Abstract
Over the last 10 years the continual discovery of novel forms of encephalitis associated with antibodies to cell-surface or synaptic proteins has changed the paradigms for diagnosing and treating disorders that were previously unknown or mischaracterized. We review here the process of discovery, the symptoms, and the target antigens of twelve autoimmune encephatilic disorders, grouped by syndromes and approached from a clinical perspective. Anti-NMDAR encephalitis, several subtypes of limbic encephalitis, stiff-person spectrum disorders, and other autoimmune encephalitides that result in psychosis, seizures, or abnormal movements are described in detail. We include a novel encephalopathy with prominent sleep dysfunction that provides an intriguing link between chronic neurodegeneration and cell-surface autoimmunity (IgLON5). Some of the caveats of limited serum testing are outlined. In addition, we review the underlying cellular and synaptic mechanisms that for some disorders confirm the antibody pathogenicity. The multidisciplinary impact of autoimmune encephalitis has been expanded recently by the discovery that herpes simplex encephalitis is a robust trigger of synaptic autoimmunity, and that some patients may develop overlapping syndromes, including anti-NMDAR encephalitis and neuromyelitis optica or other demyelinating diseases.
Keywords: autoimmune encephalitis, limbic encephalitis, anti-NMDAR antibodies, psychosis, treatment
Introduction
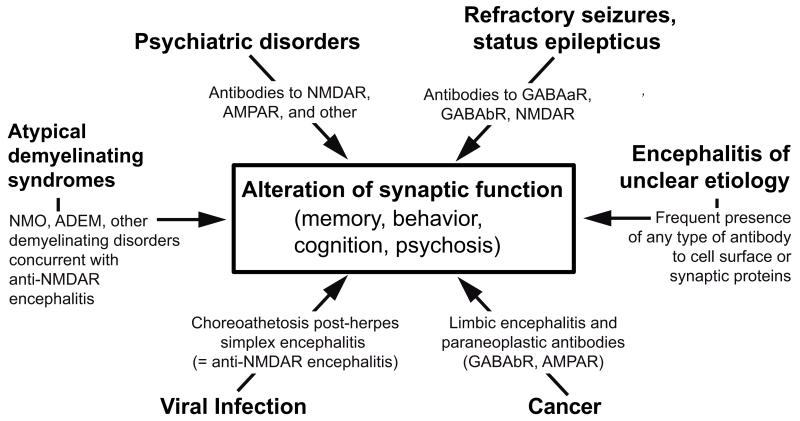
The encephalitides associated with antibodies against cell-surface or synaptic proteins are a new category of diseases that occur with focal or widespread involvement of the nervous system in association with antibodies against extracellular epitopes of neuronal cell-surface or synaptic proteins.1 The discovery of this group of disorders—referred in this review as autoimmune encephalitis—has affected many fields of medicine, resulted in changed paradigms for the diagnostic and treatment approaches to some neuropsychiatric disorders, and reclassified syndromes previously defined as idiopathic or with descriptive terms.1 In addition, the study of these disorders has revealed novel mechanisms of how antibodies might alter memory, behavior, and cognition or cause psychosis, seizures, or abnormal movements (Fig. 1).2,3
Figure 1.
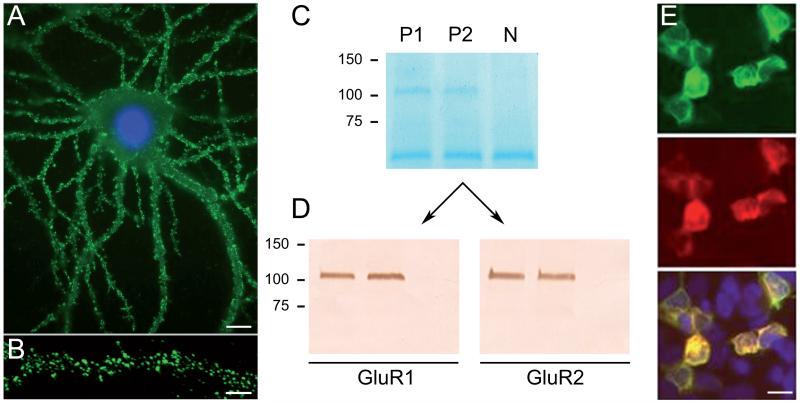
Process of discovery, antigen immunoprecipitation, and development of a diagnostic test. Dissociated rat hippocampal neurons maintained in vitro and incubated (live, non-permeabilized) with CSF of a patient. Note the intense reactivity of patient’s antibodies with cell surface antigens (A); scale bar = 10 μm. Confocal microscopy suggests that the antigens are concentrated in clusters along dendrites (B); scale bar = 5 μm. Precipitation of these antigens using two patients’ CSF antibodies is shown in a gel in which proteins are visualized with EZBlue (C). Note that patients’ antibodies (P1, P2) precipitated a single band at ~100 kDa; this band is not seen in the precipitate of a control individual (N). Analysis of the 100 kDa band by mass spectrometry demonstrated sequences derived from GluR1/GluR2 subunits of the AMPAR. The ~50 kDa band across all samples corresponds to IgG. Transfer of the proteins to nitro-cellulose and Western blot with GluR1 and GluR2 antibodies confirmed that the 100 kDa band contained both GluR1 and GluR2 subunits (panels in D). Further validation of the antigen was done in heterologous cells expressing GluR1/2, showing reactivity with patient’s antibodies (green), a monoclonal antibody (red), and the merged reactivities (yellow). Adapted from Lai and colleagues22 with permission.
The concept that autoimmunity mediated by antibodies or cytotoxic T-cell mechanisms can cause neurological symptoms is not new. The myasthenic syndromes are good examples of how autoantibodies can cause neurological symptoms; in myasthenic syndromes autoantibodies against acetylcholine receptor or voltage-gated calcium channels cause reversible muscle weakness by altering the normal function of the neuromuscular junction.4,5 As another example, some paraneoplastic syndromes such as cerebellar degeneration or limbic encephalitis are associated with highly specific antibodies against intracellular neuronal proteins and aggressive cytotoxic T cell responses that usually lead to irreversible deficits.6
In contrast to classical paraneoplastic syndromes, the novelty of the autoimmune encephalitides reviewed here is that they can affect patients of all ages, some of them preferentially occurring in children and young adults;7-9 they can develop with or without an underlying tumor; they are associated with antibodies that target extracellular epitopes of cell-surface or synaptic proteins; and for the disorders which underlying mechanisms have been investigated, the antibodies alter the structure or function of the target antigen.2,10 In addition, the associated syndromes often respond to immunotherapy, resulting in substantial or complete recovery in 70–80% of the patients.11 Owing to the severity and duration of symptoms, before these disorders were known the clinical recovery of similar patients was not expected, thus changing our concepts about supportive therapy today in cases that would have been considered futile in the past.12,13
Antibodies and target antigens
In 2005, the description of six patients with different forms of encephalitis, with antibodies against neuronal cell surface proteins, marks the beginning of the molecular identification and understanding of these disorders.14 Until then, the only cell-surface antibodies associated with autoimmune encephalitis (specifically, limbic encephalitis and Morvan’s syndrome) were attributed to antibodies specific for the Kv1.1 and Kv1.2 subunits of the Shaker family of voltage-gated potassium channels (VGKCs).15,16 (This was later shown to be incorrect; discussed below). One of the six patients had been diagnosed with the Kv1.1- and Kv1.2-specific antibodies, but in the other five cases the neuronal targets were unknown. Remarkably, all of the patients improved substantially with immunotherapy and, when appropriate, removal of the associated tumors (two had teratomas and two had tumors of the thymus).14 This improvement, when compared with the limited treatment response of classic paraneoplastic syndromes17-20 led investigators to distinguish a new category of autoimmune encephalitis. In subsequent studies, the unknown cell surface antigens were immunoprecipitated and found to be synaptic receptors, including the N-methyl-D-aspartate receptor (NMDAR),21 the α-amino-3-hydroxy-5-methyl-4-isoxazol-propionic acid receptor (AMPAR),22 or the γ-amino-butyric acid B-receptor (GABAbR).23 Similar strategies showed that the antibodies originally attributed to the Shaker family of VGKCs were in fact directed against other proteins, including leucine-rich, glioma-inactivated 1 (LGI1)24 and contactin-associated protein-like 2 (Caspr2).25,26 Since 2005, the frequency of discovery of novel syndromes and their associated autoantigens has been 1–2 per year (Table 1).
Table 1.
Autoimmune encephalitis with antibodies against cell surface and synaptic proteins
| Antigen | Clinical syndrome | Tumor |
|---|---|---|
| NMDAR (GluN1) | Anti-NMDAR encephalitis: prodromal symptoms, psychiatric, seizures, amnesia, movement disorders, catatonia, autonomic instability, coma |
Age-dependent 10–45% ovarian teratomas, infrequently carcinomas |
| AMPAR | Limbic encephalitis, psychiatric symptoms | 70% (lung, breast, thymoma) |
| GABAbR | Limbic encephalitis, prominent seizures | 50% lung, neuroendocrine |
| LGI1 | Limbic encephalitis, 60% hyponatremia, occasional focal faciobrachial seizures prior to encephalitis |
<10% (lung, thymoma) |
| CASPR2 | Encephalitis, Morvan syndrome, neuromyotonia | 0–40% thymoma |
| mGluR5 | Limbic encephalitis (reported in less than 10 patients) |
Frequently, Hodgkin lymphoma |
| D2R | Basal ganglia encephalitis, Sydenham chorea. | Infrequent |
| DPPX | Diarrhea, encephalitis with CNS hyperexcitability: confusion, psychiatric symptoms, tremor, myoclonus, nystagmus, hyperekplexia, PERM-like symptoms, ataxia. |
No tumor association |
| GABAaR | Refractory seizures, status epilepticus, or epilepsia partialis continua, stiff-person, opsoclonus |
Infrequent |
| GlyR | Stiff-person, PERM, limbic encephalitis, cerebellar degeneration, optic neuritis |
Infrequent |
| IgLON5 | Abnormal sleep movements and behaviors,obstructive sleep apnea, stridor, dysarthria, dysphagia, ataxia, chorea (reported in less than 10 patients) |
No tumor association |
Many patients with autoimmune encephalitis have a propensity to autoimmunity, including multiple serum antibodies that are unrelated to patients’ symptoms (e.g., thyroid peroxidase (TPO) antibody or antinuclear antibodies (ANA)).22,27 These encephalitis-irrelevant antibodies are usually absent in in the cerebrospinal fluid (CSF), which provides a clean source of disease-relevant antibodies for preliminary antigen characterization. Detection of CSF reactivity with the neuropil of brain along with immunolabeling of the cell surface of cultured neurons strongly suggests a pathogenic link between the antibodies and patients’ symptoms. The techniques more frequently used to eventually characterize antigens include immunoprecipitation (nine of eleven antigens shown in Table 1, Fig. 1) 1,22 and screening of candidate antigens according either to patients’ symptoms or the investigator’s hypothesis (four of eleven; two of them, LGI1 and Caspr2, also identified by precipitation).8,28
General clinical features of autoimmune encephalitis
At disease onset, there is substantial overlap of symptoms among the different types of autoimmune encephalitis. The rapid development of symptoms in a few days or weeks, sometimes with headache or mild hyperthermia, and frequent CSF pleocytosis often lead to empiric antiviral or bacterial treatments while CSF and blood work for infectious, metabolic, and toxic causes are processed.29,30 Alteration of mood, behavior, and memory, decreased level of consciousness, and seizures occur in most types of autoimmune encephalitis; however, the severity and predominance of some symptoms over others, and the presence of other features (e.g., dyskinesias, severe psychiatric manifestations, facio-brachial dystonic seizures, hyponatremia, diarrhea) along with demographic information (sex, gender, race), brain MRI findings, and presence or absence of a tumor can suggest a specific disorder. Sleep dysfunction, nearly constant in all forms of encephalitis, has been studied in detail in only two disorders, anti-LGI1 encephalitis31 and the recently reported encephalopathy with IgLON5-specific antibodies32 (discussed below).
The electroencephalogram (EEG) is almost always abnormal in all types of autoimmune encephalitis, showing focal or diffuse slow activity frequently associated with one or several foci of epileptic activity. Except for a pattern referred to as extreme delta brush that may occur in patients with anti-NMDAR encephalitis, there are no pathognomonic EEG abnormalities for any other forms of autoimmune encephalitis.33,34 Magnetic resonance imaging (MRI) of the brain is very useful in patients with limbic encephalitis (see below), usually showing increased FLAIR/T2 signal involving one or both temporal lobes, without contrast enhancement (Fig. 2). Similar findings can occur in patients with herpes simplex encephalitis (HSE) or medial temporal lobe seizures, but the pattern of highly selective medial temporal lobe involvement is limited to a few other disorders (e.g., paraneoplastic limbic encephalitis,35 rare cases of lupus,36 neurosyphilis,37 Sjogren’s syndrome,38 or HHV6 infection39).
Figure 2.
Comparison of MRIs of patients with limbic encephalitis and different cell surface autoantibodies with the MRI of a patient with multifocal encephalitis and GABAaR receptor antibodies. The MRIs of the three cases with limbic encephalitis correspond to a patient with LGI1 antibodies (A), a patient with AMPAR antibodies (B), and a patient with GABAb-R antibodies (C). In contrast, note that the MRI of the patient with GABAaR antibodies shows multifocal cortical-subcortical FLAIR abnormalities (D). Panels A–C, from Lancaster and colleagues,11 with permission; panel D from Petit-Pedrol and colleagues27 with permission.
At symptom presentation, about 80% of patients with autoimmune encephalitis have mild-to-moderate CSF lymphocytic pleocytosis (usually <100 white blood cells/μl), 30% have mild-to-moderate increase of protein concentration, and 50–60% have oligoclonal bands. These bands can be present even when routine CSF studies are normal. In contrast to most autoimmune encephalitis, the limbic encephalitis associated with LGI1-specific antibodies frequently occurs with normal or minimal CSF findings.40
Specific syndromes
Anti-NMDAR encephalitis
The encephalitis associated with NMDAR-specific antibodies has been described in large series of patients41-44 and a recent review.45 This disorder is characterized by CSF IgG antibodies against the GluN1 subunit of the NMDAR. The disease usually occurs in young adults and children, predominantly women, although it can affect patients of all ages. In a population-based study in England examining causes of autoimmune encephalitis, anti-NMDAR encephalitis was second only to acute demyelinating encephalomyelitis (ADEM).46 In a referral center focused in the study of encephalitis of unclear etiology, the incidence of anti-NMDAR encephalitis was higher than any individual viral encephalitis.30 One percent of young patients (18–35 years) admitted to the intensive care unit of a large German center for encephalitis of unknown origin were in retrospect found to have NMDAR-specific antibodies.47
In a series of 577 patients (81% female), the median age was 21 years, 37% were younger than 18 years and 5% older than 45.42 The frequency of males was higher in age groups below 12 years (39%) and above 45 (43%). Thirty eight percent of the patients (representing 46% of all women) had an underlying neoplasm. The presence of a tumor predominated in patients between 12 and 45 years. Only 6% of females younger than 12 years and 6% of males (all adults) had a tumor. Ninety four percent of the tumors were ovarian teratomas, 2% extraovarian teratomas, and 4% other tumors.
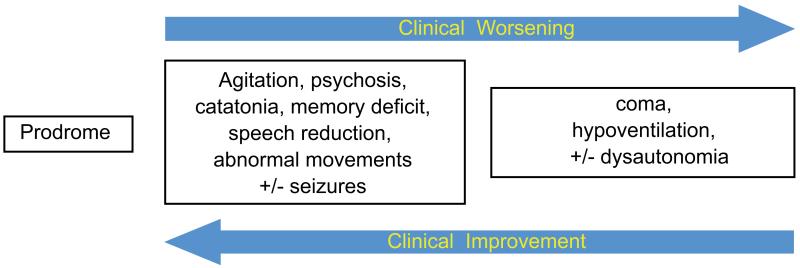
The syndrome was established in 2008 in a series of 100 patients,41 and since then the spectrum of symptoms has not changed substantially (Fig. 3).42,43 Approximately 70% of the patients develop prodromal symptoms, such as headache or fever, that are usually followed by rapid change of behavior that may include anxiety, agitation, insomnia, aggression, visual or auditory hallucinations, paranoia, grandiose delusions, hyper-religiosity, sexual disinhibition, mania, psychosis, or catatonia. The prominence of the psychiatric symptoms contributed to the initial identification of this disorder48 and has recently led to a surge of interest in psychiatry49,50 (discussed below in the section “Psychiatry”). These symptoms are usually followed by a wide range of abnormal movements that may include orofacial dyskinesias, chorea, athetosis, ballismus, myorhythmia, stereotyped movements, rigidity, or opisthotonus.7,51-53 As the symptoms progress there is decreased consciousness, stupor, coma, periods of agitation alternating with catatonia, autonomic dysregulation such as fluctuation of blood pressure, hyperthermia, sialorrhea, tachycardia, hypoventilation, and, less frequently, bradycardia, cardiac pauses, or ictal asystole that may require transient use of a pacemaker.54-56 Seizures and complex status epilepticus may occur at any stage of the disease,57 but almost always resolve after other symptoms improve, not requiring chronic antiepileptic therapy.13 Seizures, as initial presenting symptom, are more frequent in adult male patients than in women.43,58
Figure 3.
Sequence of syndrome development in patients with anti-NMDAR encephalitis. During the first month of the disease most patients with anti-NMDAR encephalitis progressively develop the sequence of symptoms shown in the graph.42 Adapted from Dalmau and colleagues.45
Brain MRI is normal in 66% of NMDAR encephalitis patients;42 the other 34% have nonspecific cortical or subcortical FLAIR/T2 abnormalities, sometimes involving posterior fossa or medial temporal regions, often with small areas of demyelination, and in a few cases with extensive demyelinating abnormalities59 (discussed in the section below “Overlapping syndromes”).
The average hospital admission is 3 months, but many patients require longer admissions in rehabilitation centers.60 During recovery and after the acute symptoms have resolved patients continue with deficits of memory and attention, impulsivity, behavioral disinhibition, and executive dysfunction that usually improve over many months. In a prolonged follow up of 501 patients, 81% had a modified Rankin scale score of 0–2 at 24 months, and some continued to improve thereafter.42 The mortality rate is approximately 7%, with most deaths occurring during the stage of intensive care support.
The presentation of NMDAR encephalitis in children with behavioral abnormalities may initially suggest autistic regression, early onset schizophrenia, or childhood disintegrative disorder.61,62 Compared with adults, children more often present initially with abnormal movements or seizures, and then in the ensuing days or weeks progress to develop a syndrome similar to that of the adults.7,34,63 In patients older than 45 years, the clinical picture is similar to that of younger patients, but the outcome is less favorable. In this age group, 23% of patients had an underlying tumor (carcinomas instead of teratomas), and delays in diagnosis and treatment were more frequent than in younger patients.64 For any age group, monosymptomatic cases such as isolated abnormal movements,65,66 psychosis,67 or seizures are extremely rare.68
Owing to the protracted clinical course, complexity of symptoms and complications, and loss of weight and deconditioning, many different specialists are involved in the care of these patients. Approximately 50% of patients respond to first line immunotherapies (intravenous immunoglobulins (IVIg), steroids, or plasma exchange) and the other 50% require second line therapies, such as rituximab or a combination of rituximab and cyclophosphamide.42 Relapses occur in 12–20% of cases (12% during the first 24 months of the disease),often presenting as fragments of the syndrome (perhaps due to prompt diagnosis), and respond to immunotherapy.69 Overall, these data, and the fact that patients that receive second line immunotherapies have fewer relapses, is leading many physicians to use rituximab initially. Patients who do not respond to treatment, or who have relapses, should be reassessed for the presence of an underlying12,13 contralateral or recurrent teratoma.70
Limbic encephalitis
Three neuronal cell–surface antibodies are associated with limbic encephalitis, anti-LGI1,24,25 anti-GABAbR,23 and anti-AMPAR.22
The encephalitis associated with LGI1 antibodies
This limbic encephalitis predominantly affects middle age or elderly patients, males more frequently than females (2:1), and presents with prominent short-term memory deficits, confusion, and frequent seizures.24,25 About 60% of the patients have hyponatremia and some patients have preceding or concomitant myoclonic-like jerks in the face, arm, or leg—described as facio-brachial dystonic or tonic seizures.71,72 Recognition and treatment of these seizures with immunotherapy may prevent development of the encephalitis and cognitive dysfunction.73 A study showed that five of six patients with limbic encephalitis and LGI1-specific antibodies had rapid eye movement (REM) sleep behavior disorder; in three patients these symptoms improved along with those of limbic encephalitis after immunotherapy, while in two the sleep disorder persisted.31 Some patients with LGI1-specific antibodies develop confusion and cognitive decline over a few months, suggesting more a rapidly progressive dementia than subacute encephalitis. Creutzfeldt-Jakob disease (CJD) has been suspected in some of these patients,74 although CJD patients do not have LGI1-specific antibodies (see below).
The presence of LGI1 rarely associates with systemic tumors; when it does there is usually an associated thymoma.24,25 Recent studies indicate the importance of differentiating LGI1 antibodies, as well as Caspr2 antibodies (both previously considered VGKC antibodies), from other antibodies referred to under the term VGKC-complex antibodies. While LGI1 and Caspr2 antibodies (discussed below) specifically associate with limited subsets of syndromes, the identity of other VGKC-complex antibodies is unknown (it is also unknown if the antigens are on the cell surface); the VGKC-complex antibodies also have limited specificity and may be found in non-autoimmune diseases, including Creutzfeldt-Jakob disease (CJD), among others.75,76 In contrast, a study of CSF from 49 patients with pathologically confirmed CJD showed that none had anti-LGI1, anti-Caspr2 or other well-defined cell-surface antibodies.77 The same study found that among 346 patients with suspected CJD, 6 (1.7%) had autoimmune disorders that responded well to immunotherapy.
The encephalitis associated with GABAbR antibodies
This limbic encephalitis usually develops as limbic encephalitis.23 Two recent series,78,79 including a total of 37 patients, showed in the majority of cases typical clinical and MRI involvement of the limbic region along with early and frequent seizures. This autoimmunity is the most frequent cause of limbic encephalitis in patients with small cell lung cancer (SCLC) and who are negative for Hu antibodies.80 About 50% of patients with GABAbR antibodies have SCLC or neuroendocrine tumors; the other 50% do not have an underlying cancer. Among patients with SCLC and limbic encephalitis, those with GABAbR antibodies have a much better outcome than those with Hu antibodies,81 reflecting the better response to immunotherapy of patients with syndromes pathogenically associated with auto-reactive antibodies rather than cytotoxic T cell mechanisms.78,82 There are only a few cases of patients with GABAbR antibodies and symptoms different from limbic encephalitis, including cerebellar ataxia83 and brainstem encephalopathy;84 and one child with overlapping GABAaR antibodies developed confusion, chorea, opsoclonus, and ataxia.85
The encephalitis associated with AMPAR antibodies
Anti-AMPAR limbic encephalitis was initially described in 2009.22 Patients develop symptoms and MRI features of limbic encephalitis, but in some cases the presentation is purely psychiatric, with confusion, agitation, and aggressive behavior. In two patients with isolated psychiatric symptoms, the CSF and MRI were normal, but the EEG was abnormal.86 In a series of 10 patients (9 women), 7 had thymoma or cancer of the lung or breast. Additional antibodies, such as anti-ANA, anti-TPO, anti-GAD65, or other autoimmune disorders (e.g., hypothyroidism) co-occur frequently.22 Symptoms usually respond to immunotherapy, although relapses are frequent. The severe memory deficits and confabulations complicate the management of these patients, who may gradually develop cumulative deficits after each relapse.
Other syndromes and autoantibodies to cell surface antigens or synaptic receptors
Morvan’s syndrome
This is a disorder that includes symptoms of encephalitis, such as amnesia, confusion, hallucinations, sleep and autonomic dysregulation, in association with peripheral nerve hyperexcitability, or neuromyotonia. Morvan’s syndrome and neuromyotonia were previously considered associated with antibodies against the Shaker family of VGKCs,15 but the serum and CSF of these patients do not react with Kv1.1 or Kv1.2 subunits of the these channels. Instead, many patients with Morvan’s syndrome,87,26 and only a few with neuromyotonia,88 have antibodies against Caspr2, a protein expressed in the CNS and at the juxtaparanodal region of myelinated nerves where it is associated with potassium channels. Patients with Caspr2 antibodies may also develop neuropathic pain, encephalitis without symptoms of peripheral neuropathy, or, rarely, limbic encephalitis.26,87 The frequency of an underlying tumor (thymoma) varies substantially (0–40%) among series, likely reflecting referral bias, and appears to depend more on the syndrome than the antibody. For example, among patients with Caspr2 antibodies, those with Morvan’s syndrome appear to more frequently have thymomas87 compared to those with encephalitis.26 Immunotherapy is usually effective.
The Ophelia syndrome
This is a form of autoimmune encephalitis that occurs in patients with Hodgkin’s lymphoma.89 The syndrome is often described as limbic encephalitis, but in fact the clinical picture and MRI studies suggest a more diffuse encephalitis. Symptoms include depression, agitation, hallucinations, memory deficits, delusions, and bizarre behavior. The disorder is extremely rare, and all cases studied recently had antibodies against the metabotropic glutamate receptor 5 (mGluR5), which is highly expressed in the hippocampus.90,91 A similar syndrome can occur without Hodgkin’s lymphoma. The importance of recognizing this disorder is that patients have complete recoveries after treatment of the tumor or immunotherapy. Patients with Hodgkin’s lymphoma can also develop cerebellar degeneration, in which case the antibodies are against mGluR1, which is highly expressed in cerebellum.92,93 It is unclear why these two mGluRs that belong to the same group of mGluR (group 1), and none of the six receptors that belong to other groups (group 2 and 3), associate with CNS autoimmune disorders and Hodgkin’s lymphoma.
Progressive encephalomyelitis with rigidity and myoclonus (PERM)
PERM is a disorder that results from predominant brainstem and spinal cord dysfunction as a result of immune-mediated disruption of inhibitory pathways. Recent studies showed that patients with PERM often have antibodies to the α1 subunit of the glycine receptor (GlyR).28,94 A few patients have been found to have thymoma or other tumors, but most patients do not have cancer. Different from patients with stiff-person syndrome who rarely have encephalopathy or prominent brainstem symptoms, 40–50% of patients with PERM have ocular movement deficits, cranial nerve paresis, brainstem dysfunction, and encephalopathy, and 30% have autonomic dysregulation. Hyperekplexia is also a frequent symptom of this disorder. In a retrospective study of 52 patients, the median age was 50 years, and four were younger than 15 years of age. After a median follow-up of 3 years most patients had substantially improved with immunotherapy, but four (9%) died during the course of the disease.95 The authors concluded that GlyR antibodies strongly associate with spinal and brainstem dysfunction. However, studies with a large number of disease control groups show that these antibodies occur in a variety of disorders, including stiff-person syndrome, limbic encephalitis, cerebellar degeneration, or optic neuritis.96 GlyR antibodies can overlap with GAD65 antibodies.97,98
The most recent neuronal cell–surface autoantigen associated with PERM is dipeptidyl-peptidase-like protein-6 (DPPX).99 Patients with antibodies to DPPX more frequently develop a syndrome of CNS hyperexcitability (discussed below).100 The three cases reported with PERM, all younger than 30 years of age, developed prominent hyperekplexia, a wide range of cerebellar-ocular movement abnormalities, ataxia, and muscle stiffness and spasms (triggered by acoustic and tactile stimuli sometimes as mild as a gentle breeze). Two of the patients had prominent gastrointestinal symptoms, a common feature of DPPX autoimmunity. Two patients responded to immunotherapy; the third patient was diagnosed retrospectively, developed spastic tetraparesis, and died of pneumonia that was a complication of severe dysphagia. PERM-like symptoms may occur in some patients with antibodies against the intracellular proteins Nova 1 and 2 (anti-Ri or ANNA2 antibodies).101 These antibodies associate with brainstem encephalitis or encephalomyelitis frequently accompanied by opsoclonus, other ocular movement disorders,102 and sometimes life threatening laryngeal spasms.103 In contrast to GlyR and DPPX antibody-associated syndromes, anti-Ri associated encephalitis is likely mediated by cytotoxic T cells.
Stiff-person syndrome
This syndrome comprises a clinical picture characterized by muscle rigidity and spasms that may occur spontaneously or are triggered by several types of stimuli (e.g., tactile, auditory, emotional upset). The disorder predominantly affects axial, low back, and proximal muscles of the extremities. Electromyography (EMG) of the involved muscles shows continuous motor unit activity as a result of dysfunction of the inhibitory GABAergic system; symptoms and EMG findings improve with diazepam. Most patients have high titers of GAD65 antibodies and may develop overlapping cerebellar symptoms in association with endocrinopathy (diabetes, thyroid dysfunction).104 The reason why antibodies against GAD65 (an intracellular protein) associate with different symptoms, including seizures or limbic encephalitis, is unknown.105,106 Patients with GAD65 antibodies usually do not have underlying tumors.
In addition to GAD65 antibodies, some patients with stiff-person syndrome develop GlyR antibodies.98 In children the disorder is frequently misdiagnosed.97 Another study identified four patients with stiff-person syndrome and low titer serum antibodies to GABAaR; three of the patients had been previously considered seronegative and one had coexisting GAD65 antibodies.27 High CSF or serum GABAaR antibody titers associate with prominent seizures and status epilepticus (discussed below).
About 5% of patients with stiff-person syndrome have SCLC or breast cancer; these patients usually develop amphiphysin antibodies instead of GAD65 or GlyR antibodies.107,108 Amphiphysin is a vesicular synaptic protein involved in endocytosis; although the protein is not expressed on the neuronal cell surface, it has been suggested that during the process of synaptic vesicle uptake amphiphysin is exposed on the cell surface and can be accessed by antibodies. Amphiphysin antibodies also occur with paraneoplastic myelitis or encephalomyelitis.109
Encephalitis associated with DPPX antibodies
Anti-DPPX encephalitis results in symptoms of CNS hyperexcitability, including agitation, hallucinations, paranoid delusions, tremor, myoclonus, nystagmus, seizures, and, sometimes, hyperekplexia.100 The disorder was initially reported in four patients (45–76 years old, 2 males), three of whom had prodromal diarrhea that led to substantial loss of weight. The target antigen, DPPX, is a cell surface auxiliary subunit of the Kv4.2 Shal potassium channel family. DPPX increases the function of Kv4.2 channels, which are involved in somatodendritic signal integration and attenuation of dendritic back propagation of action potentials. Although the encephalopathy of patients with DPPX antibodies does not conform to a specific syndrome, the symptoms of CNS hyperexcitability are consistent with the increased excitability noted in electrophysiological studies of Dppx-deficient (Dpp6−/−) mice.110 The cause of the diarrhea and other gastrointestinal symptoms is unknown, but DPPX is expressed in neurons of the myentheric plexus that strongly react with patients’ antibodies. Overall, the disorder is severe, resulting in lengthy hospitalization or multiple relapses that usually occur while immunotherapy is decreased.
Encephalitis with antibodies to γ-amino-butyric acid A-receptor (GABAaR)
Anti-GABAaR encephalitis associated with prominent seizures and status epilepticus with extensive cortical and subcortical FLAIR/T2 signal abnormalities that may appear, while other abnormalities resolve (Fig. 2D).27 These MRI findings are rarely encountered in the other autoimmune encephalitis mentioned above. In a recent study of 18 patients, six with high CSF or serum antibody titers (ages 3–63 years, median 22, five males) developed refractory seizures, status epilepticus, or epilepsia partialis continua, four of them required pharmacological coma. Patients responded to immunotherapy, but two died of sepsis during intensive care management of the seizures. The 12 patients without CSF antibodies (all with low serum titers) developed a broader spectrum of symptoms likely indicative of coexisting autoimmune disorders; six patients had encephalitis with seizures, two opsoclonus, and four stiff-person syndrome (discussed above).27
Basal ganglia encephalitis and antibodies to dopamine (D2) receptor
This encephalitis and anti-D2 antibodies were recently reported in 12 children who developed several types of movement disorders sometimes co-existing in the same patient, including dystonia, tremor, oculogyric crises, parkinsonism, and/or chorea.8 Agitation, anxiety, psychosis, and sleep dysfunction were frequent accompaniments. The brain MRI was normal in 50% of the patients; when abnormal, the findings were localized to the basal ganglia. Responses to immunotherapy were observed, but residual motor, cognitive, and psychiatric deficits were common. Antibodies to the D2 receptor were also identified in a subset of patients with Sydenham chorea, as reported by other investigators.111 The frequency of these antibodies in patients with encephalitis associated with abnormal movements is low; in our experience with 120 patients, mostly children, with encephalitis and abnormal movements or basal ganglia MRI abnormalities all were negative for D2 receptor antibodies (Armangue et al., data unpublished).
A novel link between autoimmunity and neurodegenerative disorders: encephalopathy and IgLON5 antibodies
This disorder differs in many ways from the autoimmune encephalitides described above. In contrast to the subacute presentation of most encephalitis, the clinical picture of patients with IgLON5 antibodies may evolve over years.32 The syndrome was recently described in eight patients (age 52–76 years of age, median 59 years; 5 women) and includes abnormal sleep movements and behaviors, with obstructive sleep apnea, and stridor. Accompanying symptoms preceding or developing after the core syndrome included dysarthria, dysphagia, ataxia, or chorea. In six patients, the median duration of the disease was 5 years (2–12 years); the other two patients had a subacute presentation and died within 6 months of symptom onset. In all cases studied, polysomnography revealed abnormal sleep architecture, undifferentiated non-REM sleep or poorly structured stage N2, simple movements and finalistic behaviors, normalization of non-REM sleep by the end of the night, and REM sleep behavior disorder. All patients had antibodies (IgG4) against IgLON5, a neuronal specific cell adhesion molecule with unknown function. Four of four Spanish patients had the HLA-DQB1*0501 and HLA-DRB*1001 alleles that are very uncommon among the Spanish population. Symptoms did not respond to immunotherapy, and six patients had died by the time of publication. Two patients had autopsy that showed an atypical neuronal tauopathy with predominant involvement of the hypothalamus and tegmentum of the brainstem, without signs of inflammation. Moreover, no inflammatory changes were identified in the patients’ CSF, leaving it unclear at whether there is an inflammatory response that is subtle and transient or there is no inflammatory process at all. This disorder suggests an intriguing link between autoimmunity and neurodegeneration.
Mechanisms of disease
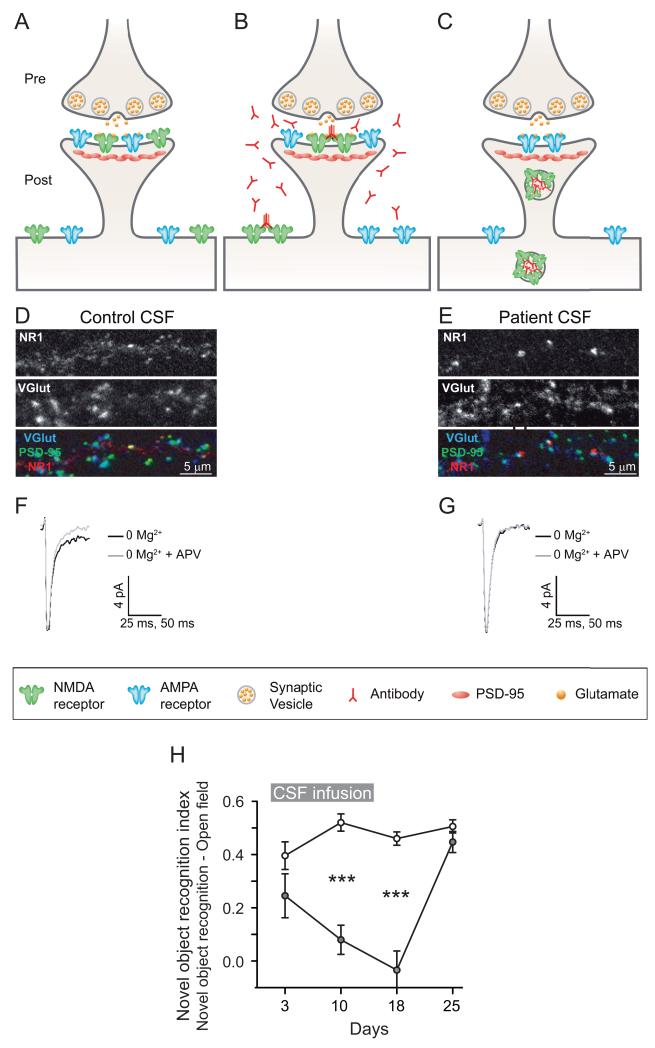
For anti-NMDAR encephalitis the pathogenic role of GluN1 IgG antibodies has been established in cultures of neurons and after hippocampal and cerebroventricular infusion into rodents of patients’ antibodies (Fig. 4).41,112 Using cultured neurons, patients’ antibodies caused crosslinking and selective internalization of NMDARs that correlated with the antibody titers; these effects were reversible after removing the antibodies.113,114 In contrast to the intense effects on NMDAR, patients’ antibodies did not alter the localization or expression of other synaptic proteins, number of synapses, dendritic spines, dendritic complexity, or cell survival. In other experiments, the density of NMDAR was also significantly reduced in the hippocampus of rats infused with patients’ antibodies, a finding similar to that observed in the hippocampus of autopsied patients.113 More recently, Mannara and colleagues developed a murine model of passive transfer of memory and behavioral deficits using continuous ventricular infusion (14 days via osmotic pumps) of CSF from patients with anti-NMDAR encephalitis. Analyses of hippocampus from the mice showed progressive antibody binding over time and a decrease of total and synaptic NMDARs, without affecting PSD95 or AMPAR. These effects gradually improved after stopping the antibody infusion, with reversibility of symptoms accompanied by restoration of NMDAR levels, establishing the pathogenicity of the antibodies (Fig. 4H).115
Figure 4.
Synaptic and behavioral effects of antibodies from patients with anti-NMDAR encephalitis. (A–C) AMPA and NMDA receptors are localized in the postsynaptic membrane and are clustered at the postsynaptic density A. Patient antibodies in the CNS bind selectively to NMDA receptors at the synapse as well as extrasynaptic receptors. This binding leads to receptor cross-linking B. NMDA receptors that have been bound and cross-linked by antibodies are internalized, resulting in a decrease of surface, synaptically localized NMDA receptors. Other synaptic components, such as postsynaptic AMPA receptor clusters, PSD-95, as well as presynaptic terminals, dendrite branches, dendritic spines and cell viability, are unaffected C. Thus patient anti-NMDA receptor antibodies lead to a rapid, selective excision of NMDA receptors from neuronal membranes. This effect is titer-dependent and reverses after antibody titers are reduced (not shown). (D–E) Rodent cultured neurons treated with control CSF or patient’s CSF for 3 days, and subsequently stained for postsynaptic GluN1 to label NMDA receptor clusters, VGlut to label presynaptic sites, and PSD-95 as a postsynaptic marker. Note that patient’s CSF cause a decrease in dendritic GluN1 cluster density, while VGlut and PSD-95 cluster density remain unchanged. (F–G) Whole-cell patch recordings of miniature excitatory postsynaptic currents (mEPSCs) that consist of a fast AMPA receptor–mediated component and a slower later component mediated by NMDA receptors and is APV sensitive. Compared with neurons incubated with CFS control F, those treated with CSF from a patient with NMDA receptor antibodies show a loss of the APV-sensitive NMDA receptor component E. H: Progressive memory deficit assessed by standard object recognition test, in a group of 5 mice treated with cerebroventricular infusion of CSF from patients with anti-NMDAR encephalitis (grey circles) compared with a group of 5 mice treated with CSF from control subjects without NMDAR antibodies (white circles). Antibodies were continuously infused for 14 days; the peak of memory deficit was on day 18, followed by progressive clinical recovery. Panels (A–G) obtained from Moscato and colleagues,2 with permission. Panel H provide by Planaguma and colleagues (unpublished data).
For the disorders associated with AMPAR or GABAaR antibodies, studies on pathogenicity have been conducted with cultured neurons, each antibody producing selective internalization of the corresponding receptor. Patients’ AMPAR antibodies caused a decrease of the receptors at synaptic and extrasynaptic sites, along with reduction of AMPAR-mediated miniature excitatory postsynaptic currents using whole-cell patch recordings.22,116 On the other hand, patients’ GABAaR antibodies selectively eliminated the receptors from synapses but did not alter the number of extrasynaptic receptors.27
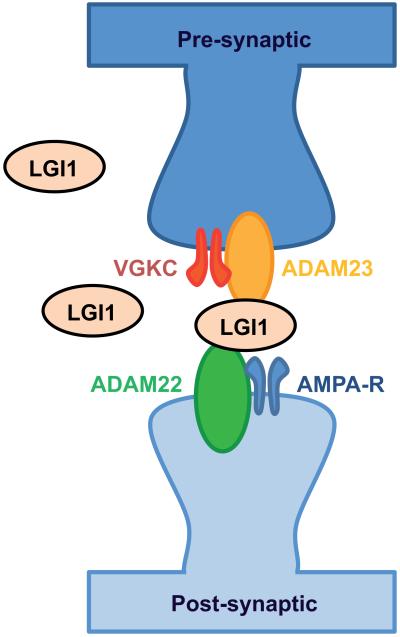
Different from the above cell membrane and synaptic receptors, LGI1 is a secreted protein that interacts with the presynaptic receptor ADAM23 and the postsynaptic receptor ADAM22, organizing a trans-synaptic complex that includes presynaptic Kv1.1 potassium channels and postsynaptic AMPAR (Fig. 5).24 Lgi1-deficient (Lgi−/−) mice develop a lethal epileptic phenotype characterized by myoclonic seizures.117 In these mice, the increase of neuronal excitability has been attributed to a decrease in AMPAR function in inhibitory neurons, and to an increase in glutamate release.117,118 Although it is unknown how LGI1 antibodies result in limbic encephalitis, a recent study using cultured neurons showed that patients’ antibodies interfered with the LGI1–ADAM interaction and decreased the levels of postsynaptic AMPARs.119
Figure 5.
Interaction of LGI1 with synaptic proteins. Secreted LGI1 interacts at the presynapse with ADAM23 and at the postsynapse with ADAM22. The figure is based on studies indicating that LGI1 co-precipitates with other proteins including the presynaptic Kv1 potassium channels and a variety of pre- and post-synaptic scaffolding proteins. Adapted from Lancaster and colleagues,11 with permision.
The effects of patients’ GlyR and dopamine D2 receptor antibodies have been examined in vitro in HEK cells expressing these receptors; the studies showed antibody-mediated internalization of the corresponding receptors.8,95 It is not currently known if similar effects apply to neurons in vivo.
Autoimmune encephalitis in other disciplines
Information on autoimmune encephalitis in other disciplines is summarized in Figure 6.
Figure 6.
Associations, trigers, and common symptoms of autoimmune encephalitis: impact on multiple disciplines
Psychiatry
That patients with anti-NMDAR encephalitis and other autoimmune encephalitis frequently present with psychosis, affective dysregulation, and other behavioral abnormalities has fueled interest in autoimmunity as potential cause of some psychiatric illness.49,50,120 One study reported finding NMDAR antibodies in serum of 3 of 46 (6%) patients with first onset of schizophrenia; the target subunit (e.g., GluN2 versus GluN1) was not determined and only one of the patients appeared to improve after immunotherapy.121 Another study included serum obtained at symptom presentation by 80 patients with first onset psychosis who, one year later, met the DSM-IV-TR criteria for schizophrenia-spectrum illness. GluN1 IgG antibodies were not detected in any of the 80 cryopreserved sera;122 these findings were consistent with those of another smaller study of patients with schizophrenia.123
Another study examined the prevalence of NMDAR antibodies in the sera from 121 patients with an initial diagnosis of schizophrenia, 108 with other psychiatric disorders, and 230 healthy subjects.124 Two patients had GluN1 IgG antibodies, and retrospectively both were diagnosed with anti-NMDAR encephalitis (both had prominent dyskinesias, abnormal movements, autonomic dysfunction, and EEG abnormalities). None of the other patients had IgG GluN1 antibodies, though they did have other antibodies, mostly IgA or IgM against GluN1 identified in 8% of patients with schizophrenia and 3% with major depression. In a subsequent study, the same investigators found IgA or IgM antibodies in 16% of patients with several types of dementia and 7% of normal subjects.125 More recently, Hammer and colleagues examined 2817 subjects, including 1325 healthy subjects and 1492 patients with schizophrenia, Parkinson disease, or affective-disorders, for the presence of serum antibodies against GluN1 subunit of the NMDAR.126 In this study, 10.5% of all subjects (patients and healthy controls) were seropositive for IgA or IgM antibodies; only 0.6% had IgG antibodies. The authors hypothesized that schizophrenic individuals with history of blood–brain barrier (BBB) disturbance (e.g., neurotrauma at birth or thereafter) were more likely to have more pronounced neurological symptoms when NMDAR antibody positive; however, the authors did not show evidence of BBB disruption or the presence of antibodies in the CSF.127
Overall, these studies demonstrate limited clinical significance of serum IgA or IgM NMDAR antibodies in patients with psychiatric illness, given that a similar proportion of healthy subjects or disease controls had the same antibodies. In contrast, the absence or rare detection of GluN1 IgG antibodies suggests the specificity of these antibodies for anti-NMDAR encephalitis. Future investigations should include CSF analysis, comprehensive autoantigen characterization, and determination of antibody isotypes and subunit targets (e.g., GluN1 versus GluN2). The importance of antigen specification is shown in patients with antibodies attributed to VGKC-complex proteins but whose target antigens are in fact unknown. Antibodies to these unknown antigens have been detected in a few patients with schizophrenia,121 though they were also found in a number of non-autoimmune disorders, raising the question of significance.75
Herpes simplex encephalitis (HSE) as trigger of synaptic autoimmunity
The clinical course of HSE is usually monophasic, but 14–26% of patients develop relapsing symptoms a few weeks after the infection has resolved.128,129 These relapsing symptoms have been attributed to (1) viral relapse characterized by the demonstration of HSV by polymerase chain reaction (HSV-PCR) in the CSF, MRI findings of new necrotic lesions, and response to acyclovir or (2) a disorder of unclear etiology without evidence of HSV reactivation, absence of new necrotic lesions, and no response to acyclovir.130 This complication occurs more frequently in children than in adults, and the clinical picture is usually different from that related to the initial episode of viral encephalitis. In fact, most children develop chorea, orofacial dyskinesias, dystonia, or ballismus, accompanied by agitation, aggression, sleep dysfunction, seizures, or decrease of level of consciousness.131 This constellation of symptoms led physicians to coin the term choreoathetosis post-HSVE to describe this complication.2,132 A similar disorder occurs in adults, but abnormal movements are infrequent.132 The observation that some patients with HSE developed IgG antibodies against the GluN1 subunit of NMDAR,133 and subsequent studies of children with choreoathetosis post-HSVE,34 revealed that this complication is in fact anti-NMDAR encephalitis.134 A recent study showed that patients who developed this complication were NMDAR antibody negative at the time HSE was diagnosed and then developed serum and CSF antibodies in the ensuing 2–6 weeks, leading to anti-NMDAR encephalitis.135 The same study demonstrated that HSE is a robust trigger of autoimmunity to other cell surface or synaptic proteins (antigens still unknown), and another study showed antibodies to dopamine D2 receptor in two patients.136 The identification of HSE as trigger of anti-NMDAR encephalitis has been confirmed by multiple investigators over a short period of time,137-140 suggesting this complication may be more common than previously reported as choreoathetosis post-HSVE. Diagnosing anti-NMDAR encephalitis post-HSE is important because patients usually respond to immunotherapy.
Reclassification of disorders
In addition to choreoathetosis post-HSE, other disorders such as encephalitis lethargica with dyskinesias or abnormal movements141 or encephalitis of unclear etiology with abnormal movements29 are now identified as anti-NMDAR encephalitis. Archived serum or CSF samples of these patients confirm the presence of IgG antibodies to these receptors and retrospective assessment of the clinical picture is consistent with anti-NMDAR encephalitis.
Hashimoto’s encephalitis is a term that continues to be in use even though the disorder was renamed steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT). The diagnosis is considered in patients who develop any type of encephalopathy of unclear etiology, with TPO antibodies, and response to steroids.142 However, TPO antibodies are present in ~10% of healthy subjects143,144 and they are also frequently encountered in patients with well-defined autoimmune encephalitis (e.g., NMDAR, GABAbR, and GABAaR, among others).7,23,27,145 In our experience, patients with autoimmune encephalitis and relevant cell surface or synaptic antibodies are frequently misdiagnosed as Hashimoto’s encephalitis, while patients without relevant autoantibodies that fulfill criteria of SREAT do not have antibodies reacting with the cell surface of neurons (J. Dalmau, personal observation). This indicates that Hashimoto’s encephalitis or SREAT should be a diagnosis of exclusion.
Overlapping syndromes: anti-NMDAR encephalitis and demyelinating disorders
Many patients with anti-NMDAR encephalitis have normal MRI scans (see anti-NMDAR encephalitis). A study of 691 patients with the disorder identified 23 (range 4–62 years of age, median 27; nine cases younger than 18 years of age), with clinical or MRI features suggesting a demyelinating disorder.59 Twelve of these 23 patients developed anti-NMDAR encephalitis preceded or followed by one or more episodes of demyelination, and the other 11 developed both disorders simultaneously. Among the 12 patients with sequential development of syndromes, the demyelinating episode of five patients fulfilled criteria of neuromyelitis optica spectrum disorder (NMOSD) or NMO (four with aquaporin 4 antibodies); the other seven patients developed a a brainstem syndrome or multifocal demyelinating features (all with myelin-oligodendrocyte (MOG) antibodies). Among the 11 patients with concurrent anti-NMDAR encephalitis and demyelinating symptoms, five had aquaporin 4 antibodies (3 with features of NMOSD), and two had MOG antibodies, both with infratentorial abnormalities.
Most patients improved with immunotherapy, but compared with the episodes of anti-NMDAR encephalitis, the episodes of demyelination required more intensive therapy and resulted in more residual deficits.59 Similar overlapping syndromes or autoantibodies have been identified by other investigators.146-148
Caveats in the diagnosis and treatment of autoimmune encephalitis
Autoantibodies to cell surface and synaptic proteins should be examined in both serum and CSF. A recent study showed that 14% of patients with anti-NMDAR encephalitis did not have detectable antibodies in serum using two different techniques, while all had antibodies in the CSF. For other disorders, the proportion of cases that are CSF positive and serum negative is unknown. In our experience, patients with encephalitis related to antibodies against NMDAR, AMPAR, GABAbR, DPPX, mGluR1, or mGluR5 always have antibodies in the CSF.
Other antibodies, such as anti-LGI1, anti-Caspr2, anti-GlyR, anti-GABAaR, may in rare instances be identified only in serum. In these cases the disease relevance of the serum antibodies is unclear, and sometimes the findings are not reproducible,149 suggesting false positive results. Indeed, most of the atypical clinical associations of antibodies to neuronal cell surface antigens (e.g., NMDAR in patients with CJD or schizophrenia)121,150 have been reported in patients who underwent limited serum testing using only cell-based assays without examining CSF, or whose CSF was negative. Although some investigators argue for a higher sensitivity of serum testing with the indicated cell-based assay, there is no data supporting this (e.g., CSF not tested, or comparison with other techniques not performed). In contrast, a study of Gresa-Arribas and colleagues151 examining paired serum and CSF of 250 patients with anti-NMDAR encephalitis and 100 controls with three different assays, showed that serum testing with any type of cell-based assay (live or fixed cells) leads to false negative results in at least 14% of the patients. We recently saw a patient that had been treated elsewhere for one year for anti-NMDAR encephalitis; the diagnosis had been based in a serum live cell-based assay without CSF analysis. The syndrome was surprisingly atypical for anti-NMDAR encephalitis; instead, the patient had narcolepsy-cataplexy that was confirmed by a compatible HLA and undetectable hypocretin in CSF. Comprehensive studies of the patient’s serum sample (considered NMDAR-antibody positive elsewhere) and CSF (which had not been previously tested) were both antibody negative. In this case, the serum assay was not more sensitive than the CSF and it provided a false positive result (J. Dalmau, unpublished observation). In our experience, the problems that result from assessing autoimmune encephalitis by serum testing only can be avoided by including CSF testing. Moreover, the CSF findings show specific antibody–syndrome associations, and the results are comparable among different series and laboratories.58
There are no standard immunotherapy protocols for autoimmune encephalitis. Approaches to decrease the antibody levels (with plasma exchange or IVIg) are less effective than in antibody-mediated diseases of the peripheral nervous system such as myasthenia gravis or the Lambert-Eaton syndrome. The intrathecal production of antibodies that occurs in many types of autoimmune encephalitis, and the presence of CNS inflammatory infiltrates along with plasma cells,21,152 likely explains the less frequent response to these immunotherapies. Interestingly, patients with anti-LGI1 associated symptoms (a disorder with infrequent intrathecal antibody synthesis) respond faster to steroids, plasma exchange, or IVIg than do patients with anti-NMDAR encephalitis (a disorder that associates with intrathecal antibody synthesis). However, the faster treatment response of patients with LGI1 antibodies does not lead to better long-term outcome; in fact, patients with anti-NMDAR encephalitis have full recoveries more frequently than do those with LGI1 antibodies (measured as ability to return to work or their original activities; Dalmau, personal observation).42,153
Experience with 501 patients with anti-NMDAR encephalitis demonstrated that rituximab and cyclophosphamide are usually effective in patients who do not respond to first-line immunotherapies.42 Although this approach is increasingly being used for other autoimmune encephalitis, it is unclear whether it has the same effects.
Follow-up of serum antibody titers is an unreliable biomarker of disease activity in many types of autoimmune encephalitis. A recent study showed that in patients with anti-NMDAR encephalitis, the CSF antibody titers associate better with the course of the disease and relapses than do serum titers.151 In our experience, serum antibody titers (or even CSF titers) should not be used as the main guide for treatment decisions, instead they should be predominantly based on clinical assessment. As examples, patients with anti-NMDAR encephalitis may be comatose for several months and have low or undetectable serum antibody titers, while their CSF titers are persistently elevated.151 Conversely, patients may have recovered, sometimes for several years, and still have antibodies in serum or CSF.154,155 The limited usefulness of antibody titers is not surprising if one considers that the same problem applies to other well-established antibody-mediated disorders such as myasthenia gravis.
Future directions
The discovery of autoimmune encephalitis has changed paradigms in the diagnosis and treatment of disorders that were previously unknown or mischaracterized. The increasing interest and number of studies in the field are raising interesting questions on multiple fronts. For example, it is unclear whether the clinical and basic mechanisms of anti-NMDAR encephalitis (the best studied of this group of disorders) are applicable to the other disorders. There are important differences among them, not only clinical but also in the function and structure of the target antigens (some are ion channels, others metabotropic receptors, and one is a secreted protein). A critical question is how and where the immunological response is initiated, and which mechanisms are involved in the activation and expansion of the immune response within the CNS. It is unclear why some disorders appear to be consistently associated with intrathecal synthesis of antibodies, while others less so. What is the mechanism of action of rituximab in these CNS disorders? Despite the limited ability to cross the BBB, this drug can eliminate plasmablasts (which express CD20) or interfere with the process of B cell expansion and maturation into plasma cells (which are not directly affected by rituximab). These postulated mechanisms may explain why patients with anti-NMDAR encephalitis who receive early and aggressive immunotherapy have faster improvement and better outcome than those with delayed or less aggressive therapies (perhaps allowing development of more extensive infiltrates of plasma cells in patient’s brain; Fig. 7). Other prognostic factors, such as severity of symptoms42 or, perhaps, compensatory mechanisms of synaptic function,156 should be investigated and may explain differences in patients’ outcomes.
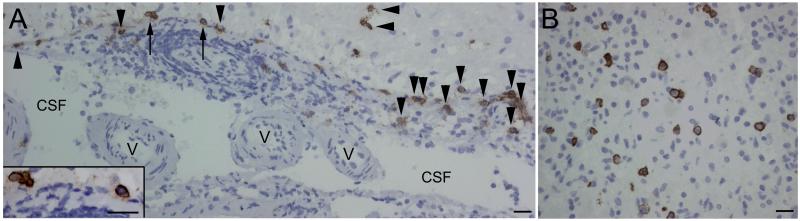
Figure 7.
Plasma cell infiltrates in the brain of a patient with anti-NMDAR encephalitis. Paraffin embedded sections of brain biopsy of a patient with anti-NMDAR encephalitis. CD138+ cells (plasma cells/plasmablasts) are present in perivascular, Virchow-Robin (A), and interstitial spaces (B). In Virchow-Robin spaces (A) the CD138+ cells are in perivascular regions (arrows) and along the tissue surface (arrow heads) that delineates spaces containing CSF and small vessels (v). The plasma cells/plasmablasts indicated with arrows are amplified in the inset in A. Scales = 20 μm. Adapted from Martinez-Hernandez and colleagues,152 with permision.
Most neuronal cell–surface or synaptic autoantibodies are IgG1 or IgG3 subtypes that potentially fix complement; but in the disorders thus far studied (mainly anti-NMDAR encephalitis) there is no evidence of complement-mediated mechanisms of cell injury.21,152,157 This is different from NMO and aquaporin 4 antibodies, where symptoms are less reversible and complement-mediated brain injury is more visible in MRI.158 On the other hand, IgLON5 antibodies are IgG4, which do not fix complement; but in general, disorders associated with IgG4 antibodies appear to respond frequently to rituximab (an observation to consider in future studies).32,159,160
The underlying mechanisms involved in antibody-mediated changes in the structure and function of the target antigen have been elucidated in some autoimmune encephalitis, but the effects at the circuit level are unknown. Upcoming animal models/studies will provide strategies on how to neutralize the antibody effects, not only using immunotherapy but perhaps optimizing compensatory synaptic mechanisms. In line with potentially novel therapeutic strategies, a study showed that Ephrin-B2 ligand prevents the destabilizing NMDAR crosslinking effects of patients’ antibodies.114
The disorders discussed here provide natural models of human disease along with unique tools (patients’ antibodies) to understand the link between selective alteration of specific cell surface proteins or receptors and plasticity, memory, learning, and behavior.
Acknowledgments
The National Institute of Health, the McKnight Neuroscience of Brain Disorders award, the Fondo de Investigaciones Sanitarias, and Fundació la Marató de TV3.
Footnotes
Conflicts of interests
Dr. Dalmau holds patents for the use of Ma2, NMDAR, and GABAbR as autoantibody tests, and has patent applications for DPPX, GABAaR, and IgLON5 as autoantibody tests; he receives royalties for Ma2, NMDAR, and GABAbR diagnostic tests. Dr. Dalmau has a research grant from Euroimmun.
References
- 1.Lancaster E, Dalmau J. Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nat. Rev. Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscato EH, Jain A, Peng X, et al. Mechanisms underlying autoimmune synaptic encephalitis leading to disorders of memory, behavior and cognition: insights from molecular, cellular and synaptic studies. Eur. J. Neurosci. 2010;32:298–309. doi: 10.1111/j.1460-9568.2010.07349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panzer JA, Gleichman AJ, Lynch DR. Glutamatergic autoencephalitides: an emerging field. J. Neural. Transm. 2014 doi: 10.1007/s00702-013-1152-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindstrom JM, Engel AG, Seybold ME, et al. Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine receptor antibodies. J. Exp. Med. 1976;144:739–753. doi: 10.1084/jem.144.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- 6.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 7.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann. Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- 9.Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J. Child. Neurol. 2012;27:1460–1469. doi: 10.1177/0883073812448838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleichman AJ, Spruce LA, Dalmau J, et al. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the glun1 amino terminal domain. J. Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–189. doi: 10.1212/WNL.0b013e318224afde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson N, Henry C, Fessler AJ, et al. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75:1480–1482. doi: 10.1212/WNL.0b013e3181f8831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleopa KA, Elman LB, Lang B, et al. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129 doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 16.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 17.Graus F, Keime-Guibert F, Rene R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 18.Shams’ili S, Grefkens J, De Leeuw B, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409–1418. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 19.Rojas I, Graus F, Keime-Guibert F, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55:713–715. doi: 10.1212/wnl.55.5.713. [DOI] [PubMed] [Google Scholar]
- 20.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 21.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann. Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann. Neurol. 2011;69:303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson M, Waters P, McHugh J, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71:1291–1292. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- 29.Gable MS, Gavali S, Radner A, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:1421–1429. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gable MS, Sheriff H, Dalmau J, et al. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin. Infect. Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann. Neurol. 2006;59:178–181. doi: 10.1002/ana.20693. [DOI] [PubMed] [Google Scholar]
- 32.Sabater L, Gaig C, Gelpi E, L., et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–586. doi: 10.1016/S1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt SE, Pargeon K, Frechette ES, et al. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–1100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armangue T, Titulaer MJ, Malaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J. Pediatr. 2013;162:850–856. doi: 10.1016/j.jpeds.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 36.Stubgen JP. Nervous system lupus mimics limbic encephalitis. Lupus. 1998;7:557–560. doi: 10.1191/096120398678920523. [DOI] [PubMed] [Google Scholar]
- 37.Scheid R, Voltz R, Vetter T, et al. Neurosyphilis and paraneoplastic limbic encephalitis: important differential diagnoses. J. Neurol. 2005;252:1129–1132. doi: 10.1007/s00415-005-0812-1. [DOI] [PubMed] [Google Scholar]
- 38.Collison K, Rees J. Asymmetric cerebellar ataxia and limbic encephalitis as a presenting feature of primary Sjogren’s syndrome. J. Neurol. 2007;254:1609–1611. doi: 10.1007/s00415-007-0596-6. [DOI] [PubMed] [Google Scholar]
- 39.Wainwright MS, Martin PL, Morse RP, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann. Neurol. 2001;50:612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 40.Jarius S, Hoffmann L, Clover L, et al. CSF findings in patients with voltage gated potassium channel antibody associated limbic encephalitis. J. Neurol. Sci. 2008;68:74–77. doi: 10.1016/j.jns.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viaccoz A, Desestret V, Ducray F, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82:556–563. doi: 10.1212/WNL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 44.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect. Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 47.Pruss H, Dalmau J, Harms L, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 48.Vitaliani R, Mason W, Ances B, et al. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann. Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kayser MS, Dalmau J. Anti-NMDA Rereptor encephalitis in psychiatry. Curr. Psychiatry Rev. 2011;7:189–193. doi: 10.2174/157340011797183184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollak TA, McCormack R, Peakman M, et al. Prevalence of anti-N-methyl-d-aspartate (NMDA) antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol. Med. 2013:1–13. doi: 10.1017/S003329171300295X. [DOI] [PubMed] [Google Scholar]
- 51.Baizabal-Carvallo JF, Stocco A, Muscal E, et al. The spectrum of movement disorders in children with anti-NMDA receptor encephalitis. Mov. Disord. 2013;28:543–547. doi: 10.1002/mds.25354. [DOI] [PubMed] [Google Scholar]
- 52.Kleinig TJ, Thompson PD, Matar W, et al. The distinctive movement disorder of ovarian teratoma-associated encephalitis. Mov. Disord. 2008;23:1256–1261. doi: 10.1002/mds.22073. [DOI] [PubMed] [Google Scholar]
- 53.Uchino A, Iizuka T, Urano Y, et al. Pseudo-piano playing motions and nocturnal hypoventilation in anti-NMDA receptor encephalitis: Response to prompt tumor removal and immunotherapy. Intern. Med. 2011;50:627–630. doi: 10.2169/internalmedicine.50.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sansing LH, Tuzun E, Ko MW, et al. A patient with encephalitis associated with NMDA receptor antibodies. Nat. Clin. Pract. Neurol. 2007;3:291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millichap JJ, Goldstein JL, Laux LC, et al. Ictal asystole and anti-N-methyl-D-aspartate receptor antibody encephalitis. Pediatrics. 2011;127:e781–e786. doi: 10.1542/peds.2010-2080. [DOI] [PubMed] [Google Scholar]
- 56.Lee M, Lawn N, Prentice D, et al. Anti-NMDA receptor encephalitis associated with ictal asystole. J. Clin. Neurosci. 2011;18:1716–1718. doi: 10.1016/j.jocn.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 57.Bayreuther C, Bourg V, Dellamonica J, et al. Complex pa rtial status epilepticus revealing anti-NMDA receptor encephalitis. Epileptic. Disord. 2009;11:261–265. doi: 10.1684/epd.2009.0266. [DOI] [PubMed] [Google Scholar]
- 58.Titulaer MJ, Dalmau J. Seizures as first symptom of anti-NMDA receptor encephalitis are more common in men. Neurology. 2014;82:550–551. doi: 10.1212/WNL.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 59.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-NMDA receptor encephalitis. Ann. Neurol. 2014;75:411–428. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tham SL, Kong KH. A case of anti-NMDAR (N-methyl-D-aspartate receptor) encephalitis: a rehabilitation perspective. NeuroRehabilitation. 2012;30:109–112. doi: 10.3233/NRE-2012-0733. [DOI] [PubMed] [Google Scholar]
- 61.Scott O, Richer L, Forbes K, et al. Anti-N-methyl-d-aspartate (NMDA) receptor encephalitis: An unusual cause of autistic regression in a toddler. J. Child Neurol. 2014;29:691–694. doi: 10.1177/0883073813501875. [DOI] [PubMed] [Google Scholar]
- 62.Creten C, van der Zwaan S, Blankespoor RJ, et al. Late onset autism and anti-NMDA-receptor encephalitis. Lancet. 2011;378:98. doi: 10.1016/S0140-6736(11)60548-5. [DOI] [PubMed] [Google Scholar]
- 63.Goldberg EM, Titulaer M, de Blank PM, et al. Anti-N-methyl-D-aspartate receptor-mediated encephalitis in infants and toddlers: case report and review of the literature. Pediatr. Neurol. 2014;50:181–184. doi: 10.1016/j.pediatrneurol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Titulaer MJ, McCracken L, Gabilondo I, et al. Late-onset anti-NMDA receptor encephalitis. Neurology. 2013;81:1058–1063. doi: 10.1212/WNL.0b013e3182a4a49c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubio-Agusti I, Dalmau J, Sevilla T, et al. Isolated hemidystonia associated with NMDA receptor antibodies. Mov. Disord. 2011;26:351–352. doi: 10.1002/mds.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hacohen Y, Dlamini N, Hedderly T, et al. N-methyl-D-aspartate receptor antibody-associated movement disorder without encephalopathy. Dev. Med. Child. Neurol. 2014;56:190–193. doi: 10.1111/dmcn.12321. [DOI] [PubMed] [Google Scholar]
- 67.Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013;70:1133–1139. doi: 10.1001/jamaneurol.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niehusmann P, Dalmau J, Rudlowski C, et al. Diagnostic value of N-methyl-D-aspartate receptor antibodies in women with new-onset epilepsy. Arch. Neurol. 2009;66:458–464. doi: 10.1001/archneurol.2009.5. [DOI] [PubMed] [Google Scholar]
- 69.Gabilondo I, Saiz A, Galan L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77:996–999. doi: 10.1212/WNL.0b013e31822cfc6b. [DOI] [PubMed] [Google Scholar]
- 70.Kumar MA, Jain A, Dechant VE, et al. Anti-N-methyl-D-aspartate receptor encephalitis during pregnancy. Arch. Neurol. 2010;67:884–887. doi: 10.1001/archneurol.2010.133. [DOI] [PubMed] [Google Scholar]
- 71.Andrade DM, Tai P, Dalmau J, et al. Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology. 2011;76:1355–1357. doi: 10.1212/WNL.0b013e3182152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann. Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 73.Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013;136:3151–3162. doi: 10.1093/brain/awt212. [DOI] [PubMed] [Google Scholar]
- 74.Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking creutzfeldt-jakob disease. Arch. Neurol. 2008;65:1341–1346. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paterson RW, Zandi MS, Armstrong R, et al. Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. J. Neurol. Neurosurg. Psychiatry. 2014;85:625–630. doi: 10.1136/jnnp-2013-305218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujita K, Yuasa T, Watanabe O, et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J. Neurol. 2012;259:2249–2250. doi: 10.1007/s00415-012-6554-y. [DOI] [PubMed] [Google Scholar]
- 77.Grau-Rivera O, Sanchez-Valle R, Saiz A, et al. Determination of neuronal antibodies in suspected and definite Creutzfeldt-Jakob disease. JAMA Neurol. 2014;71:74–78. doi: 10.1001/jamaneurol.2013.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeffery OJ, Lennon VA, Pittock SJ, et al. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81:882–887. doi: 10.1212/WNL.0b013e3182a35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boronat A, Sabater L, Saiz A, et al. GABAB receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alamowitch S, Graus F, Uchuya M, et al. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120(Pt 6):923–928. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 82.Kim TJ, Lee ST, Shin JW, et al. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J. Neuroimmunol. 2014;270:45–50. doi: 10.1016/j.jneuroim.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 83.Jarius S, Steinmeyer F, Knobel A, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J. Neuroimmunol. 2013;256:94–96. doi: 10.1016/j.jneuroim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Mundiyanapurath S, Jarius S, Probst C, et al. GABA-B-receptor antibodies in paraneoplastic brainstem encephalitis. J. Neuroimmunol. 2013;259:88–91. doi: 10.1016/j.jneuroim.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Kruer MC, Hoeftberger R, Lim KY, et al. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures: The first pediatric case of gamma-aminobutyric acid type B receptor autoimmunity. JAMA Neurol. 2014;71:620–623. doi: 10.1001/jamaneurol.2013.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Graus F, Boronat A, Xifro X, et al. The expanding clinical profile of anti-AMPA receptor encephalitis. Neurology. 2010;74:857–859. doi: 10.1212/WNL.0b013e3181d3e404. [DOI] [PubMed] [Google Scholar]
- 87.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann. Neurol. 2012;72:241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 88.Rubio-Agusti I, Perez-Miralles F, Sevilla T, et al. Peripheral nerve hyperexcitability: a clinical and immunologic study of 38 patients. Neurology. 2011;76:172–178. doi: 10.1212/WNL.0b013e3182061b1e. [DOI] [PubMed] [Google Scholar]
- 89.Carr I. The Ophelia syndrome: memory loss in Hodgkin’s disease. Lancet. 1982;1:844–845. doi: 10.1016/s0140-6736(82)91887-6. [DOI] [PubMed] [Google Scholar]
- 90.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80:1349–1350. doi: 10.1212/WNL.0b013e31828ab325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sillevis SP, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N. Engl. J. Med. 2000;342:21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- 93.Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch. Neurol. 2010;67:627–630. doi: 10.1001/archneurol.2010.51. [DOI] [PubMed] [Google Scholar]
- 94.Mas N, Saiz A, Leite MI, et al. Antiglycine-receptor encephalomyelitis with rigidity. J. Neurol. Neurosurg. Psychiatry. 2011;82:1399–1401. doi: 10.1136/jnnp.2010.229104. [DOI] [PubMed] [Google Scholar]
- 95.Carvajal-Gonzalez A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137:2178–2192. doi: 10.1093/brain/awu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martinez-Hernandez E, Gresa-Arribas N, Petit-Pedrol M, et al. Autoimmunity to inhibitory synaptic proteins in Stiff-person spectrum of symptoms. Neurology; Abstract presented at the Annual Meeting of the American Academy of Neurology; Philadelphia, PA. Apr 28-29, 2014.2014. [Google Scholar]
- 97.Clardy SL, Lennon VA, Dalmau J, et al. Childhood onset of stiff-man syndrome. JAMA Neurol. 2013;70:1531–1536. doi: 10.1001/jamaneurol.2013.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McKeon A, Martinez-Hernandez E, Lancaster E, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol. 2013;70:44–50. doi: 10.1001/jamaneurol.2013.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balint B, Jarius S, Nagel S, et al. Progressive encephalomyelitis with rigidity and myoclonus: A new variant with DPPX antibodies. Neurology. 2014;82:1521–1528. doi: 10.1212/WNL.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 100.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann. Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Casado JL, Gil-Peralta A, Graus F, et al. Anti-Ri antibodies associated with opsoclonus and progressive encephalomyelitis with rigidity. Neurology. 1994;44:1521–1522. doi: 10.1212/wnl.44.8.1521. [DOI] [PubMed] [Google Scholar]
- 102.Luque FA, Furneaux HM, Ferziger R, et al. Anti-Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann. Neurol. 1991;29:241–251. doi: 10.1002/ana.410290303. [DOI] [PubMed] [Google Scholar]
- 103.Pittock SJ, Parisi JE, McKeon A, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri) Arch. Neurol. 2010;67:1109–1115. doi: 10.1001/archneurol.2010.209. [DOI] [PubMed] [Google Scholar]
- 104.Dalakas MC, Fujii M, Li M, et al. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology. 2000;55:1531–1535. doi: 10.1212/wnl.55.10.1531. [DOI] [PubMed] [Google Scholar]
- 105.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 106.Malter MP, Helmstaedter C, Urbach H, et al. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann. Neurol. 2010;67:470–478. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- 107.De Camilli P, Thomas A, Cofiell R, et al. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J. Exp. Med. 1993;178:2219–2223. doi: 10.1084/jem.178.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann. Neurol. 2005;58:96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 109.Saiz A, Dalmau J, Butler MH, et al. Anti-amphiphysin I antibodies in patients with paraneoplastic neurological disorders associated with small cell lung carcinoma. J. Neurol. Neurosurg. Psychiatry. 1999;66:214–217. doi: 10.1136/jnnp.66.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaulin YA, De Santiago-Castillo JA, Rocha CA, et al. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J. Neurosci. 2009;29:3242–3251. doi: 10.1523/JNEUROSCI.4767-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brimberg L, Benhar I, Mascaro-Blanco A, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: A novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012;37:2076–2087. doi: 10.1038/npp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manto M, Dalmau J, Didelot A, et al. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet. J. Rare. Dis. 2010;5:31. doi: 10.1186/1750-1172-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mikasova L, De Rossi P, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 115.Mannara F, Gutierrez-Cuesta J, Leypoldt F, et al. From behavior to synapse: a murine model of passive transfer of antibodies from patients with anti-NMDAR encephalitis. Neurology; Abstract presented at the Annual Meeting of the American Academy of Neurology; Philadelphia, PA. Apr 28-29, 2014.2014. [Google Scholar]
- 116.Gleichman AJ, Panzer JA, Baumann BH, et al. Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis. Ann. Clin. Transl. Neurol. 2014;1:180–189. doi: 10.1002/acn3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu YE, Wen L, Silva J, et al. Lgi1 null mutant mice exhibit myoclonic seizures and CA1 neuronal hyperexcitability. Hum. Mol. Genet. 2010;19:1702–1711. doi: 10.1093/hmg/ddq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukata Y, Lovero KL, Iwanaga T, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3799–3804. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohkawa T, Fukata Y, Yamasaki M, et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J. Neurosci. 2013;33:18161–18174. doi: 10.1523/JNEUROSCI.3506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kayser MS, Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J. Neuropsychiatry Clin. Neurosci. 2011;23:90–97. doi: 10.1176/appi.neuropsych.23.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zandi MS, Irani SR, Lang B, et al. Disease-relevant autoantibodies in first episode schizophrenia. J. Neurol. 2011;258:686–688. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Masdeu JC, Gonzalez-Pinto A, Matute C, et al. Serum IgG antibodies against the NR1 subunit of the NMDA receptor not detected in schizophrenia. Am. J. Psychiatry. 2012;169:1120–1121. doi: 10.1176/appi.ajp.2012.12050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rhoads J, Guirgis H, McKnight C, et al. Lack of anti-NMDA receptor autoantibodies in the serum of subjects with schizophrenia. Schizophr. Res. 2011;129:213–214. doi: 10.1016/j.schres.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 124.Steiner J, Walter M, Glanz W, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: Specific relevance of IgG NR1a antibodies for distinction from n - methyl-d-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 125.Busse S, Busse M, Brix B, et al. Seroprevalence of N-methyl-D-aspartate glutamate receptor (NMDA-R) autoantibodies in aging subjects without neuropsychiatric disorders and in dementia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2014 Mar 7; doi: 10.1007/s00406-014-0493-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 126.Hammer C, Stepniak B, Schneider A, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol. Psychiatry. 2013 Sep 3; doi: 10.1038/mp.2013.110. doi: 10.1038/mp.2013.110. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 127.Titulaer MJ, Dalmau J. Antibodies to NMDA receptor, blood-brain barrier disruption and schizophrenia: a theory with unproven links. Mol. Psychiatry. 2014 Mar 25; doi: 10.1038/mp.2014.25. 2014. doi: 10.1038/mp.2014.25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 128.Skoldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J. Neurol. 2006;253:163–170. doi: 10.1007/s00415-005-0941-6. [DOI] [PubMed] [Google Scholar]
- 129.Schleede L, Bueter W, Baumgartner-Sigl S, et al. Pediatric herpes simplex virus encephalitis: a retrospective multicenter experience. J. Child Neurol. 2013;28:321–331. doi: 10.1177/0883073812471428. [DOI] [PubMed] [Google Scholar]
- 130.De Tiege X, Rozenberg F, Des Portes V, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology. 2003;61:241–243. doi: 10.1212/01.wnl.0000073985.71759.7c. [DOI] [PubMed] [Google Scholar]
- 131.Hargrave DR, Webb DW. Movement disorders in association with herpes simplex virus encephalitis in children: A review. Dev. Med. Child Neurol. 1998;40:640–642. doi: 10.1111/j.1469-8749.1998.tb15431.x. [DOI] [PubMed] [Google Scholar]
- 132.Wang HS, Kuo MF, Huang SC, et al. Choreoathetosis as an initial sign of relapsing of herpes simplex encephalitis. Pediatr. Neurol. 1994;11:341–345. doi: 10.1016/0887-8994(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 133.Pruss H, Finke C, Holtje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann. Neurol. 2012;72:902–911. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Titulaer MJ, Leypoldt F, Dalmau J. Antibodies to N-methyl-D-aspartate and other synaptic receptors in choreoathetosis and relapsing symptoms post-herpes virus encephalitis. Mov. Disord. 2014;29:3–6. doi: 10.1002/mds.25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Armangue T, Leypoldt F, Malaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann. Neurol. 2014;75:317–323. doi: 10.1002/ana.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mohammad SS, Sinclair K, Pillai S, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-Methyl-D-aspartate receptor or dopamine-2 receptor. Mov. Disord. 2014;29:117–122. doi: 10.1002/mds.25623. [DOI] [PubMed] [Google Scholar]
- 137.Hacohen Y, Deiva K, Pettingill P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov. Disord. 2014;29:90–96. doi: 10.1002/mds.25626. [DOI] [PubMed] [Google Scholar]
- 138.Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis - A case report. Neurology. 2013;81:1637–1639. doi: 10.1212/WNL.0b013e3182a9f531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desena A, Graves D, Warnack W, et al. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol. 2014;71:344–346. doi: 10.1001/jamaneurol.2013.4580. [DOI] [PubMed] [Google Scholar]
- 140.Wickstrom R, Fowler A, Cooray G, et al. Viral triggering of anti-NMDA receptor encephalitis in a child - An important cause for disease relapse. Eur. J. Paediatr. Neurol. 2014;18:543–546. doi: 10.1016/j.ejpn.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 141.Dale RC, Irani SR, Brilot F, et al. N-methyl-D-aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica. Ann. Neurol. 2009;66:704–709. doi: 10.1002/ana.21807. [DOI] [PubMed] [Google Scholar]
- 142.Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch. Neurol. 2003;60:164–171. doi: 10.1001/archneur.60.2.164. [DOI] [PubMed] [Google Scholar]
- 143.Dogan M, Acikgoz E, Acikgoz M, et al. The frequency of Hashimoto thyroiditis in children and the relationship between urinary iodine level and Hashimoto thyroiditis. J. Pediatr. Endocrinol. Metab. 2011;24:75–80. doi: 10.1515/jpem.2011.115. [DOI] [PubMed] [Google Scholar]
- 144.Zois C, Stavrou I, Kalogera C, et al. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern. Greece. Thyroid. 2003;13:485–489. doi: 10.1089/105072503322021151. [DOI] [PubMed] [Google Scholar]
- 145.Tuzun E, Erdag E, Durmus H, et al. Autoantibodies to neuronal surface antigens in thyroid antibody-positive and -negative limbic encephalitis. Neurol. India. 2011;59:47–50. doi: 10.4103/0028-3886.76857. [DOI] [PubMed] [Google Scholar]
- 146.Kruer MC, Koch TK, Bourdette DN, et al. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology. 2010;74:1473–1475. doi: 10.1212/WNL.0b013e3181dc1a7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lekoubou A, Viaccoz A, Didelot A, et al. Anti-N-methyl-D-aspartate receptor encephalitis with acute disseminated encephalomyelitis-like MRI features. Eur. J. Neurol. 2012;19:e16–e17. doi: 10.1111/j.1468-1331.2011.03617.x. [DOI] [PubMed] [Google Scholar]
- 148.Hacohen Y, Absoud M, Woodhall M, et al. Autoantibody biomarkers in childhood-acquired demyelinating syndromes: results from a national surveillance cohort. J. Neurol. Neurosurg. Psychiatry. 2014;85:456–461. doi: 10.1136/jnnp-2013-306411. [DOI] [PubMed] [Google Scholar]
- 149.Becker EB, Zuliani L, Pettingill R, et al. Contactin-associated protein-2 antibodies in non-paraneoplastic cerebellar ataxia. J. Neurol Neurosurg. Psychiatry. 2012;83:437–440. doi: 10.1136/jnnp-2011-301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J. Neurol. 2012;259:1979–1981. doi: 10.1007/s00415-012-6489-3. [DOI] [PubMed] [Google Scholar]
- 151.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, et al. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cash SS, Larvie M, Dalmau J. Case records of the Massachusetts General Hospital. Case 34-2011: A 75-year-old man with memory loss and partial seizures. N. Engl. J. Med. 2011;365:1825–1833. doi: 10.1056/NEJMcpc1100924. [DOI] [PubMed] [Google Scholar]
- 154.Alexopoulos H, Kosmidis ML, Dalmau J, et al. Paraneoplastic anti-NMDAR encephalitis: long term follow-up reveals persistent serum antibodies. J. Neurol. 2011;258:1568–1570. doi: 10.1007/s00415-011-5982-4. [DOI] [PubMed] [Google Scholar]
- 155.Hansen HC, Klingbeil C, Dalmau J, et al. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2013;70:117–119. doi: 10.1001/jamaneurol.2013.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moscato EH, Peng X, Jain A, et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 2014;76:108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- 158.Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 159.Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology. 2014;82:879–886. doi: 10.1212/WNL.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khosroshahi A, Carruthers MN, Deshpande V, et al. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 2012;91:57–66. doi: 10.1097/MD.0b013e3182431ef6. [DOI] [PubMed] [Google Scholar]