Abstract
Purpose
To study the differences in protein expression profiles of follicular fluid (FF) between controlled ovarian hyperstimulation (COH) and natural ovulatory cycles.
Methods
Twelve infertile women undergoing in vitro fertilization and embryo transfer (IVF–ET), with matched clinical information, were retrospectively recruited in the IVF center of our university hospital, including six undergoing COH and another six with natural cycles. FF was sampled from dominant follicles with mature oocytes. Protein expression profiles in each FF sample were analyzed respectively using two-dimensional gel electrophoresis. Differentially expressed proteins were identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and validated by western blotting. Differentially expressed proteins were further analyzed using Ingenuity Pathway Analysis (IPA) software.
Results
Two proteins were downregulated and 11 proteins were upregulated (change ≥1.5-fold, P < 0.05) in the COH group. We identified one down-egulated and seven upregulated proteins using MALDI-TOF MS. Four differentially expressed proteins, including transferrin, complement component C3 (C3), haptoglobin and alpha-1-antitrypsin (AAT), were further validated by rate nephelometry and western blotting analyses. The IPA analysis revealed a significant network involved in the humoral immune and inflammatory responses.
Conclusions
The eight differentially expressed proteins were related to immune and inflammatory responses in the ovary. Our results provide new insights into the influence of COH on follicular (spp) development and IVF outcomes.
Keywords: Controlled ovarian hyperstimulation/COH, Follicular fluid, Proteomics, Assisted reproductive technology/ART, MALDI-TOF MS
Introduction
Ovarian follicular growth is a process involving a complex exchange of hormonal signals between the hypothalamus–pituitary–ovarian axis and by a localized para/autocrine mechanism within the ovary involving the oocyte and its adjacent somatic cells. Many studies have aimed at unraveling the complex intraovarian control mechanisms that act in concert with systemic signals, to solve clinical problems, improve the outcome of assisted reproductive technology (ART) and establish the in vitro maturation system [1, 2]. Some progress has been obtained but the mechanism of follicular development is poorly understood, especially during controlled ovarian hyperstimulation (COH).
COH is routinely used to increase the numbers of oocytes ovulated and embryos produced in ART. However, it can affect oocyte maturation and subsequent embryo development. Artificial induction of ovulation with high doses of gonadotrophins has been demonstrated to decrease the viability of embryos and induce oocyte aneuploidy [3, 4]. In animal models, COH was shown to induce the incidence of aberrant methylation patterns in mouse two-cell embryos and the placenta [5], and affect sperm methylation patterns in the second generation of offspring [6]. In humans, it has been reported that patients who produced triple pronuclear zygotes after intracytoplasmic sperm injection (ICSI) were high responders to ovarian stimulation [7]. Singleton pregnancies in infertile couples after ovarian stimulation had a higher risk of low birth weight when compared with naturally conceived offspring [8]. Other researchers have demonstrated that there were higher risks of birth defects and imprinted diseases in ART offspring [9–11]. A recent report showed that COH could lead to higher systolic blood pressure percentiles in 4-year-old children born following in vitro fertilization and embryo transfer (IVF–ET) [12]. Therefore, it is possible that COH might result in long-term impairments to the offspring, but the underlying mechanisms are unknown.
Increasing evidence suggests that systematic studies should be conducted to reveal the impact of COH on follicle development and oocyte maturation. The follicular fluid (FF), containing a wide variety of biologically active molecules, is the microenvironment of the oocyte during its development and maturation. FF originates from the circulation and from follicular secretions, which are the predominant products of metabolic processed within the follicle including the maturing oocyte and its surrounding somatic cells. The composition of FF might influence and/or be implicated with oocyte quality. Some human FF proteins have been shown to be correlated with oocyte maturation, fertilization outcome and embryo development [13–15]. Therefore, identification of specifically altered protein levels in FF during COH cycles might be helpful in determining the influence of COH on follicular and oocyte development, and to predict IVF outcomes.
Proteomics is defined as the large-scale study of proteins, modifications, complexes and interactions from a given cell line or organism [16]. High-resolution analysis of two-dimensional gel electrophoresis (2-DE) is a powerful approach to compare patterns of protein expression, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) can be utilized to identify protein spots on 2-DE gels [17]. Here we investigated protein expression profiles in FF from womenundergoing COH or natural cycles. We analyzed the physiological pathwaysinvolving the identified proteins with the ultimate aim of evaluating the effects of COH on folliculogenesis and IVF outcome.
Materials and methods
Patients
Twelve infertile women undergoing IVF–ET were retrospectively recruited in the Reproductive Center of a university affiliated hospital. The protocol was approved by the Institutional Review Board of the university, and informed consents were obtained from all participants. Women included in this study were less than 40 years of age, and had regular menstrual cycles (27–35 days), a typical double phase basal body temperature and normal levels of serum prolactin. None had used any steroidal medicines in the past 6 months. Women with endometriosis, hyperprolactinemia or polycystic ovarian syndrome (PCOS) were excluded. The causes of infertility of these women were as follows: seven cases were associated with male infertility, and five involved male infertility combined with female tubal infertility. The cause of tubal infertility was Fallopian tube obstruction caused by infection such as pelvic inflammatory disease, and was evaluated by hysterosalpingography. The women were recruited according to different protocols, COH group (n = 6) and a natural cycle group (n = 6). Patients in the natural cycle group refused to accept gonadotrophins because they were afraid of the side effects on themselves and the potential risk on the offspring. There were no significant differences in age, basal serum follicle stimulating hormone (FSH) levels or body mass index (BMI) between the groups.
Protocol and collection of follicular fluid
In the COH group, patients were stimulated with recombinant FSH (rFSH, Serono, Geneva, Switzerland) after their pituitary glands were downregulated with gonadotropin-releasing hormone agonists (GnRH-a, Serono) according to the long stimulation protocol. When ultrasonography showed that the diameter of the leading follicle reached 18 mm, or the mean diameter of three leading follicles reached 16 mm, stimulation was stopped. A total of 10,000 IU of urinary human chorionic gonadotrophin (uhCG, Serono) was administered 36 h before follicular aspiration.
Women in the natural cycle group were not treated with medications to stimulate ovaries or to block the spontaneous luteinizing hormone (LH) surge. Transvaginal ultrasound scans were used daily to monitor the follicular size, endometrial thickness and ovarian morphology from day 8 of the menstrual cycle. Serum hormone levels of estradiol, LH and progesterone were measured daily when the dominant follicle reached 12 mm in diameter. When it reached 16 mm, serum LH was examined every 12 h. When a serum LH surge was detected (>30 IU/L), ultrasound-guided transvaginal oocyte retrieval was performed 14–28 h later.
Clear FF without macroscopic blood contamination was collected from dominant follicles with transvaginal ultrasound-guided puncture by negative pressure using a vacuum aspiration pump. In the COH group, only the fluid from the first aspirated follicle of each patient was carefully collected to avoid blood contamination. Only those FF samples from follicles in which a metaphase 2 oocyte was identified were used in this study. The FF samples were centrifuged at 1300 g for 10 min at 4 °C, and stored at −70 °C prior to assay.
IVF outcome
In the natural cycle group, 4 of 6 oocytes were fertilized and all the 4 fresh embryos were transferred. Eventually, one woman was pregnant and had a live birth. In the COH group, the oocytes were not treated individually in the IVF laboratory, so the evaluation of fertilization of the oocytes from the sampled follicles could not be investigated. Totally 12 embryos were transferred in the 6 cycles and resulted in 2 clinical pregnancies and 2 live births.
Sample preparation
In order to enhance detection of low-abundance proteins from FF, each sample was processed respectively using Aurum Serum Protein Mini Kits (Bio-Rad, Hercules, CA, USA) to deplete albumin and immunoglobulin G (IgG) prior to the 2-DE [18]. As recommended by the manufacturer, 60-μL aliquots of FF samples were diluted 4-fold with the binding buffer provided in the kit, and sonicated. Two hundred microliters of diluted sample were applied to the column, and vortexed. The column was then incubated for 5 min. Vortexing and incubation were repeated three times. After these steps, the column was centrifuged at 10,000 g for 20 s. The unbound protein fraction was collected by washing the column again with 200 μL binding buffer, and the supernatant containing the albumin- and IgG-depleted FF sample was now ready for 2-DE [19].
2-DE and image analysis
Twelve FF samples were analyzed retrospectively and then compared; 2-DE was performed according to the published procedure described by Liu AX et al. [20]. Briefly, 450-μg aliquots of FF proteins were applied to immobilized pH 4–7 nonlinear gradient strips (18 cm, Amersharm Bioscience, Uppsala, Sweden), and the strips were rehydrated and focused as previously described [19]. The second-dimensional separation was performed in 12.5 % polyacrylamide gels. For compatibility with mass spectrometry, we omitted the glutaraldehyde fixation and sensitization steps in the silver staining protocol [21]. Silver-stained gels were scanned using a Bio-Rad GS-800 imaging densitometer (Bio-Rad, Hercules, CA), and image analysis was carried out using PDQuest (Version 7.1.1; Bio-Rad) as described before [20].
Protein identification and data analysis
Excised protein spots were decolorized, digested and extracted as Liu AX et al. described [17]. Peptide samples were analyzed using a Bruker-Daltonics AutoFlex TOF-TOF LIFT Mass Spectrometer (Bruker Daltonics, Bremen, Germany), and the peptide mass fingerprint data were searched by BioTools 3.0 software on MASCOT (V2.1, Matrix Science, UK) for protein identification as described [20]. Proteins matching more than four peptides and with a MSCOT score >64 were considered significant (P < 0.05). Proteins with a minimal 1.5-fold change between groups and P < 0.05 were considered significantly differentially expressed, and analyzed using the Ingenuity Pathway Analysis (IPA) software (QIAGEN, Redwood City, CA, USA). Networks of these proteins were generated algorithmically based on their connectivity.
Analysis of alpha-1-antitrypsin (AAT), complement component C3 (C3), transferrin and haptoglobin in FF
The AAT levels in FF were measured by western blotting as described by Wu YT et al. [13]. Briefly, 1.0 mL of FF was diluted in PBS and electrophoresed using 12 % sodium dodecylsulfate polyacrylamide gelelectrophoresis (SDS–PAGE). The separated proteins were transferred onto nitrocellulose membranes, and incubated with an anti-AAT antibody (1:500, Santa Cruz, CA, USA) and anti-goat immunoglobulin G (1:5000, Zhongshan Biotechnology, Beijing, China) for western blotting. The FF levels of C3, transferrin, and haptoglobin were measured by quantitative nephelometry, using an automated biochemical analyzer (Beckman Instruments, Brea, CA, USA).
Statistical analysis
Clinical and experimental data were subjected to Student’s t-tests using SPSS for IBM Statistics version 20 (IBM Corp., Armonk, NY, USA). Only proteins that demonstrated significant and consistent changes (increased or decreased) were used for further bioinformatics analysis. Right-tailed Fisher’s exact tests were used by the IPA to calculate P-values and identify statistically significant pathways and networks associated with proteins identified in the study. For all analyses, significance was set at P < 0.05.
Results
2-DE analysis of proteins in FF
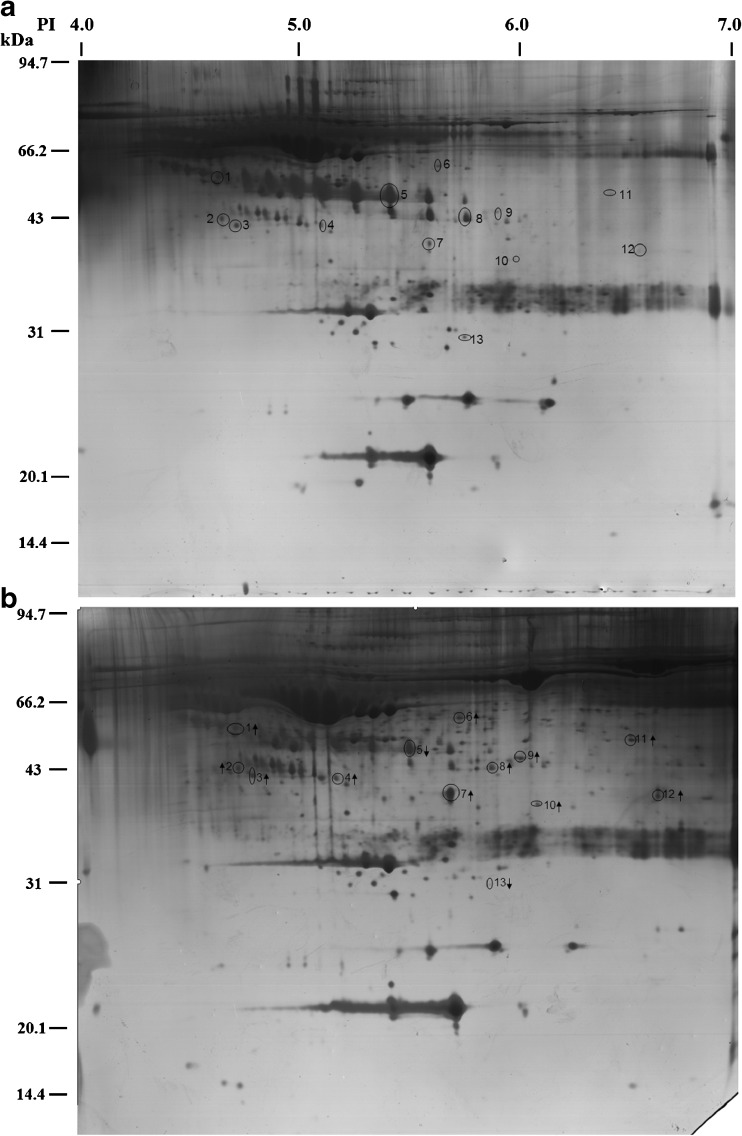
The proteins of FF were separated by 2-DE in the pH range of 4–7 as shown in Fig. 1. Compared with the natural cycle group, 13 protein spots from the FF in the COH cycle group were significantly decreased or increased ≥1.5-fold. The relative volumes of these spots and the spot volume ratios between the two groups are shown in Table 1. Among these 13 proteins, 11 were upregulated, and two proteins were downregulated as determined by spot volume (Fig. 1). Figure 1a shows 13 marked protein points in the FF of natural cycles, and Fig. 1b shows the marked corresponding proteins and changing tendencies by arrows. The 13 protein spots were excised from the gels and subsequently analyzed by MALDI-TOF MS. Using the resultant spectra, we successfully identified eight of 13 differentially expressed protein spots (Table 2), which included AAT, haptoglobin, transferrin, C3, Zn-alpha-2-Glycoprotein (ZAG), transthyretin, leucine-rich alpha-2-glycoprotein (LRG), and Fibrinogen γ. As shown in Table 2, the spot number refers to the labels in Fig. 1. The remaining five protein spots were not identified as no satisfactory spectra could be obtained.
Fig. 1.
Typical two-dimensional gel electrophoresis maps of follicular fluid (FF): a Natural cycle; b Controlled ovarian hyperstimulation (COH) cycle. Arrows show the 13 differentially expressed spots in FF from COH cycles
Table 1.
Differentially expressed protein spots in follicular fluid
| Spot No. | Single spot volume/total spot volume | p | ||
|---|---|---|---|---|
| Natural cycle group | COH group | Ratio | ||
| 1 | 0.0038 ± 0.0007 | 0.0073 ± 0.0012 | 1.92 | 0.030 |
| 2 | 0.0022 ± 0.0005 | 0.0058 ± 0.0011 | 2.64 | 0.006 |
| 3 | 0.0013 ± 0.0003 | 0.0056 ± 0.0007 | 4.31 | <0.001 |
| 4 | 0.0010 ± 0.0003 | 0.0025 ± 0.0005 | 2.5 | 0.021 |
| 5 | 0.0303 ± 0.0075 | 0.0094 ± 0.0034 | 3.22 | 0.026 |
| 6 | 0.0025 ± 0.0004 | 0.0063 ± 0.0020 | 2.52 | 0.020 |
| 7 | 0.0057 ± 0.0011 | 0.0108 ± 0.0014 | 1.89 | 0.033 |
| 8 | 0.0034 ± 0.0009 | 0.0086 ± 0.0017 | 2.53 | 0.031 |
| 9 | 0.0030 ± 0.0008 | 0.0075 ± 0.0019 | 2.5 | 0.041 |
| 10 | 0.0006 ± 0.0002 | 0.0025 ± 0.0008 | 4.16 | 0.036 |
| 11 | 0.0011 ± 0.0002 | 0.0039 ± 0.0009 | 3.55 | 0.012 |
| 12 | 0.0009 ± 0.0003 | 0.0047 ± 0.0017 | 4.4 | 0.036 |
| 13 | 0.0020 ± 0.0007 | 0.0006 ± 0.0001 | 3.33 | 0.040 |
Data are presented as the mean ± SEM and were compared using Student’s t test
Table 2.
Search results of peptides for those proteins with altered expression levels with relatively high confidence in FF samples from patients with COH
| Spot No. | Protein name | NCBI Accession No.a | Theoretical Mr (Da)/pI | Scoreb | No. matched peptides | Expression regulationc |
|---|---|---|---|---|---|---|
| 1 | Complement component C3 | gi|78101271 | 34237/4.79 | 121 | 16 | + |
| 2 | Alpha-1-antitrypsin | gi|177831 | 46677/5.43 | 142 | 11 | + |
| 3 | Transthyretin | gi|14719497 | 12614/5.26 | 156 | 3 | + |
| 4 | Zn-Alpha-2-Glycoprotein, | gi|58176763 | 32125/5.71 | 290 | 22 | + |
| 5 | Leucine-rich Alpha-2-glycoprotein | gi|72059 | 34325/5.66 | 129 | 6 | − |
| 6 | Fibrinogen γ | gi|182439 | 49450/5.61 | 450 | 15 | + |
| 9 | Haptoglobin | gi|4826762 | 45176/6.13 | 95 | 11 | + |
| 11 | Transferrin | gi|7245524 | 36328/6.49 | 157 | 7 | + |
aMASCOT result of MALDI-TOF searched from the NCBI-nr database (http://www.ncbi.nlm.nih.gov/refseq/)
bFor peptide mass fingerprint: a score of more than 64 is significant (p < 0.05)
cUpregulated +; downregulated −
Validation of differentially expressed proteins
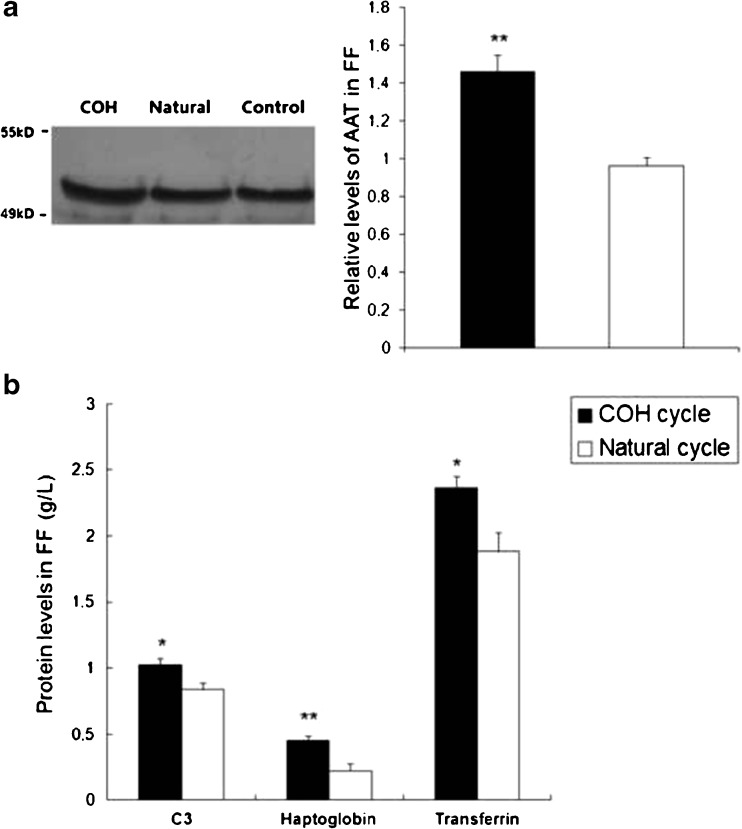
To confirm the results obtained by MALDI-TOF MS, four proteins including AAT, C3, haptoglobin and transferrin, were determined quantitatively by western blotting and rate nephelometry, in FF from the COH and natural cycle groups. Western blotting images and semi-quantitative results of FF AAT are shown in Fig. 2a and b shows the levels of haptoglobin, transferrin and C3 in FF from the COH and natural cycle groups. Consistent with the results of 2-DE, the expression levels of these four proteins in FF were significantly higher in the COH group than those in the natural cycle group.
Fig. 2.
a Western blot analysis of α1-antitrypsin levels in the FF from COH cycles and natural cycles. b Rate nephelometry analysis of complement C3, haptoglobin and transferrin concentration in FF. *P < 0.05 and **P < 0.01 versus control
Bioinformatics analysis
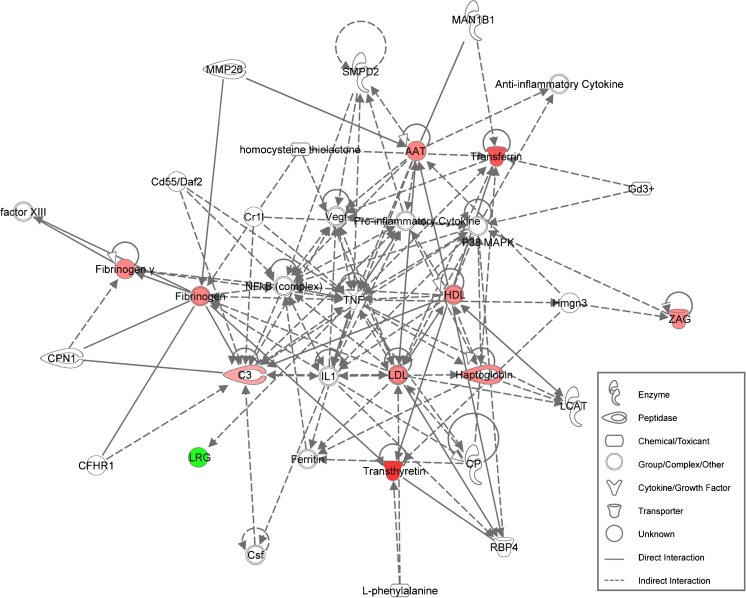
To determine the major pathways, biological functions and protein networks related to the eight differentially expressed proteins identified in the COH FF, we performed functional enrichment analysis using IPA software. We revealed a statistically significant network (p = 10−24), involved in the humoral immune response and inflammatory responses (Fig. 3). This network has all of the identified differential expressed proteins as central nodes.
Fig. 3.
Network of proteins involved in humoral immune and inflammatory responses. The network was generated using proteins differentially expressed in COH FF versus natural cycles. Red nodes indicate upregulation, and green node color indicates downregulation of differential proteins
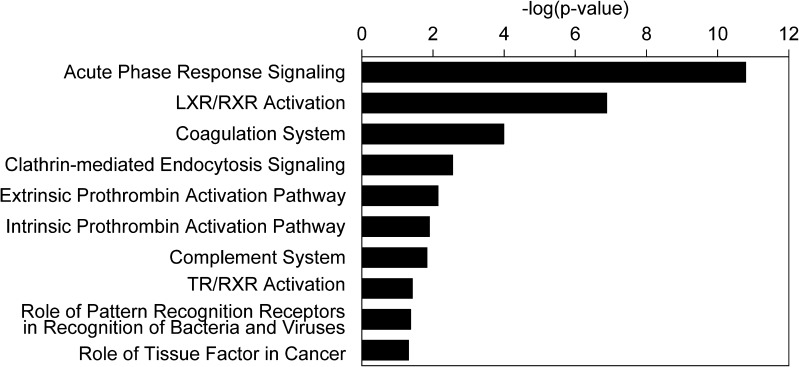
Further assessment of the differentially expressed proteins (Fig. 4) revealed several canonical pathways primarily associated with a variety of biological processes, including acute phase response signaling (p = 1.58 × 10−11), liver X receptor/retinoid X receptor (LXR/RXR) activation (p = 1.29 × 10−7), the blood coagulation system (p = 10−4), clathrin-mediated endocytosis signaling (p = 2.75 × 10−3), the extrinsic prothrombin activation pathway (p = 7.08 × 10−3), intrinsic prothrombin activation pathway (p = 1.23 × 10−2), complement system (p = 1.45 × 10−2), thyroid hormone receptor (TR/RXR) activation (p = 3.72 × 10−2), the role of pattern recognition receptors in recognition of bacteria and viruses (p = 4.17 × 10−2), and the role of tissue factors in cancer (p = 4.79 × 10−2) (Fig. 4).
Fig. 4.
Significant canonical pathways associated with differentially expressed proteins identified in COH FF samples versus natural cycles. The P-value was calculated using the right-tailed Fisher’s exact test. Bars represent − log (P-value) for significance
More detailed analyses for functional subcategories revealed proteins enriched in disease and disorder (Fig. 5a), molecular and cellular functions (Fig. 5b), and physiological system development and functions (Fig. 5c). According to overlapping p-values, the top 10 subcategories of the ‘Disease and Disorder’ class included neurological disease, organismal injury and abnormalities disorder, respiratory disease, metabolic disease, psychological disorders, hereditary disorders, skeletal and muscular disorders, endocrine system disorders, gastrointestinal diseases, and connective tissue disorders (Fig. 5a). The top 10 subcategories of the ‘Molecular and Cell Functions’ class included cellular function and maintenance, free radical scavenging, cell-to-cell signaling and interaction, molecular transport, cellular movement, cellular compromise, cell death and survival, cell signaling, small molecule biochemistry, and lipid metabolism (Fig. 5b). The top 10 subcategories of the Physiological System ‘Development and Functions’ class included organ morphology, reproductive system development and function, embryonic development, tissue development, hair and skin development and function, renal and urological system development and function, hematological system development and function, immune cell trafficking, tissue morphology, and nervous system development and function (Fig. 5c).
Fig. 5.
Graphic distribution of the most representative functional categories found for differentially expressed proteins in COH FF versus natural cycles, including (a) disease and disorder, (b) molecular and cell functions, and (c) physiological system development and functions. The top 10 enriched biological functions are shown in each functional category. The P-values were calculated using right-tailed Fisher’s exact tests. Bars represent significance as − log (P-value)
Discussion
In the present study, we found 11 upregulated proteins and two down-regulated proteins in the FF from COH cycles, compared with the FF obtained during natural cycles. We successfully identified 8 proteins: AAT, ZAG, TTR, C3, LRG, Fibrinogen γ, haptoglobin and transferrin. Some previous studies have performed proteomics analysis on FF. Fahiminiya et al. found three proteins that were associated with the preovulatory LH surge in canine FF [22]. Kushnir et al. identified 13 proteins from the FF that were respectively unique to women with viable pregnancy, miscarriage, or no pregnancy, thence suggested these FF proteins could be noninvasive markers for assessment of oocytes viability [14]. The present study identified proteomic differences in the FF between COH and natural cycles in women undergoing IVF–ET, and has provided new insights into the impact of exogenous gonadotrophins on the follicular milieu.
The main characteristic of COH cycle is the high exposure to estradiol by the gamete/embryo. A recent study showed that a supraphysiological maternal estradiol environment was correlated with an increased risk of low birth weight and small-for-gestational age neonates [23]. Moreover, many studies have shown that the developmental potential of oocytes obtained from natural cycles is better than those from COH cycles [24, 25]. However, the underlying molecular mechanisms remain elusive.
According to our bioinformatics analysis, the functions of differentially expressed proteins in COH FF emerging from the generated network were centered on the humoral immune and inflammatory responses, and most proteins in the network were upregulated. The functional enrichment analysis revealed that the differentially expressed proteins were mainly associated with embryonic development, organismal injury and free radical scavenging. It is widely accepted that the ovulatory process is a hormone-induced inflammatory process [26]. Our results are consistent with a previous study demonstrating that COH potentiates a state of systemic inflammation [27], and might injure follicular cells. Similarly, excessive ovarian stimulation has been demonstrated to induce aberrant expression of FF proteins, such as C3, associated with inflammation, iron transport and storage, blood coagulation, and innate immunity in women who developed ovarian hyperstimulation syndrome [28]. In this study, the increased levels of C3 and ZAG in the COH FF suggest that the patients might have had aberrant immune and inflammatory responses during the cycle. The level of C3 in FF has been found to be lower in the women undergoing IVF–ET with a live birth than that in the women with a failed pregnancy [29]. On the other hand, a high follicular level of ZAG was associated with failed pregnancy of the women undergoing IVF–ET [14]. ZAG is one of the most abundant proteins in human FF [30], and plays a role in immune modulation during the follicular development. The levels of ZAG in FF were correlated with the concentrations of steroids in FF [14]. The upregulated C3 and ZAG, representing enhanced immune response, might be concerned with the negative effect of COH on IVF outcome.
In this study, we revealed 10 significant canonical pathways that involved the differentially expressed proteins identified in the COH FF, including acute phase response signaling, LXR/RXR activation, the blood coagulation system, clathrin-mediated endocytosis signaling, the extrinsic prothrombin activation pathway, the intrinsic prothrombin activation pathway, the complement system, TR/RXR activation, role of pattern recognition receptors in recognition of bacteria and viruses, and the role of tissue factors in cancer. Most of the pathways identified in our study have also been reported by Kushnir et al. [14]. They tried to identify the differentially expressed proteins that could predict IVF–ET outcome as biomarkers. They found that inhibition of the complement system in oocytes was required for maintaining the viability of oocytes, and the lack of this inhibition might lead to miscarriage because women who miscarried showed a significantly higher activity of complement system than those with a live birth [14]. Here, we also found that the activity of complement system in COH FF was higher than that in the natural cycle FF, which might impair the outcome of IVF–ET. Fibrinogen is one of the acute-phase proteins, involved in maintaining early pregnancy maintaining. A previous study reported that a decreased levele of fibrinogen in FF was associated with recurrent abortion [31]. In the present study, we demonstrated a significantly increased level of fibrinogen in COH FF, suggesting that ovarian stimulation for IVF might create a state of hypercoagulability [32]. However, the effect of high level of FF fibrinogen on the IVF outcome needs to be investigated further.
It is known that AAT in the FF is not synthesized by the follicle, but arises from the circulation [33]. AAT is a kind of protease inhibitor that is supposed to finely regulate the balance of protease and anti-protease activities in the FF during follicular maturation and ovulation. Inactivation of ATT might disrupt this balance of proteolysis/inhibition-to-proteolysis, and lead to premature follicular rupture and oocyte release. Thus, AAT exerts a key role in follicular maturation and the control of mature oocyte release [34]. The FF level os AAT was increased in patients with PCOS [35]. More importantly, the AAT level in FF has been regarded as a key indicator of oocyte maturity. FF from follicles with immature oocytes showed a higher level of AAT than FF from those with mature oocytes [36]. The fertilization rate of oocytes from FF with low AAT concentrations was also significantly higher than that of oocytes from FF with high AAT concentrations [37]. Meng et al. has found that COH reduced the fertilization rate of oocytes [38]. The higher AAT concentrations in FF from the women during the COH cycle suggest that this might inhibit oocyte maturation, and could be a cause of the reduced fertilization rate in such patients.
Transferrin, mainly synthesized in the liver, is responsible for transporting iron. The main source of FF transferrin is from the blood circulation, and the concentration of transferrin in FF is usually higher than that in serum [39] because transferrin is also locally synthesized by granulosa cells [40, 41]. Transferrin was found to inhibit progesterone production and FSH-stimulated aromatase activity of granulose cells [42, 43]. During follicular development, the levels of iron and transferrin in FF are gradually increased to support the growth and maturation of oocytes. Immunohistochemical studies showed that the levels of transferrin and its receptor in mature follicles were significantly higher than in immature follicles [41], suggesting that endogenous transferrin may play a role in follicular maturation [44]. A previous study demonstrated that transferrin levels were upregulated in FF from the patients with PCOS [35]. Our data showed that COH significantly raised the FF transferrin level, which might be partly because of the increased need for iron caused by multiple dominant follicles. However, this assumption lacks evidence and needs to be validated by further investigation. An intermediate range of FF transferrin level was associated with the highest likelihood of oocyte fertilization [45]. Thus, increased FF transferrin levels in FF might impair the quality of oocytes in COH cycles.
What implications can be drawn from this study? In recent years, unstimulated cycles or COH with a low stimulation dose are increasingly being recommended by reproductive specialists. Natural cycles and mild stimulation cycles are cost-effective and show a low risk of complications such as ovarian hyperstimulation syndrome,while the pregnancy rates per embryo transfer remain acceptable [24]. Especially, mild protocol has been proven to obtain a comparable IVF outcome as long protocol for patients with expected poor ovarian response [46]. In this small sample study, the IVF outcomes in the COH group seem better than those in the natural cycle group, which is possibly due to more retrieved oocytes and lower rate of cancelled cycles. But the pregnancy rate per embryo transfer (25 %) was still acceptable in the natural cycle group. Our study supports previous results showing that COH significantly altered the follicular endocrine milieu and reduced oocyte quality [47]. The present investigations provide new evidence for the emerging trend towards reduced use of gonadotrophins.
In conclusion, using a proteomic approach we successfully identified eight differentially expressed proteins in the FF from women undergoing COH. These proteins are associated with oocyte maturationand the immune and inflammatory responses. These results partly reveal the effect of ovarian stimulation on the follicular milieu. The aberrant protein profiles indicate that aggravated immune and inflammatory responses might play important roles in adverse effects of COH on oocyte vitality, and may contribute to the poor IVF-ET outcome. Further investigations such as oocytes matured in vitro are required to determine the precise effect of these proteins on oocytes.
Acknowledgments
This work was supported by research grants from the National Science & Technology Pillar Program during the 12th Five-year Plan Period of China (No. 2012BAI32B04) and the Zhejiang Provincial Natural Science Foundation of China (No. LY13H040002).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule
To study the effect in the follicular milieu of ovarian stimulation versus no stimulation, we analyzed protein expression profiles of follicular fluid from six women undergoing COH IVF and another six with natural cycles using two-dimensional gel electrophoresis. Eight differentially expressed proteins were identified and related to immune and inflammatory responses in the ovary.
References
- 1.Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2(1):9. doi: 10.1186/1757-2215-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Kawamura K, Deguchi M, Takae S, Mulders SM, Hsueh AJ. Intraovarian thrombin and activated protein C signaling system regulates steroidogenesis during the periovulatory period. Mol Endocrinol. 2012;26(2):331–40. doi: 10.1210/me.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139(1):23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud I, Vialard F, Bergere M, Albert M, Gomes DM, Adler M, et al. Follicular fluid protein content (FSH, LH, PG4, E2 and AMH) and polar body aneuploidy. J Assist Reprod Genet. 2012;29(10):1123–34. doi: 10.1007/s10815-012-9841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortier AL, Lopes FL, Darricarrere N, Martel J, Trasler JM. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet. 2008;17(11):1653–65. doi: 10.1093/hmg/ddn055. [DOI] [PubMed] [Google Scholar]
- 6.Stouder C, Deutsch S, Paoloni-Giacobino A. Superovulation in mice alters the methylation pattern of imprinted genes in the sperm of the offspring. Reprod Toxicol. 2009;28(4):536–41. doi: 10.1016/j.reprotox.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Sachs AR, Politch JA, Jackson KV, Racowsky C, Hornstein MD, Ginsburg ES. Factors associated with the formation of triploid zygotes after intracytoplasmic sperm injection. Fertil Steril. 2000;73(6):1109–14. doi: 10.1016/S0015-0282(00)00521-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhu YM, Hu XL, Wu YT, Feng C, Huang HF. Assisted Reproductive Technology and Gamete/Embryo-Fetal Origins of Diseases. In: Huang HF, Sheng JZ, editors. Gamete and embryo-fetal origins of adult diseases. Dordrecht: Springer Netherlands; 2014. p. 197. [Google Scholar]
- 9.Wilkins-Huag L. Assisted reproductive technology, congenital malformations, and epigenetic disease. Clin Obstet Gynecol. 2008;51(1):96–105. doi: 10.1097/GRF.0b013e318161d25a. [DOI] [PubMed] [Google Scholar]
- 10.Shiota K, Yamada S. Assisted reproductive technologies and birth defects. Congenit Anom (Kyoto) 2005;45(2):39–43. doi: 10.1111/j.1741-4520.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang AS, Moley KH, Wangler M, Feinberg AP, Debaun MR. Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients. Fertil Steril. 2005;83(2):349–54. doi: 10.1016/j.fertnstert.2004.07.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seggers J, Haadsma ML, La Bastide-Van GS, Heineman MJ, Middelburg KJ, Roseboom TJ, et al. Is ovarian hyperstimulation associated with higher blood pressure in 4-year-old IVF offspring? Part I: multivariable regression analysis. Hum Reprod. 2014;29(3):502–9. doi: 10.1093/humrep/det396. [DOI] [PubMed] [Google Scholar]
- 13.Wu YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM, et al. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod. 2007;22(6):1526–31. doi: 10.1093/humrep/dem029. [DOI] [PubMed] [Google Scholar]
- 14.Kushnir MM, Naessen T, Wanggren K, Rockwood AL, Crockett DK, Bergquist J. Protein and steroid profiles in follicular fluid after ovarian hyperstimulation as potential biomarkers of IVF outcome. J Proteome Res. 2012;11(10):5090–100. doi: 10.1021/pr300535g. [DOI] [PubMed] [Google Scholar]
- 15.Von Wald T, Monisova Y, Hacker MR, Yoo SW, Penzias AS, Reindollar RR, et al. Age-related variations in follicular apolipoproteins may influence human oocyte maturation and fertility potential. Fertil Steril. 2010;93(7):2354–61. doi: 10.1016/j.fertnstert.2008.12.129. [DOI] [PubMed] [Google Scholar]
- 16.Tyers M, Mann M. From genomics to proteomics. Nature. 2003;422(6928):193–7. doi: 10.1038/nature01510. [DOI] [PubMed] [Google Scholar]
- 17.Liu AX, Jin F, Zhang WW, Zhou TH, Zhou CY, Yao WM, et al. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol Reprod. 2006;75(3):414–20. doi: 10.1095/biolreprod.105.049379. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed N, Barker G, Oliva K, Garfin D, Talmadge K, Georgiou H, et al. An approach to remove albumin for the proteomic analysis of low abundance biomarkers in human serum. Proteomics. 2003;3(10):1980–7. doi: 10.1002/pmic.200300465. [DOI] [PubMed] [Google Scholar]
- 19.Gupta N, Shankernarayan NP, Dharmalingam K. Serum proteome of leprosy patients undergoing erythema nodosum leprosum reaction: regulation of expression of the isoforms of haptoglobin. J Proteome Res. 2007;6(9):3669–79. doi: 10.1021/pr070223p. [DOI] [PubMed] [Google Scholar]
- 20.Liu AX, He WH, Yin LJ, Lv PP, Zhang Y, Sheng JZ, et al. Sustained endoplasmic reticulum stress as a cofactor of oxidative stress in decidual cells from patients with early pregnancy loss. J Clin Endocrinol Metab. 2011;96(3):E493–7. doi: 10.1210/jc.2010-2192. [DOI] [PubMed] [Google Scholar]
- 21.Swain M, Ross NW. A silver stain protocol for proteins yielding high resolution and transparent background in sodium dodecyl sulfate-polyacrylamide gels. Electrophoresis. 1995;16(6):948–51. doi: 10.1002/elps.11501601159. [DOI] [PubMed] [Google Scholar]
- 22.Fahiminiya S, Reynaud K, Labas V, Batard S, Chastant-Maillard S, Gerard N. Steroid hormones content and proteomic analysis of canine follicular fluid during the preovulatory period. Reprod Biol Endocrinol. 2010;8:132. doi: 10.1186/1477-7827-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu XL, Feng C, Lin XH, Zhong ZX, Zhu YM, Lv PP, et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab. 2014;99:2217–24. doi: 10.1210/jc.2013-3362. [DOI] [PubMed] [Google Scholar]
- 24.Aanesen A, Nygren KG, Nylund L. Modified natural cycle IVF and mild IVF: a 10 year Swedish experience. Reprod Biomed Online. 2010;20(1):156–62. doi: 10.1016/j.rbmo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JD, DiMattina M, Reh A, Botes A, Celia G, Payson M. Utilization and success rates of unstimulated in vitro fertilization in the United States: an analysis of the Society for Assisted Reproductive Technology database. Fertil Steril. 2013;100(2):392–5. doi: 10.1016/j.fertnstert.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 26.Murdoch WJ, Murphy CJ, Van Kirk EA, Shen Y. Mechanisms and pathobiology of ovulation. Soc Reprod Fertil Suppl. 2010;67:189–201. doi: 10.7313/upo9781907284991.017. [DOI] [PubMed] [Google Scholar]
- 27.Orvieto R, Chen R, Ashkenazi J, Ben-Haroush A, Bar J, Fisch B. C-reactive protein levels in patients undergoing controlled ovarian hyperstimulation for IVF cycle. Hum Reprod. 2004;19(2):357–9. doi: 10.1093/humrep/deh089. [DOI] [PubMed] [Google Scholar]
- 28.Jarkovska K, Kupcova Skalnikova H, Halada P, Hrabakova R, Moos J, Rezabek K, et al. Development of ovarian hyperstimulation syndrome: interrogation of key proteins and biological processes in human follicular fluid of women undergoing in vitro fertilization. Mol Hum Reprod. 2011;17(11):679–92. doi: 10.1093/molehr/gar047. [DOI] [PubMed] [Google Scholar]
- 29.Estes SJ, Ye B, Qiu W, Cramer D, Hornstein MD, Missmer SA. A proteomic analysis of IVF follicular fluid in women < or = 32 years old. Fertil Steril. 2009;92(5):1569–78. doi: 10.1016/j.fertnstert.2008.08.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanrieder J, Nyakas A, Naessén T, Bergquist J. Proteomic analysis of human follicular fluid using an alternative bottom-up approach. J Proteome Res. 2008;7(1):443–9. doi: 10.1021/pr070277z. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Kim MS, Lee SH, Choi BC, Lim JM, Cha KY, et al. Proteomic analysis of recurrent spontaneous abortion: Identification of an inadequately expressed set of proteins in human follicular fluid. Proteomics. 2006;6(11):3445–54. doi: 10.1002/pmic.200500775. [DOI] [PubMed] [Google Scholar]
- 32.Aune B, Høie KE, Oian P, Holst N, Osterud B. Does ovarian stimulation for in-vitro fertilization induce a hypercoagulable state? Hum Reprod. 1991;6(7):925–7. doi: 10.1093/oxfordjournals.humrep.a137461. [DOI] [PubMed] [Google Scholar]
- 33.Andersen CY. Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. J Clin Endocrinol Metab. 1993;77(5):1227–34. doi: 10.1210/jcem.77.5.7521343. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi L, Gagliardi A, Campanella G, Landi C, Capaldo A, Carleo A, et al. A methodological and functional proteomic approach of human follicular fluid en route for oocyte quality evaluation. J Proteomics. 2013;90:61–76. doi: 10.1016/j.jprot.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Dai G, Lu G. Different protein expression patterns associated with polycystic ovary syndrome in human follicular fluid during controlled ovarian hyperstimulation. Reprod Fertil Dev. 2012;24(7):893–904. doi: 10.1071/RD11201. [DOI] [PubMed] [Google Scholar]
- 36.Nagy B, Pulay T, Szarka G, Csomor S. The serum protein content of human follicular fluid and its correlation with the maturity of oocytes. Acta Physiol Hung. 1989;73(1):71–5. [PubMed] [Google Scholar]
- 37.Imoedemhe D, Shaw R. Follicular fluid alpha 1-antitrypsin–correlation with fertilizing capacity of oocytes. Br J Obstet Gynaecol. 1986;93(8):863–8. doi: 10.1111/j.1471-0528.1986.tb07996.x. [DOI] [PubMed] [Google Scholar]
- 38.Meng QX, Gao HJ, Xu CM, Dong MY, Sheng X, Sheng JZ, et al. Reduced expression and function of aquaporin-3 in mouse metaphase-II oocytes induced by controlled ovarian hyperstimulation were associated with subsequent low fertilization rate. Cell Physiol Biochem. 2008;21(1–3):123–8. doi: 10.1159/000113754. [DOI] [PubMed] [Google Scholar]
- 39.Angelucci S, Ciavardelli D, Di Giuseppe F, Eleuterio E, Sulpizio M, Tiboni GM, et al. Proteome analysis of human follicular fluid. Biochim Biophys Acta. 2006;1764(11):1775–85. doi: 10.1016/j.bbapap.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Aleshire SL, Osteen KG, Maxson WS, Entman SS, Bradley CA, Parl FF. Localization of transferrin and its receptor in ovarian follicular cells: morphologic studies in relation to follicular development. Fertil Steril. 1989;51(3):444–9. doi: 10.1016/s0015-0282(16)60551-4. [DOI] [PubMed] [Google Scholar]
- 41.Briggs DA, Sharp DJ, Miller D, Gosden RG. Transferrin in the developing ovarian follicle: evidence for de-novo expression by granulosa cells. Mol Hum Reprod. 1999;5(12):1107–14. doi: 10.1093/molehr/5.12.1107. [DOI] [PubMed] [Google Scholar]
- 42.Li YD, Zhang ZW, Li WX. Transferrin inhibits aromatase activity of rat granulosa cells in vitro. J Endocrinol. 1991;131(2):245–50. doi: 10.1677/joe.0.1310245. [DOI] [PubMed] [Google Scholar]
- 43.Kawano Y, Narahara H, Miyamura K, Mifune K, Miyakawa I. Inhibitory effect of transferrin on progesterone production in the granulosa cell of humans in vivo and porcine granulosa cell in vitro. Gynecol Obstet Invest. 1995;40(1):1–4. doi: 10.1159/000292290. [DOI] [PubMed] [Google Scholar]
- 44.Ducolomb Y, Gonzalez-Marquez H, Fierro R, Jimenez I, Casas E, Flores D, et al. Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology. 2013;79(6):896–904. doi: 10.1016/j.theriogenology.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Entman SS, Maxson WS, Bradley CA, Osteen K, Webster BW, Vaughn WK, et al. Follicular fluid transferrin levels in preovulatory human follicles. J In Vitro Fert Embryo Transf. 1987;4(2):98–102. doi: 10.1007/BF01555447. [DOI] [PubMed] [Google Scholar]
- 46.Revelli A, Chiado A, Dalmasso P, Stabile V, Evangelista F, Basso G, et al. “Mild” vs. “long” protocol for controlled ovarian hyperstimulation in patients with expected poor ovarian responsiveness undergoing in vitro fertilization (IVF): a large prospective randomized trial. J Assist Reprod Genet. 2014;31(7):809–15. doi: 10.1007/s10815-014-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Wolff M, Kollmann Z, Fluck CE, Stute P, Marti U, Weiss B, et al. Gonadotrophin stimulation for in vitro fertilization significantly alters the hormone milieu in follicular fluid: a comparative study between natural cycle IVF and conventional IVF. Hum Reprod. 2014;29(5):1049–57. doi: 10.1093/humrep/deu044. [DOI] [PubMed] [Google Scholar]