Abstract
AIM
Medical Emergency Teams (MET) activations are more frequent during daytime and weekdays, but whether due to greater patient instability, proximity from admission time, or caregiver concentration is unclear. We sought to determine if instability events, when they occurred, varied in their temporal distribution.
METHODS
Monitoring data were recorded (frequency 1/20Hz) in 634 SDU patients (41,635 monitoring hours). Vital sign excursion beyond our MET trigger thresholds defined alerts. The resultant 1,399 alerts from 216 patients were tallied according to clock hour and time elapsed since admission. We fit patient ID (n=216), clock hour, time since SDU admission, and alert present into a null model and three mixed effect logistic regression models: clock hour, hours elapsed since admission, and both clock hour and time elapsed since admission as fixed effect covariates. We performed likelihood ratio tests on these models to assess if, among all alerts, there were proportionally more alerts for any given clock hour, or proximity to admission time.
RESULTS
Only time elapsed since admission (p<0.001), and not clock hour adjusting for time elapsed since admission (p=0.885), was significant for temporal disproportion. Results were unchanged if the first 24 hours following admission were excluded from the models.
CONCLUSION
Although instability alerts are distributed most frequently within 24 hours after SDU admission in unstable patients, they are otherwise not more likely to distribute proportionally more frequently during certain clock hours. If MET utilization peaks do not coincide with admission time peaks, other variables contributing to unrecognized instability should be explored.
INTRODUCTION
Medical Emergency Teams (MET) are a portion of the efferent arm of Rapid Response Systems (RRS).1 METs are meant to be activated to support patients outside of intensive care units when they become unstable, and their needs exceed what the ward or step-down unit (SDU) can offer. The afferent arm of the RRS is based upon bedside caregivers “tracking” of patients’ conditions and then activating the MET based upon locally agreed upon “triggering” criteria.1 Though commonly used, MET efficacy in improving outcomes and decreasing mortality is still unproven.2,3 This lack of mortality benefit has been postulated to be due to RRS afferent arm failure, 4 even in ward and SDU environments where patients are continuously monitored.
In support of this hypothesis, MET activation is widely reported to be more frequent during weekdays than on weekends5, 6 and during daylight rather than early evening and nighttime hours.5-7 However, it is not known if such MET activation clustering is due to true temporal variation in the distribution of instability. We sought to determine if instability events, when they occurred, varied in their temporal distribution according to clock hour, or day of week. We examined instability according to our local MET track and trigger abnormal vital sign (VS) criteria for a cohort of SDU patients with continuously monitored VS. Lack of temporal variation in instability distribution would suggest that mechanisms other than continuous single VS monitoring are needed to enhance instability detection and support the RSS afferent arm.
METHODS
Following Institutional Review Board approval we collected continuous VS data streams, including HR (3-lead ECG), RR (bioimpedance signaling), SpO2 (pulse oximeter Model M1191B, Phillips, Boeblingen, Germany; clip-on reusable finger sensor), and intermittent noninvasive BP (minimum frequency two hours), from all patients over two sequential but separate 8 week periods in a 24-bed adult surgical-trauma SDU (Level-1 Trauma Center). This yielded monitoring data on 642 patient admissions, and a total of 41,635 hours, or 4.72 years of patient monitoring hours, with each patient having a mean of 80 and a median of 55 monitoring hours.
Noninvasive VS monitoring data were recorded at a 1/20Hz frequency for HR, RR, systolic (SysBP) and diastolic (DiaBP) blood pressure, and SpO2. VS excursion beyond our MET trigger thresholds (HR< 40 or >140, RR< 8 or >36, SysBP < 80 or >200, DiaBP>110, SpO2< 85%) were defined as alert events and occurred 634,137 times. We additionally required that events had to persist initially for a tolerance of 40s, and a minimum duration of 4 minutes continuously, or a cumulative duration of 4 out of 5 minutes if intermittent to screen for events with clinical relevance. The event period under analysis was from the time the first VS crossed threshold and fulfilled the additional persistence criteria, until the time the first VS moved back into the stability range. Next, all VS events were provided as graphical time plots and visually adjudicated by two expert clinician reviewers, who annotated each event as a real alert or artifact based on inspection of the real-time VS time plots varying values, and artifact then excluded from further analyses.
Next, each discrete alert was noted according to both clock hour and day of week of occurrence. Additionally, each alert was assigned according to the number of hours elapsed since the unstable patient’s admission time. To determine temporal event distribution according to time of day using a 24 hour clock, all the alerts were allocated to a clock hour according to time of onset, with the hour lasting from 00:00 minutes to 59:59 minutes. Alerts that lasted more than one hour were allocated only to the initial hour of onset. To determine temporal event distribution according to the day of a 7-day week, all the alerts were allocated to the day associated with time of alert onset, with a day lasting from 0.00 to 23.59h. Alerts lasting across the 00.00h of the next day were allocated only to the day of onset.
R open source statistical software (Version 2.15.2) was used. All alerts were tallied according to clock hour during unstable patient’s full length of stay (LOS), and for only the first 24 hours after admission. All alerts were also tallied per day of the week. To determine if there was temporal variation in instability distribution across clock hours, we employed a mixed effect logistic regression model.8 This approach was chosen due to the observation that, of patients who become unstable, some patients may have multiple hour instances as well as multiple alert events during his/her LOS, leading to possible inter-independence of measures for the same patient. The mixed-effects model accounts for multiple hour instances and multiple alerts for an individual patient as they occur. The models were fit to a data set with variables describing clock hour and/or number of hours elapsed since the time of SDU admission, with a binary response indicating whether a particular patient had an alert during a particular clock hour during his/her LOS, or a particular number of hours elapsed since admission. If a patient had multiple alerts during the same hour, only one alert was accounted. We then fit three types of mixed effect logistic regression models to these data. In all models, patient identification (ID) (n=216 patients with at least one instability alert) served as the grouping factor, and was treated as a random effect. Model 0 was the null model with only the intercept parameter. In Model 1 clock hour; in Model 2 hours elapsed since SDU admission, and in Model 3 both clock hour and elapsed time since SDU admission were the fixed effects. Likelihood ratio tests were performed comparing pairs of these models in the mixed effect logistic regression for unstable patients to determine if, taking all alerts into consideration, there were proportionally more alerts distributed according to certain clock hours. All model types were built for three LOS subsets for the unstable alerted patients: 1) entire SDU LOS, 2) only hours 0-24 after SDU admission, and 3) time from hour 25 through SDU discharge. Our approach was intended to identify the risk of the frequency of alerts occurring within a specific temporal unit (clock hour or day of week) among all alerts occurring (a proportion), and not the risk of having an alert among all monitored patients (a prevalence). Statistical significance was set at a p value of 0.05.
RESULTS
The demographics of the sample and instability events are listed in Table 1. The total sample of 642 patients admissions was primarily male (59%) and white (73%), with a mean age of 58 years, and a mean SDU LOS of 3.1±3.1 days. There were 634,137 events, which was reduced to 2,333 qualifying events after the tolerance and duration requirements were applied. Forty percent of qualifying events were then rejected as artifact, leaving 1,399 events as real alerts, which occurred in 216 patients having at least one alert. Of real alerts, 50% were RR, 30% were SpO2, 10% were BP, and 10% were HR. For alerts wherein multiple VS crossed threshold simultaneously, the alert was labeled based on the first VS crossing threshold. The monitoring hour profiles for the distribution of accrued total alerts for each of the 3 LOS subsets are listed in Table 2.
Table 1.
Summary of the step-down unit (SDU) patient, monitoring, and event data for the total sample (All Patients) and for only those patients who ever became unstable even once (Unstable Patients).
| Variable | Total |
|---|---|
|
| |
| All Patients | |
| Total | 642 |
| % male (N, %) | 371 (58.5%) |
| Age (mean years ± SD) | 57.68 ± 19.95 |
| Race (N, %) | |
| White | 461(72.7%) |
| Black | 85 (13.4%) |
| Charlson Deyo Comorbidity Index (mean ± SD) | 1.09 ± 1.53 |
| SDU length of stay (mean days ± SD) | 3.31 ± 3.31 |
| Hospital length of stay (mean days ± SD) | 9.05 ± 13.76 |
|
| |
| Unstable Patients | |
| Total | 216 |
| % male (N, %) | 126 (58%) |
| Age (mean years ± SD) | 58.9 ± 19.5 |
| Race (N, %) | |
| White | 162 (75%) |
| Black | 28 (13%) |
| Charlson Deyo Comorbidity Index (mean ± SD ) | 1.34 ± 1.7 |
| SDU length of stay (mean days ± SD) | 4.2 ± 4 |
| Hospital length of stay (mean days ± SD) | 12.5 ± 20 |
|
| |
| Instability Events without persistence requirement | |
| Total events | 634,137 |
| Total events by subtype | |
| HR | 20,381 (3%) |
| RR | 155,689 (25%) |
| SpO2 | 172,348 (27%) |
| Systolic BP | 147,095 (23%) |
| Diastolic BP | 138,624 (22% |
|
| |
| Instability Events with persistence requirement | |
| (Tolerance 40 sec, length 240 sec, duty cycle 80%) | |
| Total events | 2,333 |
| Total events by subtype | |
| HR | 150 (6%) |
| RR | 1,002 (43%) |
| SpO2 | 907 (39%) |
| BP | 274 (12%) |
|
| |
| Instability Event Annotation by Experts | |
| Total Events | 2,333 |
| Real alerts | 1,399 (60%) |
| Artifact | 934 (40%) |
| Real alerts total | 1,399 |
| Real alerts by subtype | |
| HR | 137 (10%) |
| RR | 693 (50%) |
| SpO2 | 425 (30%) |
| BP | 144 (10%) |
Key: HR=heart rate; RR=respiratory rate; BP = blood pressure; SpO2=oxygen saturation of peripheral arterial blood; BP = blood pressure
Table 2.
Patient Monitoring Hours Profiles in the Stepdown Unit (SDU) at various length of stay (LOS) levels)
| Monitoring Hours subset | Total SDU patient length of stay |
First 24 hours of patient SDU admission |
>24 hours since SDU admission LOS |
|---|---|---|---|
|
Total number of patient
monitoring hours |
47,977 hours | 7,077 hours | 40,270 hours |
|
Unstable patients monitoring
hours |
916 hours | 253 hours | 663 hours |
|
Percentage of total monitoring
hours associated with unstable patients |
1.9% | 3.3% | 1.6% |
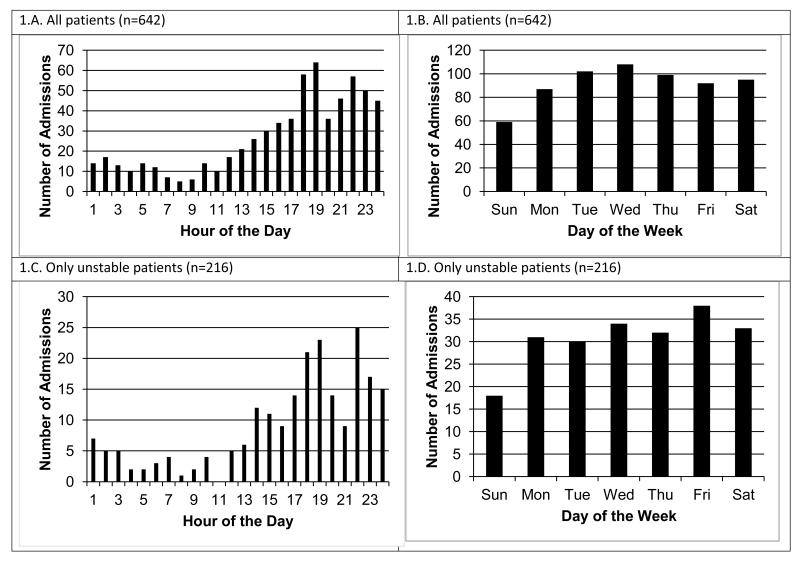
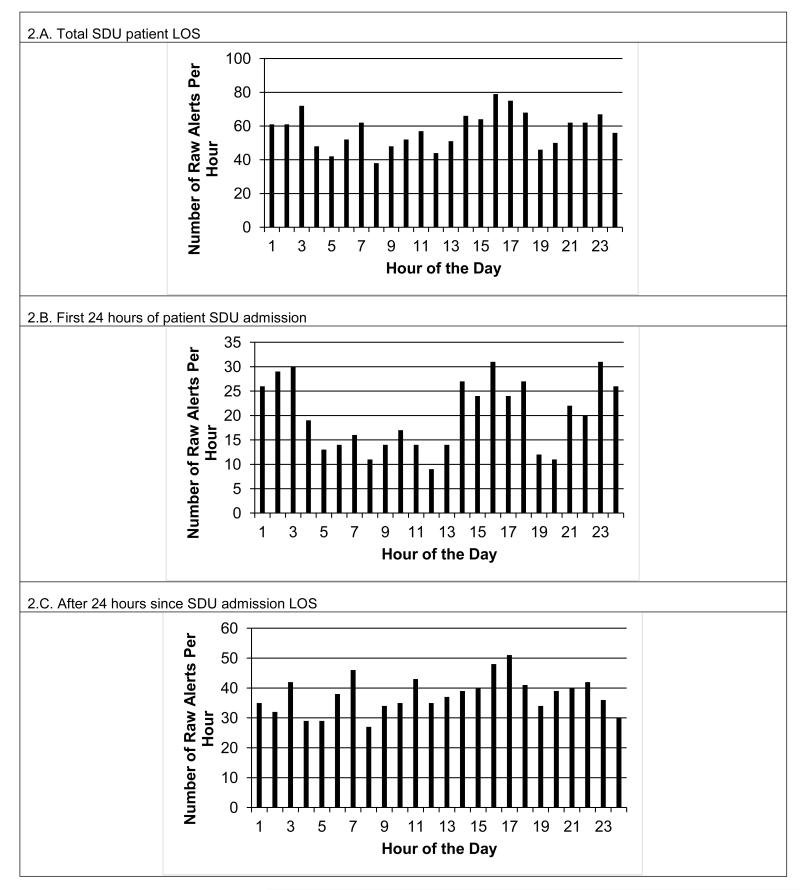
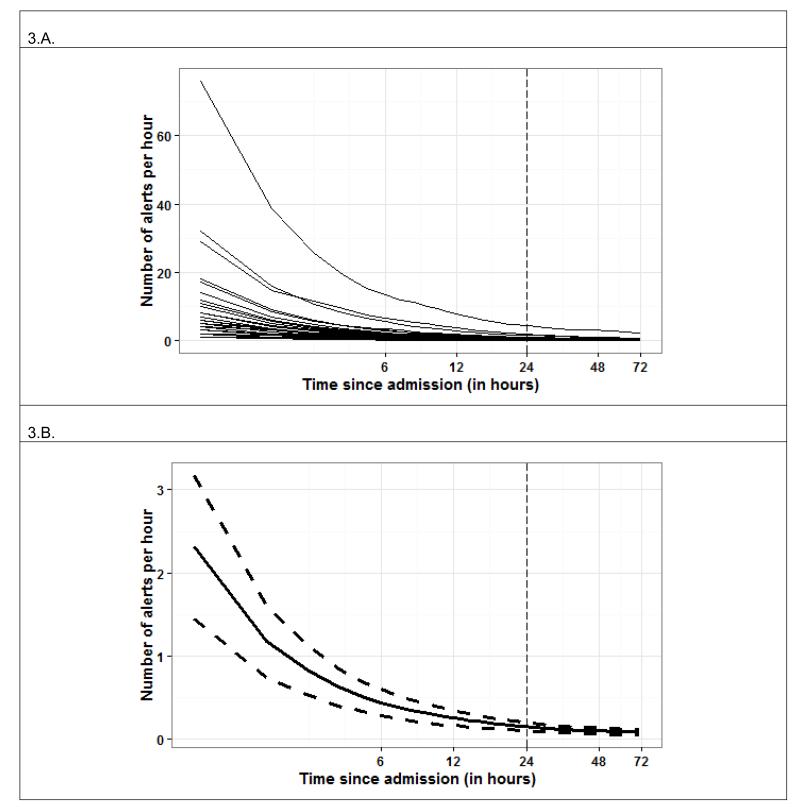
Figure 1 shows the number of patient admissions for the total 642 patient sample by clock hour (Fig. 1.A) and day of week (Fig. 1.B), and the subset of 216 patients with true alerts by clock hour (Fig. 1.C) and day of week (Fig. 1.D). For both the total sample, and sub-sample of only unstable patients, admissions tended to be highest from 16.00 to 24.00h, as might be anticipated for a surgical trauma unit. The lowest day of admission was Sunday, but was essentially the same for the remainder of days. Figure 2.A shows the total raw number of alerts distributed according to clock hour for the unstable patient’s entire LOS. Figure 2.B shows the total raw number of alerts per clock hour for only their first 24 hours of SDU admission, while Figure 2.C shows the same for their post-admission hour 25 through SDU discharge. We next examined alert distribution relative to the number of hours elapsed since the unstable patients time of SDU admission with the admission time as Time Zero (Figure 3), and alerts occurring during minutes 0-59 attributed to Hour 0. Of total alerts, 34% (n=481) happened during the first 24 hours of the 216 unstable patient’s admission.
Figure 1.
Number of patient admissions for the total sample (n=642) by clock hour (Panel 1.A.) and day of week (Panel 1.B.), as well as for the sample of only those patients with true alerts (n=216) by clock hour (Panel 1.C.) and day of week (Panel 1.D).
Figure 2.
Panel 2.A. shows the total number of raw alerts per clock hour for unstable step-down unit (SDU) patients (n=216) length of stay (LOS). Panel 2.B. shows the total number of raw alerts per clock hour for only the first 24 hours of their SDU admission, and Panel 2.C shows the alerts for post-admission hour 25 through SDU discharge.
Figure 3.
Panel 3.A. shows the accumulated alert rates for each unstable patient (n=216) (i.e. the number of alerts observed since admission time divided by the number of hours elapsed since admission) as a function of time since admission for the first 72 hours elapsed (admission events coincide with zeros on the time axes). Panel 3.B shows the mean accumulated alert rate for unstable patients during their first 72 hours following admission (solid curve) with 95% confidence limits (dashed curves). Time axes are displayed in base 2 logarithmic scales..
Table 3 presents the mixed effect logistic regression likelihood test results for effects from clock hour and hours elapsed since admission. When the complete SDU LOS for each unstable patient was modeled, the effect of clock hour was not significant (Model 1 vs. Model 0, p=0.855), but the effect of hours elapsed since admission was (Model 2 vs. Model 0, p<0.001). The effect of clock hour after controlling for hours elapsed since admission was also not significant (Model 3 vs. Model 2, p=0.885). When using only the first 24 hours of unstable patients SDU admission, the effect of clock hour was marginally significant (Model 1 vs. Model 0, p=0.046), and the effect of hours elapsed since admission was highly significant (Model 2 vs. Model 0, p<0.001), but the effect of clock hour was not significant when controlled for hours elapsed since admission (Model 3 vs. Model 2, p=0.199). When using only the distribution of alerts accrued from hour 25 since unstable patients admission until SDU discharge, hours elapsed since admission remained a significant effect (Model 2 vs. Model 0, p<0.001), but clock hour remained insignificant (Model 3 vs. Model 2, p=0.959).
Table 3.
Results of the likelihood ratio test comparing pairs of the mixed effect logistic regression models built for unstable patients (n=216). We present the model comparison for entire SDU Length of Stay (LOS), first 24 hours after SDU admission, and for LOS >24 hours since SDU admission. In all models, patient ID is the grouping factor, and is treated as random effect; different fixed effects are entered into Models 1, 2, and 3. In Model 1, it is hour of day, in Model 2, hours elapsed since SDU admission is the fixed effect, and in Model 3, both hour of the day and the hours elapsed since SDU admission are fixed effects. Model 0 is the null model with only the intercept parameter. The Effect column lists the effect that this pair of models is set to test. The Chi-square p-values from the Likelihood ratio test are significant at p<0.05.
| Model comparison pairs |
Effect | Total SDU patient LOS |
First 24 hours of patient SDU admission |
>24 hours since SDU admission LOS |
|---|---|---|---|---|
| Model 1 vs. Model 0 | Hour of day | 0.855 | 0.046 | 0.916 |
| Model 2 vs. Model 0 | Time since SDU admission |
<0.001 | <0.001 | <0.001 |
| Model 3 vs. Model 2 | Hour of day after adjusting for time since admission |
0.885 | 0.199 | 0.959 |
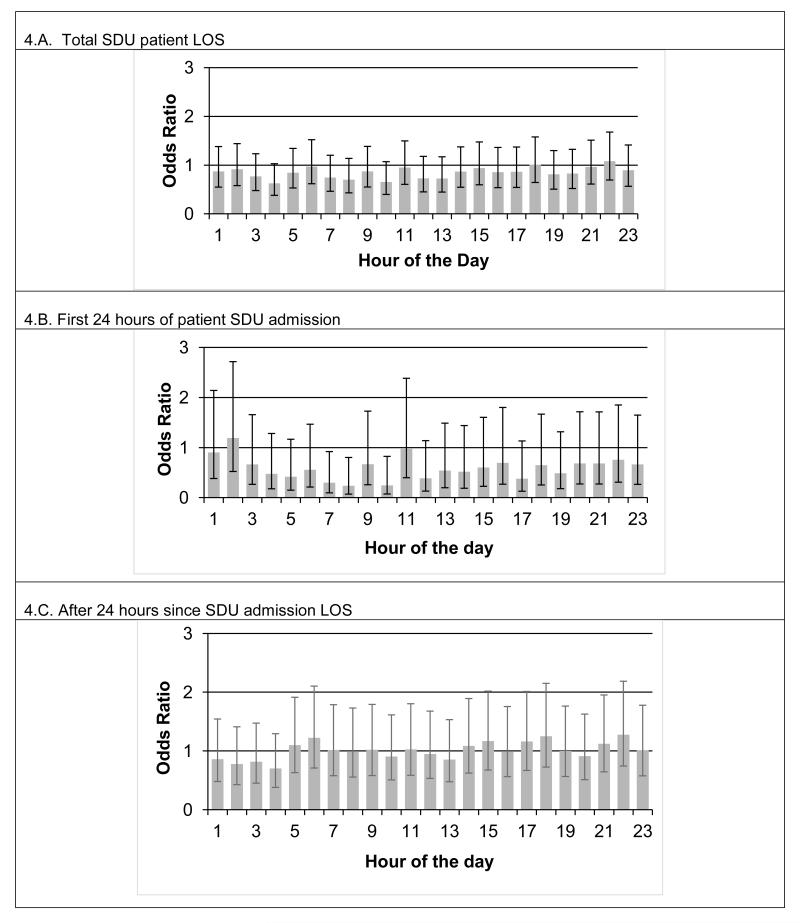
Fig. 4.A shows the odds ratios for clock hours as compared to hour zero (i.e. the 0000-0059 minute block), together with 95% confidence intervals for the fixed effect Model 3. There is no significant association between clock hour and likelihood of having an alert after controlling for elapsed time since admission. Fig 4.B shows the results when Model 3 is applied to only the first 24 hours of unstable SDU patients’ stay. Although three time intervals (hours 7, 8, and 10) display significantly low odds ratios, they are isolated and do not form systematic temporal groupings. We saw no significant clock hour clustering using the same model when only SDU post-admission hours >24 are included (Fig 4.C).
Figure 4.
Plot of the odds ratios for mixed effect logistic regression that uses both clock hour and time elapsed since SDU admission as fixed effects for the unstable patients (n=216). Panel 4.A shows the odds ratio as compared to hour zero (i.e. the 0000-0059 minute block), together with 95% confidence intervals (CIs). The CIs are largely overlapping, and spanning across the 1.0 odds ratio line, suggesting that after controlling for time since admission, alerts are equally likely to occur around the clock hour. Panel 4.B shows the same model applied to only the first 24 hours of unstable SDU patient admission. Again, the CIs are largely overlapping. Although there are three intervals (hours 7, 8 and 10) with significantly lower odds; these sporadic occurrences do not form a systematic temporal pattern. Panel 4.C shows the same model applied to SDU post-admission hour 25 through remainder of the LOS. CIs all largely overlap and also span across 1.0, suggesting no significance of the clock hour effect after controlling for time since admission.
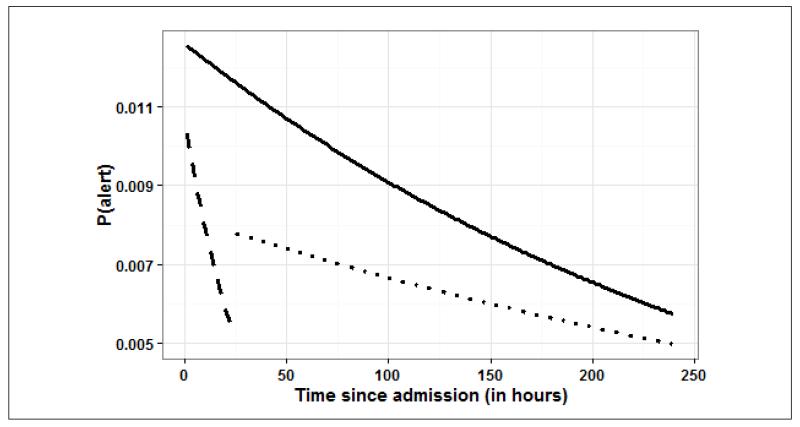
Finally, Figure 5 depicts a plot derived from the mixed effects logistic regression model illustrating the estimated probability of an alert as a function of hours elapsed since SDU admission at a fixed hour of the day (e.g. hour 12 of the day), for all unstable patients for the three different models when fitted to the different subsets of SDU LOS. The estimates are from the saturated mixed effect model (Model 3) that includes both the hours elapsed since SDU admission and the hour of day effect. After controlling for hour of the day, the likelihood of alert decreased with time since admission. Also, the effect of time elapsed since admission is stronger in the first 24 hours than afterward.
Figure 5.
Plot derived from the mixed effects logistic regression model illustrating the estimated probability of an alert occurring among all defined alerts as a function of time elapsed since SDU admission at a fixed hour of the day (e.g. hour 12). The estimates are obtained from the saturated mixed effect model (Model 3) that accounts for both the time elapsed since SDU admission and the hour of day effects. Results are shown for three different time periods (solid line: overall length of stay [LOS], dashed line: only the first 24 hours following admission, dotted line: only the part of LOS past the initial 24 hours through discharge). After controlling for hour of the day, the likelihood of alert decreases with time since admission. During the initial 24 hours of the SDU stay, the effect of time elapsed since admission is stronger than what is observed after the first 24 hours. (Note: alerts under consideration were contributed by only the 216 unstable patients)
DISCUSSION
This study reports upon what is to our knowledge the largest continuously monitored SDU patient population with instability annotation in the literature. We demonstrate that, for those patients who become unstable according to single VS parameter MET triggering criteria, and considering all alerts, there is an elevated likelihood for the distribution of alerts to be proportionally higher in the hours closest to time of admission, but there is not a noticeably higher likelihood for alerts to distribute proportionally more frequently in any given clock hour or day. If dissonance between temporal distribution of instability events and a unit’s MET activation distribution exists, then it suggests missed instability and RRS afferent arm breakdown.
There are several studies that note the irregularity in MET calls throughout the day and across the week. Galhotra et.al 5 reported upon 4,722 MET calls (single parameter triggers) over four years in a single center. MET calls were higher during the day (07.00-19.00h; 63%) than at night (19.00-07.00h; 37%, p<0.001).The proportion of daytime to nighttime MET calls was significantly higher during weekdays (65% day vs. 35% night; p<0.001), and on weekends (56% day vs. 43% night, p<0.001). The temporal variance in calls was present on both unmonitored and monitored units (62% day vs. 38% night, and 59% day vs. 41% night respectively, both p<0.001) but not in ICUs (47% day vs. 53% night, p=0.20). In a multicenter study conducted over 1 month in seven hospitals by the Medical Emergency Team End-of-Life Care Investigators,9 there were 652 MET activations in 5,198 patients. MET activations were more likely on Mondays (p=0.018) and during daylight hours (p<0.001) and less likely on weekends (p=0.003) or overnight (p<0.001). Schmid et al.7 made similar observations. All three studies suggested that spikes in MET activations seemed to cluster around time of scheduled activities like physician or nursing rounds, or scheduled vital sign acquisition
Our findings support a proposal that MET utilization success or failure must be reviewed within the context of a care unit’s temporal admission characteristics, since instability, when it occurs, is more highly distributed in the hours closest to patient’s admission times. For units where admissions are more likely to occur during daylight and midweek hours, for example an elective surgical unit, then a congruent temporal variation in MET activations might represent adequate RRS utilization. However, a daylight and weekday MET activation pattern on a unit where late day and evening admissions and equal numbers of admissions across days of week, as in a trauma unit such as ours, might represent RRS afferent arm failure and missed instability.
Our data supports matching density of resources to the density of patient admission times and days to ensure that instability is not missed. Even so, it still becomes difficult to match resource to need, since up to 75% of patients on a SDU never become unstable, 10 making it difficult to target which patients would benefit from a higher concentration of caregivers. In the absence of continuous monitoring, Bellomo et al.11 demonstrated that providing an automated alert to clinicians obtaining intermittent VS when the VS had exceeded MET activation criteria resulted in more MET calls, increased survival at both the time of MET event and at discharge, and decreased LOS. Huh et al.12 demonstrated that using auto-triggered MET activation when a VS entry crosses MET trigger threshold in the electronic health record resulted in more MET activations (p<0.001), especially with respiratory distress. Respiratory distress is historically the VS most commonly associated with MET activation, but also associated with staff lack of recognition for MET need or hesitation in activating MET calls.6, 13-17 Using aggregated warning scoring systems can objectify the unstable state and the need for RRS activation,18-22 but seem to be more helpful when alerting systems do not depend upon a caregiver.11, 19 Other solutions include integrated monitoring systems embedded into continuous monitoring systems, which move from single VS parameter alarms to indexed instability value and automated alarm. We demonstrated that using such an alert system resulted in increasing MET activations from only one for every eight patients with serious instability, to one out of every two.23 This and other analytic approaches to aid clinical decisions can be incorporated into intelligent and integrated monitoring systems.24, 25 In addition, they may help the bedside caregiver to decide to call for help in a more objective and “defensible” manner, since there are human factors which also serve as barriers to recognizing instability and increased failure-to-rescue.26 In one study of 118 ward nurses, 30% reported hesitancy to call a MET. The reasons were patient severity was unclear (31%), they were discouraged from doing so by another staff member (14%) or by a physician (57%).26 Even receiving criticism from the arriving MET team that the patient was not sufficiently unwell has been cited by nurses as a barrier to activation.27
Our study has three major limitations. First, our study window was short. Although 16 weeks of data is ample to assess instability occurrence, the timeframe was too short to assess our MET call prevalence. Second, this study was of only one surgical trauma SDU, and the patient type and admission characteristics may not extrapolate to other specialty or general hospital populations. However, if anything our findings of lack of temporal distribution of instability events according to clock hour but higher probability relative to time elapsed since admission speaks to the robustness of the data as generalizable as long as one accounts for their units own population-based admission time characteristics. Furthermore, it is impractical to monitor VS continuously on an entire hospital population. Thus, our well-characterized census monitoring of all SDU patients over the study period reflects a realistic compromise. Third, we judged instability according to MET activation criteria specific to our institution. Still, these criteria are similar to most reported MET activation criteria based on single VS parameters, and should be generalizable to other centers using similar approaches but slightly different VS threshold values for MET activation.
CONCLUSION
Although temporal variation in the distribution of MET activation is well documented, we demonstrate that true instability alerts, as defined by excursion of VS across MET activation threshold, are distributed in equal proportions according to clock hour, but are more likely to occur at higher proportion in times closest to an unstable patient’s admission time. The relationship between a unit’s temporal MET utilization must be examined in the context of its admission times of day and days of week to determine if the afferent arm of the RRS is working well. Mechanisms to improve RRS afferent arm function by improving instability recognition and the need for help regardless of clock hour or day of week are imperative to improve patient safety and outcomes, and assess RRS efferent arm efficacy.
ACKNOWLEDGEMENTS
Funding from NIH NINR 1R01NR013912, and NSF awards 0911032 and 1320347.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
No authors have financial and personal relationships with other people or organizations that could inappropriately bias this work.
Contributor Information
Marilyn Hravnak, School of Nursing, University of Pittsburgh, 336 Victoria Hall; 3500 Victoria Street, Pittsburgh, PA 15261-6314.
Lujie Chen, Auton Lab, The Robotics Institute, Carnegie Mellon University, 5000 Forbes Avenue, Pittsburgh, PA 15213-3890.
Artur Dubrawski, Auton Lab, The Robotics Institute, Carnegie Mellon University, 5000 Forbes Avenue, Pittsburgh, PA 15213-3890.
Eliezer Bose, School of Nursing, University of Pittsburgh, 336 Victoria Hall; 3500 Victoria Street, Pittsburgh, PA 15261-6314.
Michael R. Pinsky, School of Medicine, University of Pittsburgh, 606 Scaife Hall; 3550 Terrace Street, Pittsburgh PA 15261-1616.
REFERENCES
- 1.Devita MA, et al. Findings of the first consensus conference on medical emergency teams. Critical Care Medicine. 2006;34:2463–2478. doi: 10.1097/01.CCM.0000235743.38172.6E. [DOI] [PubMed] [Google Scholar]
- 2.Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid response systems as a patient safety strategy: A systematic review. Annals of Internal Medicine. 2013;158:417–425. doi: 10.7326/0003-4819-158-5-201303051-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarani, et al. Clinical emergencies and outcomes in patients admitted to a surgical vs. a medical service. Resuscitation. 2011;82:415–418. doi: 10.1016/j.resuscitation.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 4.DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, Bonello R, Cerchiari E, Farlow B, Goldsmith D, Haskell H, Hillman K, Howell M, Hravnak M, Hunt EA, Hvarfner A, Kellett J, Lighthall GK, Lippert A, Lippert FK, Mahroof R, Myers JS, Rosen M, Reynolds S, Rotondi A, Rubulotta F, Winters B. Identifying hospitalized patients in crisis: A consensus conference on the afferent limb of Rapid Response Systems. Resuscitation. 2010;81:375–382. doi: 10.1016/j.resuscitation.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Galhotra S, et al. Impact of patient monitoring on diurnal pattern of MET activation. Crit Care Med. 2006;34:1700–1706. doi: 10.1097/01.CCM.0000218418.16472.8B. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, et al. Triggers for emergency team activation: A multicenter assessment. J Crit Care. 2010;25:359.e1–359.e7. doi: 10.1016/j.jcrc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Schmid-Mazzoccoli A, Hoffman LA, Wolf GA, Happ MB, DeVita MA. The use of medical emergency teams in medical and surgical patients: impact of patient, nurse and organizational characteristics. Quality and Safety in Health Care. 2008;17:377–381. doi: 10.1136/qshc.2006.020438. [DOI] [PubMed] [Google Scholar]
- 8.Bates MD. [Accessed August 31, 2014];lme4: Mixed-effects modeling with R. Available at: http://lme4.r-forge.r-project.org/lMMwR/lrgprt.pdf.
- 9.Medical Emergency Team End-of-Life Care Investigators The timing of rapid-response team activations: a multicentre international study. Crit Care Resus. 2013;15:15–20. [PubMed] [Google Scholar]
- 10.Hravnak M, Edwards L, Clontz A, Valenta C, DeVita M, Pinsky M. Defining the incidence of cardio-respiratory instability in step-down unit patients using an electronic integrated monitoring system. Archives of Internal Medicine. 2008;168(12):1300–1308. doi: 10.1001/archinte.168.12.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo, et al. A controlled trial of automated advisory vital signs in general hospital wards. Crit Care Med. 2012;40:2349–2361. doi: 10.1097/CCM.0b013e318255d9a0. [DOI] [PubMed] [Google Scholar]
- 12.Huh JW. Activation of a MET using an electronic medical-recording-based screening system. Crit Care Med. 2014;42:801–808. doi: 10.1097/CCM.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 13.Chen, et al. The impact of introducing MET system on the documentation of vital signs. Resuscitation. 2009;80:35–43. doi: 10.1016/j.resuscitation.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Santiano, et al. Analysis of MET calls comparing subjective to “objective” calling criteria. Resuscitation. 2009;80:44–49. doi: 10.1016/j.resuscitation.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Ludikhuize J. Identification of deteriorating patients on general wards. J Crit Care. 2012;27:424.e7–424.e13. doi: 10.1016/j.jcrc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Boniatti MM, et al. Delayed MET calls and associated outcomes. Crit Care Med. 2013;42:26–30. doi: 10.1097/CCM.0b013e31829e53b9. [DOI] [PubMed] [Google Scholar]
- 17.Lighthall G, et al. Abnormal VS are associated with an increased risk for critical events in US veteran patients. Resuscitation. 2009;80:1264–1269. doi: 10.1016/j.resuscitation.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 18.McNeil G, Bryden D. Do either early warning systems or medical response teams improve hospital patient survival? A systematic review. Resuscitation. 2103;84:1652–1667. doi: 10.1016/j.resuscitation.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Kellett J, Wang F, Woodworth S, Huang W. Changes and their prognostic implications in the abbreviated VitalPAC™ Early Warning Score (ViEWS) after admission to hospital of 18,827 surgical patients. Resuscitation. 2013;84:471–476. doi: 10.1016/j.resuscitation.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Finlay GD1, Rothman MJ, Smith RA. Measuring the Modified Early Warning Score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system. J Hosp Med. 2014;9:116–119. doi: 10.1002/jhm.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143:1758–1765. doi: 10.1378/chest.12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hravnak M, DeVita MA, Clontz A, Edwards L, Valenta C, Pinsky MR. Cardiorespiratory Instability before and after implementing an integrated monitoring system. Crit Care Med. 2011;39:65–72. doi: 10.1097/CCM.0b013e3181fb7b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra V, McMahon LF. Redesigning hospital alarms for patient safety: Alarmed and potentially dangerous. JAMA. 2014;311:1199–1200. doi: 10.1001/jama.2014.710. [DOI] [PubMed] [Google Scholar]
- 25.Pinsky MR, Dubrawski A. Gleaning knowledge from data in the ICU. Am J Respir Crit Care Med. 2014 Jul 28; doi: 10.1164/rccm.201404-0716CP. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hravnak M, Schmid-Mazzoccoli A, Ott L, Pinsky MR. In Rapid Response Systems: Concept and Implementation. 2nd Ed Springer Inc; New York: 2010. Causes of failure to rescue. [Google Scholar]
- 27.Pusateri ME, et al. The role of the Non-ICU nurse on a MET: Perceptions and understanding. Am J Nursing. 2011;111:22–29. doi: 10.1097/01.NAJ.0000398045.00299.64. [DOI] [PubMed] [Google Scholar]
- 28.Bagshaw SM, et al. A survey of nurses’ beliefs about the MET System in a Canadian tertiary hospital. Am J Crit Care. 2010;19:74–83. doi: 10.4037/ajcc2009532. [DOI] [PubMed] [Google Scholar]