Abstract
Purpose
To evaluate whether the in vitro incubation of spermatozoa with myoinositol may improve the fertilization rate in ICSI cycles.
Methods
This is a prospective, bicentric, randomized study on 500 MII sibling oocytes injected in 78 ICSI cycles performed between March and October 2013. Randomization of the oocytes into two groups was performed at the time of the denudation. Fertilization rates (per oocyte injected with spermatozoa treated with myoinositol versus per oocyte injected with spermatozoa treated with placebo) were measured as primary outcome and embryo morphology as secondary outcome. Clinical outcomes were also documented.
Result (s)
Fertilization rate (78.9 ± 28.6 % vs 63.2 ± 36.7, P = 0.002) and percentage of grade A embryos on day 3 (59.8 ± 35.6 % vs 43.5 ± 41.5, P = 0.019) were significantly higher when spermatozoa were treated in vitro with myoinositol versus placebo. No differences were found for the expanded blastocyst formation rate.
Conclusion (s)
In vitro treatment of spermatozoa with myoinositol may optimize ICSI outcomes by improving the fertilization rate and embryo quality on day 3. The improvement of the number and the quality of embryos available in an ICSI cycle may have clinical utility if these findings can be confirmed.
Keywords: Myoinositol, Fertilization rate, Embryo quality, ICSI outcomes
Introduction
Despite their small size and structural simplicity, mounting evidence indicates that spermatozoa possess a sophisticated mechanism for regulation of cytoplasmic Ca2+ concentration [1, 2]. Recent studies speculate about the presence of two intracellular Ca2+ stores and one sperm-specific Ca2+-permeable channel (CatSper) in the plasma membrane of the flagellar principal piece [3–7]. One of the Ca2+-permeable channels present in the Ca2+ storage organelles of spermatozoa is the inositol 1,4,5-triphosphate-sensitive Ca2+ channel [commonly called Ins [1, 4, 5] P3R] that has been studied extensively in a variety of cell types including sperm [8]. This channel binds the second messenger inositol 1,4,5-triphosphate [Ins [1, 4, 5] P3], which leads to elevation of intracellular Ca2+ concentration [9]. Immunolocalization experiments showed that two Ins [1, 4, 5] P3R-containing Ca2+ stores are present within the sperm, one in the acrosome and the other, a much smaller Ca2+ store, located within the redundant nuclear envelope at the back of the head [4, 10–12].
Myoinositol, the most abundant steroisomer of inositol, is an important precursor for the phosphatidyl-inositol (PtdIns) signaling pathway. Inositol incorporated into PtdIns is converted successively to the polyphosphoinositides, PtdInsP and PtdIns [4, 5] P2. Under specific stimuli, PtdIns [4, 5] P2 is hydrolyzed to produce two second messengers, diacylglycerol (DAG) and Ins [1, 4, 5] P3 which respectively modulate specific protein phosphorylation process and intracellular Ca2+ concentration [13].
The most important contributions of myoinositol to human reproduction are summarized in a review by Carlomagno et al. [14]. More specifically for male gametes, recent evidence tend to suggest that the incubation with myoinositol of spermatozoa from patients with OAT significantly enhances sperm motility and increases the percentage of spermatozoa with high inner mitochondrial membrane potential (MMP), probably increasing cytosolic Ca2+ and consequently inner mitochondrial Ca2+ [15]. The number of spermatozoa with high MMP has been shown to correlate positively with sperm concentration, progressive and total motility and to be associated with fertilization rate after in vitro fertilization (IVF) [16–18].
For all these reasons, we have speculated that incubation of spermatozoa with myoinositol may impact the embryologic outcomes of IVF cycles. In order to prove our hypothesis, a prospective comparison was designed in a population of infertile patients attending two different IVF Centers. With the intent to avoid any possible bias regarding the effect of myoinositol on the oocytes during the overnight incubation with a solution of spermatozoa containing myoinositol in a conventional IVF, we have performed our study in ICSI cycles.
Sibling oocytes fertilization rates after ICSI (per oocyte injected with spermatozoa treated with myoinositol versus per oocyte injected with spermatozoa treated with placebo) were measured as primary outcome. Embryo morphology was the secondary outcome. Clinical outcomes were also documented.
Materials and methods
Study design, randomization and outcome measures
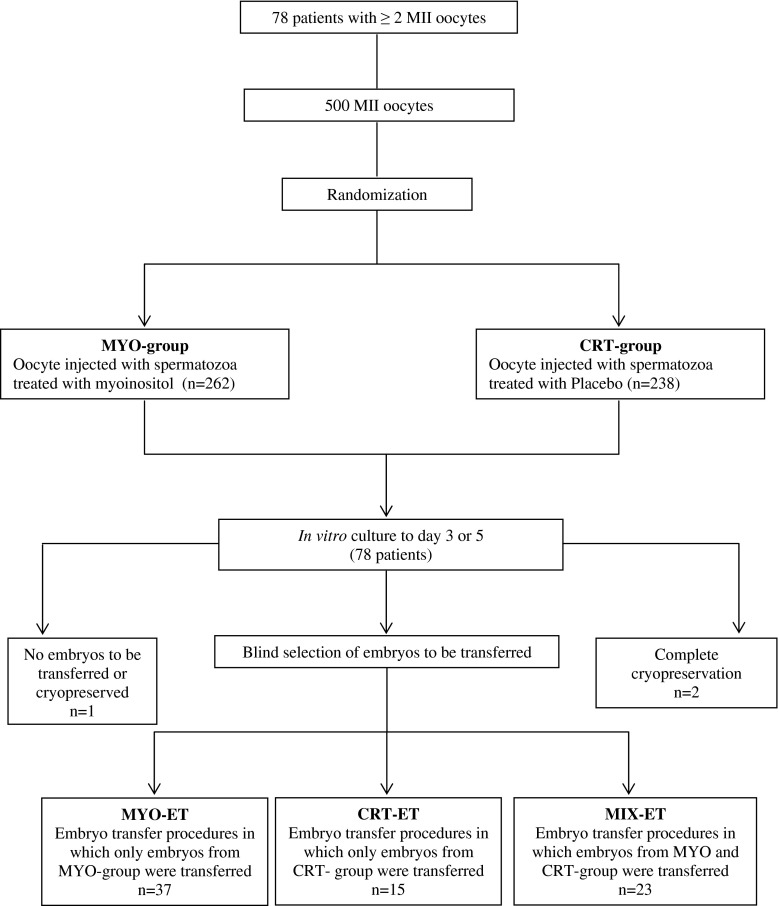
The study design is summarized in Fig. 1.
Fig. 1.
Schematic presentation of study design
A prospective randomized study on sibling oocytes was started to evaluate the effectiveness of myoinositol in an in vitro treatment of spermatozoa. Randomization of the oocytes into two groups was performed at the time of the denudation by an operator (first operator) different from the one who performed the ICSI (second operator). After the denudation, oocytes were allocated in two different ICSI dishes from the first operator, without any identification. If an odd number of mature oocytes was obtained, the first operator allocated the odd group of oocytes completely by chance in one of the unidentified dishes. The second operator added the spermatozoa treated with myoinositol (MYO-spermatozoa) in one dish and spermatozoa treated with placebo (CRT-spermatozoa) in the other dish by matter of chance. This second operator was not blinded for the sperm treatment but was blinded for the dish content. ICSI was started with MYO-spermatozoa or CRT-spermatozoa in alternating patients in order to avoid the bias of ICSI timing. A third blind operator evaluated the normal pronuclear appearance, the embryo morphology and selected the embryos for transfers.
The primary efficacy end-point was an increment in fertilization rates calculated per oocyte injected with MYO-spermatozoa or CRT-spermatozoa. Secondary end-points were the embryo morphologies. In addition clinical outcomes were documented.
Target population
Between March and October 2013, consecutive couples undergoing ICSI treatment at Tecnolab-Casa di Cura la Madonnina in Milan (MI), and at Physiopathology of Reproduction Unit, ‘Cervesi’ Hospital in Cattolica (RN), were enrolled in the study. Inclusion criteria were female age ranged between 25 and 42 years, serum follicle-stimulating hormone (FSH) level on day 3 ≤ 20 IU/L, more than 2 normal-appearing MII oocytes and availability of ejaculated sperm. Indication for ICSI were oligoasthenoteratozoospermia, advanced maternal age, previous IVF failure and immunological factors. Only fresh cycles were included in the analyses. Both Centers performed IVF laboratory procedures following similar protocols during the study period, including embryo grading. The protocol was approved by the Institutional Review Board of both Institutions and registered with ClinicalTrials.gov (Identifier number NCT02050672). All subjects provided written informed consent before participation.
Ovarian stimulation, oocyte collection, denudation, injection and embryo assessment
All patients received a recombinant FSH preparation. Pituitary suppression was achieved either by a conventional GnRH agonist protocol (long o flare) or GnRH antagonist protocol. Upon confirmation of at least three dominant follicles (mean diameter, 18 mm), recombinant or urinary hCG was administered, with oocytes retrieval occurring 35 to 36 h later. Oocyte denudation from the cumulus oophorus was performed between 39 and 41 h post-hCG administration by a brief exposure to 40 IU/ml hyaluronidase solution in medium with Hepes supplemented with 5 % of Human Serum Albumin (Sage In Vitro Fertilization, CooperSurgical, Pasadena CA), followed by mechanical removal of the corona radiata with the use of plastic pipettes of defined diameters (COOK Medical, Bloomington, IN). MII oocyte were evaluated under stereomicroscope and separated from immature oocytes. Dismorphic oocytes were excluded from randomization. The selected oocytes were randomly allocated to injection with MYO-spermatozoa or CRT-spermatozoa, both performed immediately after denudation. To be able to follow the developmental progression of individual oocyte, each inseminated oocyte was cultured separately in microdrops of 25 μl of Protein Plus Cleavage Medium (Sage In Vitro Fertilization, CooperSurgical, Pasadena CA) under mineral oil up to day 3, and eventually in microdrop of Protein Plus Blastocyst Medium (Sage In Vitro Fertilization, CooperSurgical, Pasadena CA) up to day 5/6. The culture was done in a humidified atmosphere containing 5 % O2 and 6 % CO2. Depending of number and quality of embryos available on day 3, the embryo transfer was performed on day 3 or 5. All the supernumerary viable embryos were cryopreserved on day 5 to 7. Embryos were evaluated on day 1 (16–18 h), day 2 (42–44 h) and day 3 (64–65 h) after ICSI, with the use of a cumulative embryo classification scheme taking into account cleavage speed, blastomere symmetry, extent of fragmentation, and presence or absence of multinucleated blastomeres as reported [19]. Fertilization was defined by the presence of two pronuclei and two polar bodies and the blastocyst quality was assessed according to criteria presented by Gardner and Schoolcraft (1999) [20].
Sperm preparation
We used a commercially available solution of myoinositol called Andrositol-Lab (Lo.Li.Pharma-Roma) specifically developed to reduce sperm viscosity and improve sperm motility in human semen preparation for assisted reproductive technology. Andrositol Lab was provided as a stock solution of myoinositol 133 mg/ml in sodium chloride.
Semen samples were collected after 48 to 120 h of abstinence. After liquefaction and an accurate mixing, the sperm was divided into two identical aliquots. One aliquot was supplemented with myoinositol (Andrositol-Lab, Lo.Li.Pharma-Roma) at a dose of 2 mg/ml corresponding to 15 μl/ml according to the vial composition (MYO-spermatozoa), and the other one with the same volume of Sperm Washing Medium (Sage In Vitro Fertilization, CooperSurgical, Pasadena CA) as placebo (CTR-spermatozoa) and both incubated for 20 min. All the media that we used for the preparation of MYO-spermatozoa, including PVP, were supplemented with the same concentration of myoinositol or placebo.
Swim-up technique was used to prepare the sperm for ICSI. After 20 min of incubation with myoinositol or placebo, the whole semen of the 2 aliquots was diluted with 1:2 volume of Sperm Washing Medium (Sage In Vitro Fertilization, CooperSurgical, Pasadena CA) supplemented with myoinositol or placebo respectively and then centrifuged at 1,500 rpm for 10 min. The pellets were resuspended in 50 to 250 μl of Protein Plus Fertilization Medium and layered gently under 0.5 to 1 ml of Protein Plus Fertilization Medium (Sage In Vitro Fertilization, CooperSurgical, Pasadena CA) supplemented with myoinositol or placebo according to semen quality. The tubes were incubated for 30 min to 1 h at 37 °C in a humidified atmosphere containing 5 % O2 and 6 % CO2. The swim-up portions were then incubated in the same atmosphere until the ICSI time. If no spermatozoa were retrieved in the swim-up fraction, the bottom layers were used for ICSI.
Semen analysis
Semen analysis was performed according to Manual on Basic Semen Analysis. ESHRE Monographs. Oxford: Oxford University Press, 2002 [21].
Sample specification and statistical analysis
To demonstrate the superiority of myoinositol addition to sperm preparation procedures on fertilization rate based on a maximum absolute difference of 15 % with a power of 90 % and a confidence of 95 % (comparative test with two-sided tests performed) a minimum of 198 oocytes was required per group (396 total). The superiority margin of this study was set at 15 % because this threshold was considered to represent a clinically important difference, in particular on the number of embryos available for transfer or for preimplantation genetic screening in our setting. Considering a mean fertilization rate of 65 % with ICSI procedure in our Laboratory, the higher confidence limit would be superior to 80 % for the injection performed with spermatozoa treated with myoinositol.
Paired-sample two-tailed Student’s t-tests were used to compare oocyte fertilization rate, percentage of grade A embryos, percentage of expanded blastocyst formation rate, sperm concentration and motility after swim up between experimental and control group. Differences in ongoing pregnancy and implantation rates, were evaluated with Fisher’s exact test. Differences in the mean number of embryos transferred and mean age of females between groups were compared by ANOVA. The Bonferroni’s Multiple Comparison test was used as post-test to determine significant differences between groups. Data are reported as mean ± SD and odd ratio (OR), 95 % confidence interval (CI), and P value as appropriate. P < 0.05 was considered as statistical significant.
Outcome variable definition
A pregnancy was confirmed by serial rise in serum βCG concentrations on two consecutive occasions 12 days after embryo transfer. The absence of an identifiable pregnancy on ultrasound examination was named ‘Biochemical pregnancy loss’. A clinical pregnancy was defined as the presence of an intrauterine gestational sac with positive heartbeat on ultrasound. Miscarriage was classified as ‘early’ (before 12 weeks) or ‘late’ (after 12 weeks). The implantation rate was defined as the number of gestational sacs per transferred embryo, and the ongoing implantation rate as fetuses with heartbeat beyond 12 weeks of gestation per transferred embryo.
Results
Primary and secondary outcome measures: fertilization rates and embryo morphology
The results obtained are shown in Table 1.
Table 1.
Primary and secondary outcome measures
| MYO group oocytes | CTR group oocytes | p | |
|---|---|---|---|
| Number of cycles | 78 | 78 | |
| Female mean age ± SD | 37.4 ± 4.0 | 37.4 ± 4.0 | |
| Number of injected MII oocyte | 262 | 238 | |
| Fertilization rate ± SD | 78.9 ± 28.6 % | 63.2 ± 36.7 % | 0.002 |
| Percentage of grade A embryos on day 3 ± SD | 59.8 ± 35.6 % | 43.5 ± 41.5 % | 0.019 |
| Percentage of expanded blastocysts ± SD * | 56.5 ± 31.2 % | 61.6 ± 61.5 % | NS |
* includes only cycles where embryo culture was extended at blastocyst stage and represents fraction of day 3 embryos that reached an expanded blastocyst stage
During the study period, 78 cycles from 78 patients meet the inclusion criteria and were enrolled in the study. The mean age of patients included in the study was 37.4 ± 4.0 year. The total number of injected MII oocytes were 262 in MYO group (mean number per patient ± SD 3.36 ± 1.59) and 238 in the CTR group (mean number per patient ± SD 3.05 ± 1.56). The fertilization rate obtained with MYO-spermatozoa (78.9 ± 28.6 %) was significantly higher than fertilization rate obtained with CRT-spermatozoa (63.2 ± 36.7 %, P = 0.002).
The percentage of grade A embryos on day 3 in the MYO group was also significantly higher than that of the CTR group (59.8 ± 35.6 % vs 43.5 ± 41.5 %, P = 0.019). In cycles where embryo culture was extended to blastocyst stage, the fraction of day 3 embryos that reached the expanded blastocyst stage on day 5 or 6 were similar in both groups (56.5 ± 31.2 % in MYO group vs 61.6 ± 61.5 % in CTR group).
A total of 105 embryos were transferred in 54 embryo transfers on day 3 (mean ± SD 1.94 ± 0.59) and 29 embryos in 21 embryo transfers on day 5 (mean ± SD 1.38 ± 0.58). One embryo transfer was cancelled because no embryos were available and two transfers were cancelled for high progesterone level. In 37 embryo transfer procedures, only embryos from MYO group were transferred (mean ± SD 1.64 ± 0.64), in 15 only embryos from CTR group (mean ± SD 1.46 ± 0.52) and in 23 cycles, embryos from both groups were transferred (mean ± SD 2.13 ± 0.34, P = 0.010).
No significant difference in women’s age, level of basal serum FSH, endometrial thickness on ET day and presence of history of recurrent implantation failure were recorded between patients in whom only embryos from MYO group or CTR group were transferred (data not shown).
Clinical outcomes
Twenty-six pregnancies were recorded, including two biochemical pregnancy losses and an ectopic pregnancy. Twenty-seven sacs with fetal heartbeat were observed by ultrasound examination.
Considering only embryo transfer procedures in which embryos from MYO group were transferred, the female mean age was 36.4 ± 4.1 and we documented 13 ongoing pregnancies (35.1 %) with an ongoing implantation rate of 21.3 %. Considering embryo transfer procedures in which embryos from CRT group were transferred, the female mean age was 36.3 ± 4.2 and we reported 3 ongoing pregnancies (20.0 %), with an ongoing implantation rate of 13.6 %. Considering embryo transfer procedures in which embryos from MYO and CRT groups were transferred, the female mean age was 39.4 ± 3.1 and we reported 7 ongoing clinical pregnancies (30.4 %), with an ongoing implantation rate of 21.6 %.
No statistical differences were found between the groups in terms of female age, ongoing pregnancy and ongoing implantation rate: OR (95 % CI) for ongoing pregnancy rate 2.17 (0.52–9.09); OR (95 % CI) for ongoing implantation rate 1.72 (0.44–6.71).
Sperm parameters
Sperm concentration and motility were recorded and reported in Table 2. The mean sperm concentration ± SD of the samples before the swim up procedures was 38.5 ± 43.2 (range 0.1–200) million/ml with a mean progressive motility ± SD of 33.5 ± 18.1 % (range 1–70). After sperm preparation, the mean sperm concentration ± SD in the samples with and without myoinositol was 10.1 ± 17.8 (range 0.05–65) million/ml and 8.5 ± 13.8 (range 0.05–80) million/ml respectively (p = 0.18), with a mean progressive motility ± SD of 72.9 ± 24.3 % (range 1–95) vs 67.8 ± 24.2 % (range 10–95, p = 0.004).
Table 2.
Sperm concentration and motility before and after swim up procedure
| Starting Sperm Samples N = 78 | MYO-spermatozoa N = 78 | CRT-spermatozoa N = 78 | P | |
|---|---|---|---|---|
| Sperm concentration before swim up (million/ml) ± SD | 38.5 ± 43.2 | |||
| Sperm progressive motility before swim up (%) ± SD | 33.5 ± 18.1 | |||
| Sperm concentration after swim up (million/ml) ± SD | 10.1 ± 17.8 | 8.5 ± 13.8 | NS | |
| Sperm progressive motility after swim up (%) ± SD | 72.9 ± 24.3 | 67.8 ± 24.2 | 0.004 |
Discussion
For the first time we report that the oocyte fertilization rate after ICSI can be significantly increased when spermatozoa are treated and prepared with myoinositol compared with placebo supplemented media. We have expected these results based on previous studies from Condorelli and coworkers [15] and Marchetti and coworkers [18]. Condorelli et al. observed that myoinositol significantly increased the number of spermatozoa with high MMP in OAT patients and Marchetti et al. observed that the percentage of spermatozoa with high MMP positively correlated with fertilization rate in IVF cycles [18].
We have also observed a statistically significant higher percentage of grade A embryos on day 3 in the MYO group. This result is in line with the observation by Marchetti et al., who described a better embryo quality in IVF cycles when the spermatozoa had higher MMP, although the difference observed in the study by Marchetti et al. was not statistically significant [18].
Conversely, we did not observe any difference in expanded blastocyst rate formation between the group of oocytes injected with MYO-spermatozoa and the oocytes from the control group suggesting a transient effect.
This seems to fit with the evidence that paternal mitochondria are degraded inside the zygote following the penetration of the entire male gamete into the oocyte. Indeed, sperm mitochondria are ubiquitinated inside the cytoplasm, leading to a selective proteolysis during early embryonic development [22, 23]. Thus, the proteolytic destruction of sperm mitochondria in the early embryo might explain why the functionality of sperm mitochondria is able to affect more the fertilizing capacity and the early post-fertilization development than the late development. This hypothesis needs however to be further investigated, because other mechanisms of action cannot be excluded, as an antioxidative effect of myoinositol during sperm preparation procedures.
Indeed, contrary to what normally supposed, the selection of sperm preparation techniques for the elimination of seminal plasma and the collection of functional spermatozoa represents a still controversial area of investigation. Evidence suggests that the current techniques, due to the multiple centrifugations and the long time during which the samples remain in an incubator at 37 °C, allow the production of reactive oxidative species (ROS) and induce spermatozoa DNA damage [24, 25]. Several authors agree that the production of ROS is increased rather than decreased after separation, especially when the swim up methods are used, with the consequent development of DNA abnormalities among spermatozoa [26]. It has been demonstrated that MMP and ROS levels are inversely correlated. Such relationship could be due to two mutually interconnected phenomena: on one hand, ROS causing damage to the mitochondrial membrane and, on the other hand, the damaged mitochondrial membrane causing increased ROS production [27]. Due to its potential effect in increasing the percentage of spermatozoa with high MMP [15], we can hypothesize that myoinositol can be effective in breaking down this loop, thus potentially affecting the embryo quality.
In our study, the swim up method was used to prepare the sperm samples. In order to shed more light on advantages and disadvantages of various sperm preparation techniques, it would be interesting to investigate whether the positive effect of myoinositol on fertilization rate could be replicated using sperm gradient separation was used for sperm preparation,.
Finally, we cannot exclude that myoinositol was minimally injected with the sperm, producing the positive effect on oocyte and hence, on the fertilization rate and embryo quality. Indeed, it has been demonstrated that mouse oocytes had the capacity to release Ca2+ following injection of Ins [1, 4, 5] P3, leading to meiotic maturation [28].
Some limitations of our study should be recognized. Firstly, we have enrolled all consecutive couples with ejaculated spermatozoa undergoing ICSI in our Centers with few limitations only for the female partner. We may expect that the effect of the Myoinosiyol on fertilization rate and embryo quality rate could be different according to different parameters of spermatozoa, especially MMP. Indeed, Condorelli and coworkers observed that myoinositol incubation could significantly increase the percentage of spermatozoa with high MMP in patients with OAT, but not in normozoospermic men [15]. Since sperm with high MMP correlates in a positive manner with sperm number and motility, we can expect some differences in MMP between various semen samples selected for ICSI solely according to number and motility, because nowadays ICSI is more frequently performed independently of traditional sperm parameters. As a consequence, myoinositol treatment of spermatozoa with different MMP level might produce different results on fertilization rate.
It could also be considered that myoinositol treatment might be used as a test in order to evaluate which samples may benefit of incubation with this compound based on motility improvement: patients with a certain responsiveness in terms of sperm motility might be those that can have benefits from the treatment.
Secondly, a portion of women undergoing ICSI for the partner’s infertility may have oocytes with a good capacity to repair DNA damage even if the injected spermatozoon has poor quality. This is supported by findings by Meseguer et al. [29], indicating that high-quality oocytes from donors can offset the negative impact of sperm DNA damage on pregnancy rate. As a consequence, a better stratification of the couples according to female reproductive background should be considered in order to better understand the effectiveness of myoinositol supplementation of sperm preparation media in improving ICSI outcomes.
Finally, according to literature and manufacturer’s instructions we used myoinositol at a final concentration of 2 mg/ml. Further studies are necessary to clarify if the specific effect on sperm fertilization ability is indeed dose-dependent.
To our knowledge, this is the first clinical trial designed in a prospective randomized way and involving two Centers clearly demonstrating the superiority of the myoinositol-based in vitro treatment of human spermatozoa on fertilization rate and embryo quality rate compared to placebo-treated spermatozoa in a population of infertile patients. The exact mechanism of action of myoinositol can only be hypothesized and further studies are necessary to clarify if the sperm mitochondria might be involved.
However, our results may encourage further research on all the possible benefits of myoinositol supplementation of culture media for human in vitro fertilization procedures as well as it has been observed in animal models including mice. Indeed, the addition of myoinositol to culture media of preimplantation mouse embryos (a key model system for studies concerning experimental approaches to human assisted reproduction) resulted in an increase of developmental activity of the embryos [30]. The improvement of culture conditions still represents one of the main goals of human ART research.
Acknowledgments
Dr Paola Viganò (San Raffaele Scientific Institute, Obstetrics and Gynecology Unit, Milan) is gratefully acknowledged for critically reading and commenting on the manuscript.
We also acknowledge the statistical support of Dr Luca Pagliardini (San Raffaele Scientific Institute, Division of Genetics and cell Biology, Milan).
Funding
Lo.Li.pharma supplied myoinositol free of charge.
Conflict of interest
G. Carlomagno is a Lo.semployee.
Author’s role
P.R. conceived the idea of the study and its design. She was also responsible for the collection, analysis and interpretation of data, for writing the first version of this manuscript, and for reviewing and approving the final version of the manuscript. G.C. and S.P. contributed for the analysis and interpretation of the data and approved the final version of the manuscript. S.C., A.Q., S.D.S were responsible for sperm preparation and data collection and for approving the final version of the manuscript. C.B. supervised the study and approved the final version of the manuscript.
Footnotes
Capsule
In vitro treatment of spermatozoa with myoinositol in ICSI cycles improves the fertilization rate andembryo quality on day 3.
References
- 1.Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Hum Reprod Update. 2006;12(3):253–67. doi: 10.1093/humupd/dmi050. [DOI] [PubMed] [Google Scholar]
- 2.Alasmari W, Barratt CL, Publicover SJ, Whalley KM, Foster E, Kay V, et al. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod. 2013;28(4):866–76. doi: 10.1093/humrep/des467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65(5):1606–15. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 4.Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod. 2003;68(5):1590–6. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- 5.Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating. J Biol Chem. 2004;279(44):46315–25. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- 6.Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, et al. Ca2 + −stores in sperm: their identities and functions. Reproduction. 2009;138(3):425–37. doi: 10.1530/REP-09-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012;74:453–75. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell / Auspices Eur Cell Biol Organ. 2004;96(1):3–17. doi: 10.1016/j.biolcel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Michelangeli F, Mezna M, Tovey S, Sayers LG. Pharmacological modulators of the inositol 1,4,5-trisphosphate receptor. Neuropharmacology. 1995;34(9):1111–22. doi: 10.1016/0028-3908(95)00053-9. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda Y, Kaneko S, Yoshimura Y, Nozawa S, Mikoshiba K. Are there inositol 1,4,5-triphosphate (IP3) receptors in human sperm? Life Sci. 1999;65(2):135–43. doi: 10.1016/S0024-3205(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 11.Walensky LD, Snyder SH. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J Cell Biol. 1995;130(4):857–69. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naaby-Hansen S, Wolkowicz MJ, Klotz K, Bush LA, Westbrook VA, Shibahara H, et al. Co-localization of the inositol 1,4,5-trisphosphate receptor and calreticulin in the equatorial segment and in membrane bounded vesicles in the cytoplasmic droplet of human spermatozoa. Mol Hum Reprod. 2001;7(10):923–33. doi: 10.1093/molehr/7.10.923. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- 14.Carlomagno G, Nordio M, Chiu TT, Unfer V. Contribution of myo-inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):267–72. doi: 10.1016/j.ejogrb.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Condorelli RA, La Vignera S, Bellanca S, Vicari E, Calogero AE. Myoinositol: does it improve sperm mitochondrial function and sperm motility? Urology. 2012;79(6):1290–5. doi: 10.1016/j.urology.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17(5):1257–65. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 2004;19(10):2267–76. doi: 10.1093/humrep/deh416. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti P, Ballot C, Jouy N, Thomas P, Marchetti C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia. 2012;44(2):136–41. doi: 10.1111/j.1439-0272.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 19.Rienzi L, Ubaldi F, Iacobelli M, Ferrero S, Minasi MG, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod. 2002;17(7):1852–5. doi: 10.1093/humrep/17.7.1852. [DOI] [PubMed] [Google Scholar]
- 20.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Kvist U, Bjorndahl L, Manual on Basic Semen Analysis, ESHRE Monographs. Oxford: Oxford University Press; 2002. p. 2002. [Google Scholar]
- 22.Ramalho-Santos J. A sperm’s tail: the importance of getting it right. Hum Reprod. 2011;26(9):2590–1. doi: 10.1093/humrep/der200. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura Y, Yoshinari T, Naruse K, Yamada T, Sumi K, Mitani H, et al. Active digestion of sperm mitochondrial DNA in single living sperm revealed by optical tweezers. Proc Natl Acad Sci U S A. 2006;103(5):1382–7. doi: 10.1073/pnas.0506911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Zhou Y, Liu R, Lin H, Liu W, Xiao W, et al. Effects of semen processing on the generation of reactive oxygen species and mitochondrial membrane potential of human spermatozoa. Andrologia. 2012;44(3):157–63. doi: 10.1111/j.1439-0272.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell M, McClure N, Powell LA, Steele EK, Lewis SE. Differences in mitochondrial and nuclear DNA status of high-density and low-density sperm fractions after density centrifugation preparation. Fertil Steril. 2003;79(Suppl 1):754–62. doi: 10.1016/S0015-0282(02)04827-6. [DOI] [PubMed] [Google Scholar]
- 26.Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, et al. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod. 2000;15(5):1112–6. doi: 10.1093/humrep/15.5.1112. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003;80(Suppl 2):844–50. doi: 10.1016/S0015-0282(03)00983-X. [DOI] [PubMed] [Google Scholar]
- 28.Lowther KM, Weitzman VN, Maier D, Mehlmann LM. Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biol Reprod. 2009;81(1):147–54. doi: 10.1095/biolreprod.108.072538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meseguer M, Santiso R, Garrido N, Garcia-Herrero S, Remohi J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95(1):124–8. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 30.Colazingari S, Fiorenza MT, Carlomagno G, Najjar R, Bevilacqua A. Improvement of mouse embryo quality by myo-inositol supplementation of IVF media. J Assist Reprod Genet. 2014;31(4):463–9. doi: 10.1007/s10815-014-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]