), which we interpret to reflect maintenance of local coherence among briefly available passage segments. The implications of this research is to demonstrate how the brain system for text comprehension adapts to varying perceptual and knowledge conditions.
Keywords: fMRI, comprehension, speed reading, speed listening, working memory, topic familiarity
1.1 Introduction
Language comprehension involves the interaction of several different types of processes, including lower-level cognitive processes such as phonological and lexical analyses, and higher-level cognitive processes such as inference-making and inter-sentence integration (Mason & Just, 2013). It thus relies on a combination of local, word and sentence-level processes (local coherence), and more global, text-level processes (global coherence). Maintaining local coherence involves making associations between smaller units of information in the text passage (words and phrases) as well as monitoring coherent transitions from one clause to another. In contrast, sustaining global coherence involves establishing associations between ideas in the text and some overarching theme.
The higher-level processes of inference-making and integration of discourse information are especially reliant on prior familiarity with the text content. Inferential processes help establish global coherence by relating information in the text with prior knowledge (e.g., Graesser, Singer, & Trabasso, 1994; Kintsch &van Dijk, 1978; Long & Prat, 2008; Long et al., 2006). The more prior knowledge a reader possesses about the topic of a passage, the more likely it is that they will be able to recall information from the text (Bartlett, 1932; Bransford & Johnson, 1972; Long & Prat, 2002; Long et al., 2006). Many brain imaging studies have shown that these higher-level processes are underpinned by a combination of cortical networks that support the integration of information and comprehension (see Mason & Just, 2006; Mason & Just, 2011; Prat, Mason, & Just, 2011). One goal of the present study was to investigate these higher-level cognitive processes associated with reading and listening comprehension of text passages about topics that are more familiar or less familiar to the participants. The study aimed to contribute to the understanding of higher-level cognitive processes that underpin comprehension of different types of passages.
Another important aspect of comprehension is the cognitive and brain workload involved. For example, the workload associated with the comprehension processes may be influenced by time pressure for reading a passage. The study also investigated the higher-level cognitive processes associated with this time pressure for understanding text; we simulated speed reading and speed listening situations by speeding up the presentation of visual and auditory information.
Speed reading is a type of skilled reading in which readers attempt to increase their rate of reading without a commensurate loss in comprehension. However, reading at a faster pace may come at a cost of not only poorer comprehension but also a greater consumption of certain types of mental resources. Speed reading may require readers to engage comprehension strategies that trade away comprehension accuracy for speed (Just & Carpenter, 1987). Early studies of eye-fixations during speed reading reported that speed readers skipped large portions of the text and that their eye fixations traced a path different from the traditional left-to-right path of normal English readers (McLaughlin, 1969; Taylor, 1962). Just and Carpenter (1987) found that trained speed readers showed better speeded comprehension of high-level information than untrained speed readers, but only when the rapid reading was of a text on a familiar topic. Trained speed readers were better able than untrained speed readers to use their previous knowledge to bridge the information gaps that occur during speed reading (Just & Carpenter, 1987). Thus, speed reading may evoke strategies that focus on global coherence at the expense of local coherence, but such strategies may only be effective for familiar topics. Untrained readers, when faced with the novel task of speed reading, might rely more on executive control processes. In one fMRI study of trained and untrained speed readers of Japanese, trained speed readers' activation of the left inferior frontal gyrus (LIFG, or Broca's Area) and the left posterior superior temporal gyrus (Wernicke's Area) decreased during speed reading, in comparison with normal reading (Fujimaki, Hayakawa, Munetsuna, & Sasaki, 2004). According to the authors, the results suggest that trained speed readers bypass phonological processes during speed reading.
1.2 Neural substrates of discourse comprehension: the extended language network
The “language network” is a left-hemisphere-dominant cortical network traditionally implicated in the processing of language, and it centrally includes the Left Inferior Frontal Gyrus (LIFG, Broca's area), and the superior and middle areas of the posterior temporal lobe (Constable et al., 2004; Keller, Carpenter, & Just, 2001; Michael, Keller, Carpenter, & Just, 2001). In addition to these two classical language areas, various other areas of the brain have been associated with discourse processing, with the network constituency depending on the particular task. The dorsomedial prefrontal cortex and the anterior temporal lobes are part of this “extended language network” (Ferstl, Neumann, Bogler, & von Cramon, 2008). The anterior temporal lobe (aTL) areas (bilaterally) together with the left inferior frontal gyrus, have been associated with text integration processes in discourse comprehension (Mason & Just, 2006). Text integration, the construction of a meaning-based, integrated representation of the text, has been shown to activate the aTL when readers encounter an inconsistency in the text (Ferstl, Rinck, & von Cramon, 2005). In sum, both the dmPFC and aTL are activated in association with high-level, global comprehension processes. In the reading of familiar passages, readers should have sufficient background knowledge to perform an adequate level of text integration. Hence, we hypothesized that passage familiarity would modulate the activation in the brain areas associated with maintaining global coherence.
1.3 Working memory and local coherence processes in discourse comprehension
Local coherence processes depend on the reader's ability to establish connections between successive segments of information in text. To maintain local coherence, short-term maintenance of the text information is required. This maintenance of information may load on areas involved in therehearsal of information in working memory, including temporoparietal and frontal cortex (e.g. Buchweitz, Mason, Hasegawa, & Just, 2009; Buchweitz, Mason, Tomitch, & Just, 2009). Increased working memory load for both letters and words has been associated with activation in prefrontal and parietal areas of the brain (Crottaz-Herbette, Anagnoson, & Menon, 2004; Smith & Jonides, 1998). When information from different parts of a sentence or from adjacent sentences has to be related to each other, the earlier-occurring information has to be maintained in working memory until the later occurring information is encountered. For example, a person's name might have to be maintained until a subsequent pronoun occurs in order for the correct deictic reference to be made. The cortical areas associated with maintenance and rehearsal of information include the left inferior parietal lobe (LIPL), the left inferior frontal gyrus (LIFG), and the dorsolateral prefrontal cortex (DLPFC). LIPL and DLPFC form a frontoparietal loop that plays an important role in storage and manipulation of information in verbal working memory (Crottaz-Herbette et al., 2004; Petrides, Alivisatos, Evans, & Meyer, 1993).
The processes that support short-term coherence may be disrupted in speeded reading and listening. A fast rate of incoming information may result in a sampling of the text rather than an exhaustive intake of the information. In that case, information may have to be maintained for an unspecified amount of time until a segment of related information occurs or until the missing information is provided by making an inference or by tolerating the lack of coherence. We hypothesized that speeded comprehension would result in more activation in areas associated with maintaining local coherence processes.
Two experiments were carried out, one with listening comprehension and one with reading comprehension, with both studies comparing speeded and normal comprehension. Participants were college students untrained in the skill of speed reading. It was hypothesized that comprehending texts on unfamiliar topics would result in increased activation in brain regions involved in higher-level integration of text information. It was also hypothesized that increasing the speed of presentation for the passages would increase the activation levels in brain regions associated with working memory processes. These two hypotheses were expected to apply in both reading and listening, and modality-specific activation was expected in sensory/perceptual regions.
2. Material and Methods
2.1 Design
Two experiments were conducted (each using a 2 × 2 within-subjects factorial design) in which the independent variables were passage type (Familiar vs. Unfamiliar) and presentation rate (Fast vs. Normal). Participants read (Experiment 1) or listened to (Experiment 2) 16 passages in each experiment, including four passages per experimental condition.
2.2.1 Stimuli
The familiar passages were adapted from U.S. News and World Report articles on current technical topics, such as nutrition and health or forest fires. They were written in a way that made prior knowledge of the topic unnecessary for comprehension. The unfamiliar passages were adapted from an introductory physiology textbook (Sheeler, 1996) and focused on physiological information and principles, such as inheritance patterns of sex-linked diseases and information transfer within and between neurons. Although these passages dealt with less familiar topics, they were written in a straightforward, easy-to-understand style, consistent with the introductory nature of the textbook. The stimulus passages are included in the Supplementary Material.
The passages were the same in both experiments to facilitate comparisons across presentation modalities. The topics in the four conditions were as follows. Familiar/Normal rate: AIDS in elderly, Eating disorders, Fires, Secret Service; Familiar/Fast rate: Carbohydrates and fats, Food history, Caesarian section, Obesity; Unfamiliar/Normal rate: Carbon dioxide, Glands and hormones, Hemostasis, Osmosis; Unfamiliar/Fast rate: Impulse transmission, Pancreas, Pituitary Gland, Inheritance of sex-linked diseases. It is noteworthy that the measures of reading ease are similar between the familiar and unfamiliar passages (Flesch reading ease: familiar = 45.7, unfamiliar = 47.1; with both sets of passages being at grade 11 level). The content words in the familiar text passages had a higher mean lexical frequency (Kucera-Francis frequency: M = 217.13; SE = 35.74) than those in the unfamiliar text passages (M = 172.49; SE = 28.27) (Kucera & Francis, 1967). The mean content word length in the familiar passages (5.23 characters, SD = 0.22) did not significantly differ from the mean for the unfamiliar passages (5.26 characters, SD = 0.16). Additional text measures, such as the number of words and characters per passage, as well as summary measures (e.g., sentences per passage, words per sentence, readability measures and mean imagability per word) are provided in Supplementary Table 1 (word count and length for familiar passages), Supplementary Table 2 (word count and length for unfamiliar passages), and Supplementary Table 3 (summary statistics by condition). Imagability is also similar across the familiarity variable as well as presentation rate. Following the reading of each passage, participants were presented true-false questions that probed comprehension of the information in the passage. (The probes are presented in the Supplementary Material).
2.2.2 Speed of Presentation
Text and speech were presented in Normal and Fast presentation rates. In the Fast condition, more linguistic information (text in Experiment 1; speech in Experiment 2) was presented in the same amount of time, rather than presenting the same amount of information in a shorter amount of time. As shown in Supplementary Table 3, the texts in the Fast condition were on average 140 words long, whereas those in the Normal condition were on average 94 words long. We chose this design to provide comparable numbers of brain images, and hence statistical power, across conditions. This choice also means that both speed of presentation and amount of information differ between the presentation rate conditions.
2.2.3 Topic Familiarity Norming
Seven participants who did not take part in the fMRI experiments rated the passages on how much specific background knowledge was required to understand each passage; these participants used a 7-point scale: 1 = no specific domain knowledge needed for comprehension, 7 = a lot of specific domain knowledge needed for comprehension. As expected, the unfamiliar passages were rated as requiring more background knowledge (M = 4.31; SD = 1.03) than the familiar passages (M = 2.15; SD = 0.53); t (6) = 10.52, p < .001.
2.3 Procedure
One or two days prior to the scan, each participant was familiarized with the experimental task, and with the fMRI scanner environment and procedure in a simulator. At the beginning of the fMRI scan, participants were additionally given two practice trials (using passages different from the experimental stimuli) to re-acquaint them with the presentation modes. Participants were instructed to read (Experiment 1), or listen to (Experiment 2) each passage carefully, and to respond with a button-press to a visually presented true-or-false comprehension probe that followed each passage. Half of the probes were true in each of the four conditions.
2.4.1 fMRI acquisition parameters
The data were collected using a Siemens Allegra 3.0 T scanner with a commercial birdcage, quadrature-drive radio-frequency head coil. Data acquisition was conducted at the Brain Imaging Research Center jointly established by Carnegie Mellon University and the University of Pittsburgh. The studies were performed with a gradient echo, echo planar pulse sequence with TR = 1000 ms, TE = 30 ms and a 60° flip angle. Sixteen oblique-axial slices were imaged. Each slice was 5-mm thick with a gap of 1-mm between slices. The acquisition matrix was 64 × 64 with 3.125 × 3.125 × 5-mm voxels. The total number of volumes collected was 1184 (duration = 19:44 min) for Experiment 1 and 1114 volumes (duration = 18:34 min) for Experiment 2.
2.4.2 fMRI analyses
The data were analyzed using SPM2 (Wellcome Department of Cognitive Neurology). Images were corrected for slice acquisition timing, motion-corrected, normalized to the Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm voxels, and smoothed with an 8-mm Gaussian kernel to decrease spatial noise. Statistical analysis was performed on individual and group data by using the general linear model as implemented in SPM2 (Friston et al., 1995). The model for each participant included regressors for each of the four conditions of interest (Familiar and Unfamiliar text passage, and Normal and Fast speed of presentation) convolved with the canonical SPM2 hemodynamic response function. The model included the duration of each passage (from onset of text or speech, until the end of the passage). Additional regressors were included for the probe periods (time to read and respond to the comprehension probes) for each passage type, and for a “fixation” condition consisting of six 24-s presentations of an “X” included in each run of each experiment. (As detailed below in the methods for each experiment, 12-s “rest” intervals were also included after every trial but these were not explicitly modeled in the design matrix).
To compare the distribution of activation across the four experimental conditions, two methods were used. First, whole-brain, voxel-wise, 2 × 2 analyses of variance (ANOVAs) s (with repeated measures and participants treated as a random effect) were conducted to identify areas responsive to the main effects of familiarity, rate of presentation, and the interaction of the two variables, within each presentation modality; a similar 2 × 2 × 2 mixed ANOVA was conducted to compare across presentation modalities (a between-subjects variable). Second, t-test analyses were performed using a random-effects model because the sign of the t-value allows one to easily spatially segregate positive and negative differences in activation. For each condition, activation was assessed with t-tests using passage versus fixation contrast images (one per subject, per contrast). For the contrasts between familiar and unfamiliar passages, the brain activation for all passages for the Familiar condition (collapsing across Fast and Normal conditions) was compared with the brain activation for all passages for the Unfamiliar condition (collapsing across Fast and Normal conditions). For the contrasts between fast and normal speed of presentation, the brain activation for all passages for the Fast condition (collapsing across Familiar and Unfamiliar conditions) were compared with the brain activation for all passages for the Normal condition (collapsing across Familiar and Unfamiliar conditions).
All t-maps and F-maps were calculated for the entire cortical volume, thresholded at an uncorrected height threshold of p < .001 and an extent threshold of 20 voxels. Labels for coordinates of activation were confirmed in MNI space (Tzourio-Mazoyer et al., 2002) and the Talairach Daemon (Lancaster et al., 2000) as implemented in AFNI (Cox, 1996).
3.1 Experiment 1: Reading comprehension
3.1.1 Participants
Eleven English-speaking college students (4 females) contributed data to this experiment. Data from an additional four participants were excluded from the final analyses due to excessive head motion (> 3.0 mm) or poor comprehension performance (less than 75.0% accuracy on the comprehension questions). The eleven participants were aged 18 to 27 years (M = 21.64; SD = 2.54) and all were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Participants in both experiments gave signed informed consent approved by the University of Pittsburgh and the Carnegie Mellon University Institutional Review Boards.
3.1.2 Procedure
The passages were presented one word at a time using rapid serial visual presentation (RSVP), with each word centered on the screen during presentation. RSVP allows for control over the speed of reading of the participant (Potter, 1984). Words in the Normal presentation rate passages were displayed for 290.0 ms plus 8.0 ms for each character comprising the word (for example, the word “the” was presented for 290 ms + [3 × 8.0 ms] = 314.0 ms). Words in the Fast presentation rate passages (Speed Reading) were presented for 160.0 ms plus 8.0 ms per character (for example, the word “the” was presented for 160.0 ms + [3 × 8.0 ms] = 184.0 ms). These parameters were derived from previous studies of reading rates. On average, passages in the Normal condition were presented at a rate of 181 words per minute (wpm), and passages in the Fast (Speed Reading) condition were presented at 298 wpm. Passages in both Normal and Speed Reading conditions were presented for a total of approximately 31 seconds. The Speed Reading passages were longer (139-140 words) than the Normal Reading passages (93-94 words). The presentation speeds for both the rapid and normal rates were within the reading speed for the average college student (Potter, 1984).
The comprehension probe was presented on the screen 5.0 s after the passage. Participants had up to 7.5 s to respond to the probe. A 12-s rest interval followed each probe. The 12-s rest interval was either followed by another passage or by a 24-s fixation interval. During fixation, participants were asked to clear their minds and fixate on an “X” on the center of the screen. The 24-s fixation periods were used to provide a baseline measure of each participant's brain activation and were explicitly modeled in the analysis. The shorter 12-s rest intervals were included to allow the hemodynamic response from the last trial to decrease back toward the baseline level and to give the participant a chance to relax. These shorter rest intervals were not included as regressors in the analysis. There were a total of six fixation intervals. The passages and the 24-s fixation intervals were presented in a pseudo-randomized order.
3.1.3 Results and Discussion
3.1.3.1 Behavioral Results
At the normal presentation rate, participants were reliably more accurate and responded reliably faster to the probes for familiar than for unfamiliar passages (t (10) = 2.88, p < .05 for accuracy; t (10) = 4.36; p < .01 for reaction time). In contrast, at the faster presentation rate there was no advantage of familiarity for either behavioral measure. Table 1 shows mean comprehension accuracies and response times.
Table 1. Comprehension response times and accuracies.
| Normal Speed | Fast | |||
|---|---|---|---|---|
|
| ||||
| Reading | Familiar | Unfamiliar | Familiar | Unfamiliar |
| Mean RT ms (SD) | 3743 (708) | 4375 (1016) | 4135 (902) | 4249 (838) |
| Accuracy | 1.00 | 0.89 (0.13) | 0.98 (0.08) | 0.93 (0.12) |
|
| ||||
| Listening | ||||
| Mean RT ms (SD) | 4026 (441) | 4440 (502) | 3958 (455) | 4217 (684) |
| Accuracy | 0.94 (0.11) | 0.91 (0.12) | 0.97 (0.08) | 0.88 (0.131) |
3.1.3.2 fMRI results
An extensive set of regions were activated during the processing of the texts, varying as a function of both the speed and the familiarity of the text; in contrast, very few regions exhibited a reliable interaction of the two variables. We present the full results of the voxel-wise ANOVA in Supplementary Table 4, but for clarity of presentation we focus here on the results of group paired t-tests among conditions.
3.1.3.3 fMRI results: reading familiar versus unfamiliar text
Comprehension of familiar passages activated a network that included the dorsomedial prefrontal cortex, and anterior temporal and temporoparietal areas; these areas of the brain have been described in previous studies as part of an extended language network (Ferstl et al., 2008; Mason & Just, 2006, 2013). Unfamiliar passages activated the dorsolateral prefrontal cortex, left insula, and left inferior parietal cortex. The results are presented in Figure 1a and Table 2.
Figure 1.
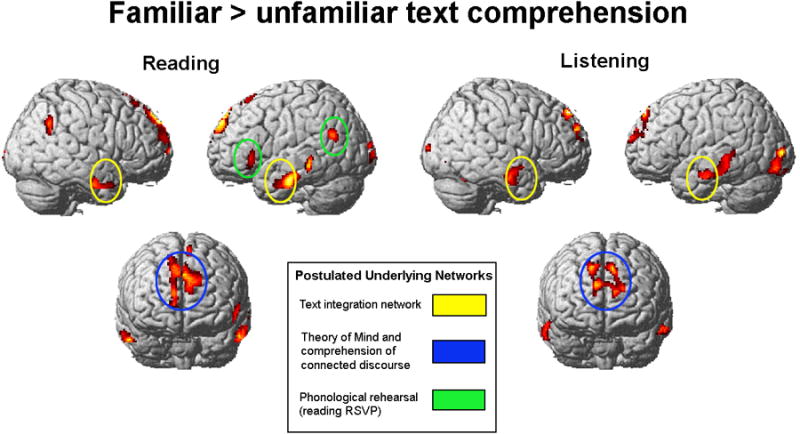
Comprehension of familiar text. Familiar passages > unfamiliar passages contrast for reading (Experiment 1) and listening comprehension (Experiment 2), collapsed across speeds of presentation. Familiar passages show more activation in the postulated language network and in bilateral medial frontal gyri. Yellow ellipses highlight the anterior and middle temporal lobes, and blue ellipses the dmPFC and superior frontal lobe activation common to reading and listening comprehension of familiar passages. Green ellipses highlight the left inferior frontal and left angular gyri network of activation found only for reading familiar passages. SPM2; clusters significant at p < .001, uncorrected, extent threshold = 20 voxels, t = 4.14 for reading comprehension (n = 11); t = 4.50 for listening comprehension (n = 9). Postulated networks: (1) text integration network and (2) theory of mind and comprehension of connected discourse (e.g. Ferstl et al., 2005, Mason & Just, 2006; Mason & Just, 2011; Prat et al., 2011); (3) phonological rehearsal (e.g. Buchweitz et al., 2009)
Table 2. Familiar > unfamiliar passages (collapsed across speeds).
| Cluster centroid | Cluster | t(12) | MNI coordinates | ||
|---|---|---|---|---|---|
|
| |||||
| Reading Comprehension | x | y | z | ||
| Frontal | |||||
| L sup med frontal gyrus | 1005 | 10.20 | -2 | 58 | 40 |
| L inf frontal gyrus (triang) | 86 | 7.99 | -56 | 26 | -2 |
| L sup med frontal gyrus | 64 | 6.78 | -6 | 28 | 60 |
| R sup frontal gyrus | 24 | 4.96 | 14 | 32 | 56 |
| Temporal | |||||
| L mid temporal gyrus | 459 | 6.75 | -56 | -14 | -24 |
| R inf temporal gyrus | 189 | 6.04 | 56 | 4 | -32 |
| L mid temporal gyrus | 146 | 6.23 | -68 | -38 | 0 |
| Parietal | |||||
| L precuneus | 342 | 6.83 | -4 | -56 | 30 |
| R angular gyrus | 143 | 6.12 | 52 | -56 | 40 |
| L angular gyrus | 171 | 5.39 | -58 | -66 | 20 |
| Occipital | |||||
| L mid occipital gyrus | 97 | 6.26 | -16 | -106 | 4 |
| L mid occipital gyrus | 43 | 5.70 | -26 | -100 | 12 |
|
| |||||
| Listening Comprehension | x | y | z | ||
|
| |||||
| Frontal | |||||
| L sup med frontal gyrus | 1205 | 10.11 | 12 | 50 | 46 |
| L medial frontal | 39 | 5.05 | -4 | 40 | -10 |
| Temporal | |||||
| R mid temporal gyrus | 278 | 7.63 | 66 | -10 | -22 |
| L mid temporal gyrus | 624 | 7.60 | -50 | -42 | 4 |
| Parietal | |||||
| R mid cingulate gyrus | 29 | 6.64 | 6 | -16 | 42 |
| Occipital | |||||
| L fusiform + lingual gyri | 95 | 10.75 | -26 | -70 | -8 |
| L mid occipital gyrus | 472 | 9.39 | -40 | -84 | -16 |
| R calcarine | 168 | 8.38 | 6 | -70 | 20 |
| R inf occipital gyrus | 88 | 8.37 | 38 | -86 | -10 |
| L lingual gyrus | 83 | 7.80 | -12 | -46 | -6 |
| L lingual gyrus | 226 | 7.59 | -12 | -84 | -14 |
| R lingual gyrus | 143 | 7.49 | 18 | -82 | -10 |
| R calcarine | 33 | 7.23 | 26 | -70 | 10 |
| L sup occipital gyrus | |||||
| Subcortical | |||||
| R + L parahipp gyrus | 827 | 8.21 | 14 | -30 | -14 |
| R parahippocampal gyrus | 39 | 5.05 | 22 | -10 | -24 |
Clusters significant at p < .001, uncorrected, extent threshold = 20 voxels. Region labels apply to the entire extent of the cluster with peak maxima designated by first locale cited. T-values and MNI coordinates are for the peak activated voxel in each cluster only.
Reading familiar passages also produced more activation in left inferior frontal gyrus and bilateral angular gyri, as well as a large activation cluster in the left precuneus (including some right precuneus and left posterior cingulate gyrus activation, as shown in Table 2).
3.1.3.4 Effects of speed reading
Fast relative to normal presentation speed resulted in more activation in a frontal-parietal network (LIFG and right angular gyrus). The right angular gyrus activation cluster overlaps with a large cluster that was also observed in a familiarity by speed interaction (where it had a volume of 122 voxels, centroid [32, -60, 48]; this result is listed in Supplementary Table 4). It is likely that the interaction effect was driven by the activation in familiar texts being greater during the fast presentation. Speed reading also produced more activation in the bilateral inferior occipital lobes. There were no significant differences for the normal > speed reading contrast. Figure 3 shows the brain activation for speed reading > normal reading (see also Table 4).
Figure 3.
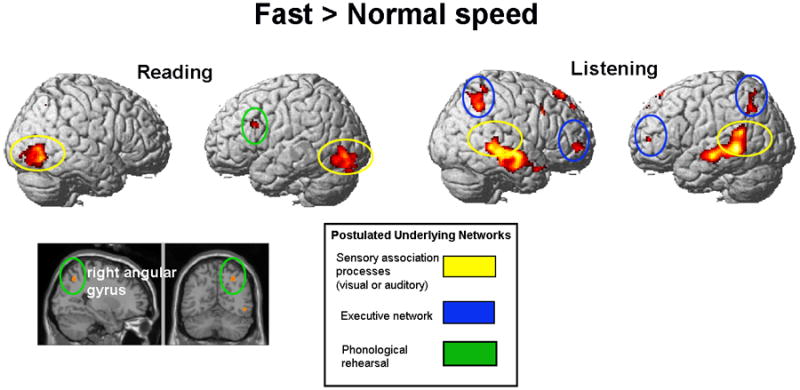
Faster comprehension: increased activation in visual and auditory association cortices. Fast presentation > normal presentation contrast for reading and listening comprehension (collapsed across familiarity). Speed reading (Experiment 1): left inferior frontal and right angular gyri, and bilateral occipital lobe. Speed listening (Experiment 2): bilateral dorsolateral prefrontal, parietal, and temporal cortices. SPM2; clusters significant at p < .001, uncorrected, extent threshold = 20 voxels, t = 4.14 for reading comprehension (n = 11); t = 4.50 for listening comprehension (n = 9). Postulated networks: (1) sensory association processes, visual or auditory (e.g. Michael et al., 2001); (2) executive network: (Crottaz-Herbette et al., 2004; Mason & Just, 2006; Smith et al., 1998; Smith & Jonides, 1998); (3) phonological rehearsal (e.g. Buchweitz et al., 2009).
Table 4. Speed reading > normal reading and speed listening > normal listening (collapsed across familiar and unfamiliar passages).
| Cluster centroid | Cluster | t(12) | MNI coordinates | ||
|---|---|---|---|---|---|
|
| |||||
| Speed Reading | x | y | z | ||
| Frontal | |||||
| L inf frontal gyrus | 504 | 9.47 | -56 | 20 | 28 |
| Parietal | |||||
| R angular gyrus | 25 | 5.23 | 28 | -62 | 46 |
| Occipital | |||||
| R inf occipital lobe | 504 | 9.47 | 56 | -68 | -8 |
| L inf occipital lobe | 550 | 7.10 | -42 | -70 | -10 |
|
| |||||
| Speed Listening | x | y | z | ||
| Frontal | |||||
| R sup medial frontal gyrus | 126 | 6.77 | 12 | 38 | 56 |
| R sup frontal gyrus (orb) | 44 | 5.87 | 20 | 44 | -10 |
| R sup medial frontal gyrus | 32 | 5.61 | 14 | 50 | 42 |
| R inf frontal gyrus | 37 | 5.05 | 54 | 16 | 38 |
| Temporal | |||||
| L mid temporal gyrus | 1395 | 12.41 | -54 | -46 | -2 |
| R mid temporal gyrus | 1595 | 9.98 | 56 | -10 | -10 |
| R mid temporal pole | 44 | 8.23 | 54 | 10 | -24 |
| Parietal | |||||
| R sup parietal lobe | 41 | 9.41 | 40 | -48 | 66 |
| L inf parietal lobe | 143 | 7.44 | -56 | -58 | 44 |
| L post cingulate gyrus | 44 | 7.40 | -2 | -36 | 28 |
| R inf parietal lobe | 327 | 7.26 | 48 | -50 | 44 |
Clusters significant at p < .001, uncorrected, extent threshold = 20 voxels. Region labels apply to the entire extent of the cluster. T-values and MNI coordinates are for the peak activated voxel in each cluster only.
3.2 Experiment 2: Listening comprehension
3.2.1 Participants
Nine right-handed English-speaking college students (3 females, ages 18 to 25 years (M = 20.54; SD = 2.06)), none of whom participated in Experiment 1, contributed data to this experiment. Data from four additional participants were excluded from the final analysis due to poor comprehension performance (less than 75% accuracy in the comprehension questions).
3.2.2 Materials and procedure
The passages were auditorily presented through pneumatic headphones. The recorded passages were digitized and processed using Gold Wave software to equate presentation amplitude and to modify the rate of presentation without excessively altering the frequency of the speech. On average, audio file presentation rate was 175 wpm for the Normal speed, and 270 wpm for the Fast condition (Speed Listening). The duration of the recorded files were equated to the nearest second. Each trial lasted approximately 32 sec (+/- 1 sec).
The comprehension probe was presented on the screen 5.0 s after the passage. Participants had up to 6.5 s to respond to the probe. A 12-s rest interval followed each probe. The 12-s rest interval was either followed by another passage or by a 24-s fixation interval, with the instructions for this condition identical to those in the reading experiment and the same pseudo-randomized order of the passages and the six fixation intervals used in that experiment.
3.2.3 Results and discussion
3.2.3.1 Behavioral results
At the normal presentation rate, participants responded to probe questions more accurately and response times were more rapid for the familiar than for the unfamiliar passages. These results partially replicate the behavioral results in the reading experiment. Although this difference was not reliable for the accuracy measure, it was for the response time measure (t (8) = 3.84; p < .01). At the faster presentation rate, there were no significant differences in comprehension accuracy or response time results for the comparison of unfamiliar and familiar passages. As in Experiment 1, the advantage of familiar passages over unfamiliar passages at the normal presentation rate was not found with more rapid presentation. Table 1 shows the behavioral results for Experiments 1 and 2.
A mixed ANOVA (with modality as a between-subjects variable and speed and familiarity as a within-subject variables) was conducted to evaluate whether familiarity and rate of presentation had different behavioral effects across the two modalities. There was no effect of modality (all F's < 1.0) nor were there any significant interactions among the three factors (all p's >.10). Table 1 shows the behavioral results for Experiments 1 and 2.
3.2.3.2 fMRI results: Listening to familiar versus unfamiliar text
The fMRI listening comprehension results of Experiment 2 were consistent with those of Experiment 1 with respect to the effects of topic familiarity. For contrasts between the familiar and unfamiliar passages, activation of the same two condition-specific networks of cortical areas was observed. Familiar passages resulted in more activation than unfamiliar passages in the dmPFC and the aTL network. For unfamiliar passages, the contrast with familiar passages showed activation of DLPFC and parietal cortex. Comprehension of unfamiliar relative to familiar passages also produced more activation in a network of areas that included the left inferior frontal gyrus (including pars triangularis) and left inferior parietal lobe.
3.2.3.3 Effects of speeded listening
As expected, listening to the faster rate of speech resulted in more activation than listening to speech at the normal speed in auditory sensory association cortex (recall that speed reading resulted in more activation in visual sensory association cortex than reading at the normal rate in Experiment 1). Speed listening also produced more activation in the bilateral inferior parietal lobe, and superior and medial frontal lobes. There were no significant differences for the normal > speed listening contrast (Figure 3, Table 4).
3.3 Comparison of effects across modalities
As shown in Figures 1 and 2, the networks of voxels responding to the familiarity variable (familiar versus unfamiliar texts) were similar in the two modalities. In fact, when the familiarity effect was compared across the two modalities, only one cluster of differential activation in the right supramarginal gyrus was present. This cluster was not present in either the reading or listening tasks, and occurs only in this contrast.
Figure 2.
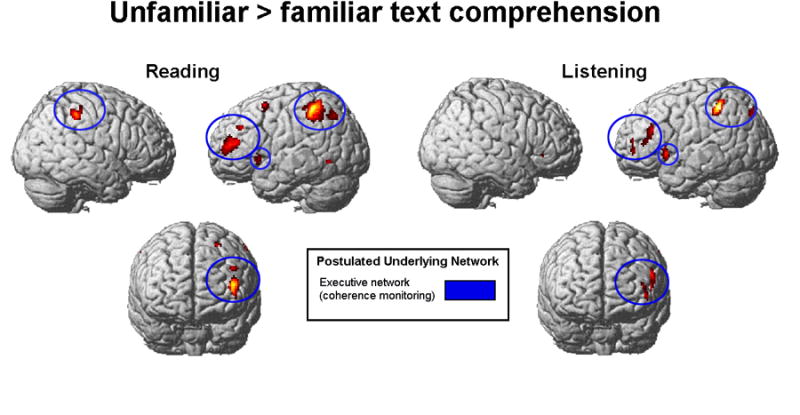
Comprehension of unfamiliar text. Unfamiliar passages > familiar passages contrast for reading (Experiment 1) and listening (Experiment 2) comprehension, collapsed across speeds. Unfamiliar passages show more activation in middle frontal gyrus (including DLPFC), in left insula, and in left parietal lobe. Yellow ellipses highlight the inferior frontal-parietal network of activation, blue ellipses highlight the middle frontal gyrus activation, both common to reading and listening comprehension of unfamiliar passages. Green ellipses highlight posterior parietal activation. SPM2; clusters significant at p < .001, uncorrected, extent threshold = 20 voxels, t = 4.14 for reading comprehension (n = 11); t = 4.50 for listening comprehension (n = 9). Postulated networks: executive network (coherence monitoring) (e.g. (Crottaz-Herbette et al., 2004; Mason & Just, 2006; Smith, Jonides, Marshuetz, & Koeppe, 1998; Smith & Jonides, 1998).
The results for speed listening were not identical to those for speed reading. The activation in bilateral middle temporal gyri, two small right frontal clusters, and a right precentral gyrus cluster were significantly different in direct contrasts of modalities. This interaction in the bilateral temporal clusters is of course expected, and reflects the sensitivity of auditory association areas to increased presentation rate in the auditory modality, but not in reading. The two frontal clusters showing this interaction also reflected a simple main effect of speed in the listening modality but not in the visual, activating more strongly as speech rate increased, but not as reading rate increased. In contrast, the right precentral gyrus cluster did not show a simple main effect of speed in either modality, and interpretation of the speed by modality interaction here must be tempered by the fact that it reflects subthreshold differences in the modulation of activation by speed of presentation. Finally, there were no significantly active voxels in a familiarity by speed of presentation by modality interaction. The full set of reliable effects and locations from this voxel-wise 3-way mixed ANOVA are presented in Table 5.
Table 5. Cross modality analysis of whole brain data.
| Cluster centroid | Cluster | F(1,30) | MNI coordinates | ||
|---|---|---|---|---|---|
|
| |||||
| Cross Modality | x | y | z | ||
| Familiarity × Modalitiy | |||||
| Parietal | |||||
| R supramarginal | 90 | 21.62 | 66 | -22 | 22 |
|
| |||||
| Speed × Modality | |||||
| Frontal | |||||
| R med sup frontal gyrus * | 27 | 22.94 | 12 | 32 | 58 |
| R sup frontal gyrus * | 45 | 20.79 | 20 | 46 | -10 |
| R precentral | 26 | 15.76 | 26 | -14 | 52 |
| Temporal | |||||
| R mid temporal gyrus * | 645 | 53.00 | 58 | -10 | -12 |
| L mid temporal gyrus * | 502 | 24.50 | -60 | -24 | -4 |
|
| |||||
| Familiarity × Speed × Modality | |||||
| No significant clusters | |||||
Clusters significant at p < .001, uncorrected, extent threshold = 20 voxels. Region labels, T-values and MNI coordinates are for the peak activated voxel in each cluster.
indicates clusters which remain when masked by within modality effects.
4. Discussion
The study reveal show the comprehension of expository information about topics that are either more or less familiar is reflected in the modulation of activation of different networks of the brain. The activation findings suggest that comprehension of familiar topics relies more on high-level, global coherence-building processes, and that comprehension of unfamiliar topics relies more on local coherence maintenance processes. The proposed account of the results presents a unifying view of the pattern of activation across the two experiments. We argue below that the underlying cognitive processes that we postulate are consistent with prior findings on brain activation in text comprehension. However, as is the case whenever applying a “reverse inference” (inferring the processes from the activation locations based on previous findings of activation in that location), alternative accounts may exist and future experiments will be needed to strengthen or reject the proposed account.
4.1 Comprehension of familiar text: Neural networks for maintaining global coherence
The cortical network activated during the comprehension of a passage on a more familiar topic relative to a less familiar one included the dorsomedial prefrontal cortex and bilateral anterior temporal lobe, and the activation of this network was consistent across both presentation modalities. This network of areas is well-known for its association with executive functions; in discourse comprehension studies, it has been associated with coherence monitoring processes (Ferstl et al., 2008; Mason & Just, 2006). The dmPFC activates during inference processes in text comprehension (Ferstl & von Cramon, 2001; Ferstl et al., 2005; Mason, Williams, Kana, Minshew, & Just, 2008). The similarity of the activation for our familiar expository passages to activation observed during the reading of narrative texts suggests that familiar expository texts are processed using the extended language network. This potentially entails generating inferences and making associations between the information in the text and the reader's knowledge of the world. In both experiments, according to our interpretation, the increased activation during familiar topic comprehension in areas associated with inference processes reflects the increased involvement of such processes to establish global coherence under those circumstances.
4.2 Comprehension of unfamiliar text: Neural networks for maintaining local coherence
The comprehension of unfamiliar topic passages, relative to familiar topic passages, resulted in activation in a network including dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, inferior frontal gyrus, and left inferior and posterior parietal lobe. The ventrolateral prefrontal cortex activation is similar to the activation found in controlled access to stored conceptual representations (Badre & Wagner, 2007). This controlled access to individual concepts in unfamiliar texts may have precluded generating inferences about that information (consistent with the lack of activation in the dmPFC-aTL network). The results also show some similarity in the networks activated for reading and listening comprehension of unfamiliar passages; the similarity may suggest that the source of the additional activation for unfamiliar passages was common across modalities.
4.3 Speed reading and speed listening effects on brain activation
Increased speed of presentation resulted in increased activation in both listening and reading comprehension. The left inferior frontal gyrus was more active for speed reading than for reading text presented at a normal rate. For speed listening, faster speech presentation resulted in increased activation in the bilateral inferior parietal lobe, and superior and medial frontal lobes. While these are different regions, none of them survived a direct contrast across modalities. It may be that the regions activated during fast presentation, though in different locations, were reflective of additional demands on phonological working memory processes for the maintenance of the visually presented information. The activation of bilateral parietal and portions of the frontal lobe has been previously associated with the maintenance of information in a phonological form (Newman, Just, & Carpenter, 2002; Petrides, 1995). In both experiments, according to our interpretation, the increased activation during faster comprehension in brain areas associated with strategic and working memory processes reflects the greater draw on such processes that maintain local coherence in those circumstances.
Although the speech compression altered the acoustic signal in listening in a way that speeded RSVP did not alter the visual stimulus in reading, as the results show, the comparison between modalities can be meaningful. For example, participants were able to maintain a high level of comprehension in both modalities (93% in reading, 88% in listening). Second, a commonality of the results for speed reading and speed listening was that both resulted in greater activation in the corresponding modality-specific association areas of the brain: secondary visual areas (occipital lobe and inferior temporal lobe) for reading and secondary auditory areas (superior temporal lobe) for listening. The higher presentation rate (information per unit time) resulted in an increase in the activity-dependent blood-oxygenation-level-dependent signal measured in the corresponding sensory association cortices for the two modalities.
5. Conclusion
The study shows that familiar passages presented in either modality trigger increased activity associated with high-level cognitive processing of connected discourse, such as semantic integration of sentence-level information and building a conceptual representation of the passage. We postulate that passages on familiar topics evoke increased computation of global coherence, whereas passages on unfamiliar topics evoke increased computation of local coherence. Fast presentation rates in either modality resulted in more activation in regions associated with working memory and in sensory processing regions. Together, the findings show the exquisite adaptability of the comprehending brain to variations in the availability of knowledge and time.
Supplementary Material
Table 3. Unfamiliar > familiar passages (collapsed across speeds).
| Cluster centroid | Cluster | t(12) | MNI coordinates | ||
|---|---|---|---|---|---|
|
| |||||
| Reading | x | y | z | ||
| Frontal | |||||
| L mid frontal gyrus | 86 | 7.00 | -20 | 10 | 52 |
| L mid frontal gyrus | 221 | 6.88 | -42 | 46 | 16 |
| L mid frontal gyrus | 29 | 5.10 | -42 | 36 | 32 |
| L insula | 111 | 5.10 | -36 | 18 | 2 |
| Temporal | |||||
| L inf temporal gyrus | 20 | 4.87 | -58 | -60 | -6 |
| Parietal | |||||
| L inf parietal lobe | 717 | 8.60 | -38 | -46 | 40 |
| R inf parietal lobe | 147 | 6.43 | 46 | -36 | 46 |
| R precuneus | 89 | 5.09 | 14 | -58 | 50 |
|
| |||||
| Listening | x | y | z | ||
|
| |||||
| Frontal | |||||
| L inf frontal gyrus | 59 | 8.69 | -52 | 32 | 26 |
| L insula | 84 | 8.11 | -38 | 16 | 2 |
| L mid frontal gyrus | 27 | 7.35 | -46 | 52 | 2 |
| R insula | 74 | 3.87 | 36 | 30 | -2 |
| Parietal | |||||
| L inf parietal lobe | 247 | 9.65 | -52 | -44 | 56 |
| L sup parietal lobe | 27 | 5.27 | -22 | -78 | 44 |
Clusters significant at p < .001, uncorrected, extent threshold = 20 voxels. Region labels apply to the entire extent of the cluster. T-values and MNI coordinates are for the peak activated voxel in each cluster only.
Acknowledgments
This research was funded by the National Institute of Mental Health Grant MH029617.
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bartlett FC. Remembering : A Study in Experimental and Social Psychology. Cambridge: Cambridge Univ Press; 1932. [Google Scholar]
- Bransford JD, Johnson MK. Bransford-Johnson-1972-context-and-memory.pdf. Journal of Verbal Learning and Verbal Behavior. 1972;11:717–726. [Google Scholar]
- Buchweitz A, Mason RA, Hasegawa M, Just MA. Japanese and English sentence reading comprehension and writing systems: An fMRI study of first and second language effects on brain activation. Bilingualism Cambridge England. 2009;12(January):141–151. doi: 10.1017/S1366728908003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchweitz A, Mason R, Tomitch L, Just M. Brain activation for reading and listening comprehension: An fMRI study of modality effects and individual differences in language comprehension. Psychology and Neuroscience. 2009;2(2):111–123. doi: 10.3922/j.psns.2009.2.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni W, Shankweiler D. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. NeuroImage. 2004;22(1):11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8812068. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. NeuroImage. 2004;21(1):340–351. doi: 10.1016/j.neuroimage.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Human Brain Mapping. 2008;29(5):581–93. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl EC, Rinck M, von Cramon DY. Emotional and temporal aspects of situation model processing during text comprehension: an event-related fMRI study. Journal of Cognitive Neuroscience. 2005;17(5):724–39. doi: 10.1162/0898929053747658. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. The role of coherence and cohesion in text comprehension: an event-related fMRI study. Brain Research Cognitive Brain Research. 2001;11(3):325–40. doi: 10.1016/s0926-6410(01)00007-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11339984. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J, Heather JD, Frackowiak RSJ. Spatial Registration and Normalization of Images. 1996;189(1995) [Google Scholar]
- Fujimaki N, Hayakawa CAT, Munetsuna S, Sasaki T. Neural activation dependent on reading speed during covert reading of novels. 2004;15(2) doi: 10.1097/01.wnr.0000097385.12920.79. [DOI] [PubMed] [Google Scholar]
- Graesser aC, Singer M, Trabasso T. Constructing inferences during narrative text comprehension. Psychological Review. 1994;101(3):371–95. doi: 10.1037/0033-295x.101.3.371. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7938337. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. Speed Reading. In: Just MA, Carpenter Pa, editors. The Psychology of Reading and language Comprehension. Newton: Allyn and Bacon; 1992. pp. 425–452. [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11(3):223–37. doi: 10.1093/cercor/11.3.223. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11230094. [DOI] [PubMed] [Google Scholar]
- Kintsch W, Dijk TA Van. Psychological Review. 1978;(5) [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-Day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10912591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DL, Prat CS. Memory for Star Trek : The role of prior knowledge in recognition revisited. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28(6):1073–1082. doi: 10.1037//0278-7393.28.6.1073. [DOI] [PubMed] [Google Scholar]
- Long DL, Prat CS. Individual differences in syntactic ambiguity resolution: Readers vary in their use of plausibility information. Memory & Cognition. 2008;36(2):375–391. doi: 10.3758/MC.36.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DL, Wilson J, Hurley R, Prat CS. Assessing text representations with recognition: The interaction of domain knowledge and text coherence. Journal of Experimental Psychology Learning, Memory, and Cognition. 2006;32(4):816–27. doi: 10.1037/0278-7393.32.4.816. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. Neuroimaging contributions to the understanding of discourse processes. In: Traxler M, Gernsbacher MA, editors. Handbook of Psycholinguistics. Amsterdam: Elsevier; 2006. pp. 765–799. [Google Scholar]
- Mason RA, Just MA. Differentiable cortical networks for inferences concerning people's intentions versus physical causality. Human Brain Mapping. 2011;32(2):313–29. doi: 10.1002/hbm.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA. Identifying component discourse processing from their fMRI time course signatures. In: Britt MA, Goldman SR, Rouet JF, editors. Reading-from works to multiple texts. New York: Routledge; 2013. pp. 147–159. [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–80. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin GH. Reading at impossible speeds. Journal of Reading. 1969;12:449. ff. [Google Scholar]
- Michael EB, Keller TA, Carpenter PA, Just MA. fMRI investigation of sentence comprehension by eye and by ear: modality fingerprints on cognitive processes. Human Brain Mapping. 2001;13(4):239–52. doi: 10.1002/hbm.1036. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11410952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Just MA, Carpenter PA. The Synchronization of the Human Cortical Working Memory Network. 2002;822:810–822. doi: 10.1006/nimg.2001.0997. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinborgy inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional Organization of the Human Frontal Cortex for Mnemonic Processing. Annals of the New York Academy of Sciences. 1995;769(1 Structure and):85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(3):873–7. doi: 10.1073/pnas.90.3.873. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=45772&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC. Rapid Serial Visual Presentation (RSVP): A Method for Studying Language Processing. In: Kieras DE, Just MA, editors. New methods in reading comprehension research. Hillsdale: Lawrence Earlbaum; 1984. pp. 91–118. [Google Scholar]
- Prat CS, Mason RA, Just MA. Individual differences in the neural basis of causal inferencing. Brain and Language. 2011;116(1):1–13. doi: 10.1016/j.bandl.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeler P. In: Essentials of human physiology. 2nd. Sheeler P, editor. Dubuque: Wm. C. Brown Publishers; 1996. [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(20):12061–8. doi: 10.1073/pnas.95.20.12061. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=21765&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe Ra. Components of verbal working memory: evidence from neuroimaging. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):876–82. doi: 10.1073/pnas.95.3.876. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=33811&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. An evaluation of forty-one trainees who had recently completed the “Reading Dynamics” program. In: Bliesmer EP, Staiger RC, editors. Problems, programs, and projects in college adult reading Eleventh yearbook of the National Reading Conference. Milwaukee: National Reading Conference; 1962. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


