Abstract
Purpose
In Vitro Fertilization is an effective treatment for infertility; however, it has relatively low success in women of advanced maternal age (>37) who have a high risk of producing aneuploid embryos, resulting in implantation failure, a higher rate of miscarriage or birth of a child with chromosome abnormalities. The purpose of this study was to compare the implantation, miscarriage and live birth rates with and without preimplantation genetic screening (PGS) of embryos from patients aged 40 through 43 years.
Methods
This is a retrospective cohort study, comparing embryos screened for ploidy using trophectoderm biopsy and array comparative genomic hybridization to embryos that were not screened. We compared pregnancy outcomes for traditional fresh IVF cycles with day 5 embryo transfers, Frozen Embryo Transfer (FET) cycles without PGS and PGS-FET (FET of only euploid embryos) cycles of patients with maternal ages ranging from 40 to 43 years, undergoing oocyte retrievals during the period between 1/1/2011 and 12/31/2012.
Results
The implantation rate of euploid embryos transferred in FET cycles (50.9 %) was significantly greater than for unscreened embryos transferred in either fresh (23.8 %) or FET (25.4 %) cycles. The incidence of live birth per transferred embryo for PGS-FET (45.5 %) was significantly greater than for No PGS fresh (15.8 %) or No PGS FET (19.0 %) cycles. The incidences of live birth per implanted sac for PGS FET cycles (89.3 %), No PGS fresh cycles (66.7 %) and No PGS FET cycles (75.0 %) were not significantly different.
Conclusions
The present data provides evidence of the benefits of PGS with regard to improved implantation and live birth rate per embryo transferred.
Keywords: Advanced maternal age, Comparative genomic hybridization, Frozen embryo transfer, Implantation, Trophectoderm biopsy
Introduction
In Vitro Fertilization (IVF) is an effective treatment for infertility; however, it has relatively low success in women 40 and older. The decline of fertility with advanced maternal age (AMA) has been shown to be associated with an age-dependent aneuploidy increase [1] and may depend on other age-dependent phenomena, as well. Women of AMA (>37) have a high risk of producing aneuploid embryos, resulting in implantation failure, a higher risk of miscarriage or birth of a child with chromosome abnormalities such as Down syndrome or Turner’s Syndrome. The objective of this study was to compare the implantation, miscarriage and live birth rates with and without preimplantation genetic screening (PGS) for patients aged 40 through 43 years.
Background
The prevalence of spontaneous abortion and birth of a child with chromosomal abnormalities increases dramatically with AMA. Likewise, the incidence of failed implantation in IVF increases with AMA. An increased incidence of aneuploidy is believed to be a major reason for the decrease of fertility in IVF [1–3]. For women over the age of 40 in the United States, roughly half of all clinical pregnancies established through IVF are lost, failing to result in live births (SART CORS Clinic Summary Report, 2012). It has been reported that approximately 50–80 % of all first trimester pregnancy losses result from chromosomal abnormalities in the developing fetus [4–7]. These abnormalities are a concern for couples seeking IVF treatment to conceive a healthy viable pregnancy. In addition, when miscarriage occurs, this will further delay attempts to achieve their goal. This delay is especially detrimental in older women where precious time is lost while their fertility is rapidly declining.
Traditionally the assisted reproductive technology (ART) community has used morphology assessment alone to select embryos for transfer. However, morphology alone only poorly predicts potential chromosomal abnormalities in embryos selected for transfer. Numerous studies show a significant proportion of aneuploid embryos are capable of achieving the highest morphology score [1, 8–12] and there is no clear distinction made between chromosomally normal and abnormal embryos by morphology assessment alone [13]. In most recent studies of good prognosis patients, aneuploidy is reported to be as high as 44.9 % of good quality blastocysts biopsied [14]. Further, the timing of morphological events determined using time lapse microscopy (morphokinetics) is insufficient to distinguish euploid embryos [15]. Hence, morphology assessment alone is an inefficient means of selecting the best embryo for transfer.
Numerous studies indicate that undergoing PGS for aneuploidy assessment has a beneficial effect on clinical outcomes in women of AMA [3, 16–18]. In the most recent randomized controlled trials (RCT), Yang et al. and Scott et al. showed that adding 24 chromosome aneuploidy screening to embryo selection for transfer, significantly improves outcomes by avoiding the transfer of aneuploid embryos. In turn, this increases the chance for implantation and decreases the likelihood of miscarriage associated with aneuploidy [14, 19]. Being able to select the euploid embryo that has higher implantation potential will allow us to limit the numbers of embryos transferred per cycle, thereby decreasing the chance of twins and high order multiple gestation while increasing overall pregnancy rate [20]. Other studies have also shown that implantation rates remain similar regardless of maternal age following embryo biopsy and PGS when only euploid embryos were transferred [3, 21].
The purpose of this study was to determine the efficacy of PGS for women ages 40–43; whether PGS and transfer of euploid embryos improves implantation, reduces the frequency of spontaneous loss, and increases the delivery rate per transferred embryo.
Materials and methods
This was a retrospective study designed to compare the embryo implantation, spontaneous abortion and live birth rates with and without PGS in women aged 40 through 43 years old at the time of oocyte retrieval. We compared pregnancy outcomes for traditional fresh IVF cycles with day 5 embryo transfer (ET), Frozen Embryo Transfer cycles (transferring blastocysts) without PGS (FET) and Frozen Embryo Transfer cycles following PGS (trophectoderm (TE) biopsy, cryopreservation and FET of only euploid embryos) in patients 40 through 43 years, undergoing oocyte retrievals during the period between 1/1/2011 and 12/31/2012. Only patients using their own oocytes were included (donor oocyte cycles were excluded)
Controlled Ovarian Hyperstimulation (COH) was performed using gonadotropin-releasing hormone (GnRH) down regulation, microdose leuprolide acetate, or GnRH-antagonist protocols and exogenous gonadotropins (recombinant follicle stimulating hormone (FSH) and human menopausal gonadotropin (hMG)). When lead follicles reached 17-mm mean diameter, final oocyte maturation was induced using an intra-muscular injection of 10,000 IU hCG (Pregnyl: Organo Oss. The Netherlands) or subcutaneous injection of leuprolide acetate. Oocyte retrieval via transvaginal ultrasound-guided needle aspiration of ovarian follicular fluid was performed 36 h later. Sperm was processed for insemination or intracellular sperm injection (ICSI) as previously described [22, 23]. ICSI was performed for patients diagnosed with male factor infertility or a history of failed fertilization. Normally fertilized zygotes exhibiting two pronuclei (PN) were cultured to day 3 in 30 ul droplets of culture media (Quinn’s Cleavage Medium, Cooper Surgical) and monitored for embryonic development as previously described [24]. Both the fresh IVF and PGS group had same criteria for culture to day 5/6. Only patients with at least 3–5 good quality 6–8 cell embryos on day 3 underwent extended culture to day5/6 for transfer or biopsy. The stages of blastocyst development were assessed using the criteria of Gardner and Lane [25].
In the IVF group, embryos were selected for fresh transfer on day 5/6 based on morphologic criteria alone (IVF-ET). Embryo transfer was performed using the Sureview Wallace catheter (Smith’s Medical International Ltd., Hythe, UK) under ultrasound guidance. Progesterone supplementation for luteal phase support was either by injection of progesterone in oil (Watson Pharma, Parsippany, NJ) or vaginally (Crinone, Endometrin or suppositories) beginning on the day following oocyte retrieval.
Excess embryos of suitable good quality (Grades 2Bc, 2Cb or better) were vitrified using the Irvine vitrification kit (Irvine, Santa Ana, CA) and Cryolock device (BioTech, Cummin, GA).
Patients undergoing FETs were administered oral estradiol tablets (Estrace, Warner Chilcott, Rockaway, NJ) beginning on day 2 of the menstrual cycle to prepare the uterus for embryo transfer and implantation. Once the uterine lining was at least 7 mm with a ring pattern, injections of progesterone in oil (Watson Pharma, Parsippany, NJ) or vaginal suppositories (Crinone, Endometrin or suppositories) were administered. On the 6th day of progesterone, embryos were warmed using Irvine (Santa Ana, CA) warming kits and then transferred to the patient’s uterus under ultrasound guidance.
Trophectoderm biopsy and genetic screening
Patients having PGS performed on their embryos underwent COH and IVF just as IVF patients. TE biopsy and genetic screening were performed as described previously [26]. For blastocyst biopsy, all viable embryos were subjected to zona perforation on day 3. A small opening in the zona pellucida (ZP) was created using Cronus 3 Laser (400 mW 0.357mS pulse width: Cronus Research Instrument Ltd. Falmouth, United Kingdom). Following this treatment, the embryos were returned to culture (Quinn’s Blastocyst Medium, Cooper Surgical). The embryos were then assessed on day 5 and day 6 and if deemed necessary again on day 7. Those embryos that developed to the expanded blastocyst stage with differentiation of ICM and TE cells (Grade 2Bc or 2Cb or better) were biopsied. TE cells herniating through the zona perforation were grasped with gentle aspiration applied to a biopsy pipette and the cells were pulled gently away from the remainder of the blastocyst. Several strategically directed laser pulses (400 mW 0.6mS pulse) and further traction helped to separate the TE biopsy from the blastocyst. The biopsied cells were immediately rinsed in PBS washing buffer, loaded into a PCR tube with 2ul of washing buffer. Each of the PCR tubes containing the TE biopsy were labeled with the number corresponding to the embryo from which it was removed and shipped on dry ice to the reference genetic lab for analysis.
All blastocysts subjected to TE biopsy were vitrified immediately following the biopsy procedure using the Irvine Vitrification Kit (Irvine Scientific, Santa Ana, CA) and were loaded onto Cryolock (BioTech, Cummin, GA). All biopsy specimens derived from blastocyst stage embryos were analyzed using 24 chromosomes array comparative genomic hybridization (aCGH) by Reprogenetics, LLC (Livingston, NJ, 07039) as described previously [3].
Once the aCGH results were obtained, patients having a euploid embryo were scheduled for an FET cycle after her next menses. FETs of euploid blastocysts were performed in the same manner as FET’s of unbiopsied/ unscreened embryos. On the 6th day of progesterone, the desired number, (generally one and sometimes two) euploid embryos were warmed using Irvine (Santa Ana, CA) warming kits and then transferred to the patient’s uterus under ultrasound guidance.
Pregnancy tests (blood Beta hCG levels) were carried out on day 28 of the cycle and if positive again on day 35. Patients having a positive pregnancy test were scanned on day 42 to 49 for presence of intrauterine sac and fetal heart activity.
Statistics
Clinical pregnancy was defined as the presence of an intrauterine gestational sac(s) with fetal cardiac activity as documented by ultrasound. Outcome parameters of Implantation rate (IR: number of intrauterine gestational sacs / total number of embryos transferred), live birth (babies) per embryo transferred (DelR: number of babies born / number of embryos transferred) and incidence of live delivery per fetal sac (LDPS: babies born/ gestational sacs) were determined for each treatment group. For statistical comparison between two groups, chi square, and student’s t-test were used as appropriate. Statistical analysis was performed using Microsoft Excel, SPSS software and/or Graphpad online. p < 0.05 was defined as statistically significant.
Results
A total of 620 cycles in patients 40 through 43 years of age underwent ovarian stimulation between January, 2011 and December, 2012: Four hundred fifty IVF cycles were planned without PGS (No-PGS group) and 170 were planned with TE biopsy and PGS for ploidy determination (PGS group). Baseline characteristics of the patients are summarized in Table 1. There were no significant differences in age, maximum d2/d3 FSH or incidence of most of diagnoses or stimulation protocols between the PGS and No-PGS groups. Patients in the PGS group had higher gravidity, higher incidence of prior pregnancy loss (biochemical pregnancies, spontaneous losses) and higher incidence of both full-term and pre-term deliveries than those in the No-PGS group. In addition, patients in the PGS group had a lower incidence of Tubal Factor and a lower incidence of unexplained infertility.
Table 1.
Parameters describing patients who initiated cycles
| Parameter | PGS | No PGS | Significance |
|---|---|---|---|
| Number of Patients | 170 | 450 | |
| Age (years) | 41.2 ± 1.1 | 41.3 ± 1.1 | NS |
| Gravidity | 2.1 ± 1.6 | 1.5 ± 1.5 | 0.0000002 |
| Prior Full Term Births | 0.53 ± 0.72 | 0.33 ± 0.60 | 0.0014 |
| Prior Pre-Term Births | 0.07 ± 0.32 | 0.03 ± 0.20 | 0.02 |
| Prior SAbs | 0.94 ± 1.0 | 0.48 ± 0.77 | 4.8E-09 |
| Prior Biochemical Pregnancies | 0.29 ± 0.53 | 0.18 ± 0.46 | 0.01 |
| Prior Gonadotropin Cycles w/o IVF | 0.5 ± 1.4 | 0.5 ± 1.1 | NS |
| Prior ART Cycles | 1.7 ± 2.1 | 1.5 ± 1.9 | NS |
| Prior Frozen Embryo Transfers | 0.34 ± 0.91 | 0.16 ± 0.66 | 0.003 |
| Day 2/3 FSH (mIU/mL) | 9.1 + 4.8 | 9.2 + 5.1 | NS |
| PCO | 0.05 (8) | 0.03 (15) | NS |
| DOR | 0.19 (32) | 0.21 (95) | NS |
| Tubal factor | 0.09 (16) | 0.21 (93) | <0.001 |
| Endometriosis | 0.06(10) | 0.07 (32) | NS |
| Male Factor | 0.14 (23) | 0.15 (65) | NS |
| Uterine | 0.16 (28) | 0.15 (65) | NS |
| Unexplained | 0.03 (5) | 0.08 (34) | <0.05 |
| Patients undergoing retrieval | 84.1 % (143/170) | 80.8 % (364/450) | NS |
| Cancelled cycles | 14.7 % (25/170) | 19.1 % (86/450) | NS |
Oocyte retrievals were cancelled for poor ovarian response in 19.1 % (86/450) of cycles in the No-PGS group and 14.7 %(25/170) of cycles in PGS group (not significant). Of the remaining 509 cycles that proceeded to oocyte retrieval; 364 intended to undergo IVF-ET (No-PGS) and 145 intended to pursue IVF with PGS (PGS). GnRH agonist triggers were used in 10 patients in the PGS group and 21 patients in the No PGS group. The two proportions of the cycles 6.9 and 5.8 %, respectively, were not significantly different (X2 comparison). Although patients in the PGS group on average received more total gonadotropins (p > 0.03) the total number of eggs retrieved, number fertilized, embryos cultured beyond day 3, day 3 embryo quality, total number of blastocysts and blastocyst quality (Table 2) were not significantly different between the 2 groups. Patients undergoing PGS had significantly higher total gonadotropin, FSH and/or hMG administered.
Table 2.
Stimulation parameters for patients who had oocyte retrievals
| Parameter | PGS | No PGS | Significance |
|---|---|---|---|
| Number of Retrievals | 145 | 364 | |
| Cycle Type: Luteal Downregulation | 0.06 (8) | 0.09 (34) | NS |
| Cycle Type: u Dose Flare | 0.13 n | 0.20 (71) | NS |
| Cycle Type: Antagonist | 0.82 (119) | 0.82 (300) | NS |
| IU gonadotropin | 4410 ± 1390 | 4120 ± 1360 | 0.0344 |
| IU FSH | 2730 ± 1000 | 2950 ± 1170 | 0.044404 |
| IU hMG | 1680 ± 920 | 1170 ± 970 | 1.05E-07 |
| E2 on day of trigger | 2360 ± 1040 | 2250 ± 1110 | NS |
| Oocytes Retrieved | 13.1 ± 6.8 | 12.8 ± 6.9 | NS |
| Oocytes with 2 pronuclei | 7.5 ± 4.4 | 7.7 ± 4.6 | NS |
| Avg. Number of Cells on Day 3 | 6.5 ± 1.7 | 6.5 ± 1.1 | NS |
| Number of embryos for extended culture | 8.5 ± 4.4 | 8.3 ± 4.8 | NS |
| Blastocysts on Day 5 | 3.3 ± 3.1 | 3.3 ± 2.7 | NS |
| Blastocysts on Day 5 and 6 | 4.3 ± 3.3 | 4.2 ± 3.4 | NS |
| Grade 2 or better Blastocysts | 3.6 ± 3.1 | 3.4 ± 3.3 | NS |
| Number of patients with TE Biopsy | 87 | N/A |
Ploidy results
Of the 145 retrievals performed on patients initially desiring PGS, 120 retrievals with PGS were performed. 25 retrievals ultimately had no PGS, either because embryos did not reach a suitable blastocyst stage for biopsy (11 retrievals = 7.6 % of the retrievals) or because patients chose to undergo fresh ET without PGS (14 retrievals = 9.7 % of the retrievals) usually because the numbers of embryos were small enough that selection of 1–2 embryos for transfer did not require ploidy results (See Fig. 1a). Fourteen patients initially planned PGS then declined PGS and underwent fresh transfer. One of these patients achieved live birth and another suffered a clinical pregnancy loss of an aneuploid fetus.
Fig. 1.
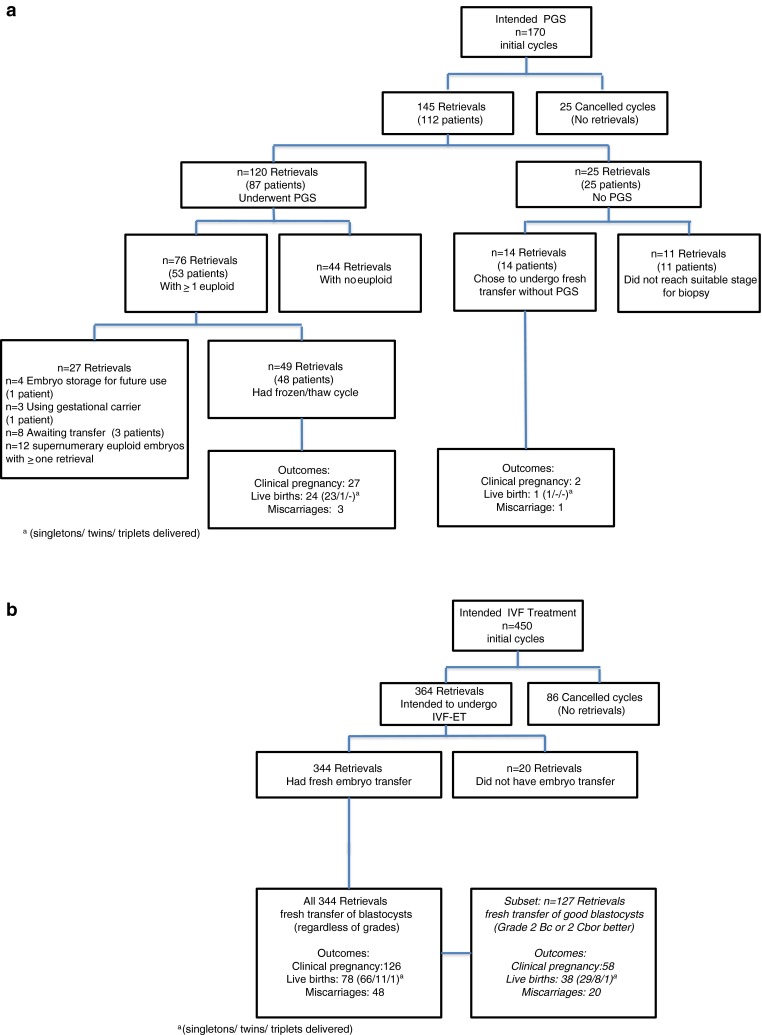
a Patients who intended to perform trophectoderm biopsy and preimplantation genetic screening. b Patients who intended IVF for Fresh Embryo Transfer
In total, 451 blastocysts were biopsied over this 2-year period of which 58.6 % were performed on day 5, 38.6 % on day 6 and 2.8 % on day 7 blastocysts. Of the embryos biopsied, ploidy analysis using aCGH revealed that 20.4 % (n = 92) were euploid, 74.3 % were aneuploid (n = 335), 2.2 % (n = 10) had chaotic profiles after amplification and 3 % (n = 14) had no diagnosis. Among the aneuploid blastocysts, 23.3 % (n = 78) displayed single chromosome monosomy, 21.2 % (n = 71) displayed single chromosome trisomy and 24.8 % (n = 83) of the aneuploid blastocysts had two chromosomes affected (either monosomy, trisomy or both) while 30.7 % (n = 103) had more than two chromosomes affected (complex abnormal).
Availability of embryos for transfer
Embryos were available for transfer in the No-PGS group in 344 of the 364 (95 %) retrievals because embryo selection was based solely on morphology. When embryos were not available it was due to either failed fertilization or poor embryo development. In contrast, in the PGS group, blastocysts were available for transfer in 76 of the 145 (52.4 %) retrievals since cycles where there was no biopsy (25 retrievals listed above) or when none of the biopsied embryos were euploid (44 retrievals) were not considered from that point forward (see Fig. 1a and b).
Transfer and pregnancy outcome
Embryos were transferred either fresh (when no PGS was performed) or in an FET cycle subsequent to the retrieval cycle when a euploid embryo was available. Incidence of clinical pregnancy per transfer was 36.6 % (126/344) for the No-PGS fresh embryo transfers and 55 % (27/49) for PGS FET. Incidence of live birth per transfer was 22.7 % (78/344) for No-PGS fresh and 51 % (24/49) for PGS FET. The IR’s were 16.3 % (150/919) for No-PGS fresh transfers and 51 % (28/55) for PGS FET. The incidence of pregnancy loss was 38.1 % (48/126) for No-PGS fresh and was 10.7 % (3/27) for PGS. The incidence of multiple gestations was 26.2 % (33/126) for No-PGS Fresh transfers and 3.7 % (1/27) for PGS FETs. Moreover, the incidence of multiple gestations was significantly greater in the No-PGS groups in which significantly more embryos were transferred.
Implantation and delivery per embryo: are improvements in implantation due to selection of euploid embryos or due to uterine preparation in FET cycles?
In order to compare implantation and delivery per embryo in the PGS and No-PGS groups we restricted our analysis in both groups to only include embryos that would have been biopsied (Graded 2Bc or 2Cb or better). Therefore, we considered only fresh transfers of blastocysts when blastocysts of this quality or better were transferred. Fresh transfers involving embryos of poorer quality were excluded from consideration.
An additional difference exists between the patients with PGS and those with No-PGS. Patients with PGS underwent FETs whereas patients in the No-PGS group underwent fresh ET (IVF). We have compared outcomes in these two groups with a third group of patients in order to attempt to discern whether the FET paradigm or the transfer of only euploid embryos was the major reason for the significant difference between the PGS and the No-PGS groups. This third group (No-PGS FET) underwent FET with embryos cryopreserved during the No-PGS cycles examined herein but that had been neither biopsied nor genetically screened for aneuploidy (Table 3). Our criterion for selection of blastocysts for cryopreservation was the same as our criterion for biopsy (Grade 2Bc or 2Cb or better). Therefore, since all embryos for FET were pre-selected by the cryopreservation criterion, no adjustment is necessary for comparison of the PGS FET groups with the other two groups (see Table 3).
Table 3.
Comparison of outcomes for blastocysts stage 2 or greater
| Fresh IVF (No PGS) | FET (No PGS) | Euploid FET | P value fresh IVF vs. FET (no PGS) | P value FET vs. Euploid FET | |
|---|---|---|---|---|---|
| #Patients (cycles) | 127 | 28 | 49 | ||
| Embryos transferred | 2.38 (303 / 127) | 2.25 (53 / 24) | 1.12 (55 / 49) | 0.6543 | <0.0001 |
| Implantation Rate (IR, sacs) | 23.8 % (72 / 303) | 25.4 % (16 / 63) | 50.9 % (28 / 55) | 0.7489 | 0.0072 |
| Live Delivery per Xfrd Embryo (DelR) | 15.8 % (48 / 303) | 19 % (12 / 63) | 45.5 % (25 / 55) | 0.5748 | 0.0028 |
There were 127 fresh transfers in the No-PGS group, 28 FETs in the No-PGS Group and 49 FETs in PGS group. The numbers of embryos per transfer were 2.38, 2.25 and 1.12 for the No-PGS fresh, No-PGS FET and PGS FET groups, respectively. The numbers of embryos transferred were significantly less in the PGS group (P < 0.0001) than in either of the other two groups. Estimates of live birth rates for each of the three groups were calculated below. For the remainder of this manuscript, we will focus our comparisons of outcomes on a per embryo rather than a per patient basis.
Implantation rates were 23.8, 25.4 and 50.9 % for the No-PGS fresh, No-PGS FET and PGS FET groups, respectively (Fig. 1, Table 3). These rates were significantly different (Contingency X2 = 42.7 with 2° of freedom, p << 0.001). IR’s were not significantly different when the No-PGS fresh and No-PGS FET groups were compared. However, there was a significant difference in IR’s between the No-PGS FET group and the PGS FET groups (Contingency X2 = 8.1, with 1° of freedom, 0.025 < p < 0.05).
There were significant differences in the incidences of live delivery per embryo transferred (DelR) for the three groups: 15.8 % (48/303) for No-PGS fresh transfers, 19 % (12/63) for No-PGS FET, and 45.5 % (25/55) for PGS FETs (Contingency X2 = 64.3 with 2° of freedom, p << 0.001). Incidences of DelR were not significantly different when the No-PGS fresh and No-PGS FET groups were compared. Of note, DelR were significantly different between the No-PGS FET group and the PGS FET groups (Contingency X2 = 9.5, with 1° of freedom, 0.01 < p < 0.0025).
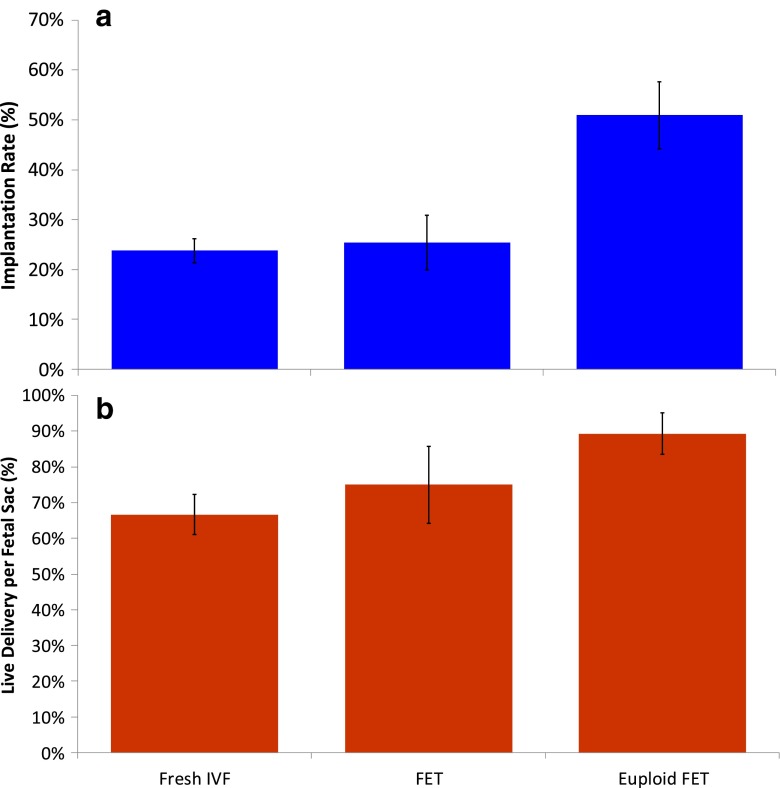
The incidences of live delivery per fetal sac (LDPS) (100 % minus the incidence of loss of an implanted sac [SABs plus vanishing twins] were 66.7 % (48 / 72) for No-PGS IVF, 75 % (12/ 16) for No-PGS FET, and 89.3 % (25/28) for PGS FET (Fig. 2). These incidences of LDPS were not significantly different when considering all three groups together (Contingency X2 = 3.1, with 2° of freedom, 0.15 < p < 0.20).
Fig. 2.
Implantation rate and live delivery per fetal sac for Fresh IVF (No PGS), FET (No-PGS) and Euploid FET (PGS). Only embryos of grade 2Bc or 2Cb or better were included. Figure 2a IR = implanta1on rate; 2b. LDPS = live delivery per fetal sac
Discussion
It is imperative that IVF treatment paradigms are designed so as to offer patients the most effective and efficient means of attaining their goal of a healthy pregnancy and child. Traditionally, embryo selection for transfer has been to select the most advanced stage embryo on the day of transfer. With the advent of TE biopsy and 24 chromosome screening it is now possible to identify chromosomally normal embryos that have reached an advanced stage of development and preferentially transfer those embryos one by one so that we can achieve similar pregnancy and live births rates more efficiently.
In this study, it is shown that acceptable rates for clinical pregnancy and delivery were obtained using transfer of fresh unscreened blastocysts, FET of unscreened blastocysts and FET of blastocysts selected for their euploid status. However we must also consider that the number of embryos transferred was significantly different in the PGS and No PGS groups. Therefore, we chose not to focus on these rates because the numbers of embryos transferred were significantly different when comparing PGS and No PGS groups but rather, we chose to compare outcomes per embryo and per sac in order to determine if euploid blastocysts are superior to unscreened blastocysts in achieving pregnancy and live birth.
Infertility diagnoses and patient characteristics prior to COH were markedly similar when comparing the group of patients whether planning to undergo PGS or not. Patients undergoing PGS had higher gravidity, more prior full term births pre-term births, spontaneous losses and biochemical pregnancies than those patients opting for fresh transfer without chromosomal screening of their embryos. This is not surprising since PGS is of more interest to patients who have experienced prior IVF failure and losses. The higher incidence of tubal factor in the No-PGS group may, in part, be a selection bias associated with the higher incidence of pregnancy in the PGS group, many of whom had achieved pregnancy without the use of assisted reproductive techniques.
There were also significant differences in gonadotropin administration between the two groups of patients who underwent oocyte retrieval. However, despite these differences in gonadotropin administration, there were no significant differences in patient response, fertilization, or embryonic progression that could be associated with outcome. The impact of these differences, apparent in the comparison groups, on outcomes, is not clear and may or may not bear any relation to the improved implantation seen in the PGS group.
Live births per cycle or per retrieval: attempt to account for unexplained attrition of patients
It is difficult to compare success per cycle or per retrieval between the patients undergoing PGS and those not undergoing PGS, largely because patients with frozen embryos do not necessarily return to our facility to undergo FET soon after cryopreservation. While 53 patients had euploid embryos cryopreserved, to date just 48 have returned for FETs. Some patients have removed their euploid embryos to other facilities for a variety of reasons, most frequent being the need to use the services of a gestational carrier. Other patients opt to store their embryos for future use. In addition, as described already a significant number of patients had no embryos available for transfer either because of a failure to develop to a stage suitable for biopsy or because none of the embryos were euploid. Since this is not an unusual occurrence in this population of patients it presents difficulty when calculating success rates per retrieval or per initiated cycle. The difficulty arises from inability to account for two groups of patients: 1) those who decide not to undergo biopsy when they know how many oocytes are fertilized or when the number of embryos is small, and 2) those who after biopsy and genetic screening have no euploid embryos. The former group often opts to go ahead and have an embryo transfer despite the low chances of success while the latter group will have no transfer since there are no euploid embryos available. Prior to the availability of PGS, these patients would have had embryos transferred and may have experienced clinical pregnancy. However, assuming that the genetic screening of TE cells is representative of the cells remaining in the embryo (blastocyst), there is virtually no chance of a chromosomally normal live birth from aneuploid embryos.
If we adjust for patients who do not return for euploid embryos, we can estimate the clinical pregnancy rates and live birth rates per retrieval and per initiated cycle by assuming that the same pregnancy and delivery rates would occur for all patients with embryos available. When this is done, the Clinical Pregnancy Rates (CPR) per retrieval (28.8 %) and per initiated cycle (24.6 %) in the PGS group can be compared with the CPR per retrieval (34.6 %) and per initiated cycle (29.3 %) for the No PGS group. Similarly, the live birth rates per retrieval (25.6 %) and per initiated cycle (21.9 %) in the PGS group can be compared with the live birth rates per retrieval (21.4 %) and per initiated cycle (18.1 %) for the No PGS group. Numerically, the live birth rates for the PGS group are ~20 % greater than the comparable live birth rates in the No PGS group, but significance cannot be determined in this example that relies on assumptions and extrapolations.
Implantation
The incidence of implantation for blastocysts found to be euploid (51.0 %) was approximately twice the incidence of implantation for embryos that were not screened (23.8 % [fresh ET] and 25.4 % [FET]) (Fig. 2, Table 3). The superiority of implantation potential for euploid embryos regardless of patient age has been reported previously [3, 16, 19, 27, 28]. Moreover, when embryos were not screened for ploidy status the IR’s were comparable in the fresh and FET cycles. This suggests that the selection of euploid embryos was the primary reason for the increase in implantation rates indicating that the uterine environment during preparation for FET was comparable to the uterine environment during COH in this study.
There was no significant difference in LDPS regardless of whether they were unscreened or euploid. This suggests that the significant difference in DelR was primarily driven by the significant differences in implantation rather than the differences in sac losses. We observed that there was no difference in implantation or delivery between Fresh IVF and FET in this cohort of patients. This does not support the observations of Shapiro et al. [29], although it does indicate that FETs are not inferior to IVF. Unfortunately, our study includes small numbers of FET’s in this group of patients with advancing age. It should also be acknowledged that embryos for No-PGS FET were predominantly those embryos that were not the first choices for transfer, since the first choice embryos were transferred in fresh cycles. This may have limited our data’s ability to support Shapiro’s conclusions regarding improved outcomes following FET. Therefore, we caution ourselves and temper our conclusions regarding the comparison of No-PGS FET and No-PGS fresh results.
In addition, it must be stated that the number of FETs with No PGS were small and may not be sufficient to accurately estimate the incidence of implantation and the rate of sac loss.
Delivery per embryo transferred
The incidence of delivery per embryo transferred was significantly greater for PGS than for IVF or FET with No-PGS (Table 3). Since the incidence of aneuploidy for products of conception is high, 50–80 % [4–7] it is expected that a reduction of aneuploid embryos might lead to a decreased incidence of pregnancy loss and an increased incidence of LDPS (Fig. 2).
Sacs lost per implanted embryo
We would expect that the incidence of loss of implanted sacs would be significantly less in the PGS FET group as suggested by Munne et al. [1] and others [28, 30]. The incidence of loss of implanted sacs was not significantly different among the three groups in this study (Fig. 2). Whether this was due to small numbers of cases or from no fundamental difference between euploid and unselected blastocysts will require further investigation.
Advantages to the avoidance of sac losses include the avoidance of the emotional distress of achieving pregnancy and then losing the pregnancy (as many of our patients suffering from recurrent pregnancy loss have previously experienced, repeatedly), the avoidance of additional invasive procedures (dilation and curettage’s, treatment with methotrexate, repeated blood draws to affirm the disappearance of any trophectodermal tissue), and the avoidance of these associated delays in progressing to another retrieval cycle. While patients may suffer emotionally from finding that they have of no euploid blastocysts, it is possible to minimize this emotional stress by managing patient expectations prior to and throughout the cycle for retrieval, biopsy and PGS. Many patients in this 40+ age group who have no euploid blastocysts in their first cycle are successful in subsequent cycles in cryopreserving euploid blastocysts and achieving live birth after undergoing repeat cycles for biopsy and PGS. If unsuccessful, the knowledge they gain can help them to consider alternative options such as donor egg
Multiple gestations
It is widely accepted that the incidence of multiple gestations is directly related to the number of embryos transferred [31, 32]. As expected, in our study the incidence of multiple gestations was significantly greater in the groups with a higher average numbers of embryos transferred (No-PGS fresh and No-PGS FET) than in the group with a lower average number of embryos transferred (PGS FET). Screening for ploidy allowed us to identify the euploid embryos with presumably the highest potential for implantation and delivery to term thus allowing for the majority of the euploid embryos to be transferred in single embryo transfers resulting in a lower incidence of multiple pregnancy.
Conclusion
Performance of TE biopsy and PGS using aCGH for patients aged 40 through 43 permits the identification and selection of euploid embryos for transfer. By employing these technologies it is possible to achieve higher implantation and pregnancy rates per embryo transferred because it avoids the transfer of aneuploid embryos, not identified in unscreened cohorts that are not capable of achieving ongoing pregnancy and live birth. In addition, the incidences of miscarriage and chromosomally abnormal pregnancies are reduced. Moreover, TE biopsy, PGS and aCGH allow for the transfer of a single embryo thereby reducing the risks associated with multiple gestations. All of these consequences are beneficial to patients desiring to achieve live birth of a healthy child using IVF, providing patients, even patients of advancing age, with a win-win situation. The validity of these benefits may be more meaningfully demonstrated in randomized controlled trial. In addition, avoiding the time delay from a miscarriage and the psychological benefit of knowing the IVF cycle failed solely due to the lack of a euploid embryo empowers patients with more knowledge to make better future treatment decisions.
Footnotes
Capsule Euploid embryos from women (40–43 years) in FET cycles had greater implantation and live birth rates than unscreened embryos in fresh or FET cycles.
References
- 1.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64(2):382–91. [PubMed] [Google Scholar]
- 2.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):703–8. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 4.Hassold TJ, Matsuyama A, Newlands IM, Matsuura JS, Jacobs PA, Manuel B, et al. A cytogenetic study of spontaneous abortions in Hawaii. Ann Hum Genet. 1978;41(4):443–54. doi: 10.1111/j.1469-1809.1978.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 5.Marquard K, Westphal LM, Milki AA, Lathi RB. Etiology of recurrent pregnancy loss in women over the age of 35 years. Fertil Steril. 2010;94(4):1473–7. doi: 10.1016/j.fertnstert.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case–control study. Hum Reprod. 2002;17(2):446–51. doi: 10.1093/humrep/17.2.446. [DOI] [PubMed] [Google Scholar]
- 7.Werner M, Reh A, Grifo J, Perle MA. Characteristics of chromosomal abnormalities diagnosed after spontaneous abortions in an infertile population. J Assist Reprod Genet. 2012;29(8):817–20. doi: 10.1007/s10815-012-9781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munne S, Chen S, Colls P, Garrisi J, Zheng X, Cekleniak N, et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod Biomed Online. 2007;14(5):628–34. doi: 10.1016/S1472-6483(10)61057-7. [DOI] [PubMed] [Google Scholar]
- 9.Rubio C, Rodrigo L, Mercader A, Mateu E, Buendia P, Pehlivan T, et al. Impact of chromosomal abnormalities on preimplantation embryo development. Prenat Diagn. 2007;27(8):748–56. doi: 10.1002/pd.1773. [DOI] [PubMed] [Google Scholar]
- 10.Sandalinas M, Sadowy S, Alikani M, Calderon G, Cohen J, Munne S. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage. Hum Reprod. 2001;16(9):1954–8. doi: 10.1093/humrep/16.9.1954. [DOI] [PubMed] [Google Scholar]
- 11.Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15(8):1781–6. doi: 10.1093/humrep/15.8.1781. [DOI] [PubMed] [Google Scholar]
- 12.Marquez C, Sandalinas M, Bahce M, Alikani M, Munne S. Chromosome abnormalities in 1255 cleavage-stage human embryos. Reprod Biomed Online. 2000;1(1):17–26. doi: 10.1016/S1472-6483(10)61988-8. [DOI] [PubMed] [Google Scholar]
- 13.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–4. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24. doi: 10.1186/1755-8166-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer YG, Kofinas JD, Melzer K, Noyes N, McCaffrey C, Buldo-Licciardi J, et al. Assessing morphokinetic parameters via time lapse microscopy (TLM) to predict euploidy: are aneuploidy risk classification models universal? Journal of assisted reproduction and genetics. 2014. PubMed PMID: 24962789. [DOI] [PMC free article] [PubMed]
- 16.Rubio C, Bellver J, Rodrigo L, Bosch E, Mercader A, Vidal C, et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril. 2013;99(5):1400–7. doi: 10.1016/j.fertnstert.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril. 2009;92(1):157–62. doi: 10.1016/j.fertnstert.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Munne S, Chen S, Fischer J, Colls P, Zheng X, Stevens J, et al. Preimplantation genetic diagnosis reduces pregnancy loss in women aged 35 years and older with a history of recurrent miscarriages. Fertil Steril. 2005;84(2):331–5. doi: 10.1016/j.fertnstert.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Scott RT, Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97(4):870–5. doi: 10.1016/j.fertnstert.2012.01.104. [DOI] [PubMed] [Google Scholar]
- 20.Grifo JA, Hodes-Wertz B, Lee HL, Amperloquio E, Clarke-Williams M, Adler A. Single thawed euploid embryo transfer improves IVF pregnancy, miscarriage, and multiple gestation outcomes and has similar implantation rates as egg donation. J Assist Reprod Genet. 2013;30(2):259–64. doi: 10.1007/s10815-012-9929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platteau P, Staessen C, Michiels A, Van Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in women older than 37 years. Fertil Steril. 2005;84(2):319–24. doi: 10.1016/j.fertnstert.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Palermo GD, Schlegel PN, Sills ES, Veeck LL, Zaninovic N, Menendez S, et al. Births after intracytoplasmic injection of sperm obtained by testicular extraction from men with nonmosaic Klinefelter’s syndrome. N Engl J Med. 1998;338(9):588–90. doi: 10.1056/NEJM199802263380905. [DOI] [PubMed] [Google Scholar]
- 23.Keegan BR, Barton S, Sanchez X, Berkeley AS, Krey LC, Grifo J. Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil Steril. 2007;88(6):1583–8. doi: 10.1016/j.fertnstert.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 24.Grifo JA, Flisser E, Adler A, McCaffrey C, Krey LC, Licciardi F, et al. Programmatic implementation of blastocyst transfer in a university-based in vitro fertilization clinic: maximizing pregnancy rates and minimizing triplet rates. Fertil Steril. 2007;88(2):294–300. doi: 10.1016/j.fertnstert.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3(4):367–82. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Adler A, Lee HL, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod Biomed Online. 2014;28(4):485–91. doi: 10.1016/j.rbmo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online. 2012;24(6):614–20. doi: 10.1016/j.rbmo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, Scott RT., Jr Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96(3):638–40. doi: 10.1016/j.fertnstert.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–8. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 30.Hodes-Wertz B, Grifo J, Ghadir S, Kaplan B, Laskin CA, Glassner M, et al. Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil Steril. 2012;98(3):675–80. doi: 10.1016/j.fertnstert.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Elsner CW, Tucker MJ, Sweitzer CL, Brockman WD, Morton PC, Wright G, et al. Multiple pregnancy rate and embryo number transferred during in vitro fertilization. Am J Obstet Gynecol. 1997;177(2):350–5. doi: 10.1016/S0002-9378(97)70197-2. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds MA, Schieve LA, Jeng G, Peterson HB, Wilcox LS. Risk of multiple birth associated with in vitro fertilization using donor eggs. Am J Epidemiol. 2001;154(11):1043–50. doi: 10.1093/aje/154.11.1043. [DOI] [PubMed] [Google Scholar]