Abstract
Purpose
This study evaluates the effect of low oxygen conditions (5 Vs 20 %) on buffalo embryo development. Expression patterns of key glucose metabolism genes (HK, PFK, LDH, PDH, G6PDH and Glut1) were assessed in buffalo oocytes and embryos cultured at 5 and 20 % oxygen and correlated with development rate.
Methods
Maturation rate was observed by determining MII stages by Aceto-orcein method and blastocyst formation was observed at 7 day post insemination (dpi). Expression levels of genes were determined by real time PCR in oocytes / embryos at 5 and 20 % O2.
Results
Oocyte maturation and blastocyst formation rates were significantly higher at 5 % O2 as compared to 20 % O2 (P < 0.05). The expression pattern of glycolytic genes (HK, PFK and G6PDH) indicated that oocytes and embryos under 5 % O2 tend to follow anaerobic glycolysis and pentose phosphate pathways to support optimum embryo development. Under 20 % O2, oocytes and embryos had high expression of PDH indicating higher oxidative phosphorylation. Further, less G6PDH expression at 20 % O2 was indicative of lower pentose phosphate activity. Higher expression of LDH was observed in oocytes and embryos under 20 % O2 indicating sub-optimal culture conditions. High Glut1 activity was observed in the oocytes / embryos at 5 % O2, indicative of high glucose uptake correlating with high expression of glycolytic genes.
Conclusion
The expression patterns of glucose metabolism genes could be a valuable indicator of the development potential of oocytes and embryos. The study indicates the importance of reduced oxygen conditions for production of good quality embryos.
Keywords: Buffalo, Oocytes, Embryos, Oxygen, Glucose metabolism
Introduction
Studies in the past had shown that mammalian oocytes/embryos develop better under reduced oxygen tension compared to atmospheric oxygen in vitro resulting in higher blastocyst formation rates, less apoptotic cells and more inner cell mass cell numbers [1–3]. The oxidative stress on oocytes / embryos had been shown to be directly proportional to their metabolism status where the aerobic metabolism results in the production of co-factors which impact the cellular redox status [4]. A reduced reactive oxygen species (ROS) content was observed in embryos cultured under the 5 % oxygen environment and was associated with a reduction in DNA fragmentation and significantly higher blastocyst formation rate [3, 5]. Aerobic metabolism (i.e. oxidative phosphorylation, OXPHOS) was inhibited by low oxygen tension and accordingly, ATP production shifted from the tricorboxylic acid (TCA) cycle to glycolysis [6, 7], which led to the decreased ROS production and increased embryonic development during hypoxia [5]. The decrease in ATP production on the other hand was compensated by an increase in expression of glucose transporters and glycolytic enzymes [8, 9]. Glucose was thought to be a major energy substrate for supporting the meiotic maturation of bovine oocytes under low oxygen tension [5]. Reduced oxygen tension increased the catabolic utilization of glucose by mouse morulae and blastocysts in vitro [10, 11]. Other studies in different species had also shown that the low O2 concentration and high glucose were beneficial for in vitro maturation, fertilization, and pre-implantation embryonic development [5, 12, 13]. It had been observed that under reduced oxygen level, glucose metabolism pattern of in vitro embryos was similar to in vivo counterparts [4]. Also the global gene expression pattern was found to be different under 5 % O2 than 20 % O2 and the expression pattern under 5 % O2 was found closer to their in vivo counterparts [14]. It reflects that the oxygen concentration in a culture environment had consequences on the glucose utilization pattern and a low oxygen environment could mimic better the in vivo conditions towards better developmental fate of oocytes/embryos.
Keeping in view the problems with the buffalo in vitro fertilization (IVF) system [15] and possible beneficial effect of reduced oxygen environment, the present study was aimed at investigating the effect of reduced oxygen level (5 % O2) on glucose metabolism and development rate of buffalo oocytes and embryos. The associated expression patterns of glucose metabolism genes were studied to infer on the glucose metabolism behavior and correlated with development rate of oocytes/embryos.
Material and methods
All media and chemicals were procured from Sigma Aldrich, St. Louis, MO, USA unless otherwise indicated. Disposable plastic wares used were from Falcon NJ, USA and Nunc, Denmark. Fetal bovine serum used was from Hyclone, Canada.
Experimental design
The effect of reduced oxygen level (5 %) in combination with glucose levels during IVM (5.6 and 20 mM) was investigated on in vitro maturation rate of the oocytes and subsequent blastocyst development rate of IVF produced buffalo embryos. Thus the study had 2 experimental groups w.r.t. IVM glucose level viz. 5.6 and 20 mM glucose under 5 % O2 and 5.6 mM glucose under 20 % O2 worked as a control. We have seen in our previous study that at 20 % O2, 5.6 mM glucose yielded a highest blastocyst development rate in buffalo [16]. The 20 mM glucose group under 5 % O2 was experimented during IVM to confirm whether a higher glucose under reduced O2 has any positive effect on buffalo IVF also as noted earlier in other species [11, 12]. During IVC, 5.6 mM glucose was used under both oxygen levels. Expression levels of glycolytic genes (hexokinase, HK; phosphofructokinase, PFK; lactate dehydrogenase, LDH; pyruvate dehydrogenase, PDH; glucose-6-phosphate dehydrogenase, G6PDH and glucose transporter 1, Glut1) were measured at 4 development stages viz. matured oocytes, 2-cell, 16-cell, and morula. We considered 2- and 16-cell embryos as representatives of pre- and post-embryonic genome activation (EGA) stages respectively while morula were considered as post compaction stage embryos [17]. The cumulus oocyte complexes (COC) matured under different experimental conditions were used to observe MII % using Aceto-orcein staining method [18]. For real time PCR assay, matured COC and embryos at 2-cell, 16-cell and morula stages were collected at 36hpi, 84hpi, and 144hpi respectively. The cleavage and blastocyst development rate data were recorded as a percentage of COC (initially put for IVM). All experiments included four biological replicates.
Production of buffalo IVF embryos
Buffalo embryos were produced following the procedure described earlier [16]. Briefly, buffalo ovaries were collected from the slaughterhouse and oocytes were aspirated from visible follicles (3–8 mm) in modified synthetic oviduct fluid — in vitro maturation (mSOF-IVM) medium supplemented with 0.2 % BSA, 60 μg/ml penicillin, and 100 μg/ml streptomycin. Oocytes with completely surrounded the cumulus mass of 2–5 layers and homogenous ooplasm were picked up in the oocyte handling medium (mSOF supplemented with 10 % FBS, 1 mM glutamine, 0.33 mM sodium pyruvate, 0.1 mM taurine, 3.3 mM lactate, 0.5 mM citrate, 1X MEM essential amino acid, 1X non-essential amino acid, 1X BME vitamin solution and 50 μg/ml streptomycin). Following 3 washings, 25–30 oocytes were transferred to 100 μl drops of maturation medium composed of the oocyte handling medium supplemented with 5 μg/ml FSH, 10 μg/ml LH, 50 ng/ml EGF, 0.1 mM cysteamine, 1 % ITS and either concentration of glucose (5.6 or 20 mM). COC in maturation drops were incubated in a CO2 incubator at either 20 or 5 % O2 and 38.5 °C for 24 h. After maturation, oocytes were washed 3 times in IVF medium (mSOF-IVM medium supplemented with 1.9 mg/ml caffeine sodium benzoate, 0.1 mg/ ml heparin and 0.14 mg/ml sodium pyruvate and 1 % BSA) and transferred to 50 μl fertilization drops in groups of 20–25. Frozen spermatozoa after thawing and washing in IVF medium were transferred to fertilization drops (1 × 106 sperms) containing matured oocytes and incubated in a CO2 incubator for 12 h at 38.5 °C at either 20 or 5 % O2. Following fertilization, oocytes were washed 5–6 times in the in vitro culture medium (mSOF-IVC) supplemented with 0.8 % bovine serum albumin (BSA), 5.6 / 20 mM glucose, 0.33 mM pyruvate, 3.3 mM lactate, 1 mM Glutamine, 1X MEM essential amino acid, 1X non-essential amino acid and 50ug/ml gentamycin. Zygotes were transferred to 100 μl culture drops (IVC-I containing BSA) at either 20 or 5 % O2. After 72 h, embryos were transferred to IVC-II medium (BSA in the IVC-I medium was replaced with 10 % fetal bovine serum (FBS)). Half of the culture medium was replaced every 48 h and the embryos were cultured till day 7.
RNA isolation, cDNA synthesis and real time PCR
RNA was isolated from 10 oocytes/embryos of each group using Trizol (Invitrogen, Cat# 15596–026) as per manufacturer’s instructions. RNA was precipitated using 10 μg glycogen (Fermentas, Cat# R0551) and finally the RNA pellet was washed with 70 % ethanol. RNA pellet was air-dried and dissolved in 10 μl nuclease free water and treated with DNase-I (Fermentas, Cat# EN0523) as per manufacturer’s instruction. A constant amount of RNA (100 ng) from each sample was used for cDNA synthesis using Superscript III (Invitrogen, Cat # 18080–044) following manufacturer’s instructions. Genes under study viz. HK, PFK, LDH, PDH, G6PDH and housekeeping genes viz. GAPDH and RPS15 were amplified using real time TaqMan assay. The buffalo specific TaqMan primers and probes for genes under study were designed from the deduced buffalo sequences (GenBank accession no GU324290 to 99) (Table 1). TaqMan probes were labeled with 5′ FAM – 3′ TAMRA and synthesized from Sigma-Aldrich (St. Louis, Missouri, USA). Respective primer and probe sequences are described in Table 1. All real time PCR reactions were of 10 μl consisted of 5 μl of 2X real-time TaqMan mix (Fermentas, Cat # K0221), 2 μl cDNA, 0.75 μl each of primers and probes (7.5 pM) and 0.75 μl water. A common thermal profile of 50 °C for 2 min, 95 °C for 10 min; 40 cycles of 95 °C for 20 s and 60 °C for 60 s was used for all genes. Standard curves were generated by serial dilution of cDNA to validate the efficiencies of the probes and primers and amplification conditions. Standard curves of all genes had efficiency in the range of 96 to 107 % and slopes in the range of −3.1 to −3.6. Real time PCR reactions were set in triplicate using 2 μl of diluted cDNA (3X) along with a negative control. All experiments included 4 biological replicates.
Table 1.
Real time PCR Taqman primer / probe sequences used
| Gene name | mRNA accession# | Primer / probe sequences |
|---|---|---|
| HK | GU324294 | F 5′ CGACGACAGTATCCTCGTGA 3′ |
| R 5′GCGGATCTTATCCACCACAG 3′ | ||
| P 5′ GTGTGTGGGGTGGTGTCCAAGAG 3′ | ||
| PFK | GU324297 | F 5′GGGCCTGGTGTTAAGGAACG3′ |
| R 5′ACGTTCTTCCTGCTGTCGAAG3′ | ||
| P 5′TGCCCTTCCCCTCCTCCGAGTACA3′ | ||
| LDH | GU324295 | F 5′TTGACAGTGCTTATGAGGTGATC3′ |
| R 5′TCATTATACTTTCTGCCAAATCGG3′ | ||
| P 5′CACTGACAGTCCAATGGCCCAGGA3′ | ||
| PDH | GU324296 | F 5′AGAAAGGCAAGGAACACACG3′ |
| R 5′TTTATCACCTCACATTCAATTCCC3′ | ||
| P 5′TGCTCATTCAAGACCTGTGGGCCA3′ | ||
| Glut1 | GU324293 | F 5′TCCACAAGCATCTTCGAGAAGG3′ |
| R 5′AATAGCGACACGACAGTGAAGG3′ | ||
| P 5′AGCAGCCCGTGTATGCCACCATCG3′ | ||
| G6PDH | GU324292 | F 5′ATGACGTGCGCGATGAGAAG3′ |
| R 5′GGGGTTCCCCACATATTGGC3′ | ||
| P 5′CCACGTTGCTCGCCTGCACCTCTG3′ | ||
| RPS15 | NM_001024541.1 | F 5′ACAACGGCAAGACCTTCAAC3′ |
| R 5′CAGGTTACTTGAGGGGGATG3′ | ||
| P 5′CTAGGCGAGTTCTCCATCACTTAC3′ | ||
| GAPDH | GU324291 | F 5′ACGTGTCTGTTGTGGATCTGAC3′ |
| R 5′CGCTGTTGAAGTCGCAGGAG3′ | ||
| P 5′TGCCGCCTGGAGAAACCTGCCAA 3′ |
F forward primer, R reverse primer, P probe sequence
Analysis of data
The maturation rate and development rate data under different experimental groups were analyzed by one-way ANOVA followed by Tukey’s multiple comparison post hoc test. Real time amplification Ct value data obtained for genes under study were normalized using the geometric mean of RPS15 and GAPDH [19]. The relative abundance values for transcripts were calculated by ∆∆CT method [20] using immature oocytes as a calibrator for gene expression data of oocytes and matured oocytes under 5 % O2 (20 mM glucose) as a calibrator for gene expression data of embryos. Relative abundance values were analyzed by two-way ANOVA followed by Bonferroni post hoc test to find out the significance of difference between different embryonic cell stages within a particular experimental group as well as for a particular cell stage between different experimental groups and control. The significance of differences was tested both at 5 and 1 % levels of significance.
Results
Effect of oxygen concentration on the developmental competence of oocytes and embryos
No significant difference was observed either in the maturation or blastocyst development rates at 20 and 5 % O2 level when the oocytes were matured in IVM medium with 5.6 mM glucose. However, the oocytes matured at reduced O2 level (5 %) and higher glucose level (20 mM) resulted in significantly more (P < 0.05) number of oocytes reaching M-II stage and a higher number of blastocysts produced (Table 2).
Table 2.
Development rate of buffalo oocytes / embryos under different levels of oxygen (5 and 20 %) and glucose (5.6 and 20 mM)
| Oxygen level (%) | Glucose level - IVM (mM) | # COCs | % M-II | % uncleaved oocyte | % 2 cell (blocked) | % 8–16 cell (blocked) | % Morula (blocked) | % Blastocyst |
|---|---|---|---|---|---|---|---|---|
| 20 | 5.6 | 232 | 87.3 ± 2.06a | 33.3 ± 1.46a | 7.52 ± 0.29a | 8.32 ± 0.64a | 29.17 ± 1.22a | 21.66 ± 0.15a |
| 5 | 5.6 | 216 | 80.69 ± 0.1a | 30.45 ± 2.5a | 8.42 ± 0.87a | 14.5 ± 2.18b | 28.23 ± 1.38a | 18.32 ± 1.37a |
| 5 | 20 | 243 | 94.8 ± 2.6b | 25.3 ± 2.87a | 4.19 ± 0.93b | 5.74 ± 1.60c | 35.80 ± 4.52b | 28.96 ± 0.91b |
Values indicate Mean ± SEM. Different superscripts indicate significant difference along column (p < 0.05). Glucose concentration at IVC-I and –II was kept at 5.6 mM both at 5 and 20 % O2
Expression pattern of glucose metabolism genes in oocytes
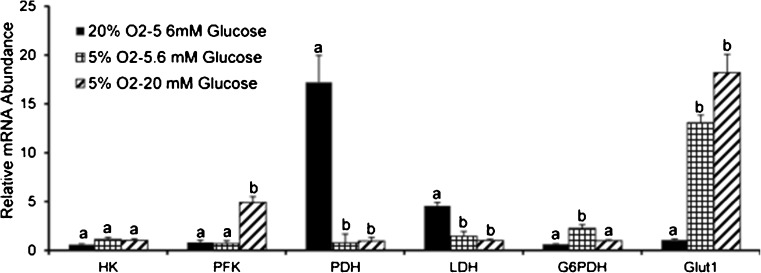
The expression of glycolytic gene HK did not significantly differ between different experimental groups but the PFK was found to be expressed at significantly higher level under 5 % O2 (20 mM glucose) as compared to 20 % O2 (5.6 mM glucose) and 5 % O2 (5.6 mM glucose) (Fig. 1). On the other hand the expression of TCA regulatory gene PDH was about 16 fold higher under 20 % O2 and 5.6 mM glucose as compared to other two groups i.e. 5 % O2 (20 and 5.6 mM glucose). Interestingly, the expression of LDH was also significantly higher in the same group (20 % O2, 5.6 mM glucose) (Fig. 1). The glucose transporter Glut1 was expressed at much higher level under 5 %O2 as compared to 20 % O2. The G6PDH expressed at moderately higher level under 5 % O2-5.6 mM glucose group over other two experimental groups (Fig. 1).
Fig. 1.
Expression pattern of glucose metabolism genes in the oocytes matured under different oxygen (5 or 20 %) and glucose (5.6 or 20 mM) levels. Oocytes were collected after 24 h of maturation and assayed for expression of glucose metabolism genes. Bars on respective columns represent standard error of mean. Columns with different superscripts indicate significant differences between different experimental groups for respective genes (p < 0.05)
Expression pattern of glucose metabolism genes in embryos
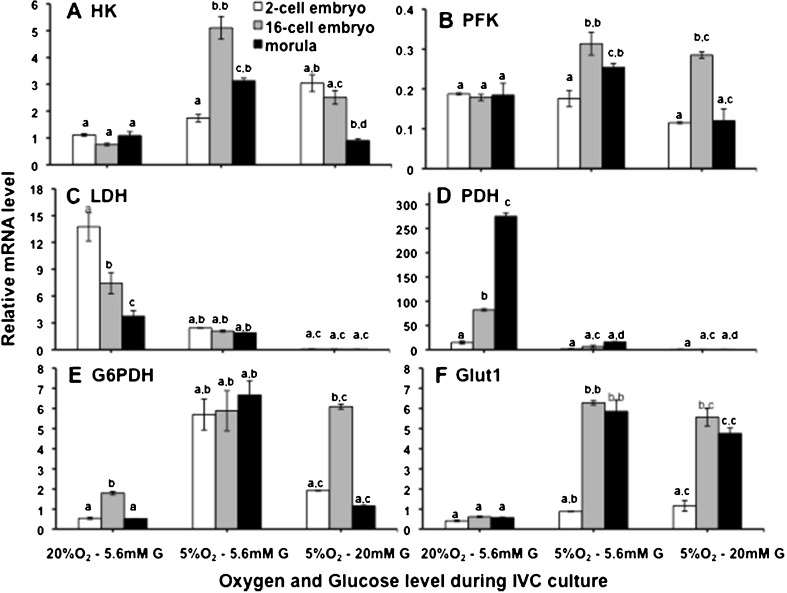
Under control conditions (20 % O2 and 5.6 mM glucose), HK didn’t show significant variation in expression through different embryonic stages (2-cell, 16-cell and morula) while at 5 % O2 and 5.6 mM glucose (during IVM as well IVC), HK expression was found to increase from 2-cell to 16 cell stage, but declined at morula stage. Interestingly however, when the glucose concentration was increased to 20 mM during IVM under 5 % O2, HK mRNA level was found declining gradually from 2-cell stage to morula, indicating a decline in glycolysis as the development of embryos progressed (Fig. 2a). Expression of PFK was found similar to HK except for the low O2 and high glucose group (5 % O2 and 20 mM glucose), where it showed a drastic increase at 16-cell stage (Fig. 2b). The expression of LDH revealed a declining trend from 2-cell to morula stage in high O2 group and its expression was not significantly different across different cell stages in 5 % O2 groups and were maintained at much lower levels than that in 20 % O2 group (Fig. 2c). The most drastic effect of lowering O2 concentration was seen in the PDH level (Fig. 2d). Sharp increase in PDH was observed from 2-cell to morula stage at 20 % O2, whereas its expression under lower O2 level (5 %) was found reduced to more than 250 times (Fig. 2d). G6PDH expression, followed similar trend in high O2 — low glucose and low O2 — high glucose groups, although their magnitude of expressions were significantly different in these two groups at all stages of embryonic development. However in low O2-low glucose group (5 % O2 and 5.6 mM glucose), it revealed non-significant variation across cell stages and were maintained at much higher levels (Fig. 2e). An aberrant increase in the level of expression of Glut1 between 2 and 16-cell stages under lower O2 level (5 % O2) was notable. Under 20 % O2, it was maintained at a significantly lower level in all embryonic stages (Fig. 2f).
Fig. 2.
Expression pattern of glucose metabolism genes in embryos at different cell stages of preimplantation development (2-cell, 16-cell and morulla). a. HK, b. PFK, c. LDH, d. PDH, e. G6PDH, f. GLUT1. Bars on respective columns represent standard error of mean. The first superscript indicates level of difference between different cell stages within a particular experimental group and the second superscript indicates difference for the same cell stage between different experimental groups. Columns with different superscripts indicate significant difference (p < 0.05)
Discussion
We have observed that developmental competence of buffalo oocytes and embryos cultured under reduced oxygen level (higher glucose) was significantly higher than normal oxygen level. The expression levels of glucose metabolism genes were less under the reduced oxygen level (5 % O2) as compared to atmospheric oxygen level, 20 % O2. Glycolytic genes were found to be maintained at low level under 5 % O2 than 20 % O2 during early embryonic development. It had been reported earlier that glucose is metabolized more through the glycolytic pathway than oxidative phosphorylation (OXPHOS) during in vivo embryonic development in pig [21]. Oocytes matured in hypoxic conditions showed less metabolic activity, more closely resembling in vivo maturation and hence improved development competence [22]. In ruminant animals the embryos produced under 20 % O2 differ in metabolism from in vivo embryos which included higher aerobic glycolysis, more lactate production and higher lactate oxidation [23, 24]. This may be a special requirement for fast proliferating growing embryos; something similar observed with cancer cells [25].
Culturing oocytes and embryos at below ambient O2 levels have been shown to be favorable for embryo development in various mammalian species including human [2, 26, 27]. Moreover the glucose level has been found to be an important factor under different oxygen concentrations in the culture environment affecting oocyte and embryo development in vitro [5]. Glucose was not considered essential during early embryonic development, however high glucose concentration proved to be detrimental to development under normal oxygen level [5, 28]. But studies have shown that high glucose is more effective for oocyte maturation and further embryonic development [5, 12]. Our results indicated that 20 mM glucose at 5 % O2 was better compared to 5.6 mM glucose for in vitro maturation of buffalo oocytes and development of resultant embryos. However, some studies have failed to demonstrate the beneficial effect of low O2 on the development of pre-implantation embryos in-vitro [23, 29]. The reason for such contradictions could be differences in basic culture systems used by different workers while manipulating the O2 levels as interplay between culture systems and O2 concentrations is a crucial factor [30]. In the current study we used a chemically defined mSOF medium throughout the IVF protocol.
In the present study expression levels of PFK and PDH indicated that the oocytes prefer an anaerobic glycolytic pathway of glucose metabolism to OXPHOS under 5 % O2 (Fig. 1), which is thought to be the preferred pathway for embryos. OXPHOS is thought to be a major source of ROS production as inhibiting OXPHOS resulted in reduced ROS and improved embryo development in bovine and porcine [31, 32]. Low oxygen and high glucose have been shown to increase development competence of bovine oocytes also by decreasing ROS and increasing ATP production via glycolysis [33]. The high expression of PDH under 20 % O2 (Fig. 2d) indicated that oocytes under high oxygen environment had higher OXPHOS activity affecting the developmental rate. The ‘Quiet Metabolism’ theory proposed for embryo development under in vivo conditions is thought to be an adaptation of the embryos to reduce ROS production through aerobic respiration [34, 35]. Reduced ROS level might help to reduce DNA damage and avoid aneuploidy that had been a major problem faced during human IVF [36, 37]. NADPH helps in counteracting the ROS by production of glutathione and thereby overcoming the deleterious effect of ROS [38, 39]. Lower expression of G6PDH under 20 % O2 in buffalo oocytes/embryos (Fig. 2e) indicated that the PPP shunt would be less active in embryos produced under this condition, which might have resulted in the decreased NADPH production unable to combat ROS production by higher OXPHOS. Also a higher expression of LDH in the embryos produced under 20 % O2 (Fig. 2c) was indicative of the stress conditions. High lactate production has been reported earlier as a result of stress response of the embryos under suboptimal culture conditions [40]. In the present study change in the culture environment from 20 to 5 % O2 resulted in drastic reduction in LDH expression both in oocytes and embryos (Figs. 1 and 2c), which correlated with reduced lactate production in other species under reduced oxygen level resembling a more in vivo situation [4].
We observed that embryos had high expression of OXPHOS genes under 20 % O2which shifted to glycolytic genes under 5 % O2 during later stages of embryonic development. OXPHOS is inhibited by low oxygen tension, and accordingly ATP production shifts from the TCA cycle to glycolysis [6, 7]. The decrease in ATP production due to decreased OXPHOS is compensated by an increase in expression of glucose transporter 1 and glycolytic enzymes [8, 9, 41]. Probably the high GLUT1 expression under 5 % O2 compensated for increased demand of glucose by embryos post maternal to zygotic transition (MZT) due to increased rate of cell proliferation. Studies in other species have also observed increased GLUT1 expression under reduced oxygen level in oocytes/embryos [42, 43]. Interestingly, increase in GLUT1 expression between 2-cell to 16-cell embryos (under 5 % O2) overlaps with the recently reported EGA event in buffalo embryos from our laboratory [17]. Facilitated glucose transporters (viz. GLUT1, 2, 3 and 8) have been identified to be responsible for glucose uptake in oocytes/early embryonic development. GLUT1 is expressed throughout preimplantation development [44], GLUT2 from 8-cell stage onward [45], while GLUT3 and GLUT8 (Insulin stimulated glucose transport) is restricted to morulla and blastocyst stage [46, 47].
We conclude that reduced oxygen level along with high glucose supplementation could improve the development rate of buffalo oocytes and embryos. Buffalo oocytes and embryos rely more on glucose under reduced oxygen level fulfilling the energy and anabolic demand of fast proliferating early embryos, a phenomenon quite similar to ‘Warburg Effect’ [25]. Further expression pattern of metabolism genes (such as G6PDH and LDH) correlated well with development competence of oocytes/embryos.
Acknowledgments
Critical inputs of Dr.R.K. Sharma, PS, Biochemistry Div, NDRI for interpreting the data, of Mr. Gian Singh, Technical Officer, NDRI computer section for statistical analysis and fund received from NAIP C-1056 and NAE projects of ICAR to the corresponding author are thankfully acknowledged.
Footnotes
Capsule
The expression pattern of glucose metabolism genes was found to be correlated with improved development of buffalo oocytes / embryos under low oxygen condition.
Contributor Information
Parveen Kumar, Email: meparveen@gmail.com.
Arpana Verma, Email: arpanandri@gmail.com.
Manish Kumar, Email: manishltu@gmail.com.
Sachinandan De, Email: sachinandan@gmail.com.
Rakesh Kumar, Email: rakeshcift@gmail.com.
Tirtha Kumar Datta, Phone: +91 184 2259506, Email: tirthadatta@gmail.com.
References
- 1.Bavister B. Oxygen concentration and preimplantation development. Reprod Biomed Online. 2004;9:484–486. doi: 10.1016/S1472-6483(10)61630-6. [DOI] [PubMed] [Google Scholar]
- 2.Van Soom A, Yuan YQ, Peelman LJ, de Matos DG, Dewulf J, Laevens H, et al. Prevalence of apoptosis and inner cell allocation in bovine embryos cultured under different oxygen tensions with or without cysteine addition. Theriogenology. 2002;57:1453–1465. doi: 10.1016/S0093-691X(01)00726-9. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62:1186–1197. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Du ZF, Wales RG. Glycolysis and glucose oxidation by the sheep conceptus at different oxygen concentrations. Reprod Fertil Dev. 1993;5:383–393. doi: 10.1071/RD9930383. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol Reprod Dev. 2000;57:353–360. doi: 10.1002/1098-2795(200012)57:4<353::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Czyzyk-Krzeska MF. Molecular aspects of oxygen sensing in physiological adaptation to hypoxia. Respir Physiol. 1997;100:99–111. doi: 10.1016/S0034-5687(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 7.Wengner RH, Gassman M. Oxygen and the hypoxia inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 8.Ebert BL, Firth JF, Ratcliffe PJ. Hypoxia and mitochondria inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J Biol Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.29.17299. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia inducible factor-1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 10.Khurana NK, Wales RG. Effects of oxygen concentration on the metabolism of [U–14C]glucose by mouse morulae and early blastocysts in vitro. Reprod Fertil Dev. 1989;1:99–106. doi: 10.1071/RD9890099. [DOI] [PubMed] [Google Scholar]
- 11.Hooper K, Lane M, Gardner D. Reduced oxygen concentration increases mouse embryo development and oxidative metabolism. Theriogenology. 2001;55:334–340. [Google Scholar]
- 12.Sandt JJ, Schroeder AC, Eppig JJ. Culture media for mouse oocyte maturation affect subsequent embryonic development. Mol Reprod Dev. 1990;25:164–171. doi: 10.1002/mrd.1080250209. [DOI] [PubMed] [Google Scholar]
- 13.Pinyopummintr T, Bavister BD. Optimum gas atmosphere for in vitro maturation and in vitro fertilization of bovine oocytes. Theriogenology. 1995;44:471–477. doi: 10.1016/0093-691X(95)00219-X. [DOI] [PubMed] [Google Scholar]
- 14.Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86:1252–1265. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Suresh KP, Nandi S, Mondal S. Factors affecting laboratory production of buffalo embryos: a meta-analysis. Theriogenology. 2009;72:978–985. doi: 10.1016/j.theriogenology.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, Verma A, Roy B, Rajput S, Ojha S, Anand S, et al. Effect of varying glucose concentrations during in vitro maturation and embryo culture on efficiency of in vitro embryo production in buffalo. Reprod Domest Anim. 2011;47:269–273. doi: 10.1111/j.1439-0531.2011.01849.x. [DOI] [PubMed] [Google Scholar]
- 17.Verma A, Kumar P, Rajput S, Roy B, De S, Datta TK. Embryonic genome activation events in buffalo (Bubalus bubalis) preimplantation embryos. Mol Reprod Dev. 2012;79:321–328. doi: 10.1002/mrd.22027. [DOI] [PubMed] [Google Scholar]
- 18.Datta TK, Goswami SL. Time dynamics and chronology of meiotic progression of buffalo (Bubalus bubalis) oocytes during in vitro maturation. Buffalo J. 1999;1:53–60. [Google Scholar]
- 19.Kumar P, Yadav P, Verma A, Singh D, De S, Datta TK. Identification of stable reference genes for gene expression studies using quantitative real time PCR in buffalo oocytes and embryos. Reprod Domest Anim. 2012;47:e88–e91. doi: 10.1111/j.1439-0531.2012.01998.x. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Swain JE, Bormann CL, Clark SG, Walters EM, Wheeler MB, Krisher RL. Use of energy substrates by various stages preimplantation pig embryos produced in vivo and in vitro. Reproduction. 2002;123:253–260. doi: 10.1530/rep.0.1230253. [DOI] [PubMed] [Google Scholar]
- 22.Preis KA, Seidel GE, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev. 2007;74:893–903. doi: 10.1002/mrd.20655. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JG. Comparison between in vivo-derived and in-vitro-produced pre-elongation embryos from domestic ruminants. Reprod Fertil Dev. 1997;9:341–354. doi: 10.1071/R96079. [DOI] [PubMed] [Google Scholar]
- 24.Khurana NK, Niemann H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo. Biol Reprod. 2000;62:847–856. doi: 10.1095/biolreprod62.4.847. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 26.Farrell PB, Foote RH. Beneficial effects of culturing rabbit zygotes to blastocyst in 5 % oxygen and 10 % carbon dioxide. J Reprod Fertil. 1995;103:127–130. doi: 10.1530/jrf.0.1030127. [DOI] [PubMed] [Google Scholar]
- 27.Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil Steril. 2009;91:1459–1461. doi: 10.1016/j.fertnstert.2008.07.1707. [DOI] [PubMed] [Google Scholar]
- 28.Iwata H, Akamatsu S, Minami N, Yamada M. Effects of antioxidants on the development of bovine IVM/IVF embryos in various concentrations of glucose. Theriogenology. 1998;50:365–375. doi: 10.1016/S0093-691X(98)00146-0. [DOI] [PubMed] [Google Scholar]
- 29.Marques MG, de Barros FR, Goissis MD, Cavalcanti PV, Viana CH, Assumpcao ME, et al. Effect of low oxygen tension atmosphere and maturation media supplementation on nuclear maturation, cortical granules migration and sperm penetration in swine in vitro fertilization. Reprod Domest Anim. 2012;47:491–507. doi: 10.1111/j.1439-0531.2011.01909.x. [DOI] [PubMed] [Google Scholar]
- 30.Voelkel SA, Hu YX. Effect of gas atmosphere on the development of one-cell bovine embryos in two culture systems. Theriogenology. 1992;37:1117–1131. doi: 10.1016/0093-691X(92)90109-5. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118:47–55. doi: 10.1530/reprod/118.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Machaty Z, Thompson JG, Abeydeera LR, Day BN, Prather RS. Inhibitors of mitochondrial ATP production at the time of compaction improve development of in vitro produced porcine embryos. Mol Reprod Dev. 2001;58:39–44. doi: 10.1002/1098-2795(200101)58:1<39::AID-MRD6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto S, Minami N, Yamada M, Imai H. An excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev. 2000;56:520–526. doi: 10.1002/1098-2795(200008)56:4<520::AID-MRD10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Leese HJ, Baumann CG, Brison D, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14:667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturmey RG, Hawkhead J, Barker EA, Leese HJ. DNA damage and metabolic activity in the preimplantation embryo. Hum Reprod. 2009;24:81–91. doi: 10.1093/humrep/den346. [DOI] [PubMed] [Google Scholar]
- 36.Kroener L, Ambartsumyan G, Jones CB, Dumesic D, Surrey M, Munne S, et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril. 2012;98:876–880. doi: 10.1016/j.fertnstert.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Scott RT, Jr, Upham K, Forman E, Hong K, Scott K, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilitzation implantation and delivery rates; a randomized controlled trial. Fertil Steril. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Brad AM, Bormann CL, Swain JE, Durkin RE, Johnson AE, Clifford AL, et al. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Mol Reprod Dev. 2003;64:492–498. doi: 10.1002/mrd.10254. [DOI] [PubMed] [Google Scholar]
- 39.Herrick JR, Brad AM, Krisher RL, Pope WF. Intracellular adenosine triphosphate and glutathione concentrations in oocytes from first estrous, multi-estrous, and testosterone-treated gilts. Anim Reprod Sci. 2003;78:123–131. doi: 10.1016/S0378-4320(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 40.Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/ blastocyst formation of bovine embryos. Theriogenology. 2000;54:741–756. doi: 10.1016/S0093-691X(00)00387-3. [DOI] [PubMed] [Google Scholar]
- 41.Kind KL, Collett RA, Harvey AJ, Thompson JG. Oxygen-regulated expression of GLUT-1, GLUT-3, and VEGF in the mouse blastocyst. Mol Reprod Dev. 2005;70:37–44. doi: 10.1002/mrd.20183. [DOI] [PubMed] [Google Scholar]
- 42.Bermejo-Álvarez P, Lonergan P, Rizos D, Gutiérrez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod BioMed Online. 2010;20:341–349. doi: 10.1016/j.rbmo.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71:1108–1119. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- 44.Morita Y, Osamu T, Iwao H. Expression and possible function of glucose transporter protein GLUT-1 during preimplantation mouse development from oocytes to blastocysts. Biochem Biophys Res Commun. 1992;188:8–15. doi: 10.1016/0006-291X(92)92342-U. [DOI] [PubMed] [Google Scholar]
- 45.Hogan A, Heyner S, Charron MJ, Copeland NG, Gilbert DJ, Jekins NA, et al. Glucose transporter gene expression in early mouse embryos. Development. 1991;113:363–372. doi: 10.1242/dev.113.1.363. [DOI] [PubMed] [Google Scholar]
- 46.Pantaleon M, Harvey MB, Pascoe WS. Glucose transporter GLUT3: ontogeny, targeting, and role in mouse blastocyst. Proc Natl Acad Sci U S A. 1997;94:3795–3800. doi: 10.1073/pnas.94.8.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carayannopoulos MO, Chi MM, Cui Y. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–7318. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]